Abstract
The relationship between the arterial and venous system in patients with impaired lower extremity blood flow remains poorly described. The objective of this secondary analysis of the Effectiveness of Intensive Lipid Modification Medication in Preventing the Progression on Peripheral Artery Disease Trial (ELIMIT) was to determine the association between femoral vein (FV) volumes and measures of peripheral artery disease (PAD). FV wall, lumen, and total volumes were quantified with fast spin-echo (FSE) proton density weighted (PDW) MRI scans in 79 PAD patients over 2-years. Reproducibility was excellent for FV total vessel (intra-class correlation coefficient [ICC] =0.924, confidence interval (CI) 0.910-0.935) and lumen volumes (ICC=0.893, CI 0.873-0.910). Baseline superficial femoral artery (SFA) volumes were directly associated with FV wall (r=0.46, p<0.0001), lumen (r=0.42, p=0.0001), and total volumes (r=0.46, p<0.0001). The 2-year change in maximum walking time (MWT) was inversely associated with the 24-month change in FV total volume (r=−0.45, p=0.03). In conclusion, FV volumes can be measured reliably with FSE PDW MRI and baseline SFA plaque burden is positively associated with FV volumes, whereas the 2-year change in FV volumes and leg function show an inverse relation.
Keywords: Peripheral artery disease, venous volumes, magnetic resonance imaging, atherosclerosis
Peripheral artery disease (PAD) is associated with reduced blood flow in the lower extremities and impaired lower extremity function.1-4 Hemodynamic dysfunction plays a central role in PAD as well as in venous disease.5-6 Venous disease and venous hypertension manifest as dilated veins which over time can result in chronic venous insufficiency (CVI), a condition seen more frequently in older patients and individuals with PAD.7 The relationship between PAD and the venous system remains poorly described. The purpose of this secondary analysis of the Effectiveness of Intensive Lipid Modification Medication in Preventing the Progression on Peripheral Artery Disease Trial (ELIMIT; NCT00687076) was to determine the association between femoral vein (FV) volumes and measures of PAD in patients with lifestyle-limiting intermittent claudication.8 We hypothesized that FV volumes are positively associated with markers of PAD including superficial femoral artery (SFA) volumes and the ankle-brachial index (ABI).
Methods
ELIMIT was a randomized, double blinded and double placebo-controlled trial which included 102 subjects with lifestyle-limiting claudication consistent with Fontaine Stage IIa/IIb or angiographically confirmed Trans-Atlantic Inter-Society Consensus A-C lesions in the SFA. The study details of ELIMIT have been reported previously.8 Briefly, patients were randomized to either triple lipid modification therapy with simvastatin (40 mg daily), ezetimibe (10 mg daily), and niacin (1500 mg daily) or mono-therapy with simvastatin (40 mg daily). Patients in the mono-therapy group received placebo ezetimibe and niacin. All patients were recruited between 2005 and 2008 with serial magnetic resonance imaging (MRI) scans of the lower extremities to assess SFA total vessel, lumen, and wall volumes. For this study, we have reanalyzed the MRI scans to measure FV volumes.
Scans were acquired with a 3.0T MRI scanner system (Signa Excite, GE Healthcare, Milwaukee, Wisconsin) using a unilateral phased array coil with a field of view of 8 cm (along z-axis) and 12 cm (in x and y axes; Pathway, Biomedical, Inc). The coil was centered 8 cm proximal to the patella. Fast spin echo (FSE) proton-density-weighted (PDW) scans were acquired (repetition time: 2575ms, echo time: 30 ms, flip angle: 90 degrees, slice thickness: 2 mm, in-plane pixel spacing: 0.43×0.43 mm, number of excitations of 2, echo train length of 8, matrix size: 384×224, and field of view: 22 cm). Images were acquired with the participant free breathing. Analysis was performed for the target limb that was defined as the non-intervened limb or the less symptomatic limb in patients who were not scheduled for revascularization. In case the target limb was revascularized during the study, the analysis was limited to MRI data up to and including the last imaging visit prior to the intervention.
Quality scores were analyzed for the PDW, T1-weighted (T1W), and T2-weighted (T2W) scans for 12 patients across 3 imaging visits by 1 reader during the primary analysis of ELIMIT. Scan quality was determined using a 4-point image quality scale (4 being best) using edge sharpness, amount of blurring, artifacts, and amount of noise. The quality scores of PDW scans were found to be significantly higher than those of T1W and T2W scans (3.10 versus 2.49 and 2.29, respectively) and thus they were used for the primary report on ELIMIT and for this analysis (Figure 1).
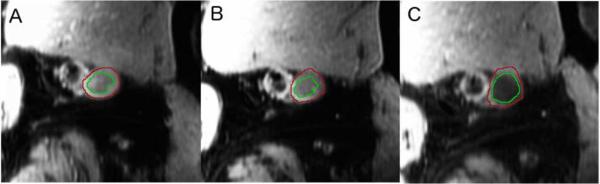
Figure 1.
Panels A-C. Cross-sectional MRI images of the femoral vein (FV) at baseline (A), 12-months (B), and 24-months (C) for a patient from the triple-therapy group.
MRI co-registration by manual identification of anatomical landmarks and acquisition of the SFA wall, lumen, and total vessel volumes were performed during the main study. For this study, reading of the FV volumes was performed by 2 readers blinded to identifiable information for each scan using VesselMASS (University of Leiden, The Netherlands). The FV was identified adjacent to the SFA and contours were drawn by freehand on the outer and inner walls of the vessel across co-registered slices with contour detection from VesselMASS. The FV total vessel, wall, and lumen volumes were then automatically calculated for each image. FV and SFA analyses were performed for the same anatomical coverage (same number of MRI slices on a per patient basis). Inter-reader variability was assessed by intra-class correlation (ICC) using a 2 way random-effects model.9
Baseline characteristics and 24-month changes in variables were expressed as mean ± standard deviation (SD). Variables were compared using the non-parametric Kruskall-Wallis rank test or parametric Student's t-test, as appropriate. Normality was checked with the Shapiro-Wilk test. Equal variances were determined with the Bartlett's test. All tests of comparison were 2-tailed with a p-value <0.05 considered statistically significant. Data from the 2 drug groups in the original study were pooled and compared to assess the relationship between venous volumes and markers of PAD. Pearson correlation coefficient regression was performed to assess the relationship between FV volumes and SFA volumes, ABI, and BMI. An ICC coefficient >0.70 was considered as excellent agreement between the 2 readers.10 All statistical analysis was performed using STATA: Stata Statistical Software: Release 12 (College Station, TX; StataCorp LP).
Results
Of the 102 patients who were randomized in ELIMIT, 87 completed the baseline visit. Eight patients opted out of baseline imaging, 6 declined other studies, and 1 withdrew from the study. Of the 87 patients with baseline MRIs, 8 did not have sufficient image quality for venous analysis. Thus, a total of 79 patients with baseline MRI images were used for the present analysis (Table 1).
Table 1.
Baseline patient characteristics
| Variable | Mono-Therapy | Triple-Therapy | P-Value |
|---|---|---|---|
| Age (years) | 64 ± 7.2 | 62 ± 7.8 | 0.16 |
| Ankle brachial index | 0.80 ± 0.22 | 0.76 ± 0.23 | 0.47 |
| Men | 40 (95%) | 34 (92%) | 0.40 |
| Body mass index (kg/m2) | 31 ± 7.3 | 31 ± 7.9 | 0.74 |
| Black | 3 (7.1%) | 10 (27%) | 0.017 |
| Smoker | 15 (36%) | 17 (46%) | 0.30 |
| Diabetes Mellitus | 17 (40%) | 15 (41%) | 0.84 |
| Hypertension (history) | 36 (86%) | 30 (81%) | 0.59 |
| Hyperlipidemia | 40 (95%) | 36 (97%) | 0.59 |
| Aspirin use | 41 (98%) | 37 (100%) | 0.32 |
| Statin use | 40 (95%) | 34 (92%) | 0.56 |
| Baseline walking pain | 17 (40%) | 16 (43%) | 0.74 |
| Baseline pain-free walking time (min) | 3.72 ± 3.22 | 2.83 ± 2.22 | 0.17 |
| Baseline FV total volume (mm3) | 126 ± 46 | 138 ± 48 | 0.26 |
| Baseline FV lumen volume (mm3) | 57 ± 27 | 64 ± 30 | 0.25 |
| Baseline FV wall volume (mm3) | 69 ± 21 | 74 ± 20 | 0.32 |
All values are presented as mean ± SD or n (%). Mono-therapy group: n=42; triple-therapy group: n=37. ABI with fewer samples. ABI mono-therapy: n=36; ABI triple-therapy: n=30. FV: femoral vein.
Inter-reader correlation was assessed for 20 PDW scans read by both readers and was found to be excellent for FV total vessel volume and FV lumen volume (Table 2). In addition, intra-reader variability was assessed by the main reader analyzing 10 PDW scans for a second time. Intra-reader variability was also found to be excellent for FV total vessel volume and FV lumen volume.
Table 2.
Inter-reader and intra-reader correlation coefficients
| Total FV | FV lumen | |||||
|---|---|---|---|---|---|---|
| N (patients) | N (slices*) | ICC | Confidence Interval (95%) | ICC | Confidence Interval (95%) | |
| Intra-reader correlation | 10 | 231 | 0.944 | 0.927 - 0.957 | 0.905 | 0.878 - 0.926 |
| Inter-reader correlation | 20 | 528 | 0.924 | 0.910 - 0.935 | 0.893 | 0.873 - 0.910 |
Number of slices differed across patients based on MRI co-registration.
ICC and confidence interval was calculated using a two-way model. FV: femoral vein. ICC: intra-class correlation.
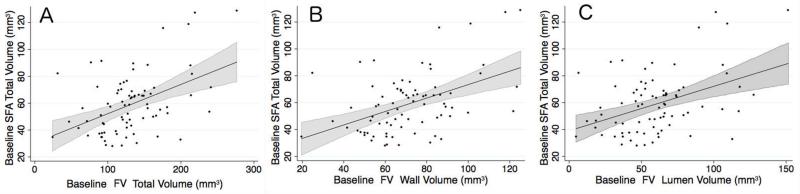
Data from both drug groups in ELIMIT were combined for pooled analyses to assess baseline association between FV volumes and parameters associated with PAD. FV volumes were correlated positively with SFA volumes (Table 3). There was a significant correlation between SFA wall volume with FV total vessel volume (r=0.429, p=0.0001), FV wall volume (r=0.448, p<0.0001), and FV lumen volume (r=0.384, p=0.0005; Figure 2). FV total volumes were significantly larger than SFA total volumes across equal lengths (131.85 ± 5.29 mm3 vs. 59.1 ± 2.51 mm3, p<0.001). FV volumes were positively correlated with ABI and BMI (Table 4).
Table 3.
Correlation between superficial femoral artery and femorval vein volumes
| SFA total volume | SFA wall volume | SFA lumen volume | ||
|---|---|---|---|---|
| FV total volume | r | 0.458 | 0.429 | 0.342 |
| p-value | <0.0001 | 0.0001 | 0.002 | |
| FV wall volume | r | 0.463 | 0.448 | 0.324 |
| p-value | <0.0001 | <0.0001 | 0.004 | |
| FV lumen volume | r | 0.422 | 0.384 | 0.331 |
| p-value | 0.0001 | 0.0005 | 0.003 | |
Correlations were calculated using the pearson correlation coefficient. n=79 for each correlation analysis. FV: femoral vein, SFA: superficial femoral artery.
Figure 2.
Linear regression with 95% confidence interval of baseline SFA total vessel volume with (A) baseline FV total volume (r=0.46, p<0.0001), (B) baseline FV wall volume (r=0.43, P=0.0001), and (C) baseline FV lumen volume (r=0.34, p=0.002). FV: femoral vein; SFA: superficial femoral artery.
Table 4.
Linear regression for femoral vein parameters with superficial femoral artery and clinical measures at baseline
| Independent Variable | β | SE | R2 | Adjusted r2 | P-value | |
|---|---|---|---|---|---|---|
| Baseline FV total volume (mm3) | Baseline SFA total volume (mm3) | 0.97 | 0.21 | 0.210 | 0.200 | <0.0001 |
| Baseline SFA lumen volume (mm3) | 1.51 | 0.47 | 0.117 | 0.106 | 0.002 | |
| Baseline SFA wall volume (mm3) | 1.31 | 0.32 | 0.184 | 0.173 | <0.0001 | |
| ABI | 0.58 | 0.25 | 0.080 | 0.065 | 0.022 | |
| BMI | 1.79 | 0.66 | 0.087 | 0.075 | 0.009 | |
| Baseline FV wall volume (mm3) | Baseline SFA total volume (mm3) | 0.43 | 0.09 | 0.214 | 0.204 | <0.0001 |
| Baseline SFA lumen volume (mm3) | 0.63 | 0.21 | 0.105 | 0.093 | 0.004 | |
| Baseline SFA wall volume (mm3) | 0.60 | 0.14 | 0.201 | 0.190 | <0.0001 | |
| ABI | 0.24 | 0.11 | 0.074 | 0.060 | 0.027 | |
| BMI | 0.82 | 0.29 | 0.094 | 0.082 | 0.006 | |
| Baseline FV lumen volume (mm3) | Baseline SFA total volume (mm3) | 0.54 | 0.13 | 0.178 | 0.167 | <0.0001 |
| Baseline SFA lumen volume (mm3) | 0.88 | 0.29 | 0.109 | 0.098 | 0.003 | |
| Baseline SFA wall volume (mm3) | 0.71 | 0.20 | 0.147 | 0.136 | 0.001 | |
| ABI | 0.34 | 0.15 | 0.073 | 0.059 | 0.028 | |
| BMI | 0.97 | 0.40 | 0.070 | 0.058 | 0.019 | |
Linear regression was obtained using pairwise correlation after normality was checked with the Shapiro-Wilk test. n=79 for each regression analysis, except for ABI for which n=66. FV: femoral vein, SFA: superficial femoral artery.
In pooled analysis, there was no significant change over 12-, and 24-months in FV total vessel volume, wall volume, or lumen volume.
There was a significant inverse correlation between 24-month change in claudication-free walking times with 24-month change in FV total vessel volume (r2=0.206, p=0.03), and 24-month change in FV lumen volume (r2=0.163, p=0.032; Table 5). However, 24-month change in claudication-free walking times was not significantly associated with 24-month change in FV wall volume (r2=0.079, p=0.10).
Table 5.
Linear regression for 24-month change in femoral vein parameters versus 24-month change in maximum walking time
| Independent Variable | β | SE | R2 | Adjusted r2 | P-value | |
|---|---|---|---|---|---|---|
| FV Δ24 month total vessel volume (mm3) | Δ24 month max. walking time | −3.75 | 1.61 | 0.206 | 0.168 | 0.03 |
| FV Δ24 month wall volume (mm3) | Δ24 month max. walking time | −1.29 | 0.76 | 0.121 | 0.079 | 0.10 |
| FV Δ24 month lumen volume (mm3) | Δ24 month max. walking time | −2.46 | 1.07 | 0.201 | 0.163 | 0.032 |
Linear regression was obtained using pairwise correlation. Δ24 month max. walking time is the 24-month change in claudication free walking time. For FV Δ24 month total vessel volume, FV Δ24 month wall volume, and FV Δ24 month lumen volume to Δ24 month max. walking time: N=23. FV: femoral vein.
Baseline characteristics did not differ between the ELIMIT drug groups except for ethnicity (p=0.017; Table 1). There were no significant differences between groups for baseline arterial and venous volumes, as expected. There was no significant 12-, and 24-month change between mono-therapy and triple-therapy in FV wall, FV lumen volume, or FV total vessel volume.
Discussion
This study sought to investigate the relationship between FV volumes and determinants of PAD. Distal FV total, lumen, and wall volumes were quantified with MR imaging in patients with lifestyle-limiting claudication. There were 3 main findings in this study. First, baseline FV volumes were found to be positively associated with baseline SFA total, wall, and lumen volumes, indicating that SFA atheroma burden may be positively associated with FV volumes. Second, intraclass correlation coefficients for inter-reader and intra-reader assessments of FV total vessel and lumen volumes were excellent, indicating high reproducibility of FSE PDW MRI based venous volume measurements. Third, an inverse association was observed between the 2-year change in claudication-free walking times and the 2-year change in FV total vessel and lumen volumes, suggesting that increased FV volumes may be associated with worsening of PAD symptoms.
Traditionally, vascular ultrasound has been used to measure venous diameters in the lower extremities. However, precise venous measurements can be variable. Haenen et al., utilized ultrasound to measure proximal vein diameters and reported a coefficient of variation of up to 15%, indicating that only large changes in diameter can be measured reliably.11 Ogawa et al. showed that procedural variability can affect results.12 The reproducibility of 2-dimensional ultrasound has been shown to be decreased for smaller, distal, and deep veins including the FV.13 MRI has been shown to be a reliable and reproducible method of measuring arterial volumes in PAD.1,14-16 Our results indicate that FSE PDW MRI is also a reliable and reproducible method to assess deep venous measurements in the lower extremities. Newer sequences have been used for MR venography since the start of ELIMIT. Direct contrast-enhanced 3D MR venography has been used to evaluate varicose veins with results similar to conventional venography.17 Steady-state free precession (SSFP) pulse sequences, which do not require contrast, have been used to provide accurate imaging of the portal venous system.18 Signal targeting alternative radiofrequency and flow-independent relaxation enhancement (STARFIRE) is another unenhanced modality that has been used to evaluate lower extremity veins.19 However, these MR sequences allow visualization of only lumen and do not directly visualize the vessel wall.
A limited number of studies have assessed the relationship of venous volumes with markers of PAD. Fronek et al. acquired ultrasound measurements of common femoral vein dimensions and found a positive correlation between venous volumes and BMI; similar results are presented in our study.20 Libertiny et al. used ultrasound to measure the popliteal vein diameter in 89 patients with PAD and compared them to 35 age-matched control subjects.21 They found a significant positive correlation between popliteal vein diameter and ABIs in the pooled analysis (r=0.35) and a negative correlation between venous flow velocity and ABIs (r=−0.24) in the PAD group.21 Our results confirm these findings of a positive correlation between venous measures and ABI with similar strengths of associations. Further, Libertiny et al.21 hypothesized that chronic ischemia resulted in constriction of the popliteal vein which can lead to increased venous flow and might provide some protection against deep vein thrombosis in PAD patients. Although, our study did not measure venous flows, we have observed an inverse association between 2-year changes in claudication-free walking times and venous volumes. Taken together, our findings and previous reports suggest that impaired leg function in PAD patients may be further attenuated by the presence of enlarged venous volumes.
Several studies have demonstrated the prevalence of concomitant PAD and venous insufficiency in contributing to patients’ symptoms. In the study by Paolini et al., the prevalence of arterial dysfunction in a population with chronic venous disease was examined.22 They found that patients with venous insufficiency had a diminished response in arterial inflow during hyperemic limb challenge, suggesting that venous claudication symptoms may be due to diminished arterial inflow. The diminished arterial blood flow has been attributed in part to a decrease in the muscle pump effect with exercise in patients with PAD.23 In a cross-sectional study, Aykan et al. examined 87 subjects with chronic venous insufficiency versus 86 healthy controls and found that the cardio-ankle vascular index scores, a measure of arterial stiffness, was higher in subjects with venous insufficiency.24 More studies are needed to elucidate the role of chronic venous insufficiency and leg function in PAD.
This study has limitations. At the time of the trial, only a unilateral phased array coil was available. Modern bilateral coils allow improved acquisition times of both SFA and FV vessels.25 Although more recent MR venous imaging techniques have become available since ELIMIT was conducted, the inter-reader and intra-reader correlation was excellent, indicating a reliable assessment of venous volumes with FSE PDW MRI. Future studies will be needed to compare the precision of measurements obtained from MRI to other traditional imaging modalities including ultrasound and computed tomography.26 ELIMIT was not designed to assess peripheral venous disease and thus CEAP (clinical, etiology, anatomy, and pathophysiology) classifications were not assessed. As this is a secondary analysis of a randomized trial, only correlations can be made, as potential confounders exist. One such confounder is the unknown prevalence of venous insufficiency in the population, as stated above. Interventions prior to baseline may have confounded baseline ABI measurements. Differences in patients’ volume statuses may have affected venous volume measurements. All limitations of the primary study apply.8
In this analysis, baseline SFA plaque burden was found to have a positive association with FV volumes. An inverse relationship was observed between the 2-year change in FV volumes and leg function. FV volumes can be measured reliably with fast spin echo MR imaging and prospective studies are needed to elucidate in detail the relationship between the venous system and leg function in PAD patients.
Acknowledgments
Grant support: ELIMIT was supported by NIH grants R01HL075824, and R01 HL085769. JDM was supported in part by NIH grant (HL63090). GB was supported in part by an American Heart Association (AHA) award (13BGIA16720014) and an NIH K25 award (K25HL121149). EYY was supported in part by AHA 15POST25080268. All supports and grants in Houston, Texas, United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: There are no conflicts of interest to disclose.
References
- 1.Lumsden AB, Rice TW, Chen C, Zhou W, Lin PH, Bray P, Morrisett J, Nambi V, Ballantyne C. Peripheral arterial occlusive disease: magnetic resonance imaging and the role of aggressive medical management. World J Surg. 2007;31:695–704. doi: 10.1007/s00268-006-0732-y. [DOI] [PubMed] [Google Scholar]
- 2.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, Browner D. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 3.Regensteiner JG, Hiatt WR, Coll JR, Criqui MH, Treat-Jacobson D, McDermott MM, Hirsch AT. The impact of peripheral arterial disease on health-related quality of life in the Peripheral Arterial Disease Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) Program. Vasc Med. 2008;13:15–24. doi: 10.1177/1358863X07084911. [DOI] [PubMed] [Google Scholar]
- 4.Criqui MH, Vargas V, Denenberg JO, Ho E, Allison M, Langer RD, Gamst A, Bundens WP, Fronek A. Ethnicity and peripheral arterial disease: the San Diego Population Study. Circulation. 2005;112:2703–2707. doi: 10.1161/CIRCULATIONAHA.105.546507. [DOI] [PubMed] [Google Scholar]
- 5.Meissner MH. Lower Extremity Venous Anatomy. Seminars in Interventional Radiology. 2005;22:147–156. doi: 10.1055/s-2005-921948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meissner MH, Moneta G, Burnand K, Gloviczki P, Lohr JM, Lurie F, Mattos MA, McLafferty RB, Mozes G, Rutherford RB, Padberg F, Sumner DS. The hemodynamics and diagnosis of venous disease. J Vasc Surg. 2007;46(Suppl S):4S–24S. doi: 10.1016/j.jvs.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 7.Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation. 2005;111:2398–2409. doi: 10.1161/01.CIR.0000164199.72440.08. [DOI] [PubMed] [Google Scholar]
- 8.Brunner G, Yang EY, Kumar A, Sun W, Virani SS, Negi SI, Murray T, Lin PH, Hoogeveen RC, Chen C, Dong JF, Kougias P, Taylor A, Lumsden AB, Nambi V, Ballantyne CM, Morrisett JD. The Effect of Lipid Modification on Peripheral Artery Disease after Endovascular Intervention Trial (ELIMIT). Atherosclerosis. 2013;231:371–377. doi: 10.1016/j.atherosclerosis.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 10.Etherington J, Innes G, Christenson J, Berkowitz J, Chamberlain R, Berringer R, Leung C. Development, implementation and reliability assessment of an emergency physician performance evaluation tool. CJEM. 2000;2:237–245. doi: 10.1017/s1481803500007260. [DOI] [PubMed] [Google Scholar]
- 11.Haenen JH, van Langen H, Janssen MC, Wollersheim H, van't hof MA, van Asten WN, Skotnicki SH, Thien T. Venous duplex scanning of the leg: range, variability and reproducibility. Clin Sci (Lond) 1999;96:271–277. [PubMed] [Google Scholar]
- 12.Ogawa T, Lurie F, Kistner RL, Eklof B, Tabrah FL. Reproducibility of ultrasound scan in the assessment of volume flow in the veins of the lower extremities. J Vasc Surg. 2002;35:527–531. doi: 10.1067/mva.2002.121564. [DOI] [PubMed] [Google Scholar]
- 13.Ranke C, Hendrickx P, Roth U, Brassel F, Creutzig A, Alexander K. Color and conventional image-directed Doppler ultrasonography: accuracy and sources of error in quantitative blood flow measurements. J Clin Ultrasound. 1992;20:187–193. doi: 10.1002/jcu.1870200305. [DOI] [PubMed] [Google Scholar]
- 14.Isbell DC, Meyer CH, Rogers WJ, Epstein FH, DiMaria JM, Harthun NL, Wang H, Kramer CM. Reproducibility and reliability of atherosclerotic plaque volume measurements in peripheral arterial disease with cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2007;9:71–76. doi: 10.1080/10976640600843330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson JD, Epstein FH, Meyer CH, Hagspiel KD, Wang H, Berr SS, Harthun NL, Weltman A, Dimaria JM, West AM, Kramer CM. Multifactorial determinants of functional capacity in peripheral arterial disease: uncoupling of calf muscle perfusion and metabolism. J Am Coll Cardiol. 2009;54:628–635. doi: 10.1016/j.jacc.2009.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu B, Zhao XQ, Saam T, Yarnykh VL, Kerwin WS, Flemming KD, Huston J, 3rd, Insull W, Jr., Morrisett JD, Rand SD, DeMarco KJ, Polissar NL, Balu N, Cai J, Kampschulte A, Hatsukami TS, Yuan C. Feasibility of in vivo, multicontrast-weighted MR imaging of carotid atherosclerosis for multicenter studies. J Magn Reson Imaging. 2005;21:809–817. doi: 10.1002/jmri.20308. [DOI] [PubMed] [Google Scholar]
- 17.Muller MA, Mayer D, Seifert B, Marincek B, Willmann JK. Recurrent lower-limb varicose veins: effect of direct contrast-enhanced three-dimensional MR venographic findings on diagnostic thinking and therapeutic decisions. Radiology. 2008;247:887–895. doi: 10.1148/radiol.2473070987. [DOI] [PubMed] [Google Scholar]
- 18.Wilson MW, LaBerge JM, Kerlan RK, Martin AJ, Weber OM, Roberts T, Vitalich C, Higgins CB, Gordon RL. MR portal venography: preliminary results of fast acquisition without contrast material or breath holding. Acad Radiol. 2002;9:1179–1184. doi: 10.1016/s1076-6332(03)80519-6. [DOI] [PubMed] [Google Scholar]
- 19.Edelman RR, Koktzoglou I. Unenhanced flow-independent MR venography by using signal targeting alternative radiofrequency and flow-independent relaxation enhancement. Radiology. 2009;250:236–245. doi: 10.1148/radiol.2493080496. [DOI] [PubMed] [Google Scholar]
- 20.Fronek A, Criqui MH, Denenberg J, Langer RD. Common femoral vein dimensions and hemodynamics including Valsalva response as a function of sex, age, and ethnicity in a population study. J Vasc Surg. 2001;33:1050–1056. doi: 10.1067/mva.2001.113496. [DOI] [PubMed] [Google Scholar]
- 21.Libertiny G, Hands L. Lower limb deep venous flow in patients with peripheral vascular disease. J Vasc Surg. 1999;29:1065–1070. doi: 10.1016/s0741-5214(99)70247-8. [DOI] [PubMed] [Google Scholar]
- 22.Paolini DJ, Comerota AJ, Jones LS. Lower extremity arterial inflow is adversely affected in patients with venous disease. J Vasc Surg. 2008;48:960–964. doi: 10.1016/j.jvs.2008.05.058. [DOI] [PubMed] [Google Scholar]
- 23.Nadland IH, Wesche J, Sheriff DD, Toska K. Does venous insufficiency impair the exercise-induced rise in arterial leg blood flow? Phlebology. 2011;26:326–331. doi: 10.1258/phleb.2011.010092. [DOI] [PubMed] [Google Scholar]
- 24.Aykan AC, Mentese S, Dogan E, Cavusoglu IG, Aykan DA, Kalaycioglu E, Gokdeniz T, Mentese U. Assessment of arterial stiffness in patients with chronic lower extremity venous disease: An observational study. Phlebology. 2015;31:349–355. doi: 10.1177/0268355515591394. [DOI] [PubMed] [Google Scholar]
- 25.Brown R, Karmonik C, Brunner G, Lumsden A, Ballantyne C, Johnson S, Wang Y, Morrisett J. Simultaneous Bilateral Magnetic Resonance Imaging of the Femoral Arteries in Peripheral Arterial Disease Patients. J Mag Reson Imag. 2011;34:150–156. doi: 10.1002/jmri.22591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee W, Chung JW, Yin YH, Jae HJ, Kim SJ, Ha J, Park JH. Three-Dimensional CT venography of varicose veins of the lower extremity: image quality and comparison with doppler sonography. AJR Am J Roentgenol. 2008;191:1186–1191. doi: 10.2214/AJR.07.3471. [DOI] [PubMed] [Google Scholar]