Abstract
Viruses infecting wild flora may have a significant negative impact on nearby crops, and vice-versa. Only limited information is available on wild species able to host economically important viruses that infect sweetpotatoes (Ipomoea batatas). In this study, Sweet potato chlorotic fleck virus (SPCFV; Carlavirus, Betaflexiviridae) and Sweet potato chlorotic stunt virus (SPCSV; Crinivirus, Closteroviridae) were surveyed in wild plants of family Convolvulaceae (genera Astripomoea, Ipomoea, Hewittia and Lepistemon) in Uganda. Plants belonging to 26 wild species, including annuals, biannuals and perennials from four agro-ecological zones, were observed for virus-like symptoms in 2004 and 2007 and sampled for virus testing. SPCFV was detected in 84 (2.9%) of 2864 plants tested from 17 species. SPCSV was detected in 66 (5.4%) of the 1224 plants from 12 species sampled in 2007. Some SPCSV-infected plants were also infected with Sweet potato feathery mottle virus (SPFMV; Potyvirus, Potyviridae; 1.3%), Sweet potato mild mottle virus (SPMMV; Ipomovirus, Potyviridae; 0.5%) or both (0.4%), but none of these three viruses were detected in SPCFV-infected plants. Co-infection of SPFMV with SPMMV was detected in 1.2% of plants sampled. Virus-like symptoms were observed in 367 wild plants (12.8%), of which 42 plants (11.4%) were negative for the viruses tested. Almost all (92.4%) the 419 sweetpotato plants sampled from fields close to the tested wild plants displayed virus-like symptoms, and 87.1% were infected with one or more of the four viruses. Phylogenetic and evolutionary analyses of the 3′-proximal genomic region of SPCFV, including the silencing suppressor (NaBP)- and coat protein (CP)-coding regions implicated strong purifying selection on the CP and NaBP, and that the SPCFV strains from East Africa are distinguishable from those from other continents. However, the strains from wild species and sweetpotato were indistinguishable, suggesting reciprocal movement of SPCFV between wild and cultivated Convolvulaceae plants in the field.
Introduction
There is evidence that wild flora acts as a reservoir of viruses causing significant losses in nearby crops and vice versa [1–7]. However, information about viruses in wild species is still quite limited. This may in part be due to the fact that viral infections in wild plants are often symptomless, even when the same infection may have obvious symptoms in cultivated plants [8–10]. Whether the same virus strains can infect wild and cultivated plants, but are better adapted to wild plants and hence cause no symptoms, is an issue requiring further study [11, 12]. The geospatial distribution and genetic variability of viruses in wild species is also poorly understood [13, 14]. Although some metagenomic surveys have explored virus diversity in wild plant communities [14–19], only a few studies have described the genetic variability of individual virus species in wild plants in relation to isolates found in cultivated plants [20–27]. Moreover, few studies have compared isolates of plant viruses from wild and cultivated hosts across broad geographical areas [22–24, 28–30]. Thus, studies comparing virus populations in weeds or wild species and crop species that share an agro-ecological interface are needed to gain insights into the evolutionary and ecological dynamics of plant virus populations, which in turn are needed to facilitate plant virus disease management [8, 31, 32].
The incidence and impact of plant viruses at the agro-ecological interface are often exacerbated in evergreen tropical environments, where susceptible cultivated and wild plants are continuously available, providing the necessary environment for viral replication and vectors for viral transmission [30, 33, 34]. Plant virus diseases not only have an economic impact but also may cause starvation, especially when the cultivated host plant constitutes a ‘food security’ crop [35–38]. An example is the sweetpotato, Ipomoea batatas (L.) Lam., the world’s third-most-important root crop and a critical food security crop in sub-Saharan Africa [38–40]. Globally, over 30 viruses are known to infect sweetpotatoes [41–43].
Sweetpotatoes are grown as a perennial crop in local cropping systems in Uganda and elsewhere in East Africa. Sources of healthy planting materials are limited [44, 45]. Perreniality and lack of healthy sweetpotato planting materials coupled with the abundance of insect vectors transmitting the viruses promotes yield losses due to virus diseases [46]. The most severe yield losses occur in sweetpotato plants co-infected with the whitefly-transmitted Sweet potato chlorotic stunt virus (SPCSV; genus Crinivirus, family Closteroviridae) and the aphid-transmitted Sweet potato feathery mottle virus (SPFMV; genus Potyvirus, family Potyviridae). Co-infection with these viruses results in so-called Sweet potato virus disease (SPVD), characterized by leaf malformation, stunted plants and nearly complete loss of yields [47–50]. Similar but milder symptoms develop in sweetpotato plants co-infected with SPCSV and Sweet potato chlorotic fleck virus (SPCFV; genus Carlavirus, family Betaflexiviridae), Sweet potato mild mottle virus (SPMMV; genus Ipomovirus; family Potyviridae) [49, 51] or sweepoviruses (genus Begomovirus, family Geminiviridae) [52]. The frequent co-infection of sweetpotatoes with SPCFV and SPFMV suggests that these viruses may be transmitted by a common vector [53, 54], but the vector of SPCFV remains to be identified [55]. Whiteflies transmit sweepoviruses and were also initially reported as vectors of SPMMV [56], but these results could not be confirmed in later studies [55].
Previous studies in Uganda have shown that SPFMV, SPMMV and SPCSV from wild plants of the family Convolvulaceae are phylogenetically similar to those found in cultivated sweetpotatoes [22–24]. SPFMV and SPMMV, respectively, were detected in 24 and 21 wild plant species and in 23 and 20 districts, respectively, surveyed in the country [23, 57]. Furthermore, 12 wild Convolvulaceae species were found to be infected with SPCSV [24], but the geographical distribution of SPCSV in wild vegetation in Uganda and the wild host species and co-infection of SPCSV with other viruses in wild plants were not reported. Similarly, information regarding SPCFV infection in wild plants of Convolulacea is lacking, even though SPCFV occurs sweetpotatoes in Uganda [58] and other East African countries such as Kenya [53, 59], Tanzania [60] and Rwanda [61], as well as western Africa [62], Asia, Australia, East Timor and Latin America [54, 63–66].
The aim of this study was to determine the incidence of SPCFV and SPCSV and their rates of co-infection with SPFMV and SPMMV, in wild species interfacing with cultivated sweetpotatoes in the major agro-ecological zones of Uganda, and to study the genetic variability of SPCFV.
Results
Virus-like symptoms in wild plants
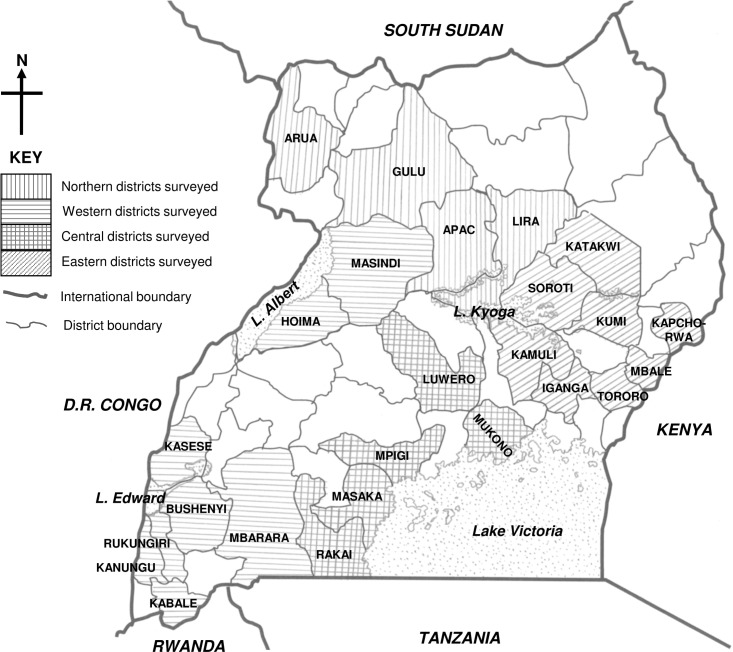
A total of 2864 wild plants of the family Convolvulaceae (genera Astripomoea, Hewittia, Ipomoea and Lepistemon) were sampled from their natural habitats in four agro-ecological zones in Uganda where sweetpotato crops are grown (Figs 1 and 2, S1 Table). The natural habitats of the wild plants surveyed were in close proximity (within 500 m) to cultivated sweetpotato fields. The wild plants were observed to trail into the sweetpotato fields, especially in the western (Fig 2A), central (Fig 2B) and eastern (Fig 2C) zones. Some wild plants grew as weeds in sweetpotato fields in the eastern zone (Fig 2D). Volunteer sweetpotato plants were found growing among wild plants in the central zone (Fig 2E).
Fig 1. Map of Uganda showing the districts surveyed for wild Convolvulaceae species and viruses in Uganda.
Fig 2. Examples of wild species of Convolvulaceae in their natural habitats in Uganda, and some virus-like symptoms.
(A) Ipomoea wightii and (B) I. acuminata (in the background) trailing into sweet potato field (foreground) in the Mbarara and Mukono districts, respectively. Wild vegetation in these districts is dominated by tall shrubs. (C) I. sinensis (dotted circle) in close proximity to sweetpotato field (edge inside solid circle) in the Soroti district, which is dominated by short grassland vegetation (background). (D) I. sinensis (white asterisks) growing as weeds in a sweetpotato field in the Katakwi district. (E) Sweetpotato plant (white o) mixed with plants of I. wightii (white asterisks) in the Mukono district. (F-J) Examples of virus-like symptoms. (F) Leaf chlorosis in H. sublobata. (G) Chlorotic spots on a leaf of I. tenuirostris. (H, I) Mild (H) and severe (I) purpling in old leaves of I. sinensis. (J) Mild chlorotic spots on a leaf of I. acuminata. Plants in F, G and J tested positive for SPCFV; plants in H and I tested positive for SPCSV.
Virus-like symptoms were observed in a total of 367 wild plants (12.8%) collected over the two sampling years (2004 and 2007); of these, 42 plants (11.4%) tested negative for all four viruses. In contrast, 132 (5.3%) of 2497 symptomless wild plants tested positive for at least one of the four viruses. The symptomless but virus-positive wild plants constituted 15.8% of all 836 wild plants that tested positive for at least one virus. In sweetpotatoes, 5 (1.3%) of the 387 plants with symptoms tested negative for all four viruses. On the other hand, 10 (31.3%) of 32 symptomless plants tested positive for at least one virus.
Leaf chlorosis was observed in H. sublobata (Fig 2F) and chlorotic spots were displayed in I. tenuirostris (Fig 2G) and I. acuminata (Fig 2J) infected with SPCFV. Mild to severe purpling of older leaves was observed in plants of I. sinensis infected with SPCSV (Fig 2H and 2I).
Incidence of SPCFV in wild plants
Plants showing a consistent and unambiguous positive reaction in three independent NCM-ELISA experiments were deemed SPCFV-infected. SPCFV was detected in 84 (2.9%) of 2864 wild plants tested, including H. sublobata, L. owariensis and 15 of the 26 Ipomoea species tested (Table 1, S1 Table). All of these 17 wild species of family Convolvulaceae represent previously unknown natural hosts for SPCFV. In eleven species (I. acuminata, I. cairica, I. eriocarpa, I. involucrata, I. obscura, I. sinensis, I. tenuirostris, I. wightii, Astripomoea hyocyamoides, H. sublobata and L. owariensis) from which over 40 plants were tested, the overall incidence of SPCFV ranged from 1.8% in I. tenuirostris and I. wightii to 5.2% in L. owariensis (Table 1). No tested plants of I. cordofana, I. eriocarpa, I. fistulosa, I. grantii, I. involucrata, I. polymorpha, I. spathulata, I. velutipes, A. grantii or A. hyocyamoides were positive for SPCFV (Table 1). The lowest incidence of SPCFV in wild plants (0.7%) was recorded in the Masindi district in western Uganda, and the highest incidence (9.0%) was found in the Katakwi district in eastern Uganda (Table 1).
Table 1. Incidence of Sweet potato chlorotic fleck virus in wild plants and cultivated sweetpotato plants from different agro-ecological zones of Uganda.
| Central zoned | Northern zoned | Eastern zoned | Western zoned | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant species | Life cyclea | Total no. of plantsc | LUW | MKN | MSK | RKI | MPG | LIR | APC | GUL | ARU | KTK | SOR | KUM | MBL | KAP | TOR | KML | IGG | RUK | KNG | KBL | BUS | MBR | KAS | MAS | HOM |
| Ipomoea acuminata | P | 157(4.5) | 0(16) | 0(11) | 0(9) | - | 0(36) | - | - | - | - | - | - | - | 6(35) | - | - | - | - | 0(9) | 0(22) | - | 1(8) | 0(6) | - | - | 0(5) |
| I. aquatica | P | 22(9.1) | - | - | - | - | - | - | - | - | - | 2(20) | 0(2) | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| I. blepharophylla | P | 14(7.1) | - | - | - | - | - | - | - | - | - | 1(14) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| I. cairica | P | 220(4.5) | 0(2) | 0(6) | 1(19) | 0(30) | 1(19) | - | - | - | - | - | - | - | 0(3) | - | 0(5) | 3(26) | - | 1(13) | - | - | 1(25) | 3(57) | - | 0(14) | 0(1) |
| I. cordofana | A | 10(0) | - | - | - | - | - | - | 0(10) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| I. crepidiformis | P | 30(3.3) | - | - | - | - | - | - | - | - | - | - | 0(20) | 1(10) | - | - | - | - | - | - | - | - | - | - | - | - | - |
| I. eriocarpa | A | 134(0) | - | - | - | - | - | 0(38) | 0(9) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0(7) | 0(47) | 0(33) | - |
| I. fistulosa | P | 25(0) | - | 0(25) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| I. grantii | P | 9(0) | - | 0(9) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| I. hederifolia | A | 47(2.1) | - | 0(11) | 0(5) | - | - | - | - | - | - | - | 1(4) | - | - | - | - | 0(8) | - | 0(9) | - | - | - | - | - | - | 0(10) |
| I. hildebrandtii | P | 17(5.9) | - | - | - | 0(2) | - | - | - | - | - | - | - | - | 0(3) | - | - | 0(7) | 1(2) | 0(1) | 0(1) | - | - | 0(1) | - | - | - |
| I. involucrata | P | 78(0) | - | - | - | - | - | - | - | - | - | - | - | 0(12) | - | - | - | - | - | - | 0(10) | 0(50) | 0(6) | - | - | - | - |
| I. obscura | P | 154(4.5) | - | 0(6) | 0(19) | 0(8) | 0(6) | - | 0(8) | - | - | - | 1(18) | 1(10) | - | 2(32) | - | - | - | 0(3) | - | 0(1) | 0(7) | 0(14) | 1(10) | 0(1) | 1(5) |
| I. polymorpha | BA | 10(0) | - | - | - | - | - | - | - | - | - | - | - | - | - | 0(10) | - | - | - | - | - | - | - | - | - | - | - |
| I. purpurea | A | 34(5.9) | - | - | 0(6) | 0(2) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 2(16) | - | - | - |
| I. repens | P | 27(11.1) | - | - | - | - | - | - | - | - | - | - | 2(4) | 1(14) | 0(9) | - | - | - | - | - | - | - | - | - | - | - | - |
| I. rubens | P | 37(2.7) | - | - | 0(2) | 0(8) | - | - | - | - | - | 1(13) | 0(8) | - | - | - | 0(1) | - | 0(5) | - | - | - | - | - | - | - | - |
| I. sinensis | A | 374(3.5) | - | - | - | - | - | - | - | 0(54) | 0(36) | 8(64) | 2(42) | 2(31) | 0(16) | 0(7) | 0(24) | 0(23) | 1(28) | - | 0(7) | - | - | - | - | 0(40) | 0(2) |
| I. spathulata | P | 42(0) | - | - | - | - | - | - | - | - | - | - | - | - | - | 0(42) | - | - | - | - | - | - | - | - | - | - | - |
| I. stenobasis | P | 23(8.7) | - | - | - | - | - | - | - | - | 0(7) | - | 1(7) | - | 0(3) | - | - | 0(6) | - | - | - | - | - | - | - | - | - |
| I. tenuirostris | P | 395(1.8) | 0(17) | 0(28) | - | 0(3) | 0(13) | 0(2) | 0(31) | - | - | - | 1(7) | - | 1(44) | 0(60) | 0(1) | 0(6) | - | 1(65) | 0(25) | 0(18) | 3(58) | 0(8) | 0(7) | 1(2) | - |
| I. velutipes | A | 10(0) | - | - | - | - | - | - | - | - | - | - | - | - | 0(2) | - | - | - | - | - | - | - | - | 0(8) | - | - | - |
| I. wightii | P | 113(1.8) | 0(9) | 0(11) | 0(12) | 0(26) | 0(1) | - | - | - | - | - | - | - | 0(2) | - | 1(4) | - | - | 0(5) | 1(18) | 0(2) | 0(6) | 0(15) | - | - | 0(2) |
| A. grantii | P | 42(0) | - | - | 0(7) | 0(15) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0(20) | - | - | - | - | - | - |
| A. hyoscyamoides | P | 64(0) | 0(12) | - | - | - | - | - | - | - | - | - | 0(5) | - | 0(38) | - | - | 0(5) | - | 0(4) | - | - | - | - | - | - | - |
| H. sublobata | P | 687(2.8) | 0(28) | 0(18) | 0(45) | 0(43) | 0(44) | 0(22) | 0(10) | 0(2) | - | - | 0(19) | - | 1(28) | 3(12) | 5(86) | 4(50) | 3(41) | 0(22) | 1(51) | - | 1(40) | 1(29) | 0(8) | 0(48) | 0(41) |
| L. owariensis | P | 97(5.2) | - | 0(22) | - | - | 0(3) | - | - | - | - | - | 0(2) | - | 1(16) | - | 2(23) | 2(25) | - | - | 0(2) | - | - | - | - | 0(1) | 0(3) |
| Total no. wild plantsb | 2864(2.9) | 0(84) | 0(147) | 1(124) | 0(137) | 1(122) | 0(62) | 0(68) | 0(56) | 0(43) | 12(113) | 8(138) | 5(77) | 9(199) | 5(163) | 8(144) | 9(156) | 5(76) | 2(131) | 2(156) | 0(71) | 6(156) | 8(161) | 1(72) | 1(139) | 1(69) | |
| SPCFV incidence (%) | 0 | 0 | 0.8 | 0 | 0.8 | 0 | 0 | 0 | 0 | 9.0 | 5.8 | 6.5 | 4.5 | 3.1 | 5.6 | 5.8 | 6.6 | 1.5 | 1.3 | 0 | 3.8 | 4.9 | 1.4 | 0.7 | 1.4 | ||
| Sweetpotato | P | 419(4.1) | - | 0(19) | 3(40) | 1(28) | 2(23) | - | - | 0(14) | 2(21) | 0(14) | 0(17) | - | 1(33) | 0(23) | 0(16) | 1(32) | - | 1(26) | 2(36) | - | 1(21) | 0(15) | - | 0(16) | 3(25) |
aLifecycle of species: A, annual; BA, biannual; P, perennial.
bNumber of SPCFV-positive plants followed (in parentheses) by total number of plants tested per district. The numbers of plants tested in each year (2004 and 2007) are shown in S1 Table.
cTotal number of plants tested per species followed (in parentheses) by percentage of plants of each species testing positive for SPCFV.
dNumber of SPCFV-positive plants followed (in parentheses) by number of plants tested per district. ‘─’ indicates that the plant species was not observed in that district. Central region districts (Lake Victoria basin): LUW = Luwero, MKN = Mukono, MSK = Masaka, RKI = Rakai, MPG = Mpigi. Northern region districts: LIR = Lira, APC = Apac, GUL = Gulu, ARU = Arua. Eastern region districts: KTK = Katakwi, SOR = Soroti, KUM = Kumi, MBL = Mbale, KAP = Kapchorwa, TOR = Tororo, KML = Kamuli, IGG = Iganga. Western region districts: RUK = Rukungiri, KNG = Kanungu, KBL = Kabale, BUS = Bushenyi, MBR = Mbarara, KAS = Kasese, MAS = Masindi, HOM = Hoima.
SPCFV was also detected in 17 (4.1%) of 419 cultivated sweetpotato plants sampled from the four agro-ecological zones. SPFMV, SPCSV and SPMMV were detected in 177 (44.6%), 112 (29.5%) and 59 (14.0%), respectively, of the tested sweetpotato plants.
To allow later sequence characterization of its genome, SPCFV isolates from wild plants and sweetpotato plants were mechanically inoculated onto sweetpotato cv. Tanzania and maintained in a greenhouse. Five SPCFV isolates were collected from wild plants, including two from I. acuminata (Mbale, eastern zone) and one each from I. acuminata (Bushenyi, western zone), I. cairica (Mbigi, central zone) and I. tenuirostris (Masindi, western zone). Four SPCFV isolates were collected from sweetpotato plants, including two from the western zone and one each from the central and northern zones.
Detection and incidence of SPCSV in wild plants
Positive reaction with SPCSV antibodies was observed in 66 (5.4%) of 1224 wild plants tested in triplicate by triple antibody sadwich-ezyme linked immunosorbent assay (TAS-ELISA) (Table 2). Only 27% of seropositive plants showed virus-like symptoms. SPCSV-infected plants belonged to 10 Ipomoea species plus H. sublobata and L. owariensis (Table 2). The SPCSV incidence in species from which at least 51 plants were tested was 9.3% in I. obscura, 8.8% in I. cairica, 7.8% in I. acuminata, 5.6% in I. sinensis, 5.5% in I. tenuirostris and 2.6% in H. sublobata (Table 2). Scions from 25 SPCSV-seropositive wild plants belonging to eight species were grafted onto I. setosa. The grafted plants developed chlorotic mottling symptoms on leaves and tested SPCSV-positive by TAS-ELISA at 4 wk post-grafting. No plants of I. aquatica (12 plants tested), I. crepidiformis (12), I. hilderbrandtii (6), I. purpurea (14), I. repens (3), A. grantii (17) or A. hyoscyamoides (14) tested positive for SPCSV (Table 2).
Table 2. Incidence of Sweet potato chlorotic stunt virus in wild plants and cultivated sweetpotato plants from different agro-ecological zones of Uganda.
| Central zoned | Northern zoned | Eastern zoned | Western zoned | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant species | Life cyclea | Total no. of plantsc | MKN | MSK | RKI | MPG | GUL | ARU | KTK | SOR | MBL | KAP | TOR | KML | RUK | KNG | BUS | MBR | MAS | HOM |
| Ipomoea acuminata | P | 102(7.8) | 0(11) | 1(9) | - | 1(22) | - | - | - | - | 4(29) | - | - | - | - | 0(17) | 1(4) | 0(5) | - | 1(5) |
| I. aquatica | P | 12(0) | - | - | - | - | - | - | 0(10) | 0(2) | - | - | - | - | - | - | - | - | - | - |
| I. cairica | P | 121(6.6) | 0(3) | 0(7) | 2(20) | 1(15) | - | - | - | - | 0(3) | - | 0(5) | 1(14) | 0(5) | - | 1(19) | 1(26) | 1(3) | 1(1) |
| I. crepidiformis | P | 12(0) | - | - | - | - | - | - | - | 0(12) | - | - | - | - | - | - | - | - | - | - |
| I. hederifolia | A | 34(8.8) | 0(11) | 0(5) | - | - | - | - | - | - | - | - | - | 2(8) | - | - | - | - | - | 1(10) |
| I. hildebrandtii | P | 6(0) | - | - | 0(2) | - | - | - | - | - | 0(3) | - | - | - | - | - | - | 0(1) | - | - |
| I. obscura | P | 75(9.3) | 1(5) | 1(10) | 0(8) | 0(6) | - | - | - | 2(10) | - | 1(14) | - | - | - | - | 0(6) | 1(10) | 0(1) | 1(5) |
| I. purpurea | A | 14(0.0) | - | 0(6) | 0(2) | - | - | - | - | - | - | - | - | - | - | - | - | 0(6) | - | - |
| I. repens | P | 3(0) | - | - | - | - | - | - | - | - | 0(3) | - | - | - | - | - | - | - | - | - |
| I. rubens | P | 10(10.0) | - | 0(2) | - | - | - | - | 0(6) | 1(1) | - | - | 0(1) | - | - | - | - | - | - | - |
| I. sinensis | A | 231(5.6) | - | - | - | - | 0(54) | 1(36) | 1(21) | 1(25) | 1(7) | - | 2(16) | 3(23) | - | 0(7) | - | - | 3(40) | 1(2) |
| I. spathulata | P | 27(3.7) | - | - | - | - | - | - | - | - | - | 1(27) | - | - | - | - | - | - | - | - |
| I. stenobasis | P | 10(10.0) | - | - | - | - | - | 1(7) | - | - | 0(3) | - | - | - | - | - | - | - | - | - |
| I. tenuirostris | P | 165(5.5) | 1(11) | - | - | 1(10) | - | - | - | - | 1(17) | 2(44) | - | - | 1(39) | 0(4) | 2(32) | 1(8) | - | - |
| I. wightii | P | 51(5.9) | - | 0(1) | 1(19) | 0(1) | - | - | - | - | - | - | - | - | 1(5) | 0(4) | 0(5) | 1(14) | - | 0(2) |
| Astripomoea granti | P | 17(0) | - | 0(7) | - | - | - | - | - | - | - | - | - | - | - | 0(10) | - | - | - | - |
| A. hyoscyamoides | P | 14(0) | - | - | - | - | - | - | - | 0(5) | - | - | - | 0(5) | 0(4) | - | - | - | - | - |
| Hewittia sublobata | P | 267(2.6) | 0(9) | 0(28) | 0(21) | 0(14) | 0(2) | - | - | 0(11) | 0(12) | - | 2(34) | 1(21) | 0(5) | 0(18) | 0(19) | 0(13) | 2(19) | 2(41) |
| Lepistemon owariensis | P | 53(9.4) | 0(10) | - | - | 0(3) | - | - | - | 0(2) | 0(3) | - | 2(18) | 0(12) | - | 1(1) | - | - | 1(1) | 1(3) |
| Total no. of wild plantsb | 1224(5.4) | 2(60) | 2(75) | 3(72) | 3(71) | 0(56) | 2(43) | 1(37) | 4(68) | 6(80) | 4(85) | 6(74) | 7(83) | 2(58) | 1(61) | 4(85) | 4(83) | 7(64) | 8(69) | |
| SPCSV incidence (%) | 3.3 | 2.7 | 4.2 | 4.2 | 0 | 4.7 | 2.7 | 5.9 | 7.5 | 4.7 | 8.1 | 8.4 | 3.4 | 1.6 | 4.7 | 4.8 | 10.9 | 11.6 | ||
| Sweetpotato | P | 419(25.1) | 4(19) | 13(40) | 7(28) | 5(23) | 0(14) | 1(21) | 2(14) | 6(17) | 6(33) | 2(23) | 3(16) | 5(32) | 10(26) | 12(36) | 10(21) | 7(15) | 5(16) | 7(25) |
aLifecycle of species: A, annual; P, perennial.
bNumber of SPCFV-positive plants followed (in parentheses) by total number of plants tested per district. Plants were tested for SPCSV only in 2007.
cTotal number of plants tested per species followed (in parentheses) by percentage of plants of each species testing positive for SPCSV.
dNumber of SPCSV-positive plants followed (in parentheses) by number of plants tested per district. ‘─’ indicates that the plant species was not observed in that district. Central region districts (Lake Victoria basin): MKN = Mukono, MSK = Masaka, RKI = Rakai, MPG = Mpigi. Northern region districts: GUL = Gulu, ARU = Arua. Eastern region districts: KTK = Katakwi, SOR = Soroti, MBL = Mbale, KAP = Kapchorwa, TOR = Tororo, KML = Kamuli. Western region districts: RUK = Rukungiri, KNG = Kanungu, BUS = Bushenyi, MBR = Mbarara, MAS = Masindi, HOM = Hoima.
Among all 1224 wild plants tested, SPCSV was more frequently detected in plants from the western (1.6–11.6%) and eastern (2.7–8.4%) zones than the central (2.7–4.2%) zone (Table 2). Only two plants from Arua, one of the two northern districts, tested positive for SPCSV (Table 2). Six species (I. acuminata, I. cairica, I. obscura, I. tenuirostis, H. sublobata and L. owariensis) were commonly found in the eastern, central and western zones and were therefore sampled in the largest numbers (64% of all plants tested). Comparison of virus incidence across these three agro-ecological zones confirmed the aforementioned spatial differences in SPCSV incidence (Table 2). SPCSV was also detected in 105 (25%) of the 419 cultivated sweetpotato plants tested.
Mixed viral infections in wild plants
Mixed infections of SPCFV with any of the three other common sweetpotato viruses, SPCSV, SPFMV and SPMMV, was not found in the wild plants tested. However, co-infections involving the other three viruses were found, including SPCSV + SPFMV and SPCSV + SPMMV in six plants each out of 1224 plants sampled in 2007. Co-infection of SPFMV and SPMMV was detected in 35 of 2864 plants sampled in 2004 and 2007. Triple infection by SPCSV, SPFMV and SPMMV was found in 5 of 1224 plants sampled in 2007 (Table 3). Single infections by SPFMV and SPMMV in wild plants have been reported elsewhere [23, 57].
Table 3. Occurrence of mixed infections with SPCFV, SPCSV, SPFMV and/or SPMMV in wild Convolvulacea species and cultivated sweetpotato plants collected in Uganda.
| Virus combinationsd | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant speciesa | Life cyclec | CF+FM | CF+CS | CF+MM | FM+MMe | FM+MMf | FM+MMg | CS+FM | CS+MM | CS+FM+MM | CS+FM+CF | CS+MM+CF | CS+FM+MM+CF |
| Ipomoea acuminata | P | - | - | - | 3(55) | 1(102) | 4(157) | 1(102) | 0(102) | 1(102) | - | - | - |
| Ipomoea cairica | P | - | - | - | 2(99) | 2(121) | 4/220 | 2(121) | 0(121) | 2(121) | - | - | - |
| Ipomoea hederifolia | P | - | - | - | 1(13) | 0(34) | 1(47) | 0(34) | 0(34) | 0(34) | - | - | - |
| Ipomoea obscura | P | - | - | - | 1(79) | 0(75) | 1(154) | 0(75) | 2(75) | 1(75) | - | - | - |
| Ipomoea repens | P | - | - | - | 0(24) | 0(3) | 0(27) | 1(3) | 0(3) | 0(3) | - | - | - |
| Ipomoea sinensis | A | - | - | - | 6(143) | 2(231) | 8(374) | 3(231) | 0(231) | 1(231) | - | - | - |
| Ipomoea spathulata | P | - | - | - | 0(15) | 0(27) | 0(42) | 0(27) | 1(27) | 0(27) | - | - | - |
| Ipomoea stenobasis | P | - | - | - | 0(13) | 0(10) | 0(23) | 1(10) | 0(10) | 0(10) | - | - | - |
| Ipomoea tenuirostris | P | - | - | - | 5(230) | 2(165) | 7(395) | 3(165) | 0(165) | 0(165) | - | - | - |
| Ipomoea wightii | P | - | - | - | 0(62) | 0(51) | 0(113) | 1(51) | 0(51) | 0(51) | - | - | - |
| Hewittia sublobata | P | - | - | - | 6(420) | 1(267) | 7(687) | 2(267) | 2(267) | 0(267) | - | - | - |
| Lepistemon owariensis | P | - | - | - | 2(44) | 1(53) | 3(97) | 2(53) | 1(53) | 0(53) | - | - | - |
| Total mixed-infected wild plantsb | - | - | - | 27(1640) | 8(1224) | 35(2864) | 16(1224) | 6(1224) | 5(1224) | - | - | - | |
| Sweetpotato | P | 3(419) | 3(419) | 2(419) | n/a | 9(419) | n/a | 60(419) | 15(419) | 5(419) | 2(419) | 1(419) | 1(419) |
aLifecycle of species: A, annual; P, perennial.
bCF = SPCFV, CS = SPCSV, FM = SPFMV, MM = SPMMV.
cAll values represent number of co-infected plants followed (in parentheses) by number of plants tested. ‘─’ indicates that the viral combination was consistently not found in the wild species but was detected in one or more sweet potato plants. n/a = not applicable.
dThe total number of wild plants tested was 1640 in 2004 and 1224 in 2007 (2864 overall). Virus combination FM+MM was detected in 2004 and 2007; all other virus combinations were detected only in 2007. CS was surveyed only in 2007. No co-infections involving CF were detected.
e,f,gFM + MM data are shown for 2004e, 2007f and 2004+2007g.
In 419 sweetpotato plants tested, SPCFV was found co-infecting with SPFMV (3 plants), SPCSV (3 plants) or SPMMV (2 plants), and with both SPFMV and SPCSV (2 plants), SPFMV and SPMMV (9 plants) or SPMMV and SPCSV (1 plant). All four viruses were detected in one sweetpotato plant (Table 3).
Molecular variability of the SPCFV coat protein (CP) and nucleic acid–binding protein (NaBP) regions
The (+)ssRNA genome of SPCFV (NCBI acc. no. AY461421) is 9104 nucleotides (nt) long, excluding the 3′-terminal poly(A) tail, and contains six open reading frames (ORFs) [67]. ORF5 encodes the coat protein (CP). ORF6 partially overlaps the 3′ end of ORF5 by 17 nt and encodes the nucleic acid–binding protein (NaBP) [67] implicated in suppression of antiviral RNA silencing [68]. The length of RT-PCR amplicons covering the 3′-proximal genomic region of SPCFV from five wild plants and four sweetpotato plants was 1578 nt. BLAST searches in the NCBI database showed that the sequences were homologous to the 3′ genomic region in the 29 SPCFV isolates previously characterized from sweetpotatoes in East Africa (Uganda, Kenya, Tanzania), Asia (China, Taiwan, South Korea), Australia, East Timor, Peru or of unknown origin (Table 4). The amplified sequences contained the ORFs for SPCFV CP (nt 242–1138, 299 aa) and NaBP (nt 1125–1523, 133 aa), and also the 3′-UTR; (nt 1527–1578). Sequences determined in this study were submitted to the NCBI database under accession numbers EF155967, EF155968 and KR086396–KR086402 (Table 4).
Table 4. Geographical origin of Sweet potato chlorotic fleck virus isolates used for comparison of their 3′-terminal genomic regions.
| Isolatea | Geographical origin | Genebank accession no. | Host | Reference |
|---|---|---|---|---|
| 4MBL | Mbale, Uganda | EF155967 | I. acuminata | This study |
| BUSH42 | Bushenyi, Uganda | KR086399 | I. acuminata | This study |
| KINT2 | Mpigi, Uganda | EF155968 | I. cairica | This study |
| MAS53 | Masindi, Uganda | KR086397 | I. tenuirostris | This study |
| MBL86 | Mbale, Uganda | KR086400 | I. acuminata | This study |
| ARU91 | Arua, Uganda | KR086402 | sweetpotato | This study |
| HOM40 | Hoima, Uganda | KR086398 | sweetpotato | This study |
| KNG92 | Kanungu, Uganda | KR086401 | sweetpotato | This study |
| RKI15 | Rakai, Uganda | KR086396 | sweetpotato | This study |
| 007VIIMS | Unknown | EU375897 | sweetpotato | [54] |
| 94-1s | Kenya | EU375900 | sweetpotato | [54] |
| AusCan | Australia | EF990647 | sweetpotato | [63] |
| AusCan | Australia | KU707475 | sweetpotato | [66] |
| B-Guangdong-11-5 | China | KC130184 | sweetpotato | Qiao et al., 2012, unpublished |
| B-Guangxi-11-1 | China | KC130186 | sweetpotato | Qiao et al., 2012, unpublished |
| B-Jiangxi-11-4 | China | KC130185 | sweetpotato | Qiao et al., 2012, unpublished |
| G-Sichuan-10-60 | China | KC130183 | sweetpotato | Qiao et al., 2012, unpublished |
| Gwangzhu1 | China | EU375901 | sweetpotato | [54] |
| HG176 | South Korea | KP715159 | sweetpotato | Kwak et al., 2015, unpublished |
| HN83 | South Korea | KP115605 | sweetpotato | Kwak et al., 2014, unpublished |
| Hoima3c | Hoima, Uganda | EU375902 | sweetpotato | [54] |
| KBL38 | Kabale, Uganda | EU375903 | sweetpotato | [54] |
| Kiboga6b | Kibonga, Uganda | EU375908 | sweetpotato | [54] |
| KY5 | Kenya | EU375904 | sweetpotato | [54] |
| Le-97-598 | Unknown | EU375905 | sweetpotato | [54] |
| Mas | Masindi, Uganda | AJ781295 | sweetpotato | Mukasa et al., 2004, unpublished |
| Mpigi6b | Mpigi, Uganda | EU375906 | sweetpotato | [54] |
| Njoro5 | Kenya | EU375910 | sweetpotato | [54] |
| Rukungiri1b | Rukungiri, Uganda | EU375907 | sweetpotato | [54] |
| SC20 | South Korea | KP115606 | sweetpotato | Kwak et al., 2014, unpublished |
| SH1 | China | KC414676 | sweetpotato | [65] |
| SPCFV | Hoima, Uganda | AY461421 | sweetpotato | [67] |
| SPCFV-CIP | Peru | EU375899 | sweetpotato | [54] |
| Tar | Tarime, Tanzania | AJ781296 | sweetpotato | Mukasa et al., 2004, unpublished |
| Tm37 | East Timor | KU720565 | sweetpotato | [66] |
| TN340 | Taiwan | EU375898 | sweetpotato | [54] |
| TN399 | Unknown | EU375909 | sweetpotato | [54] |
| UN210 | South Korea | KP115607 | sweetpotato | Kwak et al., 2014, unpublished |
a Names of seven SPCFV isolates whose complete genome sequences are currently available are shown in bold.
The nucleotide sequences of the nine SPCFV isolates were 86.1–98.2% (CP; S2 Table), 95.2–99.5% (NaBP; S3 Table) and 96.4–100% (3′-UTR; S4 Table) identical. The five isolates from wild plants were 86.2–98.2% (CP; S2 Table), 95.5–99.2% (NaBP; S3 Table), and 96.4–100% (3′-UTR; S4 Table) identical at the nucleotide level with 15 isolates from cultivated sweetpotato in East Africa. Among all 38 CP- and 32 NaBP-coding sequences of SPCFV, including those determined in this study and those available in the NCBI database, the nucleotide sequence identities were 75.0–100% for the CP (S2 Table) and 77.4–100% for the NaBP (S3 Table), and the deduced amino acid sequence identities were 88.3–100% for the CP (S2 Table) and 75.9–100% for the NaBP (S3 Table). However, identities between SPCFV isolates from East Africa and elsewhere were relatively low: 75.0–89.3% and 88.3–95.7% at the nucleotide and amino acid level, respectively, for CP (S2 Table), and 77.4–93.7% and 75.9–96.2% at the nucleotide and amino acid level for NaBP (S3 Table). NaBP of SPCFV is a cysteine-rich protein (CRP) [68] and has a zinc finger–like motif (CX2CX4CX3C) that was observed within the same protein region (aa 64–98) in all nine SPCFV isolates. The arginine-rich basic motif, RRARR, which is involved in the RNA silencing suppression activity of NaBP [68] was also observed in the same position (aa 59–63) in all nine SPCFV isolates. The CP of isolates BUSH42 and KINT2 from wild plants had unique amino acid substitutions (V/G/E/S12A and Q119H, respectively), and the NaBP of KINT2 had a unique amino acid substitution (I34V). Some amino acid sites in the CP (13E, 29E, 41I and 118A) and NaBP (3S, 7R, 23C, 74E and 95V) were conserved in isolates from East Africa but were highly variable in isolates from Asia. Overall, no consistent amino acid sequence differences were associated with geographic origin or host species.
Recombination and phylogenetic relationships in SPCFV isolates
No evidence for recombination was detected in the 1109–1761 nt-long NaBP-CP-3′-UTR region available from 35 SPCFV isolates (P = 0.999) or in the complete genomic sequences of the seven SPCFV isolates indicated in Table 4 (P = 0.071) using the six programs included in the RDP4 package and the PHI test.
Using the T92+G+I nucleotide substitution model, phylogenetic clustering of the 38 CP sequences showed no congruence with the host species (Fig 3A). However, there was phylogenetic congruence of isolates according to their geographic origin in East Africa and Asia. All isolates from East Africa (including Uganda, Kenya and Tanzania) were designated as SPCFV-EA (Fig 3A, Table 4). Isolates from Asia were clustered into two groups, designated as SPCFV-Asian1 (comprising isolates from Australia, China, South Korea and Taiwan or of unknown origin) and SPCFV-Asian2 (comprising a few isolates from China, Taiwan and East Timor or of unknown origin) (Fig 3A, Table 4). An exception was isolate SPCFV-CIP (accession no. EU375899) from Peru, which clustered with isolates from East Africa (Fig 3A). Phylogenetic clustering of isolates based on 32 NaBP nucleotide sequences (using the substitution model T92+G) was similar to that of CP (Fig 3B). The 3′-UTR sequences were too short (52 nt) for meaningful analyses and were not included in phylogenetic analyses.
Fig 3. Phylogenetic analysis of SPCFV based on the CP and NaBP nucleotide sequences.
Nine SPCFV isolates from wild plant species (▲) or sweetpotatoes (●) in this study are compared with 29 and 23 isolates, respectively, from previous studies. (A, B) Sequences for CP (A) and NaBP (B) were analyzed. Sequences cluster according to the geographical origin of the virus isolates, i.e., East Africa (SPCFV-EA) or Asia (SPCFV-Asian1 and SPCFV-Asian2). The geographical origins are unknown for isolates Le-97-598_EU375905, TN399_EU375909 and 007VIIMS_EU375897. Numbers at branches represent bootstrap values of 1000 replicates. Only bootstrap values of ≥50% are shown. Scale indicates nucleotide substitutions per site according to Tamura [69].
Nucleotide diversity and selection pressure on the SPCFV CP and NaBP
Analysis of genetic differentiation between SPCFV populations from East Africa and Asia was carried out for both the CP- and NaBP-coding sequences. FST values for CP (0.30011) and NaBP (0.30064) showed evidence of genetic differentiation, implying that for each of the CP and NaBP, 30.0% of total variance of the SPCFV population is explained by the origin of isolates in East Africa or Asia. Between-population diversity was greater than within-population diversity for CP and NaBP, further suggesting a differentiated population. For example, the SPCFV subpopulation from East Africa has a within-population diversity of 0.05007 ± 0.00590 (CP) and 0.02934 ± 0.00482 (NaBP). However, between-population diversities with the Asian subpopulation was more than two times higher, both separately (0.11003 ± 0.00751, Asian1 CP; 0.11405 ± 0.01952, Asian2 CP; 0.08593 ± 0.00886, Asian1 NaBP; and 0.07018 ± 0.001704, Asian2 NaBP) or in combination (0.15861 ± 0.01220 for the CP and 0.14723 ± 0.01336 for the NaBP). In contrast, the SPCFV subpopulation from outside East Africa had within-population diversities of 0.15861 ± 0.01220 (CP) and 0.14723 ± 0.01336 (NaBP), which are only slightly higher than the between-population diversities, indicating a subpopulation structuration in the Asian isolates. Taken together, the phylogenetic clustering of isolates (Fig 3A and 3B), gene flow estimates of FST and within- and between-population diversity indices demonstrate genetic differentiation of SPCFV according to geographical origin.
Synonymous codon usage bias was evaluated based on the effective number of codons (ENC). For the nuclear universal genetic code, the value for ENC ranges from 20 (if only one codon is used for each amino acid, i.e., codon bias is maximal) to 61 (if all synonymous codons for each amino acid are equally used, i.e., there is no codon bias). Our results showed that CP had a higher ENC value (53.9) than NaBP (50.1), suggesting that, although both coding regions had moderate bias in codon usage, NaBP had more codon bias than CP. This is consistent with the larger codon bias index (CBI) value found for NaBP (0.432) as compared with CP (0.283). CBI values range from 0 (in a gene with random codon usage) to 1 (in a gene with extreme codon bias). Thus, our CBI results suggest that codon usage is more random in CP than in NaBP.
Irrespective of the host species from which the SPCFV isolates were characterized, nucleotide diversity (π) values for each of the two protein-coding regions were relatively low (12.1% and 8.7% for CP and NaBP, respectively). The non-synonymous nucleotide diversity (πa) was 2.6% and 4.1% for CP and NaBP, respectively, whereas the synonymous nt diversity (πs) was 45.7%, and 25.2%, respectively. The ratio of πa to πs (ω = πa/πs) gives a generalized estimation of ω, which is the measure of selection pressure imposed on a given entire protein. The value of πa was 17.5- and 6.1-fold lower than the value of πs for CP and NaBP, respectively, suggesting the influence of purifying selection. Under the basic assumption that a codon is a unit of evolutionary change [70], maximum likelihood (ML) site models treat ω for any codon in a protein-coding nucleotide sequence as a random variable from a statistical distribution. Thus, selection pressures suggested by the aforementioned results and assessed by a ML framework of codon substitution under model M0, which yielded ω values of 0.044 and 0.127 for CP and NaBP, respectively, indicate purifying selection (Table 5). The heterogeneity of selective pressure was revealed by a likelihood ratio test (LRT) of M3 vs. M0, which showed that the M3 model fit the data significantly better than M0 for both CP and NaBP proteins (Table 5). M3 for NaBP suggested that 58.0% of sites were subject to strong purifying selection (ω = 0.011), 40.5% of sites were under weak purifying selection (ω = 0.278) and only 1.4% of sites were under positive selection (ω = 1.598) (Table 5). Näive empirical Bayes inference under M3 identified one amino acid (8P) as undergoing positive selection (Table 5). M3 for CP showed that all sites were under varying degrees of purifying selection as follows: 82.7% of sites were subjected to nearly lethal mutations (ω = 0.008), 14.5% were under weak purifying selection (ω = 0.177) and 2.7% of sites were under nearly neutral evolution (ω = 0.615) (Table 5). In both CP and NaBP, likelihood ratio tests (LRTs) of nested models M2a vs. M1a, M8 vs. M7 and M8a vs. M8 showed that the positive selection models (M2a, M8 and M8a) did not fit the data significantly better than the respective null models (M1a, M7 and M8; Table 5), which is consistent with purifying selection on many of the amino acid sites. Parameter estimates under each of the models are shown in Table 5.
Table 5. Parameter estimates, log-likelihood (lnL), ω-ratio (dN/dS), and likelihood ratio test (LRT) statistics under seven different maximum likelihood models of codon substitution used to investigate selection pressures exerted on NaBP and CP of SPCFV.
| Protein | Modela | Parameter estimatesb | ω-ratio (dN/dS) | Log likelihood (lnL) | LRT statisticc (2×δlnL) | Positively selected (amino acids) sitesd |
|---|---|---|---|---|---|---|
| CP | M0 | ω = 0.044 | 0.044 | −6692.464 | none | |
| M3 | p0 = 0.827, p1 = 0.145 (p2 = 0.027); ω0 = 0.008, ω1 = 0.177, ω2 = 0.615 | 0.049 | −6562.318 | p < 0.0001 (M0 vs. M3) | none | |
| M1a | p0 = 0.943 (p1 = 0.056), ω0 = 0.024 (ω1 = 1.000) | 0.079 | −6615.790 | not allowed | ||
| M2a | p0 = 0.943, p1 = 0.056 (p2 = 0.000); ω0 = 0.024, ω1 = 1.000, ω2 = 35.321 | 0.079 | −6615.790 | p > 0.05 (M1a vs. M2a) | none | |
| M7 | p = 0.136, q = 2.240 | 0.052 | −6564.566 | not allowed | ||
| M8 | p0 = 0.986 (p1 = 0.014); p = 0.155, q = 3.305, ωs = 1.000 | 0.053 | −6563.764 | p > 0.05 (M7 vs. M8) | none | |
| M8a | p0 = 0.981 (p1 = 0.018); p = 0.160, q = 3.708, ωs = 0.800 | 0.052 | −6562.619 | p > 0.05 (M8 vs. M8a) | none | |
| NaBP | M0 | ω = 0.127 | 0.127 | −2060.857 | none | |
| M3 | p0 = 0.580, p1 = 0.405 (p2 = 0.014); ω0 = 0.011, ω1 = 0.278, ω2 = 1.598 | 0.143 | −2031.819 | p < 0.001 (M0 vs. M3) | 8P** | |
| M1a | p0 = 0.893 (p1 = 0.107), ω0 = 0.077 (ω1 = 1.000) | 0.176 | −2039.934 | not allowed | ||
| M2a | p0 = 0.893, p1 = 0.074 (p2 = 0.033); ω0 = 0.078, ω1 = 1.000, ω2 = 1.000 | 0.176 | −2039.934 | p > 0.05 (M1a vs. M2a) | none | |
| M7 | p = 0.312, q = 1.818 | 0.143 | −2033.003 | not allowed | ||
| M8 | p0 = 0.991 (p1 = 0.009); p = 0.368, q = 2.431, ωs = 1.915 | 0.144 | −2031.822 | p > 0.05 (M7 vs. M8) | 8P | |
| M8a | p0 = 0.958 (p1 = 0.042),; p = 0.401, q = 3.130, ωs = 0.800 | 0.139 | −2032.608 | p > 0.05 (M8 vs. M8a) | 8P |
aModels are according to the descriptions given in the methods.
bNumbers of parameters for different models were 1 (M0), 2 (M1a), 4 (M2a), 5 (M3), 2 (M7), 3 (M8a) and 4 (M8).
cLRT statistics of M3 vs. M0 are tests of heterogeneity of selection pressures among codon sites, while those of M2a vs. M1a, M8 vs. M7 and M8 vs. M8a are tests of positive selection, all of which assess the LRT statistic (2δlnL) against a chi-square distribution with degrees of freedom (d.f.) equal to the difference in the number of parameters between the nested models under comparison.
dA positively selected amino acid site with posterior probability P > 99 (**) is shown. Identification of amino acid under positive selection is based on näive empirical Bayes (NEB) (under M3) or Bayes empirical Bayes (BEB) inference (under M2a, M8 or M8a).
Discussion
Most of the 26 tested wild species of Convolvulaceae were found to be natural hosts for SPCFV, including H. sublobata, L. owariensis and 15 Ipomoea species. Previously, I. aquatica, I. purpurea and I. wightii were shown to be infectible with SPCFV following experimental inoculation [54], but this study showed that these species can be naturally infected with SPCFV. Furthermore, SPCSV was found to infect 12 species in the field, including H. sublobata, L. owariensis and 10 wild Ipomoea species. These results significantly extend our knowledge of the natural host ranges of SPCSV and SPCFV.
Many of the wild plants tested contained double or triple infections of SPFMV, SPMMV and/or SPCSV. These mixed infections have not been reported previously. However, no wild species were co-infected with SPCFV and any of the other three viruses. This was in striking contrast to cultivated sweetpotatoes, which are frequently co-infected with SPCFV and one or more of the other viruses both in our analysis and in previous studies in East Africa [53, 58, 61]. Furthermore, our previous studies have shown that several wild Convolvulaceae species are co-infected with the SPFMV strains EA and C in the field in Uganda [22]. The C strain was proposed to be a new species [71] and was recently designated as Sweet potato virus C [72]. In sweetpotatoes, the incidence of SPFMV and SPCSV infections can be as high as 70% in Uganda [58], which in turn increases the incidence of co-infection, development of SPVD and significant yield losses.
Perennial host plants and generalist vectors of viruses could be expected to enhance mixed infection. In East Africa including Uganda, the perenniality of sweetpotato in the local cropping system favours accumulation of viruses and mixed infections are common [42, 46]. Also, mixed virus infections are known in perennial wild plants, e.g., [13, 73–75]. However, whether high incidence of mixed virus infections could be linked to the plants’ perennial or annual lifecycle requires further study. For example, an annual grass species with less resistance to virus infection showed a high potential of acting as a reservoir of a generalist plant virus that also infects perennial grass hosts growing in the same habitat [2, 76–79]. Furthermore, co-infection by a group of vectored viral pathogens is highest with abundant generalist vectors (which are able to transmit multiple virus species/strains), weak cross‐protection and co-infection–induced mortality [75, 80]. Although it is known that aphids transmit SPFMV, and whiteflies (Bemisia tabaci and Trialeurodes abutilonea) transmit SPCSV, the vectors for SPCFV and SPMMV remain to be confirmed [55]. This currently limits our ability to elucidate the impact of vectors on the contrasting incidences of mixed viral infections in wild species and sweetpotatoes. However, cross-protection between any of the virus species in our study is unlikely, because it requires high sequence homology [81]. Therefore, the most probable explanation of our observed low incidences of mixed infections may be inefficient vector transmission of viruses between the wild plants or between cultivated and wild plants [31] and/or high levels of virus resistance in wild species preventing infection or keeping virus titers at undetectable levels [12, 82]. Furthermore, synergistic or additive effects of multiple virus infections causing severe disease could have eliminated co-infected plants [1–2, 5, 83–86]. These effects can vary among populations [12, 87, 88], species [89] and environments [75, 90, 91].
Contrasting virus incidences in wild plants may be explained by community contexts and processes [92, 93]. For example, in the luteovirus complex (barley and cereal yellow dwarf viruses) in California grasslands, virus prevalence is shaped by interactions within the plant community and among host plants, insect vectors, herbivores and abiotic factors [92, 94–96]. Although general differences in natural vegetation types have been previously noted in Uganda [57, 58], empirical data on host plant community composition needs to be strengthened to warrant testable hypotheses on contrasting regional virus incidences in wild plants.
Observation of disease symptoms is the initial step in viral disease diagnosis. Although virus-like symptoms were observed, no characteristic symptoms could be associated with a particular virus for several reasons, including mixed infections and condition of the host. Furthermore, many SPCFV- and SPCSV-infected wild plants remained symptomless, which seems common among wild plants [5, 8, 13, 18, 89, 97]. In addition, some symptom-expressing plants tested negative for SPCFV, SPCSV, SPMMV and SPFMV, indicating possible infection with other viruses that could not be detected with the antibodies and PCR primers used due to assay specificities. It seems worthwhile to continue these studies using generic methods, such as small-RNA deep sequencing, that require no presumptions about the viruses present and can detect all types of viruses simultaneously [98–102].
The CP and NaBP sequences of five SPCFV isolates from three wild host species and their comparison with 11 SPCFV isolates from cultivated sweetpotato in Uganda revealed nearly identical nucleotide diversity indices and no phylogenetic evidence of diversification because of the host species. Negative selection was implicated in the evolution of CP and NaBP. Negative constraints imposed by mutations on viral CPs may be associated with multiple functions such as genome encapsidation and protection, cell-to-cell movement, transmission between plants and host and/or vector interactions. Chare and Holmes [103] analyzed selection pressures in CP-coding sequences of plant RNA viruses and found that vector-borne viruses are subjected to greater negative selection than non-vectored viruses. Negative selective pressure is usually interpreted as a mechanism of preserving the structure and function of proteins [70, 104]. The CP of SPCFV and other carlaviruses is multifunctional [104–106], whereas NaBP is a cysteine-rich protein (CRP) implicated in RNA silencing suppression, nuclear localization and viral pathogenesis [68, 107–109]. In NaBP and CP, different codon positions were subjected to varying levels of purifying selection, possibly to provide a balance between the need to maintain protein structure and function and the effectiveness of these functions. The lack of a CRP in the sweet potato C-6 carlavirus (SPC6V) [110] may also indicate that CRPs are to some extent redundant in carlaviruses.
Most of the wild plants in this study were collected from the vicinity of sweetpotato fields or grew as weeds in sweetpotato fields. This makes it easier for putative vectors to transmit viruses between wild and cultivated hosts. Indeed, the observed similarities and lack of phylogenetic congruence with wild and cultivated hosts suggests frequent exchange of SPCFV isolates between the wild plants and sweetpotatoes. Similarly, no phylogenetic association with any hosts has been found in three other carlavirus species (Shallot latent virus, Garlic latent virus and Common garlic latent virus) infecting six different Allium spp. [111]; isolates of SPMMV, SPFMV and SPCSV in Uganda [22–24]; Rice yellow mottle virus (genus Sobemovirus) in cultivated rice and wild graminaceous species in East, Central and West Africa [28, 112] and African cassava mosaic virus and East African cassava mosaic Cameroon virus (genus Begomovirus, family Geminiviridae) in cassava and various wild hosts in West Africa [113].
Phylogenetic clustering of SPCFV isolates was congruent with their geographic origin in East Africa or Asia, demonstrating diversification. This has also been shown for several other economically harmful viruses infecting sweetpotato, cassava or rice, suggesting that East Africa is a center of evolutionary diversification and emergence of many new plant viruses and virus strains. For example, the East African (EA) strain of SPFMV (SPFMV-EA) is mainly found in East Africa [22, 71, 114–117], where it is undergoing rapid molecular adaptation compared with other strains of SPFMV and Sweet potato virus C (SPVC) [22]. Until recently, an EA strain of SPCSV (SPCSV-EA) was restricted to East Africa. The SPCSV-EA isolates vary in the presence or absence of a coding region for a p22 RNA silencing suppressor, whereas SPCSV isolates from outside East Africa typically lack the p22 [24, 42, 118–120]. SPMMV is geographically restricted to East Africa [23, 71, 121], in contrast to SPCFV, which is found on many continents. Preliminary evidence suggests that SPCFV isolates from East Africa may be distinguished from those occurring elsewhere by phylogenetic analysis of CP sequences [54]. However, the inclusion of additional SPCFV isolates from East Africa and analysis of CP and NaBP sequences in this study clearly showed that SPCFV isolates from East Africa form a unique phylogenetic group. Hence, we propose the name SPCFV-EA for the strains typical of East Africa.
Other plant viruses also seem to have a center of diversification in East Africa. Cassava brown streak virus and Ugandan cassava brown streak virus occur in East Africa, where they have a modular distribution in Indian Ocean coastal areas and the mainland Lake Victoria basin [122–126]. However, they are now spreading to other areas [127, 128]. Cassava mosaic geminiviruses, including a highly virulent recombinant strain, exhibit a gradient of decreasing prevalence (100% to 38%) from eastern to southern Africa [129, 130]. Rice yellow mottle virus exhibits phylogenetic congruence with the geographical origin of isolates on an east-to-west transect across Africa and showed decreased nucleotide diversity westward across Africa [28, 112, 131, 132]. The recently emerged strain S4ug of the virus in Eastern Uganda is thought to be the outcome of singular interplay between strains in East Africa and Madagascar [133]. Although there are relatively few characterized isolates of SPCFV (n = 38), the strong phylogenetic affinity to their origin in East Africa is another piece of evidence implicating East Africa as a hot spot for diversification of important plant viruses.
Taken together, the current study further highlights wild plants as reservoirs of viruses in agro-ecosystems. The four viruses detected in wild Convolvulaceae plants in Uganda cause major constraints in sweetpotato production in East Africa. Symptomless viral infections in wild plant species were common, which is typical of viruses in wild plants and reflects adaptation [8–10, 97]. Plant viruses and their principal hosts often have common centers of origin [134–136]. The sweetpotato originated in Central and/or South America and was dispersed to Africa and other continents only during the last 300 years, although there is evidence of prehistoric cultivation in Australasia and the South Pacific [137–142]. If viruses had been dispersed along with the sweetpotato, it would be expected that identical isolates of SPFMV, SPCSV, SPMMV and SPCFV would occur worldwide. This seems to be the case for SPFMV strains RC, O and C (SPVC), but apparently not for SPFMV-EA, SPCSV-EA or SPCFV-EA, which are largely geographically confined to East Africa [22, 24, 57, 58, 71, 114, 143, 144], this study. The origin of SPMMV is likely to be East Africa, and the sweetpotato is probably not its primary host [23]. Hence, it seems that these sweetpotato viruses are undergoing unique processes of evolution and adaptation in sweetpotato landraces and wild Convolvulaceae species in East Africa.
Materials and Materials
Field surveys and sampling
Wild plants (family Convolvulaceae; genera Astripomoea, Ipomoea, Hewittia and Lepistemon) including annual, biannual and perennial species were observed for virus symptoms, and a total of 1640 and 1224 plants were collected in the four agro-ecological zones of Uganda (Fig 1) in 2004 and 2007, respectively, as described [57]. All the sampling sites in all zones were on privately owned land and the owners gave gave permission to conduct the study on these sites. The field studies did not involve endangered or protected species. Five to ten leaves (preferably with virus-like symptoms) and two to five cuttings (length, 10–25 cm) were sampled from each plant. Cuttings were planted in an insect-proof screenhouse at the Makerere University Agricultural Research Institute, Kabanyolo (MUARIK), Uganda. The plants studied were mainly in close proximity to sweetpotato cultivation or grew as weeds in sweetpotato fields. Wild plants were identified taxonomically using keys from Verdcourt [145] and by DNA barcoding (accession no. FJ795781-FJ795796) as described [22, 57]. In addition, a total of 419 cultivated sweetpotato plants were sampled from fields in whose vicinity wild plants were collected.
Serological detection of SPCFV and SPCSV in wild plants
To detect viruses, leaf discs (2 cm in diameter) were excised from 5–10 leaves of a plant, combined and tested by nitrocellulose membrane enzyme-linked immunosorbent assay (NCM-ELISA) using polyclonal antibodies as described [57, 146]. The antibodies were provided by the International Potato Center (CIP), Lima, Peru. All wild plants and sweetpotatoes were tested for SPCFV, but only wild plants and sweetpotatoes sampled in 2007 were tested for SPCSV. Leaf discs were also excised as above for triple antibody sandwich ELISA (TAS-ELISA) for serological testing [147] using polyclonal antibodies specific to the EA strain of SPCSV (antibodies provided by CIP). Testing was repeated on plants established in the screenhouse.
Scions of 25 wild plants seronegative for SPCFV, 40 plants seronegative for SPCSV (but displaying virus-like symptoms) and 30 symptomless plants seronegative for SPCFV and SPCSV were grafted onto 2-wk-old plants of I. setosa Kerr., a sensitive indicator and nearly universal host of sweetpotato–infecting viruses [148, 149]. The grafted I. setosa plants were observed for virus symptoms and tested serologically for SPCFV and SPCSV 3 and 4 wk after grafting, respectively, as described above.
The SPCSV isolates detected in wild plants were graft-transmitted to sweetpotato plants of cultivar ‘Tanzania’ for ease of maintenance and further analysis.
Molecular detection of SPCFV and SPCSV
The presence of SPCFV and SPCSV was verified in 5 and 30 seropositive samples, respectively, by RT-PCR. Total RNA was extracted from 200 mg leaf tissue using TRIzol Reagent (Invitrogen) according to the manufacturer’s instructions. First-strand cDNA was synthesized from 3 μg total RNA using an oligo-dT25 primer (for SPCFV) or random hexamers (for SPCSV) and Moloney murine leukemia virus reverse transcriptase RNase H−(Finnzymes) according to the manufacturer’s instructions. The cDNA was diluted 10-fold for use in PCR.
The 3′-proximal part of the SPCFV genome (1578 nt according to AY461421), including the CP- and NaBP-coding regions and the 3′-UTR [67], was PCR-amplified using primers designed in this study (forward primer CFVF: 5′-GTCTTTAGR(A/G)TTK(G/T)TR(A/G)AGAY(T/C)TTA-3′; reverse primer CFVR: 5′-GCTCAAAAGTACTTTAAAAC-3′). These primers were complementary to nt 7527–7547 and 9085–9104 in the triple gene block 3 (TGB3) protein-coding sequence and 3′-UTR genomic region of SPCFV (AY461421), respectively. For SPCSV, the 3′ genomic region of RNA1 was amplified using forward primer CSVR3-F2 (5′-GTGTTTCATACATTGTTTGTGTGCT-3′) and reverse primer CSVp22-R2 (5′-AGGTGTATGACTCTAGGGTATAAAC-3′) [24]. The PCR mixture and cycling parameters were those recommended for Phusion High-Fidelity DNA Polymerase (Finnzymes).
PCR products were purified using a combination of exonuclease I (ExoI) and calf intestinal alkaline phosphatase (CIAP) (Fermentas) as recommended by the manufacturer. ExoI degrades excess primers (ssDNA) and CIAP degrades unincorporated dNTPs, both of which may inhibit the dideoxy PCR sequencing reaction [150]. Purified products from two independent PCRs were sequenced directly in both directions using the Big Dye Terminator kit version 3.1 (Applied Biosystems) on an ABI automatic 3130 XL Genetic Analyzer. The sequences obtained were compared by BLAST search with existing sequences available in the National Center for Biotechnology Information (NCBI) database.
Multiple sequence alignments and fitting of nucleotide substitution models
Nucleotide sequences were aligned using CLUSTALX version 1.83 [151], examined visually and translated into amino acid sequences using the EMBOSS web translation tool (http://www.ebi.ac.uk/emboss/transeq/index.html). Percent nucleotide and amino acid identities between sequences were computed using the CLUSTALW procedure [152] as implemented in the MEGALIGN program of the DNASTAR software package.
A ML method implemented in MEGA6 [153] was used to find the best nucleotide substitution model explaining the mode of evolution. Models with the lowest Bayesian information criterion (BIC) scores were considered to best describe the substitution pattern.
Tests for recombination and phylogenetic relationships between SPCFV isolates
The presence of recombination in the sequence data was tested using the pairwise homoplasy index test [154] as implemented in SplitsTree4 version V4.14.2 [155]. Parent-like sequences and approximation of recombination breakpoints were assessed using the RDP, GENECONV, BOOTSCAN, MAXIMUM CHI SQUARE, CHIMAERA and SISTER SCAN methods as implemented in the Recombination Detection Program (RDP4) package [156].
A phylogenetic tree based on CP sequences was constructed using the neighbor joining method [157] and the Tamura three-parameter nucleotide substitution model (T92) [69] with invariant sites and gamma distribution of rates across sites (T92+G+I). Initially, the general time-reversible (GTR) models [158] with invariant sites and gamma distribution of rates across sites (GTR+G+I) or with variable sites (GTR+G) were the most appropriate models for nucleotide substitution for the CP data. However, because of problems associated with implementing the GTR model [159, 160], the T92 model with invariant sites and gamma distribution of rates across sites (T92+G+I) was thus used for the CP, because it provided the next lowest BIC score. For construction of phylogenetic tree based on NaBP sequences, T92 with gamma distribution across sites (T92+G) was used. Both substitution models were deduced by model fitting (above), which allowed modeling of evolutionary rate differences among sites. A bootstrapped consensus tree was inferred from 1000 replicates for each of the above data sets for CP and NaBP. All phylogenetic analyses were implemented using MEGA6 [153].
Nucleotide diversities and population differentiation in SPCFV
Population genetics parameters with respect to the average number of nucleotide differences between two random sequences in a population (or nucleotide diversity index, π) and the average number of nucleotide substitutions per non-synonymous (πa) and synonymous (πs) sites were computed. Synonymous codon usage bias was measured by quantifying the codon bias index (CBI) [161] and the effective number of codons (ENC) [162] used in a gene.
The extent of genetic differentiation or level of gene flow between subpopulations was evaluated by estimating FST. FST measures the degree of genetic differentiation between two putative subpopulations by comparing the agreement between two haplotypes drawn at random from each subpopulation with the agreement obtained when the haplotypes are taken from the same subpopulation. FST ranges from 0 to 1 for undifferentiated to fully differentiated populations, respectively. Population genetics parameters and gene flow estimates were calculated using DnaSP version 5 [163].
Analysis of selection pressure on CP and NaBP
The ratio of non-synonymous (dN) to synonymous (dS) nucleotide substitution rates (ω = dN/dS) provides a sensitive measure of selective constraints at the protein level. Values of ω < 1, ω = 1 and ω > 1 indicate purifying (or negative) selection, neutral evolution and diversifying (or positive) selection, respectively. Based on this, the direction and intensity of selection pressure on a functional protein can be predicted [70, 164]. The maximum likelihood (ML) approach was applied to the CP (38 sequences) and NaBP (32 sequences) used in phylogenetic analysis of SPCFV using seven site models of codon evolution implemented in the CODEML program of the PAML package (version 4.7) [165]. The models used include M0 (one-ratio), M1a (nearly neutral), M2a (positive selection), M3 (discrete), M7 (beta), M8 (beta & ω) and M8a (beta & ω = 1) as described [104, 166, 167]. The probability of observing data was computed as the log likelihood, which is the sum of probabilities over all codons in the sequence. Selection pressure was examined by assessing the value ω and comparing the log likelihoods of nested models (M0 versus M3, M1a versus M2a, M7 versus M8 and M8 vs. M8a) in likelihood ratio tests (LRTs) as described [166, 168]. Where LRTs were significant, a Bayes empirical Bayes inference [167] was used to identify the amino acid(s) under positive selection.
Supporting Information
(DOC)
(DOC)
(DOC)
(DOC)
Acknowledgments
We thank Professor Patrick R. Rubaihyo (Makerere University) for supporting the study. We also thank Allan Saasi, Joshua Okonya, Irene Yekko and Moses Ocan (Makerere University), for technical assistance during the field work. We are grateful to anonymous reviewers for their constructive comments that greatly improved the manuscript.
Data Availability
All nucleic sequence data generated in this study (for 9 isolates of SPCFV) have been submitted to the GenBank database (https://www.ncbi.nlm.nih.gov/genbank/). Their accession numbers are indexed in Table 4 in the manuscript.
Funding Statement
This work was funded by the Academy of Finland (1276136 to JPTV) and is gratefully acknowledged. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Malmstrom CM, Hughes CC, Newton LA, Stoner CJ. Virus infection in remnant native bunchgrasses from invaded California grasslands. New Phytol. 2005; 168: 217–230. 10.1111/j.1469-8137.2005.01479.x [DOI] [PubMed] [Google Scholar]
- 2.Malmstrom CM, McCullough AJ, Newton LA, Johnson HA, Borer ET. Invasive annual grasses indirectly increase virus incidence in California native perennial bunchgrasses. Oecologia. 2005; 145: 153–164. 10.1007/s00442-005-0099-z [DOI] [PubMed] [Google Scholar]
- 3.Borer ET, Hosseini PR, Seabloom EW, Dodson AP. Pathogen-induced reversal of native dominance in a grassland community. Proc Natl Acad Sci USA. 2007; 104: 5473–5478. 10.1073/pnas.0608573104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.HilleRisLambers J, Yelenik SJ, Colman BP, Levine JM. California annual grass invaders: the drivers or passengers of change? J Ecol. 2010; 98: 1147–1156. 10.1111/j.1365-2745.2010.01706.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vincent SJ, Coutts BA, Jones RAC. Effects of introduced and indigenous viruses on native plants: Exploring their disease causing potential at the agro-ecological interface. PLoS ONE. 2014; 9(3): e91224 10.1371/journal.pone.0091224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones RAC, Coutts BA. Spread of introduced viruses to new plants in natural ecosystems and the threat this poses to plant biodiversity. Mol Plant Pathol. 2015; 16: 541–545. 10.1111/mpp.12268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roossinck MJ, Garcίa-Arenal F. Ecosystem simplification, biodiversity loss and plant virus emergence. Curr Opin Virol. 2015; 10: 56–62. 10.1016/j.coviro.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper I, Jones RAC. Wild plants and viruses: under-investigated ecosystems. Adv Virus Res. 2006; 67: 1–47. 10.1016/S0065-3527(06)67001-2 [DOI] [PubMed] [Google Scholar]
- 9.Roossinck MJ. Plant virus ecology. PLoS Pathog. 2013; 9:e1003304 10.1371/journal.ppat.1003304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stobbe AH, Roossinck MJ. Plant virus metagenomics: what we know and why we need to know more. Front Plant Sci. 2014; 5: 150, 10.3389/fpls.2014.00150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraile A, García-Arenal F. Environment and evolution modulate plant virus pathogenesis. Curr Opin Virol. 2016; 17: 50–56. 10.1016/j.coviro.2016.01.008 [DOI] [PubMed] [Google Scholar]
- 12.Malmstrom CM, Alexander HM. Effects of crop viruses on wild plants. Curr Opin Virol. 2016; 19: 30–36. 10.1016/j.coviro.2016.06.008 [DOI] [PubMed] [Google Scholar]
- 13.Prendeville HR, Ye X, Morris TJ, Pilson D. Virus infections in wild plant populations are both frequent and often unapparent. Amer J Bot. 2012; 99: 1033–1042. [DOI] [PubMed] [Google Scholar]
- 14.Roossink MJ, Martin DP, Roumagnac P. Plant Virus Metagenomics: Advances in virus discovery. Phytopathology. 2015; 105: 716–727. 10.1094/PHYTO-12-14-0356-RVW [DOI] [PubMed] [Google Scholar]
- 15.Wren JD, Roossinck MJ, Nelson RS, Scheets K, Palmer MW, Melcher U. Plant virus biodiversity and ecology. PLoS Biol. 2006; 4(3): e80 10.1371/journal.pbio.0040080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muthukumar V, Melcher U, Pierce M, Wiley GB, Roe BA, Palmer MW, et al. Non-cultivated plants of the tallgrass prairie preserve of northeastern Oklahoma frequently contain virus-like sequences in particulate fractions. Virus Res. 2009; 141: 169–173. 10.1016/j.virusres.2008.06.016 [DOI] [PubMed] [Google Scholar]
- 17.Roossinck MJ. Plant virus metagenomics: biodiversity and ecology. Annu Rev Genet. 2012; 46: 357–367. [DOI] [PubMed] [Google Scholar]
- 18.Roossinck MJ, Saha P, Wiley GB, Quan J, White JD, Lai H, et al. Ecogenomics: using massively parallel pyrosequencing to understand virus ecology. Mol Ecol. 2010; 19: 81–88. 10.1111/j.1365-294X.2009.04470.x [DOI] [PubMed] [Google Scholar]
- 19.Nagano AJ, Honjo MN, Mihara M, Sato M, Kudoh H. Detection of plant viruses in natural environments by using RNA-Seq. In: Uyeda I, Masuta C, editors. Plant Virology Protocols Volume 1236, Methods in Molecular Biology; 2015. pp. 89–98. [DOI] [PubMed] [Google Scholar]
- 20.Ooi K, Ohshita S, Ishii I, Yahara T. Molecular phylogeny of geminivirus infecting wild plants in Japan. J Plant Res. 1997; 110: 247–257. [Google Scholar]
- 21.García-Andrés S, Monci F, Navas-Castillo J, Mariones E. Begomovirus genetic diversity in the native plant reservoir Solanum nigrum: evidence for presence of a new virus species of recombinant nature. Virology. 2006; 350: 433–442. 10.1016/j.virol.2006.02.028 [DOI] [PubMed] [Google Scholar]
- 22.Tugume AK, Cuéllar WJ, Mukasa SB, Valkonen JPT. Molecular genetic analysis of virus isolates from wild and cultivated plants demonstrates that East Africa is a hotspot for the evolution and diversification of Sweet potato feathery mottle virus. Mol Ecol. 2010; 19: 3139–3156. 10.1111/j.1365-294X.2010.04682.x [DOI] [PubMed] [Google Scholar]
- 23.Tugume AK, Mukasa SB, Kalkkinen N, Valkonen JPT. Recombination and selection pressure in the ipomovirus Sweet potato mild mottle virus (Potyviridae) in wild species and cultivated sweetpotato in the centre of evolution in East Africa. J Gen Virol. 2010; 91: 1092–1108. 10.1099/vir.0.016089-0 [DOI] [PubMed] [Google Scholar]
- 24.Tugume AK, Amayo R, Weinheimer I, Mukasa SB, Rubaihayo PR, Valkonen JPT. Genetic variability and evolutionary implications of RNA silencing suppressor genes in RNA1 of Sweet potato chlorotic stunt virus isolates infecting sweetpotato and related wild species. PLoS ONE. 2013; 8(11): e81479 10.1371/journal.pone.0081479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen HD, Tomitaka Y, Ho SYW, Duchene S, Vetten H-J, et al. Turnip mosaic potyvirus probably first spread to Eurasian Brassica crops from wild orchids about 1000 years ago. PLoS ONE. 2013; 8(2): e55336 10.1371/journal.pone.0055336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreno-Pérez MG, Pagán I, Aragón-Caballero L, Cáceres F, Fraile A, García-Arenal F. Ecological and genetic determinants of Pepino mosaic virus emergence. J Virol. 2014; 88: 3359–3368. 10.1128/JVI.02980-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon S-J, Choi G-S, Yoon J-Y, Seo J-K, Choi H-S. Identification of Leonurus sibiricus as a weed reservoir for three pepper-infecting viruses. Plant Pathol J. 2016; 32: 65–69. 10.5423/PPJ.NT.07.2015.0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Traoré O, Sorho F, Pinel A, Abubakar Z, Banwo O, Maley J, et al. Process of diversification and dispersion of Rice yellow mottle virus inferred from large-scale and high resolution phylogeographical studies. Mol Ecol. 2005; 14: 2097–2110. 10.1111/j.1365-294X.2005.02578.x [DOI] [PubMed] [Google Scholar]
- 29.Tomitaka Y, Ohshima K. A phylogeographical study of the Turnip mosaic virus population in East Asia reveal an 'emergent' lineage in Japan. Mol Ecol. 2006; 15: 4437–4457. 10.1111/j.1365-294X.2006.03094.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fargette D, Konaté G, Fauquet C, Muller E, Peterschmitt M, Thresh JM. Molecular ecology and emergence of tropical plant viruses. Ann Rev Phytopathol. 2006; 44: 235–260. [DOI] [PubMed] [Google Scholar]
- 31.Alexander HM, Mauck KE, Whitfield AE, Garrett KA, Malmstrom CM. Plant-virus interactions and the agro-ecological interface. Eur J Plant Pathol. 2014; 138: 529–547. [Google Scholar]
- 32.Jones RAC. Plant virus ecology and epidemiology: historical perspectives, recent progress and future prospects. Ann Appl Biol. 2014; 164: 320–347. [Google Scholar]
- 33.Thresh JM. Control of tropical plant virus diseases. Adv Virus Res. 2006; 67: 245–295. 10.1016/S0065-3527(06)67007-3 [DOI] [PubMed] [Google Scholar]
- 34.Canto T, Aranda MA, Fereress A. Climate change effects on physiology and population processes of hosts and vectors that influence the spread of hemipteran-borne plant viruses. Glob Change Biol. 2009; 15: 1884–1894. [Google Scholar]
- 35.Rybicki EP, Pietersen G. Plant virus disease problems in the developing world. Adv Virus Res. 1999; 53: 127–178. [DOI] [PubMed] [Google Scholar]
- 36.Strange RN, Scott PR. Plant Disease: a threat to global food security. Ann Rev Phytopathol. 2005; 43: 83–116. [DOI] [PubMed] [Google Scholar]
- 37.Alabi OJ, Kumar PL, Naidu RA. Cassava mosaic disease: A curse to food security in Sub-Saharan Africa. Online APSnet Features. 2011; [Google Scholar]
- 38.Mukhopadhyay SK, Chattopadhyay A, Chakraborty I, Bhattacharya I. Crops that feed the world 5. Sweetpotato. Sweetpotatoes for income and food security. Food Secur. 2011; 3: 283–305. [Google Scholar]
- 39.Low J, Lynam J, Lemaga B, Crissman C, Barker I, Thiele G, et al. Sweetpotato in Sub-Saharan Africa In: Loebenstein G, Thottapilly G, editors. The Sweetpotato. Springer Science and Business Media, Dordrecht; 2009. pp. 359–390. [Google Scholar]
- 40.Reynolds TW, Waddington SR, Anderson CL, Chew A, True Z, Cullen A. Environmental impacts and constraints associated with the production of major food crops in Sub-Saharan Africa and South Asia. Food Secur. 2015; 7: 765–822. [Google Scholar]
- 41.Valverde RA, Clark CA, Valkonen JPT. Viruses and virus disease complexes of sweetpotato. Plant Vir. 2007; 1: 116–126. [Google Scholar]
- 42.Clark CA, Davis JA, Abad JA, Cuéllar WJ, Fuentes S, Kreuze JF, et al. Sweetpotato viruses: 15 years of progress on understanding andmanaging complex diseases. Plant Dis. 2012; 96: 168–185. [DOI] [PubMed] [Google Scholar]
- 43.Gu Y-H, Tao X, Lai X-J, Wang H-Y, Zhang Y-Z. Exploring the polyadenylated RNA virome of sweetpotato through high-throughput sequencing. PLoS ONE. 2014; 9(6): e98884 10.1371/journal.pone.0098884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gibson RW, Mwanga ROM, Namanda S, Jeremiah SC, Barker I. Review of sweetpotato seed systems in East and Southern Africa. Integrated Crop Management Working Paper. International Potato Center (CIP); 2009.
- 45.Namanda S, Gibson R, Sindi K. Sweetpotato seed systems in Uganda, Tanzania, and Rwanda. J Sust Agric. 2011; 35: 870–884. [Google Scholar]
- 46.Adikini S, Mukasa SB, Mwanga ROM, Gibson RW. Sweetpotato cultivar degeneration under high and low sweet potato virus disease pressure zones in Uganda. Cad J Plant Pathol. 2015; 37: 136–147. [Google Scholar]
- 47.Karyeija RF, Kreuze JF, Gibson RW, Valkonen JPT. Synergistic interactions of a potyvirus and a phloem-limited crinivirus in sweetpotato plants. Virology. 2000; 269: 26–36. 10.1006/viro.1999.0169 [DOI] [PubMed] [Google Scholar]
- 48.Njeru RW, Mburu MWK, Cheramgoi E, Gibson RW, Kiburi ZM, Obudho E, et al. Studies on the physiological effects of viruses on sweet potato yield in Kenya. Ann Appl Biol. 2004; 145: 71–76. [Google Scholar]
- 49.Mukasa SB, Rubaihayo PR, Valkonen JPT. Interactions between a crinivirus, an ipomovirus and a potyvirus in co-infected sweetpotato plants. Plant Pathol. 2006; 55: 458–467. [Google Scholar]
- 50.Ngailo S, Shimelis H, Sibiya J, Mtunda K. Screening of Tanzanian sweetpotato germplasm for yield and related traits and resistance to sweet potato virus disease. Acta Agric Scand B Soil Plant. 2016; 66: 52–66. [Google Scholar]
- 51.Untiveros M, Fuentes S, Salazar LF. Synergistic interaction of Sweet potato chlorotic stunt virus (Crinivirus) with carla-, cucumo-, ipomo-, and potyviruses infecting sweetpotato. Plant Dis. 2007; 91: 669–676. [DOI] [PubMed] [Google Scholar]
- 52.Cuéllar WJ, Galvez M, Fuentes S, Tugume J, Kreuze J. Synergistic interactions of begomoviruses with Sweet potato chlorotic stunt virus (genus Crinivirus) in sweetpotato (Ipomoea batatas L.). Mol Plant Pathol. 2015; 16: 459–471. 10.1111/mpp.12200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ateka EM, Njeru RW, Kibaru AG, Kimenju JW, Barg E, Gibson RW, et al. Identification and distribution of viruses infecting sweetpotato in Kenya. Ann Appl Biol. 2004; 144: 371–379. [Google Scholar]
- 54.Aritua V, Barg E, Adipala E, Gibson RW, Lesemann DE, Vetten HJ. Host range, purification, and genetic variability in Sweet potato chlorotic fleck virus. Plant Dis. 2009; 93: 87–93. [DOI] [PubMed] [Google Scholar]
- 55.Tugume AK, Mukasa SB, Valkonen JPT. Transmission of the viruses commonly detected in sweetpotatoes and wild species of Convolvulaceae in East Africa: many gaps to fill In: Brown JK editor. Vector-mediated transmission of plant pathogens. St. Paul: American Phytopathological Society; 2016. pp. 447–452. [Google Scholar]
- 56.Hollings M, Stone OM, Bock KR. Purification and properties of Sweet potato mild mottle, a whitefly born virus from sweetpotato (Ipomoea batatas) in East Africa. Ann Appl Biol. 1976; 82: 511–528. [DOI] [PubMed] [Google Scholar]
- 57.Tugume AK, Mukasa SB, Valkonen JPT. Natural wild hosts of Sweet potato feathery mottle virus show spatial differences in virus incidence and virus-like diseases in Uganda. Phytopathology. 2008; 98: 640–652. 10.1094/PHYTO-98-6-0640 [DOI] [PubMed] [Google Scholar]
- 58.Mukasa SB, Rubaihayo PR, Valkonen JPT. Incidence of viruses and virus-like diseases of Sweetpotato in Uganda. Plant Dis. 2003; 87: 329–335. [DOI] [PubMed] [Google Scholar]
- 59.Opiyo SA, Ateka EM, Owuor PO, Manguro LOA, Karuri HW. Survey of sweetpotato viruses in western Kenya and detection of Cucumber mosaic virus. J Plant Pathol. 2010; 92: 797–801. [Google Scholar]
- 60.Ndunguru J, Kapinga R, Sseruwagi P, Sayi B, Mwanga R, Tumwegamire S, et al. Assessing the sweetpotato virus disease and its associated vectors in northwestern Tanzania and central Uganda. Afr J Agric Sci. 2009; 4: 334–343. [Google Scholar]
- 61.Njeru RW, Bagabe MC, Nkezabahizi D, Kayiranga D, Kajuga J, Butare L, et al. Viruses infecting sweetpotato in Rwanda: occurrence and distribution. Ann Appl Biol. 2008; 153: 215–221. [Google Scholar]
- 62.Sossah FL, Appiah AS, Oduro V, Amoatey HM, Owusu GK, Oppong A, et al. Incidence of sweetpotato viruses in the coastal savannah agro-ecological zone of Ghana. J Plant Pathol. 2015; 97: 109–117. [Google Scholar]
- 63.Jones RAC, Dwyer GI. Detection of Sweet potato chlorotic fleck virus and Sweet potato feathery mottle virus—strain O in Australia. Aust Plant Pathol. 2007; 36: 591–594. [Google Scholar]
- 64.Kwak HR, Kim MK, Shin JC, Lee YJ, Seo JK, Lee HU, et al. The current incidence of viral disease in Korean sweet potatoes and development of multiplex RT-PCR assays for simultaneous detection of eight sweetpotato viruses. Plant Pathol J. 2014; 30: 416–424. 10.5423/PPJ.OA.04.2014.0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deng XG, Zhu F, Zhu T, Lin HH, Xi DH. First report of Sweet potato chlorotic fleck virus infecting sweetpotato in Sichuan Province, China. Plant Dis. 2014; 98: 163. [DOI] [PubMed] [Google Scholar]
- 66.Maina S, Edwards OR, de Almeida L, Ximenes A, Jones RAC. Complete genome sequences of the Carlavirus Sweet potato chlorotic fleck virus from East Timor and Australia. Genome Announc. 2016; 4(3):e00414–16. 10.1128/genomeA.00414-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aritua V, Barg E, Adipala E, Vetten HJ. Sequence analysis of the entire RNA genome of a Sweet potato chlorotic fleck virus isolate reveals that it belongs to a distinct carlavirus species. Arch Virol. 2007; 152: 813–818. 10.1007/s00705-006-0891-z [DOI] [PubMed] [Google Scholar]
- 68.Deng XG, Peng XJ, Zhu F, Chen YJ, Zhu T, Qin SB, et al. A critical domain of Sweet potato chlorotic fleck virus nucleotide-binding protein (NaBp) for RNA silencing suppression, nuclear localization and viral pathogenesis. Mol Plant Pathol. 2015; 16: 365–375. 10.1111/mpp.12186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tamura K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases. Mol Biol Evol. 1992; 9: 678–87. [DOI] [PubMed] [Google Scholar]
- 70.Goldman N, Yang Z. A codon-based model of nucleotide substitution for protein coding DNA sequences. Mol Biol Evol. 1994; 11: 725–736. [DOI] [PubMed] [Google Scholar]
- 71.Tairo F, Mukasa SB, Jones RAC, Kullaya A, Rubaihayo PR, Valkonen JPT. Unravelling the genetic diversity of the three main viruses involved in sweetpotato virus disease (SPVD), and its practical implications. Mol Plant Pathol. 2005; 6: 199–211. 10.1111/j.1364-3703.2005.00267.x [DOI] [PubMed] [Google Scholar]
- 72.Untiveros M, Quispe D, Kreuze JF. Analysis of complete genomic sequences of isolates of the Sweet potato feathery mottle virus strains C and EA: molecular evidence for two distinct potyvirus species and two P1 protein domains. Arch Virol. 2010; 155: 2059–2063. 10.1007/s00705-010-0805-y [DOI] [PubMed] [Google Scholar]
- 73.Hammond J. Viruses occurring in Plantago species in England. Plant Pathol. 1981; 30: 237–243. [Google Scholar]
- 74.Raybould AF, Maskell lC, Edwards M-l, Cooper JI, Gray AJ. The prevalence and spatial distribution of viruses in natural populations of Brassica oleracea. New Phytol. 1999; 141: 265–275. [DOI] [PubMed] [Google Scholar]
- 75.Seabloom EW, Hosseini PR, Power AG, Borer ET. Diversity and composition of viral communities: Coinfection of Barley and Cereal yellow dwarf viruses in California grasslands. The Amer Nat. 2009; 173: 3. [DOI] [PubMed] [Google Scholar]
- 76.Borer ET, Mitchell CE, Power AG, Seabloom EW. Consumers indirectly increase infection risk in grassland food webs. Proc Natl Acad Sci USA. 2009; 106: 503–506. 10.1073/pnas.0808778106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cronin JP, Welsh ME, Dekkers MG, Abercrombie ST, Mitchell CE. Host physiological phenotype explains pathogen reservoir potential. Ecol Lett. 2010; 13: 1221–1232. 10.1111/j.1461-0248.2010.01513.x [DOI] [PubMed] [Google Scholar]
- 78.Cronin JP, Rua MA, Mitchell CE. Why is living fast dangerous? Disentangling the roles of resistance and tolerance of disease. Am Nat. 2014; 184: 172–187. 10.1086/676854 [DOI] [PubMed] [Google Scholar]
- 79.Hily JM, García A, Moreno A, Plaza M, Wilkinson MD, Fereres A, et al. The Relationship between host lifespan and pathogen reservoir potential: An analysis in the system Arabidopsis thaliana-Cucumber mosaic virus. PLoS Pathog. 2014; 10(11): e1004492 10.1371/journal.ppat.1004492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Power AG. Community ecology of plant viruses In: Roossinck MJ, editor. Plant virus evolution. Springer-Verlag, Berlin, Heidelberg; 2008. pp. 15–26. [Google Scholar]
- 81.Valkonen JPT, Rajamäki ML, Kekarainen T. Mapping of viral genomic regions important in cross-protection between strains of a potyvirus. Mol Plant-Microbe Interact. 2002; 15: 683–692. 10.1094/MPMI.2002.15.7.683 [DOI] [PubMed] [Google Scholar]
- 82.Karyeija RF, Gibson RW, Valkonen JPT. Resistance to sweetpotato virus disease (SPVD) in wild East African Ipomoea. Ann Appl Biol. 1998; 133: 39–44. [Google Scholar]
- 83.Friess N, Maillet J. Influence of Cucumber mosaic virus infection on the competitive ability and fitness of purslane (Portulaca oleraccea). New Phytol. 1996; 132: 103–111. [DOI] [PubMed] [Google Scholar]
- 84.Maskell LC, Raybould AF, Cooper JI, Edwards M.-L, Gray AJ. Effects of Turnip mosaic virus and Turnip yellow mosaic virus on survival, growth and reproduction of wild cabbage (Brassica oleracea). Ann Appl Biol. 1996; 135: 401–407. [Google Scholar]
- 85.Funayama S, Terashima I, Yahara T. Effects of virus infection and light environment on population dynamics of Eupatorium makinoi (Asteraceae). Amer J Bot. 2001; 88: 616–622. [PubMed] [Google Scholar]
- 86.Prendeville HR, Tenhumberg B, Pilson D. Effects of virus on plant fecundity and population dynamics. New Phytol. 2014; 202: 1346–1356. 10.1111/nph.12730 [DOI] [PubMed] [Google Scholar]
- 87.Yahara T, Oyama K. Effects of virus-infection on demographic traits of an agamospermous population of Eupatorium chinense (Asteraceae). Oecologia. 1993; 96: 310–315. [DOI] [PubMed] [Google Scholar]
- 88.Thurstom MI, Pallet DW, Cortina-Borja M, Edwards M.-L, Raybould AF, Cooper JI. The incidence of viruses in wild Brassica nigra in Dorset (UK). Ann Appl Biol. 2001; 139: 277–284. [Google Scholar]
- 89.Remold SK. Unapparent virus infection and host fitness in three weedy grass species. J Ecol. 2002; 90: 967–977. [Google Scholar]
- 90.Seabloom EW, Borer ET, Jolles A, Mitchell CE. Direct and indirect effects of viral pathogens and the environment on invasive grass fecundity in Pacific Coast grasslands. J Ecol. 2009; 97: 1264–1273. [Google Scholar]
- 91.Seabloom EW, Borer ET, Mitchell CE, Power AG. Viral diversity and prevalence gradients in North American Pacific coast grasslands. Ecology. 2010; 91: 721–732. [DOI] [PubMed] [Google Scholar]
- 92.Power AG, Borer ET, Hosseini P, Mitchell CE, Seabloom EW. The community ecology of barley/cereal yellow dwarf viruses in Western US grasslands. Virus Res. 2011; 159: 95–100. 10.1016/j.virusres.2011.05.016 [DOI] [PubMed] [Google Scholar]
- 93.Seabloom EW, Borer ET, Gross K, Kendig AE, Lacroix C, Mitchell CE, et al. The community ecology of pathogens: coinfection, coexistence and community composition. Ecol Lett. 2015; 18: 401–415. 10.1111/ele.12418 [DOI] [PubMed] [Google Scholar]
- 94.Borer ET, Seabloom EW, Mitchell CE, Power AG. Local context drives infection of grasses by vector-borne generalist viruses. Ecol Lett. 2010; 13: 810–818. 10.1111/j.1461-0248.2010.01475.x [DOI] [PubMed] [Google Scholar]
- 95.Moore SM, Borer ET. The influence of host diversity and composition on epidemiological patterns at multiple spatial scales. Ecology. 2012; 93: 1095–1105. [DOI] [PubMed] [Google Scholar]
- 96.Moore SM, Manore CA, Bokil VA, Borer ET, Hosseini PR. Spatiotemporal model of Barley and Cereal yellow dwarf virus transmission dynamics with seasonality and plant competition. Bull Math Biol. 2011; 73: 2707–2730. 10.1007/s11538-011-9654-4 [DOI] [PubMed] [Google Scholar]
- 97.Roossinck MJ. Lifestyles of plant viruses. Phil Trans R Soc B. 2010; 365: 1899–1905. 10.1098/rstb.2010.0057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kreuze JF, Perez A, Untiveros M, Quispe D, Fuentes S, Barker I, et al. Complete viral genome sequence and discovery of novel viruses by deep sequencing of small RNAs: a generic method for diagnosis, discovery and sequencing of viruses. Virology. 2009; 388: 1–7. 10.1016/j.virol.2009.03.024 [DOI] [PubMed] [Google Scholar]
- 99.Bi Y, Tugume AK, Valkonen JPT. Small-RNA deep sequencing reveals Arctium tomentosum as a natural host of Alstroemeria virus X and a new putative emaravirus. PLoS ONE. 2012; 7(8): e42758 10.1371/journal.pone.0042758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kashif M, Pietilä S, Artola K, Jones RAC, Tugume AK, Mäkinen V, et al. Detection of viruses in sweetpotatoes from Honduras and Guatemala augmented by deep-sequencing of small-RNAs. Plant Dis. 2012; 96:1430–1437. [DOI] [PubMed] [Google Scholar]
- 101.Mbanzibwa DR, Tugume AK, Chiunga E, Mark D, Tairo FD. Small RNA deep sequencing-based detection and further evidence of DNA viruses infecting sweetpotato plants in Tanzania. Ann Appl Biol. 2014; 165: 329–339. [Google Scholar]
- 102.Visser M, Burger JP, Maree HJ. Targeted virus detection in next-generation sequencing data using an automated e-probe based approach. Virology. 2016; 495: 122–128. 10.1016/j.virol.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 103.Chare ER, Holmes EC. Selection pressures in the capsid genes of plant RNA viruses reflect mode of transmission. J Gen Virol. 2004; 83: 3149–3157. [DOI] [PubMed] [Google Scholar]
- 104.Yang Z, Nielsen R, Goldman N, Pedersen AK. Codon-substitution models for heterogeneous selection pressure at amino acid sites. Genetics. 2000; 155: 431–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ivanov KI, Mäkinen K. Coat proteins, host factors and plant viral replication. Curr Opin Virol. 2012; 2: 712–718. 10.1016/j.coviro.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 106.Weber PH, Bujarski JJ. Multiple functions of capsid proteins in (+) stranded RNA viruses during plant-virus interactions. Virus Res. 2015; 196: 140–149. 10.1016/j.virusres.2014.11.014 [DOI] [PubMed] [Google Scholar]
- 107.Senshu H, Ozeki J, Komatsu K, Hashimoto M, Hatada K, Aoyama M, et al. Variability in the level of RNA silencing suppression caused by triple gene block protein 1 (TGBp1) from various potexviruses during infection. J Gen Virol. 2009; 90: 1014–1024. 10.1099/vir.0.008243-0 [DOI] [PubMed] [Google Scholar]
- 108.Senshu H, Yamaji Y, Minato N, Shiraishi T, Maejima K, Hashimoto M, et al. A dual strategy for the suppression of host antiviral silencing: two distinct suppressors for viral replication and viral movement encoded by Potato virus M. J Virol. 2011; 85: 10269–10278. 10.1128/JVI.05273-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lukhovitskaya NI, Gushchin VA, Solovyev AG, Savenkov EI. Making sense of nuclear localization: A zinc-finger protein encoded by a cytoplasmically replicating plant RNA virus acts a transcription factor, Plant Sig Beh. 2013; 8: 8, e25263, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.De Souza J, Fuentes S, Savenkov EI, Cuéllar W, Kreuze JF. The complete nucleotide sequence of Sweet potato C6 virus: a carlavirus lacking a cysteine-rich protein. Arch Virol. 2013; 158: 1393–1396. 10.1007/s00705-013-1614-x [DOI] [PubMed] [Google Scholar]
- 111.Tsuneyoshi T, Matsumi T, Deng TC, Sako I, Sumi S. Differentiation of Allium carlaviruses isolated from different parts of the world based on the viral coat protein sequence. Arch Virol. 1998; 143: 1093–1107. [DOI] [PubMed] [Google Scholar]
- 112.Fargette D, Pinel A, Abubakar Z, Traoré O, Brugidou C, et al. Inferring the evolutionary history of Rice yellow mottle virus from genomic and phylogeographic studies. J Virol. 2004; 78: 3252–3261. 10.1128/JVI.78.7.3252-3261.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alabi OJ, Ogbe FO, Bandyopadhyay R, Kumar PL, Dixon AGO, Hughes J, et al. Alternate hosts of African cassava mosaic virus and East African mosaic Cameron virus in Nigeria. Arch Virol. 2008; 153: 1743–1747. 10.1007/s00705-008-0169-8 [DOI] [PubMed] [Google Scholar]
- 114.Kreuze JF, Karyeija RF, Gibson RW, Valkonen JPT. Comparisons of coat protein gene sequences show that East African isolates of Sweet potato feathery mottle virus is a genetically distinct group. Arch Virol. 2000; 145: 567–574. [DOI] [PubMed] [Google Scholar]
- 115.Untiveros M, Fuentes S, Kreuze J. Molecular variability of Sweet potato feathery mottle virus and other potyviruses infecting sweetpotato in Peru. Arch Virol. 2008; 153: 473–483. 10.1007/s00705-007-0019-0 [DOI] [PubMed] [Google Scholar]
- 116.Ha C, Revill P, Harding RM, Vu M, Dale JL. Identification and sequence analysis of potyviruses infecting crops in Vietnam. Arch Virol. 2008; 153: 45–60. 10.1007/s00705-007-1067-1 [DOI] [PubMed] [Google Scholar]
- 117.Rännäli M, Czekaj V, Jones RAC, Fletcher JD, Davis RI, Mu L, et al. Molecular characterization of Sweet potato feathery mottle virus (SPFMV) isolates from Easter Island, French Polynesia, New Zealand, and Southern Africa. Plant Dis. 2009; 93: 933–939. [DOI] [PubMed] [Google Scholar]
- 118.Cuéllar WJ, Tairo F, Kreuze JF, Valkonen JPT. Analysis of gene content in Sweet potato chlorotic stunt virus RNA1 reveals the presence of p22 RNA silencing suppressor in only few isolates: implications to viral evolution and synergism. J Gen Virol. 2008; 89: 573–582. 10.1099/vir.0.83471-0 [DOI] [PubMed] [Google Scholar]
- 119.Cuéllar WJ, Cruzado RK, Fuentes S, Untiverso M, Soto M, Kreuze JF. Sequence characterization of a Peruvian isolate of Sweet potato chlorotic stunt virus: Further variability and a model for p22 acquisition. Virus Res. 2011; 157: 111–115. 10.1016/j.virusres.2011.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Qin Y, Wang L, Zhang Z, Qiao Q, Zhang D, Tian Y, et al. Complete genomic sequence and comparative analysis of the genome segments of Sweet potato chlorotic stunt virus in China. PLoS ONE. 2014; 9(8): e106323 10.1371/journal.pone.0106323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mukasa SB, Rubaihayo PR, Valkonen JPT. Sequence variability within the 3’-proximal part of the Sweet potato mild mottle virus genome. Arch Virol. 2003; 148: 487–496. 10.1007/s00705-002-0930-3 [DOI] [PubMed] [Google Scholar]
- 122.Mbanzibwa DR, Tian YP, Tugume AK, Mukasa SB, Tairo F, Kyamanywa S, at al. Genetically distinct strains of Cassava brown streak virus in the Lake Victoria basin and the Indian Ocean coastal area of East Africa. Arch Virol. 2009; 154: 353–359. 10.1007/s00705-008-0301-9 [DOI] [PubMed] [Google Scholar]
- 123.Mbanzibwa DR, Tian YP, Tugume AK, Mukasa SB, Tairo F, Kyamanywa S, et al. Simultaneous virus-specific detection of the two Cassava brown streak-associated viruses by RT-PCR reveals wide distribution in East Africa, mixed infections and infections in Manihot glaziovii. J Virol Meth. 2011; 171: 394–400. [DOI] [PubMed] [Google Scholar]
- 124.Mbanzibwa DR, Tian YP, Tugume AK, Patil BL, Yadav JS, Bagewadi B, et al. Evolution of cassava brown streak disease-associated viruses. J Gen Virol. 2011; 92: 974–987. 10.1099/vir.0.026922-0 [DOI] [PubMed] [Google Scholar]
- 125.Legg JP, Jeremiah SC, Obiero HM, Maruthi MN, Ndyetabula I, Okao-Okuja G, et al. Comparing the regional epidemiology of the Cassava mosaic and Cassava brown streak virus pandemics in Africa. Virus Res. 2011; 159: 161–170. 10.1016/j.virusres.2011.04.018 [DOI] [PubMed] [Google Scholar]
- 126.Ndunguru J, Sseruwagi P, Tairo F, Stomeo F, Maina S, Djikeng A, et al. Analyses of twelve new whole genome sequences of Cassava brown streak viruses and Ugandan cassava brown streak viruses from East Africa: Diversity, supercomputing and evidence for further speciation. PLoS ONE. 2015; 10(10): e0141939 10.1371/journal.pone.0141939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bigirimana S, Barumbanze P, Ndayihanzamaso P, Shirima R, Legg JP. First report of Cassava brown streak disease and associated Ugandan cassava brown streak virus in Burundi. New Dis Rep. 2011; 24: 26. [Google Scholar]
- 128.Mulimbi W, Phemba X, Assumani B, Kasereka P, Muyisa S, Ugentho H, et al. First report of Ugandan cassava brown streak virus on cassava in Democratic Republic of Congo. New Dis Rep. 2012; 26: 11. [Google Scholar]
- 129.Ndungruru J, Legg JP, Aveling TAS, Thompson G, Fauquet CM. Molecular biodiversity of cassava begomoviruses in Tanzania: evolution of Cassava geminiviruses in Africa and evidence for East Africa being a centre of diversity of cassava geminiviruses. Virol J. 2005; 2: 21 10.1186/1743-422X-2-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.De Bruyn A, Villemot J, Lefeuvre P, Villar E, Hoareau M, Harimalala M, et al. East African cassava mosaic-like viruses from Africa to Indian ocean islands: molecular diversity, evolutionary history and geographical dissemination of a bipartite begomovirus. BMC Evol Biol. 2012; 12: 228 10.1186/1471-2148-12-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Abubakar A, Ali F, Pinel A, Traoré O, N’Guessan P, Notteghem JL, et al. Phylogeography of Rice yellow mottle virus in Africa. J Gen Virol. 2003; 84: 733–743. 10.1099/vir.0.18759-0 [DOI] [PubMed] [Google Scholar]
- 132.Traoré O, Pinel-Galzi A, Sorho F, Sarra S, Rakotomalala M, Sangu E, et al. A reassessment of the epidemiology of Rice yellow mottle virus following recent advances in field and molecular studies. Virus Res. 2009; 141: 258–267. 10.1016/j.virusres.2009.01.011 [DOI] [PubMed] [Google Scholar]
- 133.Ochola D, Issaka S, Rakotomalala M, Pinel-Galzi A, Ndikumana I, Hubert J, et al. Emergence of Rice yellow mottle virus in eastern Uganda: Recent and singular interplay between strains in East Africa and in Madagascar. Virus Res. 2015; 195: 64–72. 10.1016/j.virusres.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 134.Lovisolo O, Hull R, Rosler O. Co-evolution of viruses with hosts and vectors and possible paleontology. Adv Virus Res. 2003; 62: 325–379. [DOI] [PubMed] [Google Scholar]
- 135.Anderson PK, Cunningham AA, Patel NG, Morales FJ, Epstein PR, Daszak P. Emerging infectious diseases of plants: pathogen pollution, climate change and agrotechnology drivers. Trends Ecol Evol. 2004; 19: 535–544. 10.1016/j.tree.2004.07.021 [DOI] [PubMed] [Google Scholar]
- 136.Jones RAC. Plant virus emergence and evolution: Origins, new encounter scenarios, factors driving emergence, effects of changing world conditions, and prospects for control. Virus Res. 2009; 141: 113–130. 10.1016/j.virusres.2008.07.028 [DOI] [PubMed] [Google Scholar]
- 137.Yen DE. The New Zealand kumara or sweetpotato. Econ Bot. 1963; 17: 31–45. [Google Scholar]
- 138.O'Brien PJ. The sweetpotato: its origin and dispersal. Am Anthropol. 1972; 74: 342–365. [Google Scholar]
- 139.Zhang D, Rossel G, Kriegner A, Hijmans R. AFLP assessment of diversity in sweetpotato from Latin America and the Pacific region: its implications of the dispersal of the crop. Gen Res Crop Evol. 2004; 51: 115–120. [Google Scholar]
- 140.Srisuwan S, Sihachakr D, Siljak-Yakolev S. The origin and evolution of sweetpotato (Ipomoea batatas Lam.) and its wild relatives through the cytogenetic approaches. Plant Sci. 2006; 171: 424–433. 10.1016/j.plantsci.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 141.Horrocks M, Lawlor I. Plant microfossil analysis of soils from Polynesian stonefields in South Auckland, New Zealand. J Archaeol Sci. 2006; 33: 200–217. [Google Scholar]
- 142.Roullier C, Duputie´ A, Wennekes P, Benoit L, Fernandez Bringas VM, Rossel G, et al. Disentangling the origins of cultivated Sweetpotato (Ipomoea batatas (L.) Lam.). PLoS ONE. 2013; 8(5): e62707 10.1371/journal.pone.0062707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Alicai T, Fenby NS, Gibson RW, Adipala E, Vetten HJ, Foster GD, et al. Occurrence of two serotypes of Sweet potato chlorotic stunt virus in East Africa and their associated differences in coat protein and HSP70 homologue gene sequences. Plant Pathol. 1999; 48: 718–726. [Google Scholar]
- 144.Mukasa SB, Tairo F, Kullaya A, Rubaihayo PR, Valkonen JPT. Coat protein sequence analysis reveals occurrence of new strains of Sweet potato feathery mottle virus in Uganda and Tanzania. Virus Gen. 2003; 27: 49–56. [DOI] [PubMed] [Google Scholar]
- 145.Verdcourt B. Convolvulaceae In: Hubbard CE, Milne-Redhead E, editors. Flora of Tropical East Africa. Whitefriars Press Ltd, London, Great Britain; 1963. pp. 1–161. [Google Scholar]
- 146.Gibb KS, Padovan AC. Detection of Sweet potato feathery mottle virus in sweetpotato grown in northern Australia using an efficient and simple assay. Int J Pest Manag. 1993; 39: 223–228. [Google Scholar]
- 147.Gibson RW, Mpembe I, Alicai T, Carey EE, Mwanga ROM, Seal SE, et al. Symptoms, aetiology and serological analysis of sweet potato virus disease in Uganda. Plant Pathol. 1998; 47: 95–102. [Google Scholar]
- 148.Schaefers GA, Terry ER. Insect transmission of sweetpotato disease agents in Nigeria. Phytopathology. 1976; 66: 642–645. [Google Scholar]
- 149.Brunt AA, Crabtree K, Dallwitz MJ, Gibbs AJ, Watson L. Viruses of Plants. Descriptions and Lists from VIDE Database. CAB International, Wallingford, UK; 1996. [Google Scholar]
- 150.Warle E, Schneider C, Renner M, Völker M, Fiehn W. Convenient single-step, one tube purification of PCR products for direct sequencing. Nucl Acids Res. 1994; 22: 4354–4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl Acids Res. 1997; 25: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Thompson JD, Higgins DG, Gibson TJ. CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through weighting, position-specific gap penalties and weight matrix choice. Nucl Acids Res. 1994; 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013; 30: 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bruen TC, Philippe H, Bryant D. A simple test for detecting the presence of recombination. Genetics. 2006; 172: 2665–2681. 10.1534/genetics.105.048975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Huson DH, Bryant D. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol. 2006; 23: 254–267. 10.1093/molbev/msj030 [DOI] [PubMed] [Google Scholar]
- 156.Martin DP, Murrell B, Golden M, Khoosal A, Muhire B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015; 1: 1–5, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Saitou N, Nei M. The neighbour-joining method, a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987; 4: 406–425. [DOI] [PubMed] [Google Scholar]
- 158.Tavaré S. Some probabilistic and statistical problems in the analysis of DNA sequences In: Miura RM, editor. Lectures on mathematics in the life sciences. Volume 17, Providence (RI) American Mathematica Society; 1986. pp. 57–86. [Google Scholar]
- 159.Sumner JG, Fernández-Sánchez J, Jarvis PD. Lie Markov models. J Theor Biol. 2012; 298: 16–31. 10.1016/j.jtbi.2011.12.017 [DOI] [PubMed] [Google Scholar]
- 160.Sumner JG, Jarvis PD, Fernández-Sánchez J, Kaine BT, Woodhams MD, Holland BR. Is the General Time-Reversible model bad for molecular phylogenetics? Syst Biol. 2012; 61: 1–6. [DOI] [PubMed] [Google Scholar]
- 161.Bennetzen JL, Hall BD (1982) Codon selection in yeast. J Biol Chem 257: 3026–3031. [PubMed] [Google Scholar]
- 162.Wright F. The 'effective number of codons' used in a gene. Gene. 1990; 87: 23–29. [DOI] [PubMed] [Google Scholar]
- 163.Librado P, Rozas J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009; 25: 1451–1452. 10.1093/bioinformatics/btp187 [DOI] [PubMed] [Google Scholar]
- 164.Miyata T, Miyazawa S, Yasunaga T. Two types of amino acid substitutions in protein evolution. J Mol Evol. 1979; 12: 219–236. [DOI] [PubMed] [Google Scholar]
- 165.Yang Z. PAML4: Phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007; 24: 1586–1591. 10.1093/molbev/msm088 [DOI] [PubMed] [Google Scholar]
- 166.Wong WSW, Yang Z, Goldman N, Nielsen R. Accuracy and power of statistical methods for detecting adaptive evolution in protein coding sequences and for identifying positively selected sites. Genetics. 2004; 168: 1041–1051. 10.1534/genetics.104.031153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yang Z, Wong WSW, Nielsen R. Bayes empirical Bayes inference of amino acid sites under positive selection. Mol Biol Evol. 2005; 22: 1107–1118. 10.1093/molbev/msi097 [DOI] [PubMed] [Google Scholar]
- 168.Swanson WJ, Nielsen R, Yang Q. Pervasive adaptive evolution in mammalian fertilization proteins. Mol Biol Evol. 2003; 20: 18–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability Statement
All nucleic sequence data generated in this study (for 9 isolates of SPCFV) have been submitted to the GenBank database (https://www.ncbi.nlm.nih.gov/genbank/). Their accession numbers are indexed in Table 4 in the manuscript.