Abstract
Background
There is substantial interest in identifying interventions that can protect and buffer older adults from atrophy in the cortex and particularly, the hippocampus, a region important to memory. We report the two-year effects of a randomized-controlled trial of an intergenerational social health promotion program on older men's and women's brain volumes.
Methods
The Brain Health Study simultaneously enrolled, evaluated and randomized 111 men and women (58 intervention; 53 control) within the Baltimore Experience Corps Trial to evaluate intervention impact on biomarkers of brain health at baseline and annual follow-ups during the two-year trial exposure.
Results
Intention-to-treat analyses on cortical and hippocampal volumes for full and sex-stratified samples revealed program-specific increases in volumes that reached significance in men only (p's≤0.04). Whereas men in the control arm exhibited age-related declines over two years, men in the Experience Corps arm showed a 0.7-1.6% increase in brain volumes. Women also exhibited modest intervention-specific gains of 0.3-0.54% by the second year of exposure that contrasted with declines of about 1% among women in the control group.
Conclusions
These findings showed that purposeful activity embedded within a social health promotion program halted and, in men, reversed declines in brain volume in regions vulnerable to dementia.
Keywords: randomized, controlled trial, brain aging, social activity, cognitive activity, physical activity, brain aging, cortical volume, hippocampus, MRI
1. Introduction
Research over the last decade has shown that increased physical activity impacts cognitive aging and dementia risk [1]. Although the mechanisms related to the benefits of physical activity remain to be understood [2], evidence for physical exercise, particularly in enriched environments, suggests that exercise alters the functional and structural properties of the hippocampus, a brain region critical to learning and memory throughout the life course [2, 3], vulnerable to aging [4, 5] and implicated in risk for dementia [6]. In older adults, smaller and shrinking hippocampal volumes have been correlated with decline in memory performance and with increased risk for Alzheimer's disease (AD) [7-9]. Studies have reported an inverse association between self-reported physical activity and age-related decreases in mediotemporal lobe volume [10] and with reduced declines in hippocampal volume over nine years [11]. In a one-year, randomized, controlled trial (RCT) of exercise in healthy older adults, walking led to a two percent increase in hippocampal volume relative to a one percent decrease in volume in the stretching and toning control group [12]. However, in comparison to exercise studies, neuroimaging studies examining how physical activity in the daily lives of older adults impacts brain volume have been limited [11, 13, 14].
Likewise, although a wealth of evidence shows that cognitive and social [15-17] activities reduce rates of cognitive decline and risk for dementia, similar evidence for their impact on rates of brain atrophy has been largely inferred from studies in younger adults [18], aging animal models [19-21], and indirectly from cognitive training interventions [22, 23]. Animal studies of aging provide the most direct evidence that engagement in physical, cognitive and social activity may yield measurable benefits in the hippocampus. A number of randomized, controlled trials have emerged, integrating the findings above to examine whether multi-modal, physical, cognitive and social activity impacts cognition [24-26], but we are aware of no published large-scale, randomized, controlled trials examining the impact of multi-modal, real-world activity on brain volume and rates of age-related brain atrophy.
Experience Corps® (EC) represents one such model of multi-modal activity embedded within a social health promotion model of high-intensity volunteer service. EC is a community-based program in which older adults volunteer in public schools to improve the academic performance of children in underserved urban areas by harnessing retired adults' time, skills, and wisdom to volunteer in teams in neighborhood elementary schools as mentors of children in grades Kindergarten through third grade for 15 hours a week over an academic year [27-32]. We demonstrated that, through service, volunteers showed increases in physical, cognitive, and social activities[28], suggesting the possibility that it might be a real world example of how increases in these activities might lead to better cognitive health and reduced risk for dementia[33]. In a pilot trial, we therefore compared participants in the EC program to wait-list controls and found short-term gains in a component of executive functioning and memory, particularly among those with poor executive functioning at baseline [34]. A second, smaller functional magnetic resonance imaging (fMRI) study [35] in women extended these findings and showed increases in neural activity in prefrontal regions that support executive functions in EC participants vs. controls. These pilot results suggested the potential of real world, activity-based interventions for increasing plasticity in age-vulnerable regions of the brain in older persons [35].
The pilot findings, above, were among the data that served as the basis for a large-scale randomized, controlled trial (RCT) entitled the Baltimore Experience Corps Trial (BECT) [32] and a simultaneous nested Brain Health Substudy (BHS). The BHS was designed to extend upon results observed in pilot studies of primarily [34], and solely [35], female volunteer samples by recruiting a sufficient number of men to allow for formal evaluation of mechanistic benefits of volunteer service to men and women [36, 37], given sex differences in brain morphology and risk for age-related neuropathologies, including AD [38-40] and vascular dementia [41], and given sex differences in the association between exercise and neurocognition [9].
Here, we evaluated the effectiveness of two years of exposure to Experience Corps, a productive social engagement intervention, on brain volume in a subset of participants randomized to the BHS within the BECT. The BECT represents an effort to design meaningful, socially valuable roles for older adults that will: 1) attract and engage a larger proportion of older adults; 2) demonstrate the benefits of an aging society by helping a younger generation (i.e., improving elementary school education), and; 3) serve as a vehicle for enhancing physical, cognitive, and social activity in sociodemographically diverse populations [28] to thereby promote neurocognitive health among those at greatest risk for health disparities [42][28]. Here, we evaluated whether the Experience Corps program mitigated, or even reversed, rates of age-related brain atrophy, as measured by cortical and hippocampal volumes.
2. Methods
2. 1. Participants
The BECT is a sex-stratified randomized-controlled trial designed to evaluate the effectiveness of the Experience Corps® (EC) high-intensity, senior service volunteer program on the health of older adults, the school's climate, and children. The trial is described in greater detail elsewhere [32]. Briefly, the EC Baltimore program in elementary schools is designed to support both the academic success of children in grades kindergarten through third grade and to promote the health of older volunteers by increasing their physical, social, and cognitive activity. The nested BHS simultaneously recruited eligible participants prior to randomization to evaluate the benefits of the program on their brain and biological health. From January 2006 to December 2009, 702 eligible participants were randomized to either the EC program or referred to a low-activity control. Simultaneously, 123 individuals were enrolled in the BHS. Eligibility criteria included: 1) being 60 years of age or older; 2) English speaking; 3) clearance on the criminal background check; 3) minimum sixth grade reading level on the Wide Range Achievement Test [43], and; 4) Mini-Mental State Examination (MMSE) score ≥ 24 [32, 44]. Additional criteria for enrollment in the BHS have been described previously [35] and included: 1) right-hand dominance; 2) being free of a pacemaker or other ferrous metals in the body, and; 3) no history of brain cancer, brain aneurism or stroke in the prior year. Self-reported health was assessed on a scale of 1 (Excellent) to 5 (Poor) and participants reported if their doctor told them they had hypertension or diabetes. As noted above, the BHS stratified randomization by sex to allow for stratified comparisons.
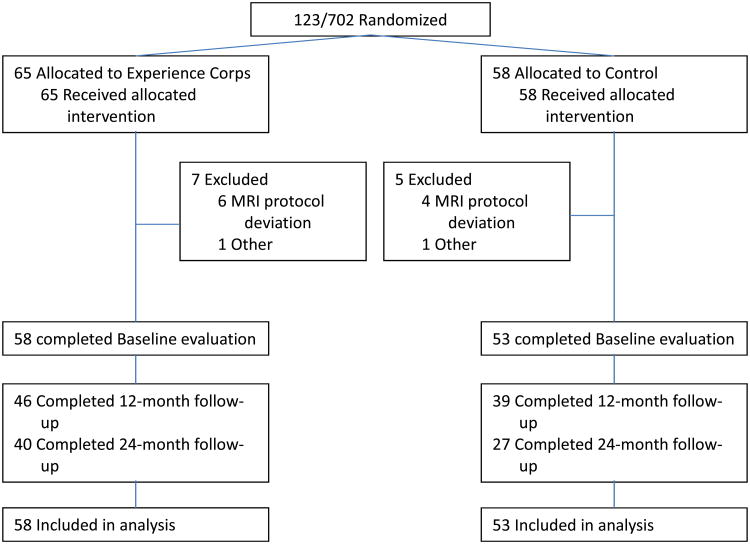
During BECT randomization, 123 participants were invited to enroll in the nested BHS, as detailed in the CONSORT Figure 1. The final baseline sample included 111 participants. Excluded participants did not vary significantly from included participants in age, MMSE, sex or education (p's>0.05). Additionally, BHS participants did not differ significantly from participants in the larger BECT in age, MMSE, self-reported health, and education (p's>0.05). BHS study visits were conducted at baseline, 12- and 24- month follow-ups in conjunction with the BECT. The study was approved by the Johns Hopkins institutional Review Board and all participants provided written, informed consent.
Figure 1.
CONSORT diagram summarizing Baltimore Experience Corps Trial participants' randomization into the Brain Health Substudy.
Intervention
Experience Corps volunteers received five days (30 hours) of formal training conducted by the staff of the Greater Homewood Community Corporation (GHCC), the community-based partner in trial and program implementation. Training includes lecture, discussion, exercises, role plays, and handouts designed to provide necessary skills in a) orientation to the school environment; b) working with today's children, and their needs; c) overview of roles for volunteers in the school; d) basic skills necessary to perform roles and e) what they can and cannot do as EC volunteers in schools (i.e., they do not run a class, either with or in the absence of a teacher, and their roles are to meet major unmet needs for children's success). A secondary purpose of training is to promote a sense of community among volunteers, assign them into teams who will work in a school together, and to train them in teamwork. Training culminates in a formal graduation ceremony attended by local dignitaries. Those randomized to the wait-list control arm were referred to the Baltimore City Commission on Aging and Retirement Education (CARE) for other low-activity volunteer opportunities in Baltimore City; these were selected to be of short duration and of low time demand, such as volunteering at health fairs, city festivals, and senior center events. Controls were wait-listed for participation in EC after two years, if interested.
2. 2. Brain magnetic resonance imaging (MRI) acquisition
MRI scans were collected at baseline and at two subsequent annual visits (12 and 24 month follow-ups). All high-resolution T1-weighted brain MRI data were collected on a 3.0T Phillips scanner (Best, the Netherlands) using MPRAGE with TR= 8 ms, TE= 3.6 ms, matrix size = 256 × 256, and the field of view (FOV) = 256 × 256 mm. Two hundred slices were contiguous with slice thickness of 1 mm. All images were processed using Free Surfer version 5.1.0 [45], a program which allows for longitudinal analysis and offers good test-retest reliability [46, 47]. Details of the procedures are published elsewhere [48, 49]. Pre-processing included motion correction, non-uniform intensity normalization and automated removal of non-brain tissue. All processed images were visually inspected and manually corrected, as needed, by blinded reviewers. Inter-rater reliability for images requiring manual correction was high (Inter-class correlations > 0.95) for cortical volume, or the sum of cortical white and gray matter volumes, and bilateral hippocampal volumes. Both measures were adjusted for intra-cranial volume (ICV) and reported in mm3.
To assess changes in brain volumes, we used Free Surfer [46] to incorporate within-subject correlations across observations. All time points were first processed cross-sectionally, as described above. Then, an unbiased template image [50] was created from three time points using robust, inverse consistent registration [48]. This template image served as an initial estimate for the segmentation and surface reconstruction and all follow-up measurements were registered to this template to ensure unbiased analysis, thereby reducing variability and increasing the stability of all longitudinal analyses [46].
2. 3. Memory measure
Verbal memory was measured by the Rey Auditory Verbal Learning Test (RAVLT) [51]. The RAVLT is widely used to measure memory in older adults [52] with good validity and reliability [53, 54]. The RAVLT is comprised of a list-learning test, an interference test and recall tests. Participants were read and recalled words from a 15-word list, five consecutive times. The target list was followed by one learning trial of an interference list and then recall of the original target list (short delay).
2. 4. Statistical Analysis
At baseline, intervention and control groups were comparable in age, MMSE, years of education, self-reported health, and MRI volumes (Table 1) (p's>0.05). Men and women were comparable on the variables, above. Women in the EC program tended to report more hours (615) than men in the program (459.4) but this group difference was not significant (p>0.10), due in part, to variation. The primary MRI outcomes were cortical volume and hippocampal volume. Total hippocampal volume was obtained by summing volumes for the left and right hemispheres.
Table 1.
Baseline characteristics of Brain Health Study participants by intervention status.
| Characteristics | Intervention (n=58) Mean (SD) | Control (n=53) Mean (SD) | Total (n=111) Mean (SD) |
|---|---|---|---|
| Age | 67.7 (6.2) | 66.7 (5.9) | 67.2 (6.1) |
| Sex (% male) | 27.6 | 30.2 | 28.8 |
| MMSE score (max=30) | 28.4 (1.4) | 28.3 (1.5) | 28.3 (1.5) |
| RAVLT short delay (max=15) | 6.6 (2.9) | 6.5 (2.6) | 6.6 (2.7) |
| Ethnic group (%) | |||
| African-American and Other | 91.4 | 96.2 | 93.7 |
| White | 8.6 | 3.8 | 6.3 |
| Education (years) | 14.1 (2.9) | 13.5 (2.6) | 13.8 (2.8) |
| Self-reported Health (1= Excellent; 5=Poor) | 2.4 (0.8) | 2.5 (0.9) | 2.5 (0.8) |
| Total Exposure Hours | 562.8 (466.5) | 0 | |
| Brain volumes (adjusted for ICV) | |||
| Cortical Volume (mm3) | 937214.9 | 929501.9 | 933532.13 |
| Hippocampal Volume (mm3) | 6636.2 | 6563.8 | 6601.63 |
Abbreviations: MMSE, Mini-Mental State Exam; RAVLT, Rey Auditory Verbal Learning Test, ICV, inter-cranial volume; SD, standard deviation
There were no significant differences between groups (at p<0.05), with the exception of exposure.
All analyses were performed using an intention-to-treat (ITT) design. Mixed effects models with random intercept were adjusted for covariates, ICV, age, centered at the mean age of 67 years, and education, given previous evidence that these variables have strong associations with brain and cognitive aging. Time of follow-up and intervention status (EC vs. Control) were treated as categorical variables, with time equal to zero at baseline and controls as the reference group. All analyses were completed for the full and sex-stratified samples. Volume estimates at 12-and 24- month follow-ups were computed with least squares means, and percent change in volumes at 12 and 24 month follow-ups between intervention and control groups were calculated from these least square means. To examine whether intervention-specific changes in brain volume were correlated with behavioral change in memory over 24 months of exposure, we calculated 24-month difference scores for brain volumes and RAVLT short delay recall. Linear scatterplots and Pearson correlations were conducted stratifying by intervention status. All analyses were conducted in SAS version 9.3 and intervention results were generated using MIXED procedure (SAS Institute, Inc, Cary, NC).
3. Results
Participant characteristics are presented in Table 1. Of the 111 participants included in this analysis, 58 were randomized to the EC intervention group and 53 to the control group. In aggregate, participants were about 67.2 years of age, 72.2% female, 93% African American, reported good health, and had 13.8 years of education (= some college). Eight EC and 12 Control participants did not contribute follow-up data for various reasons, including: failing a criminal background check, death, moving out of state, dropping out of the trial, health, and poor MRI data quality. These participants were not significantly different at baseline from those retained in mean age, sex, racial distribution, MMSE, education, or brain volumes (p's >0.05).
At baseline, older age was significantly associated with smaller cortical volume, as expected. Specifically, each additional year of age was associated with 140704 mm3 smaller cortical volume (p<0.001). A similar relationship was observed in the hippocampus where each additional year of age was associated with a 4583 mm3 reduction in volume (p<0.0001). Education was not associated with baseline brain volumes (p's> 0.15).
3. 1. Intervention Effects
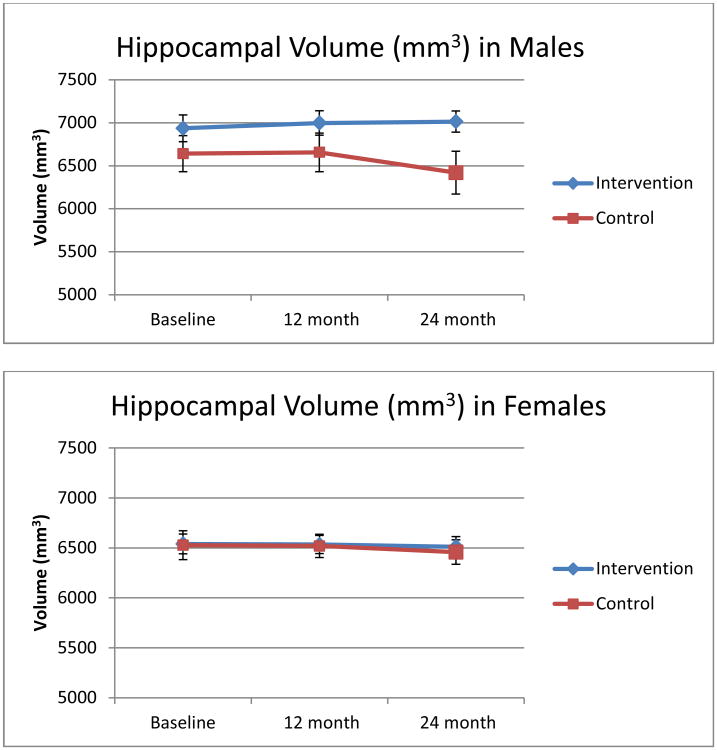
Effects of time at 12- and 24- month follow-ups were modeled relative to reference baseline volumes in full and sex stratified models. ITT analyses in the full sample revealed no significant intervention effects for either cortical or hippocampal volumes at either 12- or 24- month follow-ups (p's>0.10; data not shown) with an effect size of 0.54 (based on a 2-tailed test with 80% power). The effect size of 0.54 signifies that the mean regional brain volume of the intervention group was greater than that of about 70% of those in the control group. In analyses stratified by sex, shown in Table 2, men showed intervention-specific gains in cortical (0.67%) and hippocampal (1.56%) volumes by 24 months whereas male controls showed expected age- related declines of -0.14- 3.52%. Both differences were significant (p's=0.04) with effect sizes over 1.0. Residualized mean hippocampal volumes by intervention status are graphed in Figure 2. a. In women (Figure 2. b), intervention-related gains emerged at 24 months, but did not reach statistical significance (p>0.10) with effect sizes of 0.64-0.66. Specifically, by 24 months, women in the intervention group showed marginally less age-related declines (0.29-0.54%) than female controls (1.09-1.19%).
Table 2.
Mean percent change in brain volumes over 12 and 24 month intervals by intervention status. All models were adjusted for age (centered at 67), education, and intra-cranial volume (ICV).
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Percent change in brain volume | Control | Intervention | Control | Intervention | ||||
| 12 months-Baseline | 24 months-Baseline | 12 months-Baseline | 24 months-Baseline | 12 months-Baseline | 24 months-Baseline | 12 months-Baseline | 24 months-Baseline | |
| Cortex | 0.20% | -0.14% | -0.55% | 0.67%* | -0.37% | -1.19% | 0.02% | -0.54% |
| Hippocampus | 0.34% | -3.52% | 0.77% | 1.56%* | -0.11% | -1.09% | -0.25% | -0.29% |
Differences significant at p<0.05 in models.
Figure 2.
Annual changes over 24 months in total hippocampal volume for (a) men and (b) women by intervention status.
3. 2. Correlating Intervention-specific Changes in Cortical and Hippocampal Volumes with Intervention-specific Changes in Memory
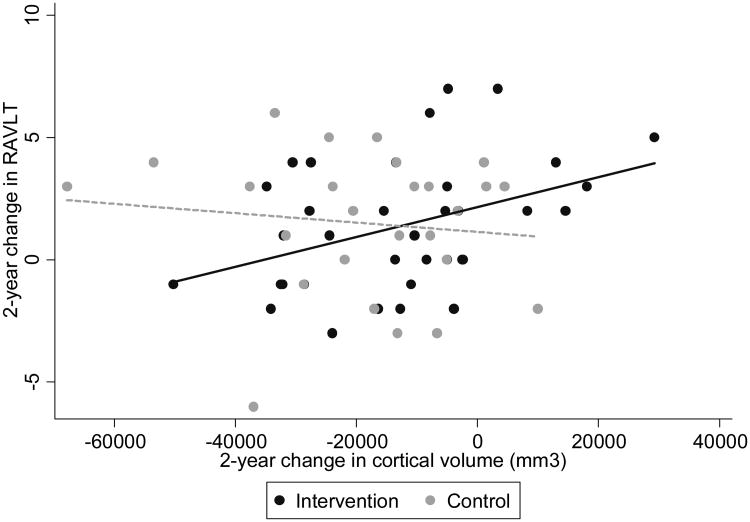
Using linear scatterplots, we examined whether intervention-specific differences from baseline to 24 months in cortical and hippocampal volumes were correlated with intervention-specific differences in short-delay recall on the RAVLT over the same period. For the Intervention group only, the 24-month difference in recall was positively correlated with the 24-month difference in cortical volume (Figure 3; r= 0.39; p=0.02). A positive correlation was also observed for hippocampal volume but did not reach significance (p > 0.10; data not presented) due, in part, to this region's small size and the corresponding small annual rates of decline expected over two years among non-demented older adults [55].
Figure 3.
Correlation between 24-month difference in RAVLT short delay and 24-month difference in cortical volume by Intervention status.
4. Discussion
Results from the BHS revealed program-specific increases in cortical and hippocampal volumes by the second year that were greater in men than in women. These results suggest that multi-modal activity embedded within a social health promotion program forestalled and possibly reversed age-related declines in annual rates of atrophy that typically range from 0.8- 2.0 percent [55, 56]. Whereas men in the control arm exhibited these expected declines over two years, men in the Experience Corps arm showed a 0.7-1.6 percent increase in brain volumes. This two-year gain corresponds to an approximate three-year reversal in brain aging. Women in Experience Corps also exhibited modest gains of 0.3-0.54 percent by the second year of exposure that contrasted with approximately 1.15 percent declines among women in the control group. In both men and women in the Experience Corps arm, two-year changes in cortical volume were positively correlated with two-year improvements in memory. These results were achieved in a sample of older adults that reflected the Baltimore City community, including minorities and a variety of socioeconomic and educational backgrounds. Together, these findings are the first from a randomized, controlled trial of a multi-modal activity intervention to show age-related brain plasticity, providing support for the brain reserve hypothesis [57]. They suggest that those men randomized to Experience Corps maintained brain volumes with increasing age, and even exhibit modest increases in brain volumes relative to the control group, a finding consistent with that observed for exercise.
Furthermore, these results were achieved through a community-based, social health promotion program embedded within a novel, complex and changing school environment. We hypothesize that this complexity provided a key source of the program's strengths in exercising many abilities that can directly impact markers of brain health (for review, see [17]). It is therefore possible that programs that combine physical, cognitive and social engagement, like Experience Corps, can serve to buffer age-related changes in brain and cognitive aging.
Duration of engagement beyond one year led to additional benefits in the maintenance, and even, increases in brain structure. If this intervention had concluded in under a year, as most cognitive interventions do, we would not have been able to assess the duration-dependent brain benefits. The two-year effects indicate that measurable and statistically meaningful changes in brain volume accrued over time. We speculate that intervention-related benefits may continue to accrue post exposure perhaps by changing behaviors and motives that lead to sustained increases in social, cognitive, and physical activity in daily life.
Experience Corps increased multi-modal activity through a social health promotion program that involved intergenerational transfer of wisdom and knowledge, raising the question of whether the desire to give back to a younger generation simply served as a motivator to increase and sustain volunteer activity or whether it offered more synergistic benefits related to the rewards associated with achieving these developmental goals [58]. In focus groups with program volunteers, they reported experiencing rewards through their perceived positive impact on children, a sense of renewed purpose post-retirement, social bonds formed with peers and teachers (networks), and through self discovery of their own learning and teaching potential [59]. It is therefore possible that these perceived benefits played an important role.
Older women as a group did not show the same magnitude of intervention-related gains in brain volumes as men, We consider several possible explanations, in turn. First, Experience Corps was hypothesized to promote novelty and social engagement, but may not have been as novel for women as for men given that women's roles over the life course often involve teaching and care-giving with peers and children. Second, women in the BHS were more socioeconomically disadvantaged than men (less education, lower income). Socioeconomic disadvantage is associated with greater age-related cognitive decline and impairment [60], The third reason for sex differences may be related to older women's elevated risk for physical and mobility difficulties [61] and tendency to be less physically active than older men [62, 63], particularly among African-American women, who comprised the majority of the sample. While Experience Corps has been shown to increase physical activity in older African-American women through daily transit to and from and within schools [27], this alone may not have been sufficient to engender brain benefits observed through exercise. However, cross-sectional examination suggests that even modestly greater rates of daily walking activity are associated with larger hippocampal volumes among older women independent of moderate to vigorous-intensity activity [64]. The role of daily walking activity remains to be understood for its relationship to neurocognitive health. We will evaluate the role of daily walking activity among men and women in mediating intervention-related benefits.
Study limitations are largely related to the effects of variable exposures and attrition over two years on the power to observe Experience Corps program benefits. This power restriction likely led to an underestimation of program benefits. In addition, the implementation of this real-world intervention precluded our ability to parse and measure intervention-specific effects of cognitive, social, and physical activity for their independent contributions. Future efforts will address this limitation through the use of accelerometry, GPS, and real-time survey methods. Strengths of this study include the two-year exposure period, which is longer than prior randomized trials of cognitive training and exercise, a period that allowed us to observe cumulative, duration-dependent effects on brain health. Second, the rationale and incorporation of MRI into this trial was evidence-based and cost-effective because it built upon prior pilot trial data [35], and, co-occurred simultaneous with randomization in the larger trial according to the same ITT design thereby mitigating selection bias and ensuring a representative subsample from the trial with which to examine mechanisms. Third, this trial addressed a limitation of prior studies of aging and lifestyle that are observational and thus, unable to disentangle selection bias associated with other healthful behaviors and with already high levels of cognitive ability. Fourth, direct examination of brain volume and atrophy provides useful intermediate biomarkers of program impact on cognitive aging [65] and risk for dementia that suggest longer-term benefits to Experience Corps volunteers post-exposure in delaying dementia onset and possibly, mortality risk. Finally, this mechanistic study nested within an RCT sets a precedent by examining the impact of multi-modal, real-world activity yoked to volunteer service, the success of which offers promise for the large-scale promotion of brain health in older adults, particularly among those at elevated sociodemographic risk for health disparities.
5. Conclusion
This is the first study, to our knowledge, to incorporate neuroimaging into a randomized, controlled trial to directly assess the impact of a multi-modal social engagement program on markers of brain health in older adults. We observed increases in cortical and hippocampal volumes by the second year that reached significance only in men. In women and men in the intervention group, two-year increases in cortical volume were positively correlated with improvements in memory. These results await replication and continued follow-up post exposure will allow us to examine whether programs like this can delay memory declines and risk of dementia. Broadly, these findings offer new directions for embedding healthful and generative activity into everyday life.
Research in Context.
Systematic Review
Previous observational studies have shown that physical, cognitive, and social activities are associated with reduced rates of cognitive decline and dementia in older adults. However, these studies are prone to selection bias and interventions have yet to evaluate the impact of lifestyle activities on biomarkers of brain health related to risk for dementia. This study addressed prior limitations through a randomized, controlled trial assessing the impact of volunteer service as a vehicle for enhancing physical, cognitive, and social activity in sociodemographically diverse older adults on cortical and hippocampal volumes in healthy older adults over two years.
Interpretation
Activity embedded within a social health promotion program, entitled Experience Corps, to improve academic achievement among elementary school children vs. activity solely for personal health promotion led to maintenance and, in men, increases in biomarkers of brain health implicated in risk for dementia. With ongoing exposure over two years, benefits continued to accrue.
Future Directions
Continued follow-up post exposure will allow us to examine whether social programs like Experience Corps can delay memory declines and risk of dementia. Broadly, these findings offer new directions for embedding healthful and generative activity into everyday life.
Acknowledgments
The authors would like to extend their appreciation and thanks to the BECT BHS study participants for their critical contributions to this research. Recruitment and baseline evaluation was supported by the Johns Hopkins Neurobehavioral Research Unit and a supplement to the National Institute on Aging (BSR grant P01 AG027735-03). Follow-up evaluation, data cleaning and analysis were supported by the Alzheimer's Drug Discovery Foundation. Dr. Kuo's data analysis was supported by the Johns Hopkins Epidemiology and Biostatistics of Aging Research Fellowship (NIA T32AG000247).
Footnotes
Clinical Trial Registration: NCT00380562
Author Contributions: Drs, Carlson and Kuo had full access to the data in this study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest Disclosures: No other disclosures were reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Erickson KI, Weinstein AM, Lopez OL. Physical activity, brain plasticity, and Alzheimer's disease. Arch Med Res. 2012;43(8):615–21. doi: 10.1016/j.arcmed.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci. 2013;17(10):525–44. doi: 10.1016/j.tics.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen Y, Specht SM, De Saint Ghislain I, Li R. The hippocampus: a biological model for studying learning and memory. Progress in Neurobiology. 1994;44(5):485–96. doi: 10.1016/0301-0082(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 4.Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16(3):250–60. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- 5.Shetty AK, Turner DA. Aging impairs axonal sprouting response of dentate granule cells following target loss and partial deafferentation. J Comp Neurol. 1999;414(2):238–54. doi: 10.1002/(sici)1096-9861(19991115)414:2<238::aid-cne7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 6.Henneman WJ, Sluimer JD, Barnes J, van der Flier WM, Sluimer IC, Fox NC, et al. Hippocampal atrophy rates in Alzheimer disease: added value over whole brain volume measures. Neurology. 2009;72(11):999–1007. doi: 10.1212/01.wnl.0000344568.09360.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desikan RS, Cabral HJ, Fischl B, Guttmann CR, Blacker D, Hyman BT, et al. Temporoparietal MR imaging measures of atrophy in subjects with mild cognitive impairment that predict subsequent diagnosis of Alzheimer disease. AJNR, American Journal of Neuroradiology. 2009;30(3):532–8. doi: 10.3174/ajnr.A1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerchner GA, Hess CP, Hammond-Rosenbluth KE, Xu D, Rabinovici GD, Kelley DA, et al. Hippocampal CA1 apical neuropil atrophy in mild Alzheimer's disease visualized with 7-T MRI. Neurology. 2010;(75):1381–1387. doi: 10.1212/WNL.0b013e3181f736a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kramer JH, Mungas D, Reed BR, Wetzel ME, Burnett MM, Miller BL, et al. Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology. 2007;21(4):412–8. doi: 10.1037/0894-4105.21.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bugg JM, Head D. Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiology of Aging. 2011;32(3):506–14. doi: 10.1016/j.neurobiolaging.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erickson KI, Raji CA, Lopez OL, Becker JT, Rosano C, Newman AB, et al. Physical activity predicts gray matter volume in late adulthood: the Cardiovascular Health Study. Neurology. 2010;75(16):1415–22. doi: 10.1212/WNL.0b013e3181f88359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America. 2010;108(7):3017–22. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlson M, Varma V. Activity and Neurocognitive Health in Older Adults. In: Waldstein S, Elias M, editors. Neuropsychology of Cardiovascular Disease. 2nd. Psychology Press; 2014. In Press. [Google Scholar]
- 14.Guiney H, Machado L. Benefits of regular aerobic exercise for executive functioning in healthy populations. Psychon Bull Rev. 2013;20(1):73–86. doi: 10.3758/s13423-012-0345-4. [DOI] [PubMed] [Google Scholar]
- 15.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurology. 2004;3(6):343–53. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 16.Barnes LL, Mendes de Leon CF, Wilson RS, Bienias JL, Evans DA. Social resources and cognitive decline in a population of older African Americans and whites. Neurology. 2004;63(12):2322–6. doi: 10.1212/01.wnl.0000147473.04043.b3. [DOI] [PubMed] [Google Scholar]
- 17.Bennett D, Arnold S, Valenzuela M, Brayne C, Schneider J. Cognitive and social lifestyle: links with neuropathology and cognition in late life. Acta Neuropathologica. 2014;127(1):137–150. doi: 10.1007/s00401-013-1226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Buchel C, et al. Temporal and spatial dynamics of brain structure changes during extensive learning. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2006;26(23):6314–7. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386(6624):493–5. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 20.Keuker JI, Luiten PG, Fuchs E. Preservation of hippocampal neuron numbers in aged rhesus monkeys. Neurobiol Aging. 2003;24(1):157–65. doi: 10.1016/s0197-4580(02)00062-3. [DOI] [PubMed] [Google Scholar]
- 21.Siwak-Tapp CT, Head E, Muggenburg BA, Milgram NW, Cotman CW. Region specific neuron loss in the aged canine hippocampus is reduced by enrichment. Neurobiol Aging. 2008;29(1):39–50. doi: 10.1016/j.neurobiolaging.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnes DE, Yaffe K, Belfor N, Jagust WJ, DeCarli C, Reed BR, et al. Computer-based cognitive training for mild cognitive impairment: results from a pilot randomized, controlled trial. Alzheimer Dis Assoc Disord. 2009;23(3):205–10. doi: 10.1097/WAD.0b013e31819c6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. The Journal of the American Medical Association. 2006;296(23):2805–14. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noice T, Noice H, Kramer AF. Participatory Arts for Older Adults: A Review of Benefits and Challenges. Gerontologist. 2013 doi: 10.1093/geront/gnt138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stine-Morrow EA, Parisi JM, Morrow DG, Greene J, Park DC. An engagement model of cognitive optimization through adulthood. The Journals of Gerontology Series B, Psychological Sciences and Social Sciences. 2007;62:62–9. doi: 10.1093/geronb/62.special_issue_1.62. Spec No 1. [DOI] [PubMed] [Google Scholar]
- 26.Park DC, Lodi-Smith J, Drew L, Haber S, Hebrank A, Bischof GN, et al. The impact of sustained engagement on cognitive function in older adults: the Synapse Project. Psychol Sci. 2014;25(1):103–12. doi: 10.1177/0956797613499592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freedman M, Fried L. Launching Experience Corps: Findings from a two-year pilot project mobilizing older Americans to help inner-city elementary schools. Oakland, CA: Civic Ventures; 1999. [Google Scholar]
- 28.Fried LP, Carlson MC, Freedman M, Frick KD, Glass TA, Hill J, et al. A social model for health promotion for an aging population: initial evidence on the Experience Corps model. Journal of Urban Health. 2004;81(1):64–78. doi: 10.1093/jurban/jth094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glass TA, Freedman M, Carlson MC, Hill J, Frick KD, Ialongo N, et al. Experience Corps: design of an intergenerational program to boost social capital and promote the health of an aging society. Journal of Urban Health: Bulletin of the New York Academy of Medicine. 2004;81(1):94–105. doi: 10.1093/jurban/jth096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parisi J, Stine-Marrow EAL, Noh SR, Morrow DG. Predispositional Engagement, Activity Engagement, and Cognition among Older Adults. Aging, Neuropsychology, and Cognition. 2009;16:485–504. doi: 10.1080/13825580902866653. [DOI] [PubMed] [Google Scholar]
- 31.Rebok GW, Carlson MC, Barron JS, Frick K, McGill S. Experience Corps®: A Civic Engagement-Based Public Health Intervention in the Public Schools. In: Hartman-Stein P, LaRue A, editors. Enhancing Cognitive Fitness in Adults. 1st. Springer; 2011. pp. 469–487. [Google Scholar]
- 32.Fried LP, Carlson MC, McGill S, Seeman T, Xue QL, Frick K, et al. Experience Corps: a dual trial to promote the health of older adults and children's academic success. Contemp Clin Trials. 2013;36(1):1–13. doi: 10.1016/j.cct.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Preventing Alzheimer's Disease and Cognitive Decline. NIH State of Science Conference; April 26-28, 2010; Bethesda, MD. Apr 26-28, 2010. [Google Scholar]
- 34.Carlson MC, Saczynski JS, Rebok GW, Seeman T, Glass TA, McGill S, et al. Exploring the effects of an “everyday” activity program on executive function and memory in older adults: Experience Corps. Gerontologist. 2008;48(6):793–801. doi: 10.1093/geront/48.6.793. [DOI] [PubMed] [Google Scholar]
- 35.Carlson MC, Erickson KI, Kramer AF, Voss MW, Bolea N, Mielke M, et al. Evidence for neurocognitive plasticity in at-risk older adults: the experience corps program. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2009;64(12):1275–82. doi: 10.1093/gerona/glp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chuang YF, Eldreth D, Erickson KI, Varma V, Harris G, Fried LP, et al. Cardiovascular risks and brain function: a functional magnetic resonance imaging study of executive function in older adults. Neurobiol Aging. 2013 doi: 10.1016/j.neurobiolaging.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jonnassaint C, Varma V, Chuang Y, Harris G, Yasar S, Polinder-Bos H, et al. Lower hemoglobin is associated with poorer cognitive performance and smaller brain volumes in older adults. Journal of the American Geriatrics Society. 2014 doi: 10.1111/jgs.12810. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alzheimer's Association. Alzheimer's disease facts and figures. Alzheimer's and Dementia. 2014;10(2) doi: 10.1016/j.jalz.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Lessov-Schlaggar CN, Reed T, Swan GE, Krasnow RE, DeCarli C, Marcus R, et al. Association of sex steroid hormones with brain morphology and cognition in healthy elderly men. Neurology. 2005;65(10):1591–6. doi: 10.1212/01.wnl.0000184512.08249.48. [DOI] [PubMed] [Google Scholar]
- 40.Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer's disease: assessing sex and gender differences. Clin Epidemiol. 2014;6:37–48. doi: 10.2147/CLEP.S37929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cherbuin N, Mortby ME, Janke AL, Sachdev PS, Abhayaratna WP, Anstey KJ. Blood Pressure, Brain Structure and Cognition: Opposite Associations in Men and Women. Am J Hypertens. 2014 doi: 10.1093/ajh/hpu120. [DOI] [PubMed] [Google Scholar]
- 42.Rowe JW, Kahn RL. Successful aging. Aging (Milano) 1998;10(2):142–4. [PubMed] [Google Scholar]
- 43.Wilkinson GS. Wide Range I. 1993. WRAT3. [Google Scholar]
- 44.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 45.Fischl B. FreeSurfer. NeuroImage. 2012;62(2):774–81. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402–18. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. NeuroImage. 2006;32(1):180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 48.Reuter M, Rosas HD, Fischl B. Highly Accurate Inverse Consistent Registration: A Robust Approach. NeuroImage. 2010;53(4):1181–1196. doi: 10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007;26:518–529. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- 50.Reuter M, Fischl B. Avoiding asymmetry-induced bias in longitudinal image processing. Neuroimage. 2011;57(1):19–21. doi: 10.1016/j.neuroimage.2011.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rey A. L'examne psychologique dans les cas d'encephalopathie tramatique. Archives de Psychologie. 1941;28:21. [Google Scholar]
- 52.Lezak M, Howieson D, DW L, HJ H, JS F. Neuropsychological Assessment. 5th. New York, NY: Oxford University Press; 2012. [Google Scholar]
- 53.Lezak M, Howieson D, DW L, HJ H, JS F. Neuropsychological Assessment. 4th. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 54.Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 2nd. New York, NY: Oxford University Press; 1998. [Google Scholar]
- 55.Barnes J, Bartlett JW, van de Pol LA, Loy CT, Scahill RI, Frost C, et al. A meta-analysis of hippocampal atrophy rates in Alzheimer's disease. Neurobiol Aging. 2009;30(11):1711–23. doi: 10.1016/j.neurobiolaging.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang J, Sachdev P, Lipnicki DM, Zhang H, Liu T, Zhu W, et al. A longitudinal study of brain atrophy over two years in community-dwelling older individuals. Neuroimage. 2014;86:203–11. doi: 10.1016/j.neuroimage.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 57.Sachdev PS, Valenzuela M. Brain and cognitive reserve. Am J Geriatr Psychiatry. 2009;17(3):175–8. doi: 10.1097/JGP.0b013e318196a661. [DOI] [PubMed] [Google Scholar]
- 58.Erikson E, Erikson JM, Kivnick H. Vital involvement in old age: The experience of old age in our time. London: W. W. Norton& Company, Ltd.; 1986. [Google Scholar]
- 59.Varma V, Carlson M, Parisi J, Tanner E, McGill S, Fried L, et al. Gerontologist. 2014. Experience Corps Baltimore: exploring the stressors and rewards of high-intensity civic engagement. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carlson M, Seplaki C, Seeman T. Reversing the impact of disparities in socioeconomic status over the life course on cognitive and brain aging. In: Wolfe B, Evans W, Seeman T, editors. The biological consequences of socioeconomic inequalities. Russell Sage Foundations Publications; 2012. pp. 215–247. [Google Scholar]
- 61.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Azevedo MR, Araujo CL, Reichert FF, Siqueira FV, da Silva MC, Hallal PC. Gender differences in leisure-time physical activity. Int J Public Health. 2007;52(1):8–15. doi: 10.1007/s00038-006-5062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Manini TM, Everhart JE, Patel KV, Schoeller DA, Cummings S, Mackey DC, et al. Activity energy expenditure and mobility limitation in older adults: differential associations by sex. Am J Epidemiol. 2009;169(12):1507–16. doi: 10.1093/aje/kwp069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Varma V, Chuang Y, Harris G, Tan E, Carlson M. Low-intensity daily walking activity is associated with hippocampal volume in older adults. Hippocampus. 2014 doi: 10.1002/hipo.22397. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steffener J, Brickman AM, Habeck CG, Salthouse TA, Stern Y. Cerebral blood flow and gray matter volume covariance patterns of cognition in aging. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]