Abstract
Vacuolar bacterial pathogens are sheltered within unique membrane-bound organelles that expand over time to support bacterial replication. These compartments sequester bacterial molecules away from host cytosolic immunosurveillance pathways that induce antimicrobial responses. The mechanisms by which the human pulmonary pathogen Legionella pneumophila maintains niche homeostasis are poorly understood. We uncovered that the Legionella-containing vacuole (LCV) required a sustained supply of host lipids during expansion. Lipids shortage resulted in LCV rupture and initiation of a host cell death response, whereas excess of host lipids increased LCVs size and housing capacity. We found that lipids uptake from serum and de novo lipogenesis are distinct redundant supply mechanisms for membrane biogenesis in Legionella-infected macrophages. During infection, the metabolic checkpoint kinase Mechanistic Target of Rapamycin (MTOR) controlled lipogenesis through the Serum Response Element Binding Protein 1 and 2 (SREBP1/2) transcription factors. In Legionella-infected macrophages a host-driven response that required the Toll-like receptors (TLRs) adaptor protein Myeloid differentiation primary response gene 88 (Myd88) dampened MTOR signaling which in turn destabilized LCVs under serum starvation. Inactivation of the host MTOR-suppression pathway revealed that L. pneumophila sustained MTOR signaling throughout its intracellular infection cycle by a process that required the upstream regulator Phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) and one or more Dot/Icm effector proteins. Legionella-sustained MTOR signaling facilitated LCV expansion and inhibition of the PI3K-MTOR-SREPB1/2 axis through pharmacological or genetic interference or by activation of the host MTOR-suppression response destabilized expanding LCVs, which in turn triggered cell death of infected macrophages. Our work identified a host metabolic requirement for LCV homeostasis and demonstrated that L. pneumophila has evolved to manipulate MTOR-dependent lipogenesis for optimal intracellular replication.
Author Summary
To evade host defenses certain bacterial pathogens establish unique membrane-bound compartments within infected cells where they replicate to large numbers. The membrane integrity of these vacuolar compartments is crucial for pathogen survival because rupture initiate host anti-microbial defenses. The causative agent of Legionnaires’ disease, Legionella pneumophila is a prototypical intracellular bacterial pathogen that establishes and replicates within a vacuolar compartment. How Legionella ensures integrity of the vacuolar compartment as it expands to accommodate hundreds of bacteria is unknown. We found that Legionella subverts the metabolism of its host by activating the central metabolic checkpoint kinase MTOR to sustain membrane production needed for the expansion of its intracellular niche. MTOR activation favored Legionella replication by increasing the housing capacity of the Legionella-occupied vacuole whereas interference with MTOR function led to destabilization, premature rupture and activation of host anti-microbial responses. Our work provides novel insight into how pathogens maximize intracellular replication by exploiting the host metabolic processes.
Introduction
Residence within a membrane-bound organelle is a survival strategy common to intracellular bacterial pathogens [1–3]. Legionella pneumophila, the etiological agent of a severe pneumonia known as Legionnaires’ disease, can establish a unique endoplasmic reticulum (ER)-derived vacuole in amoebae and also in mammalian macrophages [4–8]. The molecular strategies that Legionella has evolved to survive within a broad range of protozoan hosts allow the bacterium to replicate in alveolar macrophages during human infections [9]. The kinetics and the mechanism of LCV biogenesis are largely conserved in different host cells [4]. Within 30 min of phagocytosis, Legionella blocks endocytic maturation and initiates phagosomal membrane remodeling through the recruitment and fusion with early secretory vesicles [6,10–14]. By 4 hrs post-infection the LCV fuses with the ER and an intracellular niche that supports bacterial replication is established; however, the LCV retains features distinct form the ER [15], such as accumulation of ubiquitinated proteins [13,16]. In synchronized infections, bacterial replication starts at ~ 4 hrs and by 16 hrs a single LCV expands to and contains hundreds of bacteria [16,17]. At the end of a single replication cycle, the number of bacteria per vacuole varies widely among established LCVs [18,19], however the underlying mechanisms that support such heterogeneity are unclear. Thus, it is important to identify the processes that favor and the processes that limit bacterial replication within established LCVs.
Legionella species encode a type IVb secretion system (T4bSS), known as the Dot/Icm apparatus, which translocates over 300 bacterial effector proteins directly into the host cytosol [20–22]. The T4bSS is required for intracellular survival and deletion mutants lacking single structural components of the Dot/Icm apparatus are avirulent because they fail to block endocytic maturation [23–25]. Collectively, the Dot/Icm effector proteins facilitate niche biogenesis and homeostasis [20,22]. One example is the SdhA effector, which maintains LCV integrity by counteracting, through an unknown mechanism, the activity of the secreted Legionella phospholipase PlaA [18]. In macrophage infections, vacuoles containing ΔsdhA mutants rupture during the early stages of bacterial replication, release bacterial products in the host cytosol and trigger pyroptosis—an inflammatory host cell death that restricts bacterial replication [18,26,27]. Mutants lacking LidA, another Dot/Icm effector, also establish a rupture-prone LCV and fail to grow intracellularly, but only when LidA is deleted in combination of either WipB or MavP [28]. The evolution of multiple bacterial regulators highlights the importance of an intact LCV membrane for Legionella intracellular survival. Host regulators of LCVs stability are unknown; however, membrane biogenesis regulators likely are involved because in the course of 12 hrs the size of the pathogen-containing vacuole expands.
Membrane biogenesis in eukaryotes occurs at the ER, which is the main site of de novo synthesis of phopholipids and cholesterol from metabolic precursors [29]. Adaptive lipogenesis is mainly regulated through increase in gene expression of lipogenic enzymes mediated by the SREPB1/2 transcription factors [30–32]. In turn, SREBPs are controlled by the master metabolic checkpoint serine/threonine kinase MTOR [33–36]. MTOR responds to cues from energy and nutrient sensing pathways to initiate a global anabolic state in eukaryotic cells when conditions are favorable [37,38]. MTOR nucleates two distinct protein complexes—TORC1 and TORC2 that have unique as well as shared components, distinct substrate specificities and subcellular localizations [39,40]. Raptor and Rictor are unique scaffolding proteins that define the TORC1 and TORC2 complex, respectively [38,41]. Several pharmacological inhibitors of MTOR have been identified, including the ATP-competitive MTOR inhibitors PP242 and Torin1/2 as well as the macrolide rapamycin that inhibits phosphorylation of only a subset of TORC1 substrates [42–45]. In cell cultures, MTOR inhibitors block de novo lipogenesis and in vivo genetic models attribute lipogenic functions to both TORC complexes [34,36,37].
In addition to lipogenesis, MTOR controls immunity to infection by repressing inflammation and autophagy [46–49]. In epithelial cells infected by Salmonella and Shigella, an amino acid starvation response blocks MTOR signaling to promote autophagy [50]. Infections of primary BMMs with Legionella encoding a functional Dot/Icm system triggers a host-driven suppression of MTOR through ubiquitination-induced degradation of the upstream MTOR regulator Akt that promote inflammation [51]. In this study, we found that host-driven MTOR suppression requires Myd88 and interferes with a Legionella-induced MTOR-dependent lipogenic reprogramming mediated by one or more Dot/Icm effector proteins that control LCV homeostasis.
Results
Loss of the TLR adaptor Myd88 reveals TLR-independent MTOR signaling elicited by Dot/Icm+ Legionella
Previously, we reported that TLR-dependent activation of MTOR is suppressed when macrophages become infected with Legionella encoding a functional Dot/Icm system [51]. Thus, the role of the TLR adaptor Myd88, which is required for LPS-induced MTOR signaling [46,48], was investigated in the context of Legionella infection. To eliminate rapid pyroptosis induced by Legionella flagellin, flagellin-deletion mutants (ΔflaA) were used in this study [52]. Also, the cells were serum-starved for 10hrs prior to and for the duration of the infections to avoid growth factor-induced MTOR activation. There was no detectible decrease in cell viability caused by serum withdrawal under these conditions.
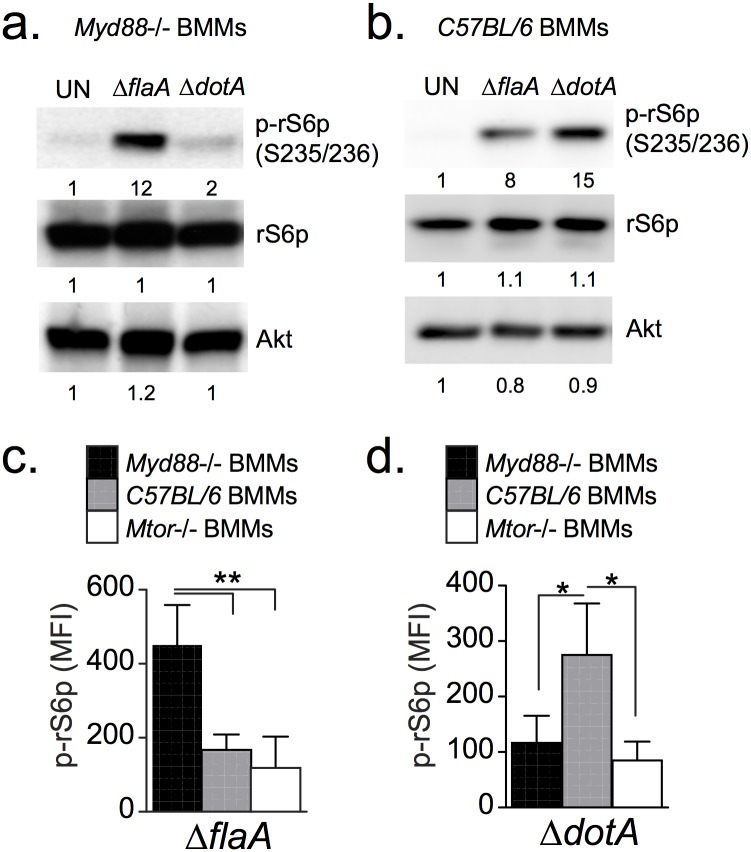
To assess mTOR signaling, Myd88-/- BMMs were infected with a pathogenic Dot/Icm+ strain (ΔflaA) or an avirulent Dot/Icm- strain (ΔdotA) and phosphorylation of the ribosomal S6 protein (rS6p) was measured by immunoblot analysis to assess MTOR activity (Fig 1a). Phosphorylation of rS6p at serine residues 235 and 236 by the S6 kinase 1 (S6K1) was used as readout for mTOR signaling because S6K1 is directly activated by TORC1 [53]. As expected, a ΔdotA strain did not induce rS6p phosphorylation in Myd88-/- BMMs because TLR signaling is defective; surprisingly, the Dot/Icm+ strain triggered a robust response (Fig 1a). These data are in sharp contrast with the attenuated rS6p phosphorylation elicited by the ΔflaA strain as compared to the ΔdotA strain in BMMs derived from C57BL/6 mice (Fig 1b). To analyze MTOR activity specifically in infected cells we quantitated phospho-rS6p fluorescence intensity using single-cell immunofluorescence analysis. These studies confirmed that robust phospho-rS6p was elicited by a ΔflaA strain in Myd88-/- BMMs and by a ΔdotA strain in C57BL/6 BMMs (Fig 1c and 1d and S1 Fig). Taken together, these data demonstrate that MTOR suppression in response to virulent Legionella requires MyD88 signaling, and that in the absence of MyD88 signaling virulent Legionella augment MTOR activation.
Fig 1. Analysis of rS6p phosphorylation in bone marrow-derived macrophages.
Immunoblot analysis of cell lysates from Myd88-/- (a) or C57BL/6 (b) BMMs that were serum-starved and infected with Legionella (MOI = 20) for 4 hrs as indicated under serum-free conditions. Quantified band intensities are normalized to uninfected conditions (UN) and listed below each blot. Single-cell analysis of rS6p phosphorylation in serum-starved BMMs infected with either ΔflaA (c) of ΔdotA (d). Graph shows means and 95% confidence intervals of phospho-rS6p fluorescent intensity signal for at least 100 infected cells for each condition. * p<0.05, ** p<0.005 (one-way ANOVA). (a-d) A representative of three biological replicates is shown for each experiment.
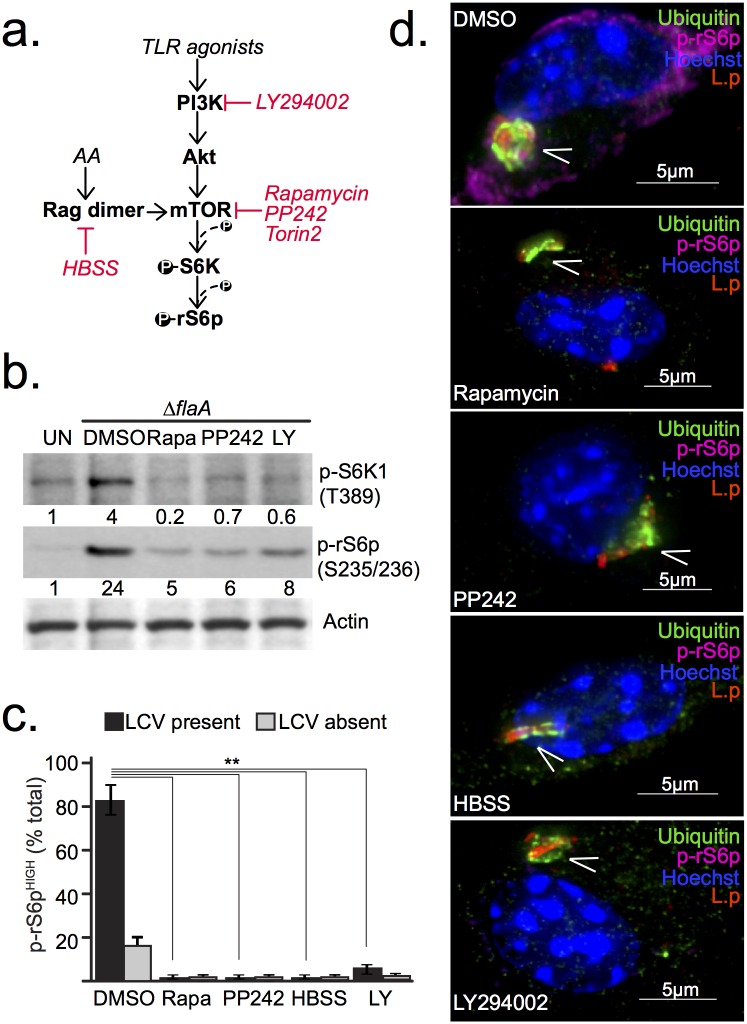
To exclude the possibility of an MTOR-independent phospho-rS6p response to the ΔflaA strain in Myd88-/- BMMs, MTOR function was inhibited in infected cells through various approaches (Fig 2a). Addition of the MTOR inhibitors PP242 or rapamycin to synchronized Legionella infections of Myd88-/- BMMs reduced phosphorylation of S6K1 and its substrate rS6p (Fig 2b). Single cells microscopy confirmed that phospho-rS6p signal was diminished in infected macrophages when MTOR inhibitors were added (Fig 2c and 2d). Amino acid starvation (HBSS treatment) or PI3K inhibition also ablated Legionella-induced phospho-rS6p in Myd88-/- BMMs (Fig 2b–2d). Under these infection conditions, MTOR inhibition was initiated after the infections were synchronized and therefore did not interfere with bacterial uptake. LCV maturation and bacterial viability were also unaffected by MTOR inhibition as evidenced by the ubiquitin accumulation on vacuoles containing bacteria with normal morphology (Fig 2d). Thus, loss of phospho-rS6p under MTOR inhibiting conditions was not a result of bacterial killing by the host cell or perturbations in LCV biogenesis. Together, these data indicate that phosphorylation of rS6p induced by Legionella in Myd88-/- BMMs requires PI3K/MTOR signaling and is an indicator of MTOR activity in the cell.
Fig 2. Legionella-induced phosphorylation of rS6p requires MTOR and PI3K activity.
(a) Simplified schematics of the PI3K/MTOR signaling axis indicating the inhibitors used in this study. PI3K is inhibited by LY294002. Rapamycin, PP242 and Torin2 act on MTOR and Hanks' Balanced Salt Solution (HBSS) blocks MTOR by starving cells for amino acids. (b-d) Analyses of serum-starved Myd88-/- BMMs unstimulated or infected with ΔflaA (MOI = 20) for 5hrs under serum-free conditions. Inhibitors—Rapamycin (200nM), PP242 (5μM), LY294002 (10μM)—or HBSS were added at the time of infection synchronization at 60 min post infection. (b) Immunoblot analysis of S6K1 and rS6p phosphorylation from cell lysates showing quantified band intensities normalized to uninfected conditions (UN). (c) Single cell immunofluorescence analysis of phospho-rS6 positive (MFI>300) Myd88-/-macrophages exposed to ΔflaA (MOI = 20). Graphed are the means and standard deviations (s.d) of technical triplicates for the two distinct groups within the cell population—infected (LCV present) and uninfected (LCV absent) for each condition. At least 100 cells were analyzed for each condition. ** p<0.005 (one-way ANOVA) (d) Immunofluorescense micrographs of representative infected cells from each condition stained with anti-L. pneumophila (L.p), anti-p-rS6p (S235/236), anti-ubiquitinated proteins (FK2) antibodies and Hoechst 33342. Arrowheads indicate Legionella-containing vacuoles, Bar = 5μm. (b-d) A representative of three biological replicates is shown for each experiment.
MTOR activation by Legionella is a Dot/Icm effector-driven process that is independent of intracellular niche biogenesis
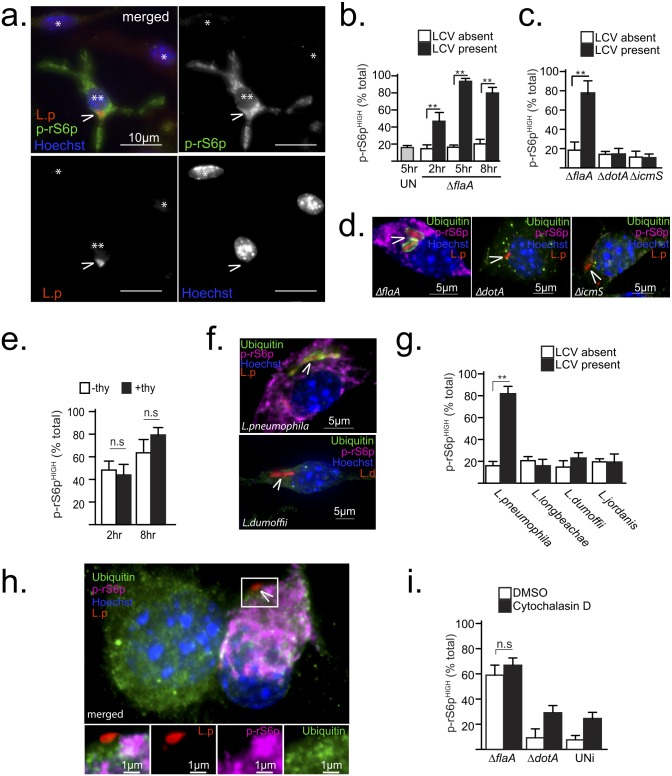
To gain insight into the mechanism by which Legionella infections activate MTOR in Myd88-/- BMMs, the kinetics of MTOR signaling was analyzed using fluorescence microscopy by quantification of phospho-rS6p positive cells at different times post-infection (Fig 3a and 3b). In synchronized infections, at 2hrs post-infection over 40% of infected cells stained positive for phospho-rS6p as compared to only 15% of neighboring uninfected cells (Fig 3b). In a population of unstimulated serum-starved BMMs only 17% of the cells were phospho-rS6p positive (Fig 3b). Over time, the number of phospho-rS6p positive infected cells further increased to over 80% at 5hrs and 8hrs post-infection. In contrast, the number of phospho-rS6p positive uninfected cells remained at basal level. Thus, a significant number of infected cells activated MTOR prior to initiation of bacterial replication and sustained high MTOR activity throughout the Legionella intracellular lifecycle.
Fig 3. Legionella-induced MTOR activation is a Dot/Icm effector-driven process independent of intracellular niche biogenesis.
(a-h) Show results from synchronized infections (MOI = 20) under serum-free conditions of serum-starved Myd88-/- BMMs treated as indicated. (a) Immonofluorescent micrograph of ΔflaA-infected (**) or neighboring uninfected (*) cells at 6 hrs post-infection stained with anti-L. pneumophila (L.p), anti-p-rS6p (S235/236) antibodies and Hoechst 33342. Arrowheads indicate Legionella-containing vacuoles, Bar = 10μm. (b-c and g) Single cell immunofluorescence analysis of phospho-rS6 positive (MFI>300) BMMs exposed to various Legionella strains for the indicated times (b) or for 8hrs (c and g). Means ± s.d of technical triplicates for the two distinct groups within the cell population—infected (LCV present) and uninfected (LCV absent) for each condition are shown. (d) Micrographs of cells infected with the indicated strains for 8hrs and stained with anti-L. pneumophila (L.p), anti-p-rS6p (S235/236), anti-ubiquitinated proteins (FK2) antibodies and Hoechst 33342. Arrowheads indicate intracellular bacteria, Bar = 5μm. (e) Quantitation of p-rS6p positive cells (MFI>300) in synchronized infections with a thyA ΔflaA strain in the presence (+thy) or absence (-thy) of thymidine for the indicated time periods. Means ± s.d of technical triplicates are shown. (f-g) Synchronized macrophages infections with different Legionella species are shown. (f) Representative micrographs of cells infected with L. pneumophila or L. dumoffii for 8hrs (MOI = 20) and stained as in (d), which are quantitated in (g). (h-i) Cells were treated with cytochalasin D (5μM) or vehicle (DMSO) for 15 min prior to and for the duration of infections (3hrs) with the indicated bacterial strains. (i) Quantitation of p-rS6p positive cells (MFI>300) in contact with bacteria (ΔflaA and ΔdotA) or uninfected cells (UNi). Means ± s.d of technical triplicates are shown. (h) Representative micrograph of macrophages treated with cytochalasin D (5μM) and infected with ΔflaA and stained as in (d). Arrowhead indicates a bacterium in contact with the macrophage, Bar = 1μm. At least 50 cells (i) or 100 cells (b, c, e and g) were analyzed for each condition. A representative of two (f-h) or three (a-g) biological replicates is shown for each experiment. (b, c, e, g and i) n.s—not significant, ** p<0.005 (unpaired T-test).
Because a functional Dot/Icm apparatus is essential for MTOR activation by Legionella (Fig 1a), a strain lacking the chaperone IcmS, which loads effectors into the Dot/Icm apparatus [54,55], was used to determine whether the apparatus or secretion of effectors through the apparatus is important for MTOR activation. The ΔicmS bacteria assemble a functional Dot/Icm apparatus but exhibits a severe defect in translocation of most effectors and therefore fails to establish an intracellular niche that supports bacterial replication in macrophages [56]. Both ΔdotA and ΔicmS strains failed to induce MTOR activity (Fig 3c and 3d) indicating that signaling is unlikely a host response to the Dot/Icm apparatus but rather efficient translocation of effectors is necessary for MTOR activation. Such a requirement indicates that Dot/Icm effectors mediate MTOR activation directly or indirectly through LCV biogenesis.
A potential indirect trigger for MTOR activation could be the need of the infected cell to offset a metabolic demand placed by a rapidly expanding membrane-bound organelle that contains replicating bacteria. Such a mechanism would require bacterial replication; however, a thymidine auxotroph strain activated MTOR irrespective of thymidine presence, indicating that organelle expansion is not the trigger (Fig 3e). Next, MTOR activation by other Legionella species was examined. L. dumoffii, L. longbeachae and L. jordanis have conserved LCV biogenesis programs but considerable diversity in their Dot/Icm effector repertoires. All the tested Legionella species established LCVs that supported bacterial replication, but only L. pneumophila infections activated MTOR (Fig 3f and 3g). In co-infections of Myd88-/- BMMs, L. dumoffii failed to repress MTOR signaling induced by L. pneumophila indicating that L. dumoffii neither induces nor suppresses MTOR (S2 Fig). Thus, MTOR is activated by a L. pneumophila-specific process and is independent of LCV expansion.
Next, the actin polymerization inhibitor cytochalasin D was used to investigate the possibility that LCV establishment could lead to MTOR induction. Treatment with cytochalasin D blocks LCV formation because phagocytosis is inhibited and the bacteria are retained on the extracellular interface of the plasma membrane. However, cell-surface associated bacteria still inject Dot/Icm effectors in the host cytosol [57]. Macrophages in contact with ΔflaA bacteria, but not ΔdotA bacteria, triggered MTOR irrespective of cytochalasin D treatment (Fig 3h and 3i). Therefore, MTOR induction by L. pneumophila is independent of LCV biogenesis altogether and strongly support the existence of Dot/Icm effector(s) that promote PI3K-dependent MTOR signaling. The pentuple deletion mutant lacking 71 Dot/Icm effector genes [58] still activated MTOR (S2c Fig), therefore a forward genetic screen would be required to identify the Legionella MTOR regulator(s).
Blocking Legionella-induced MTOR activation triggers host cell death
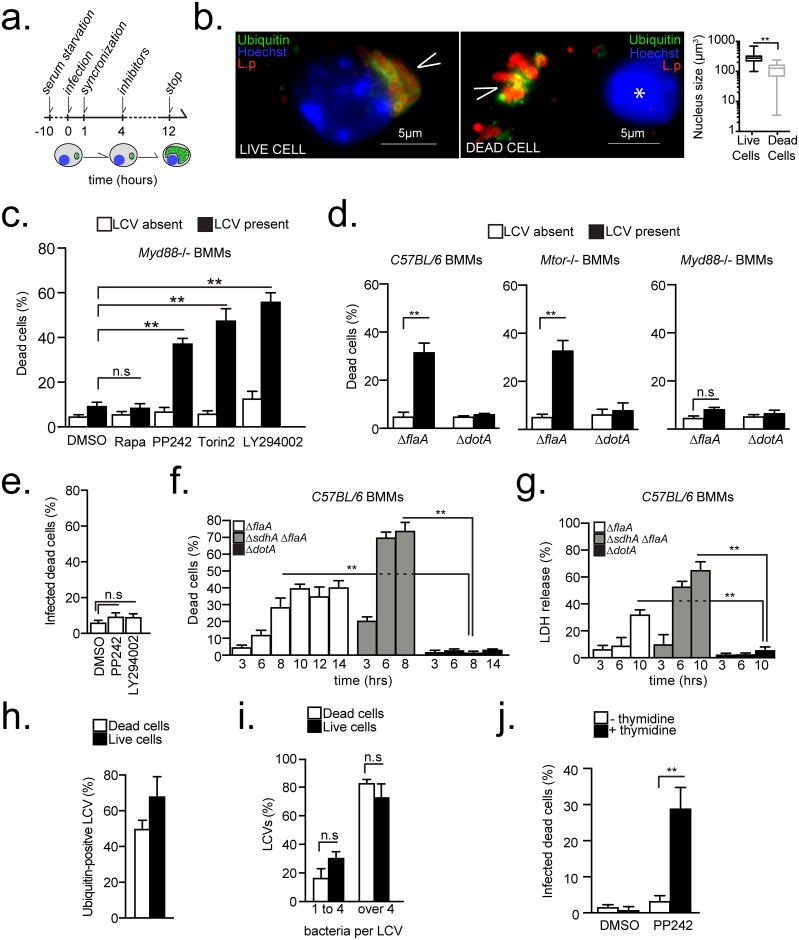
Next, the impact of sustained MTOR activation on Legionella intracellular life cycle was determined in macrophage infections under serum-free conditions that were allowed to progress for less than a single infection cycle (~16hrs) in the presence or absence of MTOR inhibitors (Fig 4a). Inhibitors were added at 4 hrs post infection and timed to coincide with the start of bacterial replication to eliminate potential interference with bacterial uptake and trafficking. A morphologically distinct population of infected cells characterized by condensed nuclei—decreased nuclear volume (from 284 to 141μm3) and increased fluorescence when stained with the DNA dye Hoechst 33342—was detected clearly under all infection conditions (Fig 4b and S3 Fig). Aberrant condensed nuclear morphology is a prototypical hallmark used to identify dying cell [59].
Fig 4. Loss of MTOR function triggers a host cell death response that requires bacterial replication.
(a) Experimental scheme for the results shown in (b-e and g-j). (b-j) BMMs were serum-starved and infected under serum-free conditions either at MOI = 20 (b-e and g-j) or MOI = 10 (f). (b) Micrographs showing representative of live and dead macrophages infected with Legionella for 12 hrs and stained with anti-L. pneumophila (L.p), anti-ubiquitinated proteins (FK2) antibodies and Hoechst 33342. Arrowheads indicate LCVs, Bar = 5μm. (*) marks the condensed nucleus of the dead cells. The mean nuclear volumes ± s.d of at least 100 live and 100 dead cells are graphed. (c-d) Quantitation of infected and neighboring uninfected Myd88-/- (c-d), C57BL/6 (d) and Mtor-/- (d) macrophages with condensed nuclei after infections with ΔflaA (c-d) or ΔdotA (d). Means ± s.d of technical replicates of dead cell as percentage of total cells in each condition are shown. (e) Quantitation of infected Myd88-/- BMMs with condensed nuclei after infection with L. dumoffii and treatment with inhibitors or vehicle as indicated. (f-g) Kinetics of the cell death response in C57BL/6 BMMs under serum starvation conditions infected as indicated. Quantitation of infected cells with condensed nuclei (f) and LDH released in the culture supernatants (g) are shown. (h-i) Analyses of ubiquitin recruitment (h) and LCV size (i) in live and dead Myd88-/- BMMs infected with ΔflaA and treated with PP242. (j) Cell death in infected Myd88-/- BMMs with thyA ΔflaA strain treated with vehicle (DMSO) or PP242 in the presence or absence of thymidine. (c, e, h-j) Rapamycin (250nM), PP242 (2.5μM), LY294002 (10μM), Torin2 (300nM). (c-j) Means ± s.d of technical triplicates for each condition are shown. At least 50 cells (e, h-j) or 200 cells (c-d) were analyzed for each condition. A representative of two (e-j) or three (c-d) biological replicates is shown for each experiment. (b-j) n.s—not significant, ** p<0.005 (unpaired T-test).
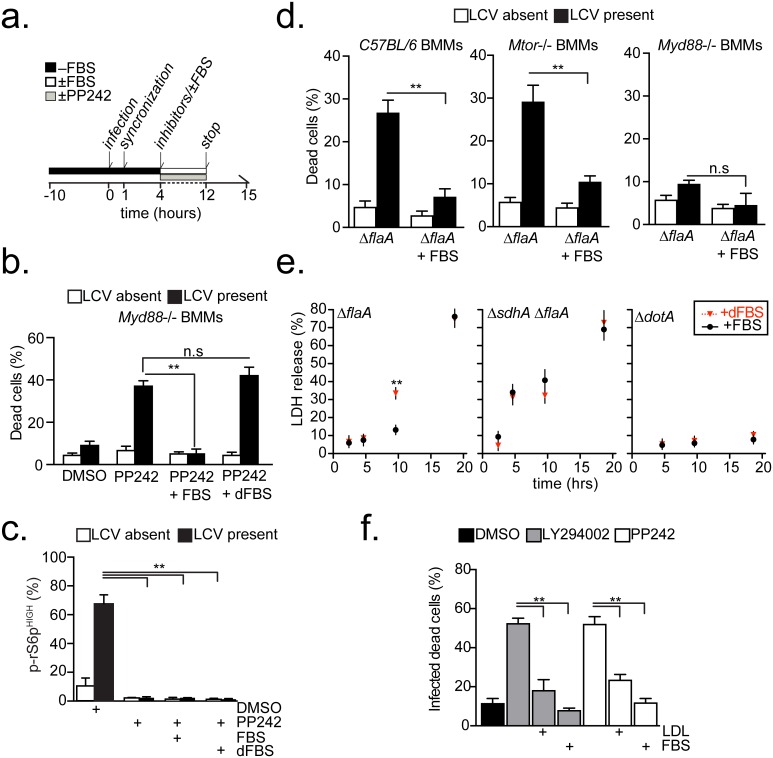
The number of dead Myd88-/- BMMs infected with Legionella, identified by their aberrant nuclear morphology, significantly increased from 8% to over 40% when PI3K (LY294002) or MTOR (PP242, Torin2) were inhibited (Fig 4c). This cell-death phenotype was not a result of inhibitor-induced cell toxicity because neighboring uninfected cells were not affected. Interestingly, rapamycin, which inhibits the phosphorylation of a subset of TORC1 substrates, such as rS6p [45], did not induce cell death in infected or uninfected cells (Fig 4c). Taken together, these data demonstrate that suppression of the Legionella-induced PI3K/MTOR signaling triggers a cell death response in infected Myd88-/- BMMs through a rapamycin-insensitive mechanism.
If Legionella-induced MTOR activity interferes with a host cell death response, it would be predicted that in the presence of TLR-signaling the host Akt/MTOR-ubiquitination pathway will phenocopy the effect of the pharmacological inhibitors in Myd88-/- BMMs infections. Indeed, a significant increase in the number of dead Legionella-infected cells was observed at 12 hrs post-infection in macrophages derived from C57BL/6 mice (Fig 4d). Similar results were obtained when macrophages derived from mice with MTOR ablation in the myeloid compartment were infected (Fig 4d). Importantly, under serum-free conditions, neighboring uninfected bystander macrophages from infections with either the ΔflaA or the ΔdotA strain did not undergo cell death. Also, Myd88-/- macrophages, which sustain MTOR activity, remained alive when infected with the ΔflaA strain. Thus, the host Akt/MTOR-ubiquitination pathway as well as pharmacological or genetic ablation of MTOR function triggered a cell-death response specifically in macrophages infected with virulent Legionella encoding functionally intact Dot/Icm system. However, the Dot/Icm apparatus itself did not trigger this cell death response because infections with L. dumoffii, which encodes a homologous Dot/Icm system, did not induce host cell death in Myd88-/- BMMs even when MTOR inhibitors were present (Fig 4e).
The host cell death in Legionella-infected macrophages triggered by MTOR suppression requires bacterial replication
Multiple bacteria within ubiquitin-positive LCVs were observed frequently within macrophages undergoing MTOR suppression-dependent cell death (similar to Fig 4b); therefore, the kinetics of the cell death response was investigated. Under MTOR suppressing conditions, BMMs with aberrant nuclear morphology infected with ΔflaA bacteria increased gradually starting at 6hrs after bacterial replication is initiated and peaked at 10-14hrs post-infection (Fig 4f). The release in the culture supernatants of the cytosolic enzyme lactate dehydrogenase (LDH) by dying macrophages also increased with similar kinetics (Fig 4g). In contrast, infections with the ΔsdhA mutant strain, which triggers cell death prior to bacterial replication [18], produced distinct kinetics that peaked at 6hrs post-infection (Fig 4f and 4g). Moreover, majority of LCVs within dead macrophages in MTOR-suppressing conditions were mature ubiquitin-positive LCVs that have supported bacterial replication (i.e. contained more than 4 bacteria) (Fig 4h and 4i). Thus, bacterial replication is likely initiated prior to host cell death. To determine whether bacterial replication is required for initiation of the MTOR-regulated host cell death, Myd88-/- BMMs were infected with a thymidine auxotroph ΔflaA strain. In those infections, host cell death was detected only in the presence of PP242 and thymidine, demonstrating that bacterial replication together with MTOR suppression are required for induction of host cell death (Fig 4j).
Serum-derived lipids rescue the host cell death phenotype brought by MTOR inhibition
The detection of a Legionella-induced host cell death response in C57BL/6 BMMs under serum starvation is intriguing because it is well established that ΔflaA L. pneumophila replicates in these macrophages without initiating a cell death response in the presence of fetal bovine serum (FBS) [52]. Therefore, the possibility that FBS could account for the distinct phenotypes was investigated by infecting macrophages for 12hrs (less than a single replication cycle) with Legionella in the presence/absence of FBS and MTOR inhibitors (Fig 5a). Under these conditions, serum supplementation effectively blocked host cell death in Legionella-infected Myd88-/- BMMs treated with the MTOR inhibitor PP242 (Fig 5b). Because, FBS did not interfere with PP242 activity (Fig 5c), these data indicate that FBS compensated for the loss of MTOR function. Supplementation with FBS also blocked the Legionella-induced cell death in BMMs derived from C57BL/6 and from Mtor-/- mice (Fig 5d) providing further evidence that FBS compensates for the reduction in MTOR function brought by multiple MTOR-suppression mechanisms.
Fig 5. Legionella-dependent cell death triggered by MTOR suppression is blocked by lipids supplementation.
(a) Experimental scheme for the results shown in (b-d, f). (b-c) Myd88-/- BMMs with condensed nuclei (b) or positive for phospho-rS6p (MFI>300) (c) produced by infections with ΔflaA (MOI = 20) and the indicated treatments. Infected and neighboring uninfected cells were quantified for each category. (d) Infected and neighboring uninfected BMMs with condensed nuclei after infections with ΔflaA or ΔdotA (MOI = 20) in the presence/absence of FBS. (e) Kinetics of LDH release by C57BL/6 BMMs infected as indicated (MOI = 10) in the presence of FBS or dFBS. (f) Infected Myd88-/- BMMs with condensed nuclei produced by ΔflaA infection (MOI = 20) and the indicated treatments. (b-f) PP242 (2.5μM), LY294002 (10μM), FBS (10%), dFBS (10%), human LDL (10mg/ml). (b-f) Means ± s.d of technical triplicates for each condition are shown. At least 50 cells (c,f) or 100 cells (b,d) were analyzed for each condition. A representative of two (e-f) or three (b-d) biological replicates is shown for each experiment. (b-f) n.s—not significant, ** p<0.005 (unpaired T-test).
Although MTOR is a global metabolic regulator, the potential loss of MTOR-dependent lipogenesis was investigated first because: (1) FBS can compensate for loss of MTOR-driven lipogenesis and when FBS is available cells default to lipid uptake over de novo synthesis to reduce energy expenditure [29]; (2) serum is a major source for cellular lipids and lipid-precursors in vertebrates [29]; (3) rapamycin failed to trigger cell death in Legionella infections and MTOR-driven lipogenesis is insensitive to rapamycin in some cell types [36,60,61]. The master lipogenic transcription factors SREBP1 and SREBP2 promote lipogenesis downstream of MTOR by increasing gene expression of lipid uptake regulators and biosynthesis enzymes [33,34,62]. Consistent with induction of a lipogenic transcriptional response, a significant increase in expression of Srebf1 as well as several of its target genes was detected in Myd88-/- BMMs infected with ΔflaA but not ΔdotA Legionella (S4 Fig). Therefore, a functional Dot/Icm apparatus is critical for initiation of MTOR signaling and the induction of a pro-lipogenic transcriptional program in Legionella-infected macrophages (S4 Fig).
If serum-derived lipids prevented host cell death in infected cells by compensating for the loss of MTOR-driven lipogenesis, the phenotype should be sensitive to depletion of serum lipids. Thus, the cell death response brought by MTOR-inhibition was investigated in the presence of delipidated FBS (dFBS) (cholesterol 2mg/dL, triglycerides 59mg/dL) or complete FBS (cholesterol 34mg/dL, triglycerides 61mg/dL) (Fig 5). Delipidated FBS failed to block the Legionella-induced host cell death brought by PP242-treatment in Myd88-/- macrophages (Fig 5b) or by the host MTOR-suppression pathway (Fig 5d and 5e), demonstrating that FBS-derived lipids compensate for the lost MTOR function in infected macrophages. Furthermore, addition of purified Low-density lipoprotein (LDL) particles—the main cholesterol transport carriers in serum—was sufficient to block cell death in Legionella-infected Myd88-/- BMMs triggered by LY294002 (PI3K inhibitor) or PP242 (MTOR inhibitor) (Fig 5f).
Next, the Legionella-induced MTOR-regulated cell death response and its dependency on lipids starvation was investigated in human macrophages. Legionella-infected phorbol-ester differentiated U937 macrophage cells were treated with PP242 in the presence of FBS or dFBS. Similar to the results with mouse BMMs, the number of dead infected U937 increased only when PP242 was added under serum-free conditions or in the presence of delipidated FBS; addition of PP242 and lipids-containing FBS blocked the PP242-induced cell death (S5 Fig). Collectively, these data link MTOR-driven lipogenesis with the Legionella-induced cells death response and indicate that MTOR activation or presence of exogenous lipids prevents a premature death of infected macrophages.
MTOR maintains LCV integrity through activation of de novo lipogenesis
Due to the unique features of the Legionella-induced cell death: (1) requirement for bacterial replication and (2) loss of host lipogenesis, it was investigated whether insufficient supply of lipids could increase membrane tension and lead to LCV rupture during expansion. Elegant infection studies with a ΔsdhA strain, which resides in a rupture-prone LCV, have demonstrated that loss of LCV integrity releases bacterial molecules in the host cytosol leading to flagellin-independent cell death of infected macrophages [18].
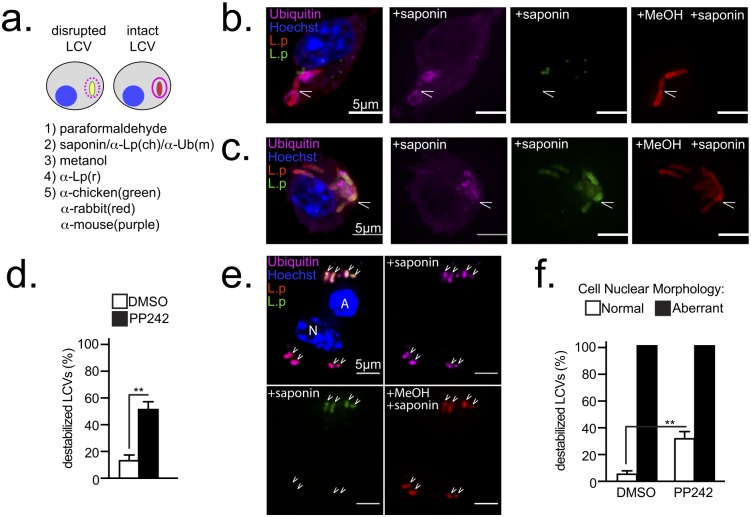
To discriminate between intact and leaky LCVs, the vacuole ability to prevent cytosolic anti-Legionella antibody from accessing the LCV lumen was investigated. First, the plasma membrane was selectively permeabilized with the amphipathic glycoside saponin and cells were stained with anti-Legionella chicken antibody as well as anti-ubiquitin antibody. Next, all membrane compartments were permeabilized with methanol and cells were stained with anti-Legionella rabbit antibody (Fig 6a). Thus, bacteria within intact LCVs are detected only after methanol incubation and remain single positive, whereas bacteria in leaky LCVs are accessible after saponin treatment and become double positive (Fig 6b and 6c). Detection of the ubiquitinated proteins that accumulate on the cytosolic interface of the LCV membrane during infection was used to control for saponin activity. In Myd88-/- BMMs infected with the ΔflaA strain for 7hrs, 16% of the LCVs were leaky as indicated by detection of the vacuole-residing bacteria after saponin treatment (Fig 6c and 6d). The number of leaky LCVs increased to 51% following PP242 treatment indicating that MTOR suppression compromises LCV integrity. Although leaky LCVs were detected in BMMs with normal nuclear morphology (Fig 6c), all LCVs harbored by cells with aberrant nuclear morphology were leaky (Fig 6e and 6f). These data indicate that MTOR suppression destabilizes LCVs.
Fig 6. MTOR inhibition destabilizes LCVs.
(a) Schematic for detection of leaky LCVs by selective plasma membrane permeabilization of infected cells. Single-positive bacteria reside in stable LCVs; double-positive bacteria reside in disrupted LCVs (b-f) Serum-starved Myd88-/- macrophages infected with ΔflaA L. pneumophila (MOI = 20) for 7hrs. PP242 was added at the time of synchronization– 60min p.i. (b-c) Micrographs of representative stable (b) or leaky (c) LCVs are shown. Cells were stained as indicated in (a). Arrowheads indicate LCVs. Bar = 5μm (d) Quantitation of destabilized LCVs in cells treated as indicated. (e) Micrographs of representative destabilized LCVs (double-positive) in BMM with aberrant (A) nuclear morphology and stable LCVs (single-positive) in a neighboring cell with normal (N) nuclear morphology Bar = 5μm (f) Quantitation of destabilized LCVs in BMMs with normal or aberrant nuclear morphology. BMMs treated as indicated. (b-f) PP242 (2.5μM). (d-f) Means ± s.d of technical triplicates for each condition are shown. At least 100 LCVs were analyzed for each condition. (b-f) A representative of three biological replicates is shown. (d and f) ** p<0.005 (unpaired T-test).
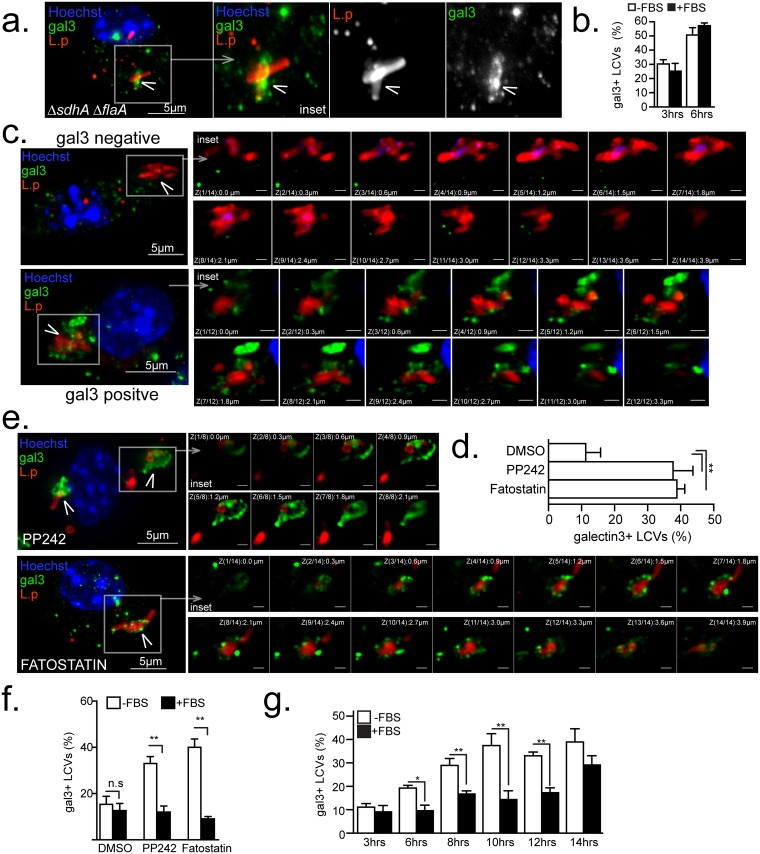
To measure LCV rupture the recruitment of the cytosolic lectin Galectin-3 was assessed. Galectin-3 binds to lumenally occluded glycoprotein epitopes and accumulates onto membrane-bound organelles only when their epitopes become exposed upon membrane disruption [18,63–65]. Unstable LCVs, such as the ones harboring the ΔsdhA mutant, become Galectin-3 positive upon rupture (Fig 7a and 7b) [18]. Galactin-3 preferentially accumulates at the sites of membrane rupture [18,66] thus produces a wide-variety of non-uniform straining pattern onto the LCVs (S6a and S6b Fig). Accumulation of Galectin-3 onto LCVs within infected Myd88-/- BMMs following MTOR inhibition was investigated. At 12 hrs post infection ~13% of the LCVs in vehicle-control treated cell were decorated with Galactin-3 (Fig 7c and 7d) and treatment with PP242 increased the number of Galactin-3+ LCVs to over 36% (Fig 7d and 7e). However, this number is likely an underestimate because Galactin3 staining was undetectable in dead macrophages and PP242-treatment increased the number of infected cells with condensed nuclei under serum-free conditions. MTOR inhibition did not cause global destabilization of pathogen-containing organelles because the number of Galactin-3+ LCVs harboring L. dumoffii did not increase after PP242 treatment (S6c and S6d Fig). In addition, MTOR inhibitors did not affect L. pneumophila replication and viability in vitro (S7 Fig), thus LCV rupture is unlikely a consequence of an interrupted LCV biogenesis program brought by an inhibitor-induced toxic effect on the residing bacteria.
Fig 7. Serum lipids, SREPB1/2 and MTOR regulate LCV stability.
Serum-starved Myd88-/- (a-f) or C57BL/6 (g) BMMs or were infected by the indicated strains (MOI = 20) in synchronized infections for 12 hrs (a-f) or for the indicated time periods (g) in the absence (a-g) or presence (f-g) of FBS. (a-b) Galectin 3 accumulation onto LCVs harboring ΔsdhA ΔflaA. (a) projection micrograph of a representative infected cell with the inset showing the LCV (b) Kinetic analysis of Galectin 3+ LCVs harboring ΔsdhA ΔflaA. (c,e) Representative projection micrographs of Galectin 3 positive (c,e) or negative (c) LCVs harboring ΔflaA after treatments with inhibitors (e) or vehicle alone (c). The insets show all individual planes of the projection image. Quantitation of Galectin 3+ LCVs after the indicated treatments in the absence (d and f) or presence (f) of FBS. (g) Kinetics of emergence of Galectin 3 positive LCV under serum starvation or replete conditions. (c-g) PP242 (2.5μM), fatostatin (4μM), FBS (10%). (b,d,f and g) Means ± s.d of technical triplicates for each condition are shown. At least 100 LCVs were analyzed for each condition. A representative of two (b, f-g) or three (a, c-e) biological replicates is shown for each experiment. (b, d, f and g) n.s—not significant, ** p<0.005 (unpaired T-test) (a, c and e) Cells were stained with anti-galectin3, anti-Legionella antibodies and Hoechst 33342. Arrowheads indicate the LCVs. Bar = 5μm.
Inhibition of lipogenesis at a step downstream of MTOR with the SREPB1/2 peptidomimetic inhibitor fatostatin, which specifically prevents the release of SREBP1/2 from the ER [67], destabilized LCV similar to PP242 treatment (Fig 7d and 7e). Importantly, addition of FBS restored LCV integrity even in the presence of PP242 and fatostatin as evidenced by the decreased number of Galactin-3+ LCVs (Fig 7f). Under MTOR suppressing condition, Galactin-3+ LCVs increased after bacterial replication was initiated and the kinetics of Galactin-3 acquisition by LCVs harboring ΔflaA bacteria mirrored the kinetics of host cell death response (Figs 7g vs. 4f and 4g). These data demonstrate that LCV membrane integrity during expansion is maintained through a continuous supply of lipids, either through uptake or de novo synthesis. Thus, interference with MTOR-driven lipogenesis results in LCV rupture during expansion and initiation of host cell death likely through the release of bacterial products in the host cytosol. Unlike MTOR suppression-induced instability, rupture of LCVs harboring ΔsdhA bacteria preceded bacterial replication and was not rescued by FBS supplementation indicating that MTOR and SdhA stabilize the LCVs via distinct mechanisms (Fig 7b).
Lipids supply determines the housing capacity of the Legionella intracellular niche
Because the lipids supply/demand ratio is important for LCV homeostasis, its effects on the housing capacity of the pathogen-containing vacuole were investigated. In Legionella-infected cells, the LCV membrane contours the residing bacteria [15] and therefore the volume of the bacterial mass in the vacuole is proportional to the volume of the LCV membrane. In previous studies, housing capacity of a LCV was determined by manually counting the residing bacteria; however, the accuracy and reliability of this approach deteriorates for vacuoles containing more than 12 bacteria, which forces investigators to group large LCVs in a single category. To overcome this obstacle, an unbiased computer-based 3D image analysis was utilized to enumerate accurately bacteria within vacuoles independent of LCV size and topology. This analysis measures the volume of the bacterial mass within a LCV from reconstructed Z-stacks (0.3μm planes distance) of infected cells. To image the whole cell, cell height determined the Z-stack depth (4.5μm to 13μm). Next, the number of bacteria that can fit within the volume of the bacterial mass was extrapolated mathematically based on an algorithm derived from volume measurements of LCVs in which the bacteria can be counted manually (S8a–S8c Fig). When the algorithm was back tested it exhibited excellent correlation between counted and calculated number of bacteria per cell (R2 = 0.9287, p = 2.11X10-38) (S8d Fig). This automated volume-to-bacteria conversion approach facilitates reliable enumeration of bacteria even in large LCVs containing over a hundred bacteria (S8e and S8f Fig and S1 Video). Most 3D-imaging systems capable of volume measurements of binary masks should be able to enumerate intracellular bacteria using this methodology.
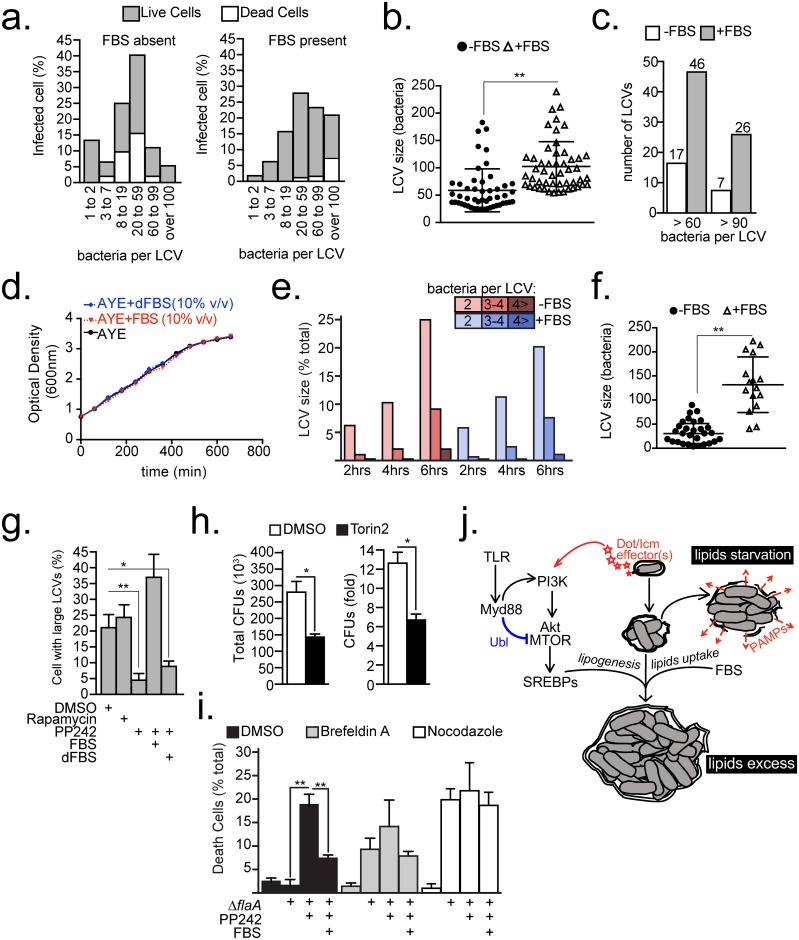
The relationship between lipid supply and LCV size was investigated in C57BL/6 BMMs, which suppress MTOR when infected by Legionella. In this system, exogenous FBS restores the lipids supply lost due to MTOR suppression. Thus, LCVs sizes were measured at the end of a single Legionella intracellular replication cycle (~16 hrs) in the presence/absence of FBS (similar to Fig 5a). In synchronized infections, the LCV sizes formed a Gaussian distribution with a peak at the 20–59 bacteria per LCV size bin irrespective of FBS supplementation (Fig 8a). However, FBS addition increased the population of large LCVs containing more than 60 bacteria (Fig 8a). Comparison between the fifty Iargest LCVs detected in each condition, revealed that FBS increased the average number of bacteria per LCV (from 58 to 102) and the number of LCVs containing more than 60 bacteria (from 17 to 46) (Fig 8b and 8c). FBS did not increase bacterial growth in axenic cultures (Fig 8d). Moreover, during the early stages of intracellular replication the number of bacteria per LCV remained similar in the presence or absence of FBS (Fig 8e) indicating that the LCV size increase observed in the later stages of infection was not a result of accelerated or prematurely initiated bacterial replication but likely due to LCV membrane biogenesis.
Fig 8. Host lipids dictate the LCV housing capacity.
(a-c, f) Size analysis of LCVs harbored by C57BL/6 BMMs infected with ΔflaA bacteria (MOI = 20) for 15 hrs after 60 min synchronization. Cells were serum-starved prior to infection for 10hrs. LCV sizes were measured through 3D microscopy analysis of infected cells as detailed in S8 Fig. (a) Relative distributions of sizes of LCV harbored by live and dead cells produced by infections in the presence/absence of FBS. Live/dead distinction was determined morphologically by nuclear condensation. (b-c) Size analysis of the 50 largest LCVs produced by ΔflaA infections shown in (a). (d) Legionella growth in axenic cultures supplemented with FBS or delipidated FBS (dFBS) (10% v/v). (e) Kinetic analysis of LCVs that support bacterial replication in C57BL/6 BMMs infected with ΔflaA bacteria. FBS was added or omitted after the infection synchronization at 30min post infection. At least 200 LCVs were scored for each condition. (f) Size analysis of LCVs harbored by cells with condensed nucleus from (a). (g) Percentage of Myd88-/- BMMs harboring large LCVs (bacteria>20) produced by 12 hrs synchronized infections with ΔflaA bacteria. Cell treatments were initiated at 4 hrs post infection as indicated. (h) L. pneumophila intracellular growth in Acanthamoeba castellanii over 48hrs in the presence of DMSO or Torin2 (300 nM), MOI = 5. (i) Percentage of Myd88-/- BMMs with condensed nuclei uninfected or infected with ΔflaA bacteria for 9hrs. Infections were synchronized at 60min and various treatments were added at 6hrs as indicated. (j) Model for MTOR-dependent regulation of LCV homeostasis through the lipogenesis and serum-derived lipids. Abbreviations: ubiquitin ligase (UBL), pathogen-associated molecular patterns (PAMPs) (b, f-g, h-i) Means ± s.d of technical triplicates for each condition are shown. (a and g) At least 100 LCVs were analyzed for each condition. A representative of two (d, h-i) or three (a-c, e-g) biological replicates is shown for each experiment. (a, g and h) PP242 (2.5 μM), Brefeldin A (17.8 μM), Nocodazole (20 μM), FBS (10% v/v), dFBS (10% v/v) (b, f-i) * p<0.05, ** p<0.005 (unpaired T-test).
Next, the size of LCVs harbored by cells with aberrant nuclear morphology was analyzed because host cell death caused by vacuole rupture is rapid and likely captures the critical inflection point of cellular lipid-exhaustion when supply becomes insufficient. In the presence of FBS, infected dead cells harbored on average 132 bacteria and most of the LCVs exceeded 100 bacteria whereas under serum-free conditions the average size was reduced to 32 bacteria and very large LCVs (bacteria>100) were not detected in the population of dead cells (Fig 8a and 8f). Under serum-free conditions, when membrane biogenesis depends on lipogenesis, the size distribution curve of LCVs harbored by dead cells was broad, indicating that the threshold for LCV rupture varied (Fig 8a and 8f). Conversely, in the presence of FBS, most of the LCVs harbored by dead macrophages were very large, consistent with delayed LCV rupture and prolonged bacterial replication when lipids supply to infected cells is sustained (Fig 8a and 8f).
To determine if Legionella-induced MTOR-dependent lipogenesis similarly prolongs intracellular replication, the number of large LCVs harbored by Myd88-/- BMMs infected for 12 hrs (as in Fig 5a) in the presence/absence of mTOR inhibitors were analyzed. Inhibition of MTOR with PP242, but not rapamycin, decreased the number of large LCVs consistent with regulation of LCV expansion and stability by Legionella-induced MTOR signaling (Fig 8g). Importantly, addition of FBS, but not delipidated FBS, sustained the number of large LCVs in the presence of PP242. Therefore, lipogenesis and lipid uptake are important functionally redundant determinants of the LCV housing capacity in Legionella-infected cells.
However, some large LCVs remained intact even under lipid-starvation conditions (Fig 8a and 8g), suggesting additional MTOR-independent mechanism can facilitate LCV expansion. Functional redundancy is a well-documented survival strategy by Legionella that facilitates a broad host range [28,58]. Indeed, MTOR inhibition with TORIN2 moderately reduced L. pneumophila intracellular replication in the protozoan host Acanthamoeba castellanii supporting MTOR-dependent and -independent LCV expansion mechanisms (Fig 8h). One potential MTOR-independent LCV expansion mechanism could be a direct membrane acquisition from other intracellular compartments. Indeed, early secretory vesicles tether and directly fuse with the LCV [6,68] thus it was tested whether the secretory compartment contributes to LCV expansion during MTOR suppression. To this end, Myd88-/- BMMs infected with ΔflaA bacteria were treated for 3hrs with PP242 and brefeldin A to disperse the Golgi compartment or nocodazole to block microtubules-dependent vasicular trafficking during the late LCV expansion phase (Fig 8i). Neither inhibitor treatment potentiated the PP242-induced cell death indicating that it is unlikely that the secretory compartment directly contributes membranes for the late LCV expansion.
Taken together, our data support a model in which an MTOR-dependent and an MTOR-independent pathway regulate LCV expansion by Legionella species. In the MTOR-dependent pathway, Legionella pneumophila manipulates MTOR function through secreted Dot/Icm effector(s) to drive lipogenesis and facilitate maximal niche expansion (Fig 8j). Insufficient host lipogenesis during Legionella pneumophila intracellular replication results in premature exhaustion of cellular lipids leading to loss of LCV membrane integrity, release of bacterial products in the cytosol of infected cells and cell death. However, serum-derived lipids can compensate for host-mediated or pharmacological MTOR suppression to facilitate maximal LCV expansion. Nevertheless, we provide evidence that L. pneumophila LCV expansion can be achieved through MTOR-independent mechanism(s) which function does not depend on the secretory pathway and are likely extensively utilized also by species other than L. pneumophila.
Discussion
The building blocks of intracellular niches harboring pathogens are host-derived, thus the metabolic state of the host cell frequently dictates replication permissiveness [1,69,70]. As a central metabolic regulator in eukaryotes, MTOR is frequently targeted by viruses for metabolic reprogramming [71]. Here, we provide evidence that a bacterial pathogen subverts MTOR function to execute its niche biogenesis program.
Previously, we reported that in macrophage infections Legionella encoding a functional Dot/Icm system elicited a host-driven ubiquitination pathway that degrades activated Akt resulting in MTOR suppression and increased inflammation due to inefficient translation of anti-inflammatory mediators [51]. In this study, we provide evidence that host-driven MTOR suppression induced by Legionella is dependent on the TLR adaptor Myd88. The loss of host-mediated MTOR-suppression in Myd88-/- BMMs revealed that Legionella pneumophila activates MTOR to reprogram infected cells metabolically for optimal expansion of its intracellular niche. The Legionella induction of MTOR required (i) a functional Dot/Icm apparatus; (ii) the Dot/Icm chaperone IcmS; and (iii) the host lipid kinase P3IK. These requirements are consistent with the existence of a Legionella Dot/Icm effector(s) that activates MTOR either directly by subverting PI3K or indirectly through LCV biogenesis. Legionella infections in the presence of cytochalasin D, a condition under which the Dot/Icm apparatus continues to translocate effectors but LCV formation is blocked, clearly demonstrates that the direct hypothesis holds true. The direct hypothesis is further supported by the observation that MTOR activation was specific to L. pneumophila and not several other Legionella species that encode diverse set of Dot/Icm effectors but establish similar ER-derived intracellular niches [72,73].
Our data indicate that the PI3K-Akt axis in Legionella-infected macrophages is subject to regulation by host-derived and bacteria-derived factors. PI3K signaling is induced by at least two regulators: (i) an activated TLR(s) [48,51] and (ii) a Dot/Icm effector protein; however, signaling is blocked downstream of PI3K by Myd88-dependent ubiquitination of Akt and MTOR [51]. This model reconciles the distinct MTOR signaling in Legionella-infected macrophages expressing or lacking Myd88. Our PI3K inhibition studies demonstrate that PI3K is required for Legionella-induced MTOR activation. In Myd88-/- macrophages, the detection of sustained MTOR signaling argues that the host Akt/MTOR ubiquitination pathway requires priming by an activated TLR. When TLR function is intact, the degradation of activated Akt by the host ubiquitin ligases likely overrides the PI3K activation by the Legionella effector, thus suppressing the Legionella-driven MTOR signaling. Although the ubiquitin ligase for Akt has to be identified before this scenario can be tested experimentally, the loss of activated Akt is sufficient to reduce MTOR activation by TLRs through the PI3K/Akt axis [51] supporting the notion that MTOR induction by Legionella would be similarly suppressed. Thus, multiple signaling inputs impinge upon MTOR function in Legionella-infected cells.
Although sustained MTOR activation leads to significant pleiotropic reprogramming of cellular metabolism [39], our data demonstrates that Legionella benefits from the increase in host lipogenesis, which maximizes the housing capacity of the LCV likely by increasing membrane biogenesis. Eukaryotic membrane biogenesis requires constant supply of phospholipids and cholesterol that are derived by either de novo lipogenesis or by uptake from the extracellular environment [29]. In mammals, the circulatory system provides a rich and constant source of lipids to distal cells through the serum; In the presence of serum, cells favor lipids uptake over de novo lipogenesis to sustain membrane biogenesis because it is less energy expensive [29]. In our experiments we cultured terminally differentiated macrophages in serum-free cell culture conditions. In the absence of FBS, membrane biogenesis in infected BMMs requires de novo lipid synthesis because Legionella inhibits autophagy [74], a process that facilitates membranes/lipids recycling [75]. We provide evidence that when membrane biogenesis is dependent on de novo lipogenesis, LCV stability and expansion is mediated by the PI3K-MTOR-SREBP1/2 axis. Providing lipids exogenously reversed the LCV instability phenotype caused by PI3K or MTOR or SREBP1/2 inhibition, which is consistent with a LCV rupture caused by halted membrane biogenesis due to insufficient lipids supply.
MTOR can activate the lipogenic transcription factors SREBP1/2 via multiple mechanisms—increased mRNA stability, trafficking from the ER and interference with protein degradation in the nucleus [76]. MTOR activation by Legionella coincided with increased gene expression of Srebf1 and its downstream target Fasn and Ldlr and preceded LCVs rupture indicating that at least partially SREBPs function in Legionella-infected cells is regulated through gene expression. However, post-transcriptional mechanisms might also contribute due to a global repression of protein synthesis that affects translation of some but not all polypeptides in infected cells [51,77,78]. Clearly, Legionella regulation of MTOR-dependent lipogenesis warrants future investigation.
Regardless of the precise mechanism, the importance of sustained MTOR activity for LCV homeostasis is evident by the premature loss of LCV integrity. Ruptured LCVs release microbial products in the host cytosol that induce flagellin-independent pyroptosis [18,26]. Similarly, we observed increased number of dead infected macrophages upon MTOR suppression either pharmacologically or genetically or by the host Akt/MTOR ubiquitination pathway. Importantly, the host cell death brought by MTOR inhibition required bacterial replication and was rescued when lipids were provided exogenously through serum or purified LDL particles further supporting the idea that MTOR activity regulates the lipid supply/demand ratio during the process of LCV expansion. Serum-derived lipids did not promote growth in axenic cultures nor in the early phase of intracellular replication, which supports a role for host-derived lipids in LCV biogenesis rather than bacterial replication and is consistent with the well documented Legionella utilization of carbohydrates and amino acids as biosynthesis and energy sources [3,79,80].
In mammalian infections, serum likely eliminates the de novo lipogenesis requirement for LCV homeostasis because inhibition of either pathway individually did not produce a LCV biogenesis defect. These data reconcile the lack of a cell death response in C57BL/6 macrophages infected with the ΔflaA strain in the presence of serum [52]. However, unicellular amoebae—the natural hosts for Legionella—inhabit aquatic environments in which lipids availability is likely similar to cell cultures under serum starvation conditions. In amoebae, such as D. discoideum and A. castellani, TORC1 metabolic functions are conserved [81], and in our studies TORIN2 moderately inhibited L. pneumophila intracellular replication in A. castellani. Because high MTOR activity overcomes premature lipids exhaustion during the late stages of LCV expansion it offers evolutionary advantage by increasing the number of bacteria released from infected cells. However, some large LCVs (>60 bacteria) were still detected upon lipid starvation thus a functionally redundant MTOR-independent pathway likely exits. In agreement, we also observed intracellular replication in an MTOR-independent manner by other Legionella species. Potentially, those species might subvert lipogenic factors downstream of MTOR or expand the LCV through fusion with membrane-bound vesicles or direct absorption of ER membranes. Disrupting the secretory pathway in the context of L. pneumophila infection did not exacerbate PP242-driven LCV instability in Myd88-/- BMMs consistent with an important role of the secretory compartment in early LCV remodeling [6] but not late LCV expansion. Considering the knowledge gap in our understanding of the LCV expansion mechanisms it would be important to investigate the diverse expansion programs encoded by Legionella species.
MTOR subversion could be a strategy tailored for survival in a specific protozoan host that otherwise offers a moderate evolutionary advantage in other host species—a phenomenon discovered through en masse effector deletion studies [58]. Because of the critical link between membrane integrity of pathogen-occupied vacuoles and host defenses, MTOR subversion benefits would likely increase in hosts with robust restrictive mechanisms triggered by LCV instability and decrease in hosts that fail to restrict Legionella replication from leaky LCVs. Such a scenario is illustrated by the loss of the LCV stability regulator SdhA that impact intracellular replication but only in BMMs and not in Dictyostelium [26]. Thus, MTOR could be important for L. pneumophila survival in protozoan species that encode cytosolic macrophage-like defense responses.
However, differences in the wiring of MTOR circuits between mammalian and protozoan hosts and even between protozoa species could impact Legionella survival. For example, TORC2 regulates the TORC1 complex in macrophages but not Dictyostelium [81]. Also, Legionella uptake in macrophages but not Dictyostelium is PI3K-dependent [82,83]. In fact, loss of PI3K promotes L. pneumophila replication in Dictyostelium due to accumulation of phosphatidylinositol 4-phosphate, which facilitates early LCV remodeling, as well as decreased fusion with Nramp+ vesicles [82]. Conversely, in primary macrophages we observed increase in host cell death upon PI3K inhibition presumably due to LCV instability. The capacity of Dictyostelium protozoa to support robust replication of L. pneumophila residing in rupture-prone LCVs [26] could reconcile the phenotypic differences of PI3K inhibition during L. pneumophila infection.
Although our data established a requirement of continuous host lipids supply for LCV stability during expansion, we cannot differentiate between the need for a bulk increase in lipid content and the need for a specific lipid species on large LCVs. Little is known regarding the nature and dynamics of the LCV membrane lipid composition as the vacuole expands. Furthermore, Legionella secretes several lipid-modifying enzymes that potentially remodel the LCV membrane during infection [84,85]. As lipid content defines physical properties of biological membranes, enzymatic modifications could alter membrane tension leading to LCV rupture. Indeed, deletion of the secreted phospholipase PlaA stabilizes the rupture-prone vacuoles of the ΔsdhA Legionella [18]. In addition to SdhA, loss of several other Dot/Icm effectors with unknown functions results in a LCV instability phenotype [28,69]. The fact that Legionella has evolved multiple mechanisms to ensure LCV integrity, including metabolic reprogramming through MTOR, highlights the importance of niche homeostasis for Legionella intracellular survival.
Because MTOR represses autophagy, increased MTOR function could be an effective autophagy-suppressing mechanism encoded by Legionella that complements the LC3 protease RavZ [74]. Similar reactivation of MTOR by Salmonella suppresses autophagy induced by a host amino acids starvation response during infection of epithelial cells [50]. Under our experimental conditions, RavZ blocks autophagy regardless of MTOR activation state; thus, autophagy unlikely contributes to the cell death response elicited by MTOR suppression. Furthermore, rapamycin failed to disrupt LCVs and trigger the Legionella-induced cell death response despite a well-documented capacity to boost autophagy in BMMs [74]. Instead, our data indicates that LCV stability is regulated by a TORC1 rapamycin-insensitive or a TORC2 substrate. The mechanism of MTOR-driven lipogenesis in macrophages is poorly understood. Although in some cell types the TORC1 substrate S6K1 functionally links MTOR and SREBP1 [36], this is unlikely the case for Legionella-infected macrophages because S6K1 activity is sensitive to rapamycin. MTOR-dependent lipogenesis has exhibited both sensitivity and resistance to rapamycin depending on the cell type [36,60,61]. Because membrane biogenesis is critical for many physiological functions performed by macrophages, such as phagocytosis and protein secretion, it is important to dissect its mechanisms. In this aspect, Legionella infection could be an invaluable model to elucidate the mechanism of MTOR-induced lipogenesis.
For vacuolar bacterial pathogens, such as Legionella, host membranes are an integral part of niche homeostasis. Filling the large knowledge gap in our understanding of the niche biogenesis process is important for the development of not only anti-microbial agents but also for deciphering metabolic regulation. Our work establishes a mechanistic link between host lipids metabolism and Legionella intracellular niche homeostasis by defining a novel host determinant for LCV membrane integrity and setting the foundation for future research in the metabolism of host-pathogen interactions.
Materials and Methods
Ethics statement
All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at the Louisiana State University Health Sciences Center-Shreveport. Approval notice (P-15-026).
Bacterial strains
The following strains were used in this study: (1) L. pneumophila serogroup 1 strain JR32 deleted for flaA (ΔflaA) or dotA (ΔdotA) [52]; Lp02 pentuple strain [58]; (2) Lp02 thymidine auxotroph strains lacking flaA (thyA ΔflaA), dotA (thyA ΔdotA), icmS (thyA ΔicmS) [23] and sdhA (thyA ΔsdhAΔflaA) (this study); (3) Lp02 pentuple ΔflaA strain (this study); (4) Legionella longbeachae (ATCC 33462), Legionella jordanis BL-540 (ATCC 33623) and Legionella dumoffii (ATCC 33279) species were kind gift from Dr. Nicholas Cianciotto (Northwestern University). Legionella strains were grown on charcoal yeast extract (CYE) plates [1% yeast extract, 1%N-(2-acetamido)-2-aminoethanesulphonic acid (ACES; pH 6.9), 3.3 mM l-cysteine, 0.33 mM Fe(NO3)3, 1.5% bacto-agar, 0.2% activated charcoal] [86]. Lp02 derived strains were grown on CYE plates containing thymidine at 100μg/ml. For all experiments, Legionella were harvested from CYE plates after growth for 2 days at 37°C.
Plasmids and strain construction
The ΔsdhA and the ΔflaA allele was generated by recombinant PCR using a ~1kb region upstream of the gene using the following primers for ΔsdhA (forward primer 5’-CGGGATCCCCTGATATAATGAAAAATATAGCCC-3’ and reverse primer 5’-CGCGGCCGCATTTCAATATTAAAAAAATTAACTTGTG-3’), a downstream region of sdhA (forward primer 5’-CGCGGCCGTAAAAATATTTGATTTAATCTTTTAATTTACC-3’ and reverse primer 5’-GCGAGCTCTGTCCATTTTTTCATACGAAAATACC-3’) and for ΔflaA (forward primer 5’-CGGGATCCTTCGTTGAAAGCCTTCTGGC-3’ and reverse primer 5’-CCGCTCGAGGGATGTCGCAATCGAAGTGC-3’), a downstream region of ΔflaA (forward primer 5’-CCGCTCGAGTCTCCTCAGACCTGAATCC-3’ and reverse primer 5’-AAACTGCAGCAGTTAATGAATTCACTCCC-3’). The resulting fragments were linked by a EagI site for ΔsdhA and XhoI site for ΔflaA and cloned into the BamHI/SacI sites of the gene replacement vector pSR47s creating pSR47s-CD0376 and pSR47s-CD1340 respectively. Strain Lp02 ΔflaA ΔsdhA was derived from LP02 ΔflaA and generated by allelic exchange of sdhA with ΔsdhA and the Lp02 pentuple ΔflaA was derived from LP02 pentuple and generated by allelic exchange of flaA with ΔflaA. The allelic exchange was confirmed by genotyping clonal isolates with the following primers in a single PCR reaction for sdhA (5’-CATTGAGCGTATCAAACACG-3’, 5’-GTAAAATGATAATTGAAGAGG-3’ and 5’-CAGTCTGGGGTTGAAATGTCC-3’) or for flaA (5’-TTGTGCAGTCATTCGTTGAG-3’, 5’-GTATTGATTATATTACAATCG-3’ and 5’-TCATCAGAAGGCATTTTACG-3’) that produces a fragment of ~330bp for sdhA/flaA or a fragment of ~500bp for ΔsdhA/ΔflaA.
Reagents
The pharmacological agents used in the study were obtained from the following suppliers Cell Signaling (LY294002), Sigma (PP242), Biolegend (Brefeldin A), Cayman Chemical Company (Torin2, Cytochalasin D and Nocodazole), CalBiochem (InSolutionRapamycin) and R&D Systems (Fatostatin). Primary antibodies were purchased from Cell Signaling– α-Akt(pan) (C67E7), α-rS6p(5G10), α-prS6p(S235/236)(D57.2.2E) and α-pS6K1(T389)(108D12); Enzo Life Sciences—α-Ubiquitinated proteins (FK2); Abcam– α-Galectin-3 (A3A12); Santa Cruz—α-Actin (C-2). The following antibodies were custom produced by Cocalico Biologicals against formalin-killed bacterial strains– α-L. pneumophila (chicken IgY), α-L. dumoffii (chicken IgY), α-L. longbeachae (rabbit IgG) and α-L. jordanis (rabbit IgG). Secondary antibodies and Hoechst 33342 (cat #H3570) were purchased from ThermoFisher Scientific—α-mouse-Alexa488 (cat #A11001), α-mouse-Alexa647 (cat #A21235), α-rabbit-Dylight550 (cat #SA5-10039), α-rabbit-Alexa647 (cat #A21244), α-chicken-IgY-FITC (cat #A16055), α-chicken-IgY-TRITC (cat #A16059). Complete FBS was purchased from Seradigm (cat #1300_500). For the studies with delipidated-serum, FBS was purchased from Gemini Bio-products: complete FBS (cat #100–106), delipidated FBS (cat #900–123). The cholesterol and triglycerides content of FBS was verified using biochemical assays. Purified human LDL was purchased from Intracel (cat #RP-032, Intracel Frederick, MD).
Mice
For these studies bone-marrow was harvested from C57BL/6J (Jackson Labs, cat # 000664), Myd88-/- (Jackson Labs, cat #009088) and mTORfl/flCre+ mice (Mtor-/-). Mice with Mtor deletion in the myeloid compartment were generated by breeding mTORfl/fl (Jackson Labs, cat # 011009) harboring loxP sites flanking exons 1–5 of the Mtor locus with LyzMPro Cre+ transgenic mice (Jackson Labs, cat #004781) [51].
BMM and U937 derivation and cell culture
To derive BMMs, bone marrow cells were isolated from the femurs and tibiae of mice, and cultured in RPMI 1640 with L-glutamine (Sigma, cat #R8758) containing 10% FBS (Seradigm), 20% macrophage colony-stimulating factor (M-CSF)-conditioned medium, and 1% primocin (Invivogen) at 37°C with 5% CO2. Equal volume of media was added on day 4. On day 7, cells were harvested and used for infection assays. M-CSF condition media was obtained from an L-929 fibroblast cell line (ATCC). U937 cells (ATCC) were cultured in RPMI 1640 containing 10% FBS (Seradigm) and 1% Pen-Strep (ThermoFicher) at 37°C with 5% CO2 and were differentiated with 10ng/ml Phorbol 12-myristate 13-acetate (Adipogen) for 24hrs followed by culture in PMA-free media for 48hrs prior to infections.
Infections with Legionella strains for immunofluorescence analysis
For infections, BMMs were seed on cover slips in re-plating media (RPMI 1640 with L-glutamine, 10%FBS, 10% M-CSF-conditioned media) for 8 hrs prior to serum starvation. U937 were seeded in 24-well plates and PMA-differentiated directly onto cover slips. For all experiments cell were serum starved for 10 hrs by culturing in RPMI 1640 with L-glutamine (serum-free RPMI). BMMs (2.5x105 per well) or PMA-differentiated U937 (5x105 per well) seeded on cover slips in 24-well plates were infected with Legionella at MOIs as indicated for each experiment. At 60 min post infections extracellular bacteria were removed by washing 5X with warm (37°C) phosphate-buffered saline (PBS) and the infected BMMs were cultured in serum-free RPMI. Inhibitors and FBS or dFBS were added as indicated for each experiment. The duration of infection is noted for each experiment.
For immunostaining, coverslips containing cells were washed with 3x PBS and fixed with 2% paraformaldehyde (PFA) for 20 min, permeabilized with cold methanol for 30 seconds, blocked with 2% BSA in PBS for 60 min. Primary antibodies dilutions were 1:500 for α-p-rS6p, 1:200 for α-ubiquitin (FK2), 1:200 for α-Galectin 3 and 1:1000 for all α-Legionella antibodies. Primary antibodies were incubated in PBS containing 1% BSA overnight at 4°C. Secondary antibodies were used at 1:500 dilution and Hoechst 33342 at 1:2000 for 60 min at room temperature. Coverslips were mounted with ProLong Gold antifade reagent (ThermoFisher) onto slides and examined by fluorescence microscopy. For detection of leaky LCVs by selective permeabilization cell were fixed with 2% PFA for 20min, incubated with α-ubiquitin (FK2) and α-L. pneumophila chicken ab in PBS with 0.1% saponin and 2% BSA overnight at 4C. Next, coverslips were extensively washed with PBS, incubated with cold 100% methanol for 30 sec, extensively washed with PBS and incubated with α-L. pneumophila rabbit ab for 2hrs. Secondary antibodies were applied as described above.
Legionella intracellular replication in Acanthamoeba castellanii
A. castellanii (ATCC) was propagated in ATCC Medium 712: PYG w/ Additives at room temperature. For infections, amoebae were seeded at 5X105 cells per well in a 24 well tissue culture plates in A.c. buffer and allowed to settle down for 60 min. Next, amoeba were infected with JR32ΔflaA Legionella in A.c. buffer at MOI = 5 in the presence of DMSO or Torin2 (300nM) in technical triplicates for each condition. Inhibitors were added at the time of infection. At 60min post infection and at 48hrs post-infection, cells were collected and mechanically disrupted by passaging 10 times through a 27G syringe needle and various dilution were plated on CYE agar plates.
Microscopy analyses of infected cells
3D images were acquired with inverted wide-field microscope (Nikon Eclipse Ti) controlled by NES Elements v4.3 imaging software (Nikon) using a 60X/1.40 oil objective (Nikon Plan Apo λ), LED illumination (Lumencor) and CoolSNAP MYO CCD camera. Image acquisition, deconvolution and analysis was completed with NES Elements v4.3 imaging software. Only linear image corrections in brightness or contrast were completed. For all analyses, three-dimensional images of randomly selected fields were acquired and image acquisition parameters were kept constant for all the cover slips from the same experiment.
For phospho-rS6p analysis in cells, the ubiquitin signal was utilized to define a binary mask for each cell from which the mean fluorescence intensity of p-rS6p was obtained. Background fluorescence signal from each acquired field was individually determined from a cell-size mask positioned in an unoccupied area and subsequently subtracted from the MFI of each cell in the field.
For imaging Galectin 3 LCV recruitment, each plane of the Z-stack was scrutinized to ensure that multiple Galectin 3 puncta localize proximal or co-localized with the bacteria. Because Galectin 3 binds exposed sugar moieties on ruptured membranes, LCVs were considered positive if the galactin 3 wrapped the bacteria or greater than 4 puncta were proximal to the bacteria (< 0.5μm).
For cell death analysis, we set binary mask using Hoechst fluorescence to define and measure individual objects larger than 5μm3. Cell with aberrant nuclear morphology were obvious even by manual scoring and were marked by decrease in nuclear volume and increase in Hoechst 33342 fluorescence.
For LCV size analysis, 3D images of infected cells stained with purified anti-Legionella IgY chicken antibody were acquired by setting the Z-distance between the immediate out-of-focus planes to capture the whole cell. The space between imaged planes was set at 0.3μm, thus depending on the cell type the number of planes in a given Z-stack varied. 3D binary masks were set for each field of cells individually based on bacterial fluorescence to define objects and to ensure out of focus signal was omitted from the mask. Due to the high fidelity of the anti-Legionella IgY antibody, images were not deconvoluted prior to binary mask definition because deconvolution did not improve data acquisition. The volume of each object was measured with the 3D image volume analysis function of NES Elements (Nikon). To derive the mathematical algorithm for volume-to-bacteria conversion the object volumes of LCVs containing bacteria that can be definitively manually enumerated were determined and plotted against the data from the bacterial count per vacuole. The best linear fit algorithm for the plot was used to derive the conversion equation (Y = 0.5842*X+0.4835, X = object volume [μm3] Y = number of bacteria). The conversion algorithm was back tested by extrapolating bacterial numbers of LCVs in which the number of bacteria can be manually counted definitively. (see S8 Fig)
Infections with Legionella strains for LDH release assay
BMMs were seeded in 48-well plates (1X105 cells per well), serum-starved for 10 hrs and infected as indicated at MOI = 10 in technical triplicates for each time point. Total supernatant volume was 200μl. For the FBS rescue experiments, the serum was added at the time of infection. Supernatants were collected and immediately frozen at -20C. Thawed supernatants (50μl) were assayed for LDH activity using the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega) according to the manufacturer’s protocol. Supernatants from media only, unifected cell and Triton X-100 lysed cells were used to calculate the dynamic range of the assay as per the manufacturer’s instructions.
Infections with Legionella strains for immunoblot analysis
BMMs were seeded in 12-well plates (8X105 cells per well), serum-starved for 10 hrs and infected as indicated. Cells were washed with cold PBS and lysed with RIPA buffer (20mM Tris-HCl pH7.5, 200mM NaCl, 1mM EDTA, 1% NP-40, 1% sodium deoxycholate, 2.5mM sodium pyrophosphate, 1mM β-glycerophosphate, 1mM Na3VO4, 1μg/ml leupeptin). Total cell lysates (20μg) were resolved by SDS-PAGE, transferred onto nitrocellulose membranes blocked with 2% BSA and incubated with primary antibody for either 3 hrs at room temperature or overnight at 4°C for phospho-antibodies. Primary antibody dilutions were used as per manufacturers’ instructions. Secondary horseradish peroxidase-conjugated antibodies (ThermoFisher) were used at 1:5000 in 2 hrs incubations at room temperature. Signals were visualized with ECL (GE Healthcare) and analyzed with ImageJ (NIH).
Quantitative PCR analysis of gene expression
Myd88-/- BMMs (1X106 cells) were serum starved and infected under serum-free conditions as indicated for 6 hrs. Total RNA and first-strand cDNA synthesis was performed with TaqMan Gene Expression Cells-To-Ct Kit (ThermoFisher) according to the manufacturer’s instructions. mRNA levels were determined by quantitative real-time PCR using the Universal ProbeLibrary (Roche, Life Science) and LightCycler 480 Probes Master (Roche, Life Science). Thermal cycling was carried out using a LightCycler 96 instrument (Roche Diagnostics) under the following conditions: 95°C for 5 min and 45 cycles at 95°C for 10 sec and 60°C for 25 sec. Gene expression was normalized to Canx (Calnexin). The fold increase is shown relative to uninfected cells (value of 1).
Statistical analysis
Calculations for statistical differences were completed by Student’s T-test or ANOVA as indicated using Prism v6 (GraphPad Software).
Supporting Information
(a-b) Images from synchronized infections under serum-free conditions of serum-starved BMMs infected as indicated. Representative single projection images of 3D microscopy images of macrophages infected by ΔflaA (a) or ΔdotA (b) for 8 hrs (MOI = 20, infections synchronized at 60min) and stained with anti-phospho-rS6p(S235/S236), anti-Legionella (L.p) antibodies and Hoechst 33342. (*) indicates Legionella infected cell, Bar = 10μm. (c-d) Frequency distribution of the phospho-rS6p mean fluorescent intensity (MFI) in individual macrophages infected by ΔflaA (c) or ΔdotA (d). Each MFI bin spans 200 fluorescence intensity units of the p-rS6p(S235/S236) fluorescent signal. At least 200 LCVs were analyzed for each condition. A representative of three (a-d) biological replicates is shown for each experiment.
(EPS)
(a-b) Images from synchronized infections under serum-free conditions of serum-starved Myd88-/- BMMs for 8 hrs (MOI = 20) as indicated. Cells were infected by either L.pneumophila (LP), L.dumoffii (LD), L.longbeachae (LL) or the indicated combinations in the co-infection (ratio 1:3) experiments. The ΔflaA of L.pneumophila was used to avoid pyroptosis. LD and LL lack the flaA gene [87]. Quantitated data is shown in (a). (b) A representative micrograph of cells stained with anti-ubiquitinated proteins (FK2), anti-p-rS6p (S235/236), anti-L.dumoffii antibodies and Hoechst 33342. Each channel is shown individually. Ubiquitin is a marker specific for LP LCVs and was used to identify LP-infected macrophages. Arrowheads indicate Legionella-containing vacuoles and (*) mark cells co-infected by LP and LD, Bar = 10μm. (c) Quantitation of p-rS6p positive Myd88-/- BMMs uninfected or infected with either ΔflaA or pentuple ΔflaA strains for 8hrs. Synchronized infections with MOI = 20 (a, c) Means ± s.d of technical triplicates for the two distinct groups within the cell population–(a) LP present and LP absent for each condition are shown. At least 50 infected cells were analyzed for each condition. A representative of two biological replicates is shown. (a and c) n.s—not significant (unpaired T-test).
(EPS)
(a-b) Panel of micrographs of representative ΔflaA infected BMMs stained with anti-L. pneumophila antibody and Hoechst 33342. Cells with aberrant (a) or normal (b) nuclear morphology are shown. Bar = 10μm.
(EPS)
(a) qPCR analysis of gene transcripts that regulate lipogenesis and lipids trafficking in serum-starved BMMs infected by either ΔflaA or ΔdotA (MOI = 10) for 6 hrs under serum-free conditions. Means ± s.d of technical triplicates for each condition are shown. The amount of each transcript was normalized to Canx (Calnexin) and is presented as fold increase over unstimulated cells (UN). A representative of two biological replicates is shown. n.s—not significant, * p<0.05, ** p<0.005 (unpaired T-test).
(EPS)
(a-b) Phorbol ester differentiated U937 cells were serum-starved and infected under serum-free conditions with ΔflaA Legionella for 16 hrs (MOI = 10, synchronized infections) in the presence/absence of PP242 (2.5μM). (a) Micrographs show representative populations of macrophages stained with anti-L. pneumophila (L.p) and Hoechst 33342. Arrowheads indicate LCVs and (*) indicate infected cells with condensed nucleus. (Bar = 10μm.) (b) Quantitation of infected and neighboring uninfected U937 macrophages with condensed nuclei after the indicated treatments. Means ± s.d of technical replicates of dead cell as percentage of total cells in each condition are shown. A representative of two biological replicates is shown. n.s—not significant, ** p<0.005 (unpaired T-test).
(EPS)
(a-c) Serum-starved Myd88-/- macrophages were infected with ΔflaA L. pneumophila for 10 hrs (a-b) or L. dumoffii for 12 hrs (c-d) in synchronized infections in the absence (a-d) or presence (d) of FBS (10% v/v). (a-c) Micrographs of representative Galectin 3 negative (a) and positive (b and c) vacuoles are shown. Cells were stained with anti-galectin3, Hoechst 33342 and anti-L. dumoffii (L.d) or anti-L. pneumophila (L.p) antibodies. Arrowheads indicate the LCVs. (a) Cell harboring Galectin 3 negative LCVs show typical dispersed Galectin 3 staining pattern, whereas cell containing Galectin 3 positive LCVs show distinct accumulation of multiple bacteria-proximal Galectin 3 puncta (b and c). (d) Quantitation of Galectin 3-positive LCVs produced by infections with ΔflaA or L.dumoffii and treatments with PP242 (2.5μM) or vehicle alone. Means ± s.d of technical triplicates for each condition are shown. At least 100 LCVs were analyzed for each condition. A representative of two biological replicates is shown. n.s—not significant, ** p<0.005 (unpaired T-test).
(EPS)
(a-b) Legionella growth in AYE liquid cultures in the presence/absence of fatostatin (40μM) (a), PP242 (25μM) (b), Torin2 (3μM) (b). Changes in the cultures’ optical densities over time are shown for each condition. A representative of two biological replicates are shown for each experiment.
(EPS)
(a) Methodological outline for the analysis of LCV bacterial size from 3D microscopy images of infected cells stained with purified anti-Legionella IgY chicken antibody. Z-stack series of a single LCV spanning 2.4 μm and containing four bacteria is shown. Each bacterium is numbered and the outline of the 3D binary mask used to measure the LCV volume (5.65 μm3) is shown. (b) Derivation of the LCV volume-to-bacteria number conversion formula. Graph plots the measured volumes of the bacterial mass for 100 LCVs in which the individual bacteria can be unambiguously identified over the manually counted number of bacteria per LCV. (c) Micrographs of image projections of individual representative LCVs used in the formula derivation protocol. Each bacterium is numbered and the volume of the bacterial mass binary mask is shown. The volume of individual bacteria varied from 0.95μm3 to 2.42μm3. A bacterium undergoing binary fission was counted as a single cell until the septum is resolved. (d) Back-testing of the conversion formula derived in (b). The number of bacteria per LCV (counted manually vs. calculated from volume measurements) is plotted for over 100 LCVs. Circles in red color denote perfect correlation. Circle size in the graph positively correlates with the number of LCVs that were assigned to each circle. (e) Z-stack series of a single large LCV from a U937 macrophage infected for 16hrs. The outline of the binary mask used to generate the volume measurement is shown for each 0.3μm Z-slice. The LCV volume (211μm3) was used to calculate the size (124 bacteria) using the formula derived in (b). The LCV Z-stack series is animated in the S1 Video. (f) Projection micrograph of the macrophage containing the LCV analyzed in (e) was stained with anti-ubiquitinated proteins (FK2), anti-Legionella antibodies (L.p) and Hoechst 33342.
(EPS)
Three-dimensional animation of the large LCV shown in S8f Fig with the edges of the binary mask used for volume analysis highlighted in green.
(AVI)
Acknowledgments
We are grateful to Dr. N. Cianciotto (Northwestern University) for the L. longbeachae, L. dumoffii and L. jordanis strains and Dr. Ralph Isberg (Tufts University) for the Lp02 pentuple strain.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from the Louisiana Board of Regents (http://www.regents.la.gov/) (LEQSF(2016-19)-RD-A-15) to SSI and the National Institute of Allergy and Infectious Diseases (https://www.niaid.nih.gov/Pages/default.aspx) (AI097847-02) to CRR. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kumar Y, Valdivia RH. Leading a sheltered life: intracellular pathogens and maintenance of vacuolar compartments. Cell Host Microbe. 2009;5: 593–601. 10.1016/j.chom.2009.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schulz F, Horn M. Intranuclear bacteria: inside the cellular control center of eukaryotes. Trends Cell Biol. 2015;25: 339–346. 10.1016/j.tcb.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 3.Manske C, Hilbi H. Metabolism of the vacuolar pathogen Legionella and implications for virulence. Front Cell Infect Microbiol. Frontiers; 2014;4: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isberg RR, O'Connor TJ, Heidtman M. The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nat Rev Microbiol. 2009;7: 13–24. 10.1038/nrmicro1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tilney LG, Harb OS, Connelly PS, Robinson CG, Roy CR. How the parasitic bacterium Legionella pneumophila modifies its phagosome and transforms it into rough ER: implications for conversion of plasma membrane to the ER membrane. J Cell Sci. 2001;114: 4637–4650. [DOI] [PubMed] [Google Scholar]
- 6.Kagan JC, Roy CR. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat Cell Biol. 2002;4: 945–954. 10.1038/ncb883 [DOI] [PubMed] [Google Scholar]
- 7.Abu Kwaik Y. The phagosome containing Legionella pneumophila within the protozoan Hartmannella vermiformis is surrounded by the rough endoplasmic reticulum. Appl Environ Microbiol. American Society for Microbiology (ASM); 1996;62: 2022–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roy CR, Tilney LG. The road less traveled: transport of Legionella to the endoplasmic reticulum. J Cell Biol. Rockefeller Univ Press; 2002;158: 415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hilbi H, Weber SS, Ragaz C, Nyfeler Y, Urwyler S. Environmental predators as models for bacterial pathogenesis. Environ Microbiol. Blackwell Publishing Ltd; 2007;9: 563–575. [DOI] [PubMed] [Google Scholar]
- 10.Kagan JC, Stein M-P, Pypaert M, Roy CR. Legionella subvert the functions of Rab1 and Sec22b to create a replicative organelle. J Exp Med. 2004;199: 1201–1211. 10.1084/jem.20031706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derré I, Isberg RR. Legionella pneumophila replication vacuole formation involves rapid recruitment of proteins of the early secretory system. Infect Immun. 2004;72: 3048–3053. 10.1128/IAI.72.5.3048-3053.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin S, Roy CR. Host cell processes that influence the intracellular survival of Legionella pneumophila. Cell Microbiol. 2008;10: 1209–1220. 10.1111/j.1462-5822.2008.01145.x [DOI] [PubMed] [Google Scholar]
- 13.Dorer MS, Kirton D, Bader JS, Isberg RR. RNA interference analysis of Legionella in Drosophila cells: exploitation of early secretory apparatus dynamics. PLoS Pathog. 2006;2: e34 10.1371/journal.ppat.0020034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy CR, Berger KH, Isberg RR. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol Microbiol. 1998;28: 663–674. [DOI] [PubMed] [Google Scholar]
- 15.Weber S, Wagner M, Hilbi H. Live-cell imaging of phosphoinositide dynamics and membrane architecture during Legionella infection. MBio. American Society for Microbiology; 2014;5: e00839–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivanov SS, Roy CR. Modulation of ubiquitin dynamics and suppression of DALIS formation by the Legionella pneumophila Dot/Icm system. Cell Microbiol. 2009;11: 261–278. 10.1111/j.1462-5822.2008.01251.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horwitz MA, Silverstein SC. Legionnaires' disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J Clin Invest. American Society for Clinical Investigation; 1980;66: 441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Creasey EA, Isberg RR. The protein SdhA maintains the integrity of the Legionella-containing vacuole. Proceedings of the National Academy of Sciences. National Acad Sciences; 2012;109: 3481–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isaac DT, Laguna RK, Valtz N, Isberg RR. MavN is a Legionella pneumophila vacuole-associated protein required for efficient iron acquisition during intracellular growth. Proceedings of the National Academy of Sciences. National Acad Sciences; 2015;112: E5208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.So EC, Mattheis C, Tate EW, Frankel G, Schroeder GN. Creating a customized intracellular niche: subversion of host cell signaling by Legionella type IV secretion system effectors. Can J Microbiol. NRC Research Press; 2015;61: 617–635. [DOI] [PubMed] [Google Scholar]
- 21.Kubori T, Nagai H. The Type IVB secretion system: an enigmatic chimera. Curr Opin Microbiol. 2016;29: 22–29. 10.1016/j.mib.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 22.Hubber A, Roy CR. Modulation of host cell function by Legionella pneumophila type IV effectors. Annu Rev Cell Dev Biol. Annual Reviews; 2010;26: 261–283. [DOI] [PubMed] [Google Scholar]
- 23.Berger KH, Isberg RR. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol. 1993;7: 7–19. [DOI] [PubMed] [Google Scholar]
- 24.Marra A, Blander SJ, Horwitz MA, Shuman HA. Identification of a Legionella pneumophila locus required for intracellular multiplication in human macrophages. Proc Natl Acad Sci USA. National Academy of Sciences; 1992;89: 9607–9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrews HL, Vogel JP, Isberg RR. Identification of linked Legionella pneumophila genes essential for intracellular growth and evasion of the endocytic pathway. Infect Immun. American Society for Microbiology (ASM); 1998;66: 950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laguna RK, Creasey EA, Li Z, Valtz N, Isberg RR. A Legionella pneumophila-translocated substrate that is required for growth within macrophages and protection from host cell death. Proc Natl Acad Sci USA. National Acad Sciences; 2006;103: 18745–18750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monroe KM, McWhirter SM, Vance RE. Identification of host cytosolic sensors and bacterial factors regulating the type I interferon response to Legionella pneumophila. PLoS Pathog. 2009;5: e1000665 10.1371/journal.ppat.1000665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Connor TJ, Boyd D, Dorer MS, Isberg RR. Aggravating genetic interactions allow a solution to redundancy in a bacterial pathogen. Science. American Association for the Advancement of Science; 2012;338: 1440–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nohturfft A, Zhang SC. Coordination of lipid metabolism in membrane biogenesis. Annu Rev Cell Dev Biol. 2009;25: 539–566. 10.1146/annurev.cellbio.24.110707.175344 [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Sato R, Brown MS, Hua X, Goldstein JL. SREBP-1, a membrane-bound transcription factor released by sterol-regulated proteolysis. Cell. 1994;77: 53–62. [DOI] [PubMed] [Google Scholar]
- 31.Hagen RM, Rodriguez-Cuenca S, Vidal-Puig A. An allostatic control of membrane lipid composition by SREBP1. FEBS Lett. 2010. [DOI] [PubMed] [Google Scholar]
- 32.Bengoechea-Alonso MT, Ericsson J. SREBP in signal transduction: cholesterol metabolism and beyond. Curr Opin Cell Biol. 2007;19: 215–222. 10.1016/j.ceb.2007.02.004 [DOI] [PubMed] [Google Scholar]
- 33.Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8: 224–236. 10.1016/j.cmet.2008.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Düvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39: 171–183. 10.1016/j.molcel.2010.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laplante M, Sabatini DM. An emerging role of mTOR in lipid biosynthesis. Curr Biol. 2009;19: R1046–52. 10.1016/j.cub.2009.09.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ricoult SJH, Manning BD. The multifaceted role of mTORC1 in the control of lipid metabolism. EMBO Rep. EMBO Press; 2013;14: 242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kennedy BK, Lamming DW. The Mechanistic Target of Rapamycin: The Grand ConducTOR of Metabolism and Aging. Cell Metab. 2016;23: 990–1003. 10.1016/j.cmet.2016.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim D-H, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110: 163–175. [DOI] [PubMed] [Google Scholar]
- 39.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149: 274–293. 10.1016/j.cell.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Betz C, Hall MN. Where is mTOR and what is it doing there? J Cell Biol. Rockefeller Univ Press; 2013;203: 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarbassov DD, Ali SM, Kim D-H, Guertin DA, Latek RR, Erdjument-Bromage H, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14: 1296–1302. 10.1016/j.cub.2004.06.054 [DOI] [PubMed] [Google Scholar]
- 42.Arriola Apelo SI, Lamming DW. Rapamycin: An InhibiTOR of Aging Emerges From the Soil of Easter Island. J Gerontol A Biol Sci Med Sci. Oxford University Press; 2016;71: 841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. Hunter T, editor. PLoS Biol. Public Library of Science; 2009;7: e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. Journal of Biological Chemistry. American Society for Biochemistry and Molecular Biology; 2009;284: 8023–8032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang SA, Pacold ME, Cervantes CL, Lim D, Lou HJ, Ottina K, et al. mTORC1 phosphorylation sites encode their sensitivity to starvation and rapamycin. Science. American Association for the Advancement of Science; 2013;341: 1236566–1236566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29: 565–577. 10.1016/j.immuni.2008.08.012 [DOI] [PubMed] [Google Scholar]
- 47.Cao W, Manicassamy S, Tang H, Kasturi SP, Pirani A, Murthy N, et al. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol. 2008;9: 1157–1164. 10.1038/ni.1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weichhart T, Hengstschläger M, Linke M. Regulation of innate immune cell function by mTOR. Nat Rev Immunol. 2015;15: 599–614. 10.1038/nri3901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abdel-Nour M, Tsalikis J, Kleinman D, Girardin SE. The emerging role of mTOR signalling in antibacterial immunity. Immunol Cell Biol. Nature Publishing Group; 2014;92: 346–353. [DOI] [PubMed] [Google Scholar]
- 50.Tattoli I, Sorbara MT, Vuckovic D, Ling A, Soares F, Carneiro LAM, et al. Amino acid starvation induced by invasive bacterial pathogens triggers an innate host defense program. Cell Host Microbe. 2012;11: 563–575. 10.1016/j.chom.2012.04.012 [DOI] [PubMed] [Google Scholar]
- 51.Ivanov SS, Roy CR. Pathogen signatures activate a ubiquitination pathway that modulates the function of the metabolic checkpoint kinase mTOR. Nat Immunol. 2013;14: 1219–1228. 10.1038/ni.2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE. Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog. 2006;2: e18 10.1371/journal.ppat.0020018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meyuhas O. Ribosomal Protein S6 Phosphorylation: Four Decades of Research. Int Rev Cell Mol Biol. Elsevier; 2015;320: 41–73. [DOI] [PubMed] [Google Scholar]
- 54.Ninio S, Zuckman-Cholon DM, Cambronne ED, Roy CR. The Legionella IcmS-IcmW protein complex is important for Dot/Icm-mediated protein translocation. Mol Microbiol. Blackwell Science Ltd; 2005;55: 912–926. [DOI] [PubMed] [Google Scholar]
- 55.Cambronne ED, Roy CR. The Legionella pneumophila IcmSW complex interacts with multiple Dot/Icm effectors to facilitate type IV translocation. PLoS Pathog. 2007;3: e188 10.1371/journal.ppat.0030188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coers J, Kagan JC, Matthews M, Nagai H, Zuckman DM, Roy CR. Identification of Icm protein complexes that play distinct roles in the biogenesis of an organelle permissive for Legionella pneumophila intracellular growth. Mol Microbiol. 2000;38: 719–736. [DOI] [PubMed] [Google Scholar]
- 57.Nagai H, Cambronne ED, Kagan JC, Amor JC, Kahn RA, Roy CR. A C-terminal translocation signal required for Dot/Icm-dependent delivery of the Legionella RalF protein to host cells. Proc Natl Acad Sci USA. 2005;102: 826–831. 10.1073/pnas.0406239101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O'Connor TJ, Adepoju Y, Boyd D, Isberg RR. Minimization of the Legionella pneumophila genome reveals chromosomal regions involved in host range expansion. Proceedings of the National Academy of Sciences. 2011;108: 14733–14740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cummings BS, Wills LP, Schnellmann RG. Measurement of cell death in Mammalian cells. Curr Protoc Pharmacol. John Wiley & Sons, Inc; 2012;Chapter 12: Unit12.8–12.8.24. [DOI] [PubMed] [Google Scholar]
- 60.Yuan M, Pino E, Wu L, Kacergis M, Soukas AA. Identification of Akt-independent regulation of hepatic lipogenesis by mammalian target of rapamycin (mTOR) complex 2. J Biol Chem. American Society for Biochemistry and Molecular Biology; 2012;287: 29579–29588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang BT, Ducker GS, Barczak AJ, Barbeau R, Erle DJ, Shokat KM. The mammalian target of rapamycin regulates cholesterol biosynthetic gene expression and exhibits a rapamycin-resistant transcriptional profile. Proceedings of the National Academy of Sciences. National Acad Sciences; 2011;108: 15201–15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gilardi F, Migliavacca E, Naldi A, Baruchet M, Canella D, Le Martelot G, et al. Genome-wide analysis of SREBP1 activity around the clock reveals its combined dependency on nutrient and circadian signals. Kramer A, editor. PLoS Genet. Public Library of Science; 2014;10: e1004155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ray K, Bobard A, Danckaert A, Paz-Haftel I, Clair C, Ehsani S, et al. Tracking the dynamic interplay between bacterial and host factors during pathogen-induced vacuole rupture in real time. Cell Microbiol. 2010. [DOI] [PubMed] [Google Scholar]
- 64.Fredlund J, Enninga J. Cytoplasmic access by intracellular bacterial pathogens. Trends Microbiol. 2014;22: 128–137. 10.1016/j.tim.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 65.Boyle KB, Randow F. The role of “eat-me” signals and autophagy cargo receptors in innate immunity. Curr Opin Microbiol. 2013;16: 339–348. 10.1016/j.mib.2013.03.010 [DOI] [PubMed] [Google Scholar]
- 66.Paz I, Sachse M, Dupont N, Mounier J, Cederfur C, Enninga J, et al. Galectin-3, a marker for vacuole lysis by invasive pathogens. Cell Microbiol. Blackwell Publishing Ltd; 2010;12: 530–544. [DOI] [PubMed] [Google Scholar]
- 67.Kamisuki S, Mao Q, Abu-Elheiga L, Gu Z, Kugimiya A, Kwon Y, et al. A small molecule that blocks fat synthesis by inhibiting the activation of SREBP. Chem Biol. 2009;16: 882–892. 10.1016/j.chembiol.2009.07.007 [DOI] [PubMed] [Google Scholar]
- 68.Sherwood RK, Roy CR. Autophagy Evasion and Endoplasmic Reticulum Subversion: The Yin and Yang of Legionella Intracellular Infection. Annu Rev Microbiol. 2016;70: 413–433. 10.1146/annurev-micro-102215-095557 [DOI] [PubMed] [Google Scholar]
- 69.Creasey EA, Isberg RR. Maintenance of vacuole integrity by bacterial pathogens. Curr Opin Microbiol. 2014;17: 46–52. 10.1016/j.mib.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eisenreich W, Heesemann J, Rudel T, Goebel W. Metabolic Adaptations of Intracellullar Bacterial Pathogens and their Mammalian Host Cells during Infection (“Pathometabolism”). Microbiol Spectr. 2015;3. [DOI] [PubMed] [Google Scholar]
- 71.Buchkovich NJ, Yu Y, Zampieri CA, Alwine JC. The TORrid affairs of viruses: effects of mammalian DNA viruses on the PI3K-Akt-mTOR signalling pathway. Nat Rev Microbiol. 2008;6: 266–275. 10.1038/nrmicro1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gomez-Valero L, Rusniok C, Rolando M, Neou M, Dervins-Ravault D, Demirtas J, et al. Comparative analyses of Legionella species identifies genetic features of strains causing Legionnaires' disease. Genome Biol. BioMed Central; 2014;15: 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burstein D, Amaro F, Zusman T, Lifshitz Z, Cohen O, Gilbert JA, et al. Genomic analysis of 38 Legionella species identifies large and diverse effector repertoires. Nat Genet. 2016;48: 167–175.: 10.1038/ng.3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choy A, Dancourt J, Mugo B, O'Connor TJ, Isberg RR, Melia TJ, et al. The Legionella effector RavZ inhibits host autophagy through irreversible Atg8 deconjugation. Science. American Association for the Advancement of Science; 2012;338: 1072–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miettinen TP, Björklund M. Mevalonate Pathway Regulates Cell Size Homeostasis and Proteostasis through Autophagy. Cell Rep. 2015;13: 2610–2620. 10.1016/j.celrep.2015.11.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jeon T-I, Osborne TF. SREBPs: metabolic integrators in physiology and metabolism. Trends Endocrinol Metab. 2012;23: 65–72. 10.1016/j.tem.2011.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Asrat S, Dugan AS, Isberg RR. The frustrated host response to Legionella pneumophila is bypassed by MyD88-dependent translation of pro-inflammatory cytokines. PLoS Pathog. Public Library of Science; 2014;10: e1004229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Copenhaver AM, Casson CN, Nguyen HT, Duda MM, Shin S. IL-1R signaling enables bystander cells to overcome bacterial blockade of host protein synthesis. Proceedings of the National Academy of Sciences. National Acad Sciences; 2015;112: 7557–7562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Häuslein I, Manske C, Goebel W, Eisenreich W, Hilbi H. Pathway analysis using (13) C-glycerol and other carbon tracers reveals a bipartite metabolism of Legionella pneumophila. Mol Microbiol. 2016;100: 229–246. 10.1111/mmi.13313 [DOI] [PubMed] [Google Scholar]
- 80.Price CTD, Richards AM, Abu Kwaik Y. Nutrient generation and retrieval from the host cell cytosol by intra-vacuolar Legionella pneumophila. Front Cell Infect Microbiol. Frontiers; 2014;4: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rosel D, Khurana T, Majithia A, Huang X, Bhandari R, Kimmel AR. TOR complex 2 (TORC2) in Dictyostelium suppresses phagocytic nutrient capture independently of TORC1-mediated nutrient sensing. J Cell Sci. The Company of Biologists Ltd; 2012;125: 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peracino B, Balest A, Bozzaro S. Phosphoinositides differentially regulate bacterial uptake and Nramp1-induced resistance to Legionella infection in Dictyostelium. J Cell Sci. 2010. [DOI] [PubMed] [Google Scholar]
- 83.Weber SS, Ragaz C, Reus K, Nyfeler Y, Hilbi H. Legionella pneumophila exploits PI(4)P to anchor secreted effector proteins to the replicative vacuole. PLoS Pathog. 2006;2: e46 10.1371/journal.ppat.0020046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Flieger A, Neumeister B, Cianciotto NP. Characterization of the gene encoding the major secreted lysophospholipase A of Legionella pneumophila and its role in detoxification of lysophosphatidylcholine. Infect Immun. 2002;70: 6094–6106. 10.1128/IAI.70.11.6094-6106.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Banerji S, Aurass P, Flieger A. The manifold phospholipases A of Legionella pneumophila—identification, export, regulation, and their link to bacterial virulence. Int J Med Microbiol. 2008;298: 169–181. 10.1016/j.ijmm.2007.11.004 [DOI] [PubMed] [Google Scholar]
- 86.Feeley JC, Gibson RJ, Gorman GW, Langford NC, Rasheed JK, Mackel DC, et al. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J Clin Microbiol. American Society for Microbiology (ASM); 1979;10: 437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Silveira TN, Zamboni DS. Pore formation triggered by Legionella spp. is an Nlrc4 inflammasome-dependent host cell response that precedes pyroptosis. Infect Immun. 2010;78: 1403–1413. 10.1128/IAI.00905-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a-b) Images from synchronized infections under serum-free conditions of serum-starved BMMs infected as indicated. Representative single projection images of 3D microscopy images of macrophages infected by ΔflaA (a) or ΔdotA (b) for 8 hrs (MOI = 20, infections synchronized at 60min) and stained with anti-phospho-rS6p(S235/S236), anti-Legionella (L.p) antibodies and Hoechst 33342. (*) indicates Legionella infected cell, Bar = 10μm. (c-d) Frequency distribution of the phospho-rS6p mean fluorescent intensity (MFI) in individual macrophages infected by ΔflaA (c) or ΔdotA (d). Each MFI bin spans 200 fluorescence intensity units of the p-rS6p(S235/S236) fluorescent signal. At least 200 LCVs were analyzed for each condition. A representative of three (a-d) biological replicates is shown for each experiment.
(EPS)
(a-b) Images from synchronized infections under serum-free conditions of serum-starved Myd88-/- BMMs for 8 hrs (MOI = 20) as indicated. Cells were infected by either L.pneumophila (LP), L.dumoffii (LD), L.longbeachae (LL) or the indicated combinations in the co-infection (ratio 1:3) experiments. The ΔflaA of L.pneumophila was used to avoid pyroptosis. LD and LL lack the flaA gene [87]. Quantitated data is shown in (a). (b) A representative micrograph of cells stained with anti-ubiquitinated proteins (FK2), anti-p-rS6p (S235/236), anti-L.dumoffii antibodies and Hoechst 33342. Each channel is shown individually. Ubiquitin is a marker specific for LP LCVs and was used to identify LP-infected macrophages. Arrowheads indicate Legionella-containing vacuoles and (*) mark cells co-infected by LP and LD, Bar = 10μm. (c) Quantitation of p-rS6p positive Myd88-/- BMMs uninfected or infected with either ΔflaA or pentuple ΔflaA strains for 8hrs. Synchronized infections with MOI = 20 (a, c) Means ± s.d of technical triplicates for the two distinct groups within the cell population–(a) LP present and LP absent for each condition are shown. At least 50 infected cells were analyzed for each condition. A representative of two biological replicates is shown. (a and c) n.s—not significant (unpaired T-test).
(EPS)
(a-b) Panel of micrographs of representative ΔflaA infected BMMs stained with anti-L. pneumophila antibody and Hoechst 33342. Cells with aberrant (a) or normal (b) nuclear morphology are shown. Bar = 10μm.
(EPS)
(a) qPCR analysis of gene transcripts that regulate lipogenesis and lipids trafficking in serum-starved BMMs infected by either ΔflaA or ΔdotA (MOI = 10) for 6 hrs under serum-free conditions. Means ± s.d of technical triplicates for each condition are shown. The amount of each transcript was normalized to Canx (Calnexin) and is presented as fold increase over unstimulated cells (UN). A representative of two biological replicates is shown. n.s—not significant, * p<0.05, ** p<0.005 (unpaired T-test).
(EPS)
(a-b) Phorbol ester differentiated U937 cells were serum-starved and infected under serum-free conditions with ΔflaA Legionella for 16 hrs (MOI = 10, synchronized infections) in the presence/absence of PP242 (2.5μM). (a) Micrographs show representative populations of macrophages stained with anti-L. pneumophila (L.p) and Hoechst 33342. Arrowheads indicate LCVs and (*) indicate infected cells with condensed nucleus. (Bar = 10μm.) (b) Quantitation of infected and neighboring uninfected U937 macrophages with condensed nuclei after the indicated treatments. Means ± s.d of technical replicates of dead cell as percentage of total cells in each condition are shown. A representative of two biological replicates is shown. n.s—not significant, ** p<0.005 (unpaired T-test).
(EPS)
(a-c) Serum-starved Myd88-/- macrophages were infected with ΔflaA L. pneumophila for 10 hrs (a-b) or L. dumoffii for 12 hrs (c-d) in synchronized infections in the absence (a-d) or presence (d) of FBS (10% v/v). (a-c) Micrographs of representative Galectin 3 negative (a) and positive (b and c) vacuoles are shown. Cells were stained with anti-galectin3, Hoechst 33342 and anti-L. dumoffii (L.d) or anti-L. pneumophila (L.p) antibodies. Arrowheads indicate the LCVs. (a) Cell harboring Galectin 3 negative LCVs show typical dispersed Galectin 3 staining pattern, whereas cell containing Galectin 3 positive LCVs show distinct accumulation of multiple bacteria-proximal Galectin 3 puncta (b and c). (d) Quantitation of Galectin 3-positive LCVs produced by infections with ΔflaA or L.dumoffii and treatments with PP242 (2.5μM) or vehicle alone. Means ± s.d of technical triplicates for each condition are shown. At least 100 LCVs were analyzed for each condition. A representative of two biological replicates is shown. n.s—not significant, ** p<0.005 (unpaired T-test).
(EPS)
(a-b) Legionella growth in AYE liquid cultures in the presence/absence of fatostatin (40μM) (a), PP242 (25μM) (b), Torin2 (3μM) (b). Changes in the cultures’ optical densities over time are shown for each condition. A representative of two biological replicates are shown for each experiment.
(EPS)
(a) Methodological outline for the analysis of LCV bacterial size from 3D microscopy images of infected cells stained with purified anti-Legionella IgY chicken antibody. Z-stack series of a single LCV spanning 2.4 μm and containing four bacteria is shown. Each bacterium is numbered and the outline of the 3D binary mask used to measure the LCV volume (5.65 μm3) is shown. (b) Derivation of the LCV volume-to-bacteria number conversion formula. Graph plots the measured volumes of the bacterial mass for 100 LCVs in which the individual bacteria can be unambiguously identified over the manually counted number of bacteria per LCV. (c) Micrographs of image projections of individual representative LCVs used in the formula derivation protocol. Each bacterium is numbered and the volume of the bacterial mass binary mask is shown. The volume of individual bacteria varied from 0.95μm3 to 2.42μm3. A bacterium undergoing binary fission was counted as a single cell until the septum is resolved. (d) Back-testing of the conversion formula derived in (b). The number of bacteria per LCV (counted manually vs. calculated from volume measurements) is plotted for over 100 LCVs. Circles in red color denote perfect correlation. Circle size in the graph positively correlates with the number of LCVs that were assigned to each circle. (e) Z-stack series of a single large LCV from a U937 macrophage infected for 16hrs. The outline of the binary mask used to generate the volume measurement is shown for each 0.3μm Z-slice. The LCV volume (211μm3) was used to calculate the size (124 bacteria) using the formula derived in (b). The LCV Z-stack series is animated in the S1 Video. (f) Projection micrograph of the macrophage containing the LCV analyzed in (e) was stained with anti-ubiquitinated proteins (FK2), anti-Legionella antibodies (L.p) and Hoechst 33342.
(EPS)
Three-dimensional animation of the large LCV shown in S8f Fig with the edges of the binary mask used for volume analysis highlighted in green.
(AVI)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.