Abstract
Urogenital schistosomiasis is a chronic infection caused by the human blood fluke Schistosoma haematobium. Schistosomiasis haematobium is a known risk factor for cancer leading to squamous cell carcinoma of the urinary bladder (SCC). This is a neglected tropical disease endemic in many countries of Africa and the Middle East.
Schistosome eggs produce catechol-estrogens. These molecules are metabolized to active quinones that cause alterations in DNA (leading in other contexts to breast or thyroid cancer). Our group have shown that schistosome egg associated catechol estrogens induce tumor-like phenotypes in urothelial cells, originated from parasite estrogen-host cell chromosomal DNA adducts and mutations.
Here we review recent findings on the role of estrogen–DNA adducts and how their shedding in urine may be prognostic of schistosome infection and/or represent potential biomarkers for urogenital schistosomiasis associated bladder cancer and infertility.
Keywords: Schistosoma haematobium, Bladder cancer, Infertility, Estrogen metabolites, Biomarkers
Graphical Abstract
Urogenital schistosomiasis
Schistosomiasis is a parasitic disease that infects an estimated 243 million people worldwide, and every year kills about 200 thousand [1,2]. It is contracted through contaminated waters, a disease of the developing world where is 2nd only to malaria in rates of infection and public health impact [3].
This infectious disease is acquired when the parasite larvae enter the skin in contact with contaminated waters. The larvae live in blood vessels for up to 5 years releasing eggs, which are excreted to the outside to infect snails and start a new parasite’s life cycle. However, many eggs are not excreted but get stuck in the patient’s tissues and organs triggering an immune response that causes chronic inflammation and destruction of these tissues [4].
The most prevalent human Schistosoma species is Schistosoma (S.) haematobium, which affects the urogenital system and may cause bladder cancer [5,6]. In women, the cervix and the uterus are often affected causing female genital schistosomiasis (FGS), which produces infertility, as well as bleeding and pain during sexual intercourse [7]. In countries with little access to information and medical care, this type of problems may result in stigmatization and other negative social consequences to women, as fertility remains central for their identity and discussion of sexual problems is a taboo in many ethnic groups of endemic areas. Around 40 million women of childbearing age are estimated to be infected [3,8].
Estrogen metabolites as biomarkers of urogenital schistosomiasis
An appalling elevation in levels of estradiol but not luteinizing hormone (LH) or testosterone in sera was observed in human cases of urogenital schistosomiasis for the study of hormones in this infection [9].
Estradiol is an hormone belonging to the steroid superfamily mainly produced by the follicles of ovaries from vertebrates. We questioned if the elevated levels in these cases could be attributed to hypothalamic-pituitary gonadal axis regulations. Instead, we hypothesized that schistosomes produced estradiol-related molecules and these molecules contributed to the increased estradiol levels.
Next, using LC-MS-MS (Liquid Chromatography coupled with Mass Detector) techniques we characterized estradiol related molecules, from sera of S. haematobium-infected persons and, noteworthy, in the parasites including the eggs [10].
As shown previously by our group, we identified and characterized by LC-MS-MS novel catechol estrogens in schistosome worms and infected person’s sera and urine.
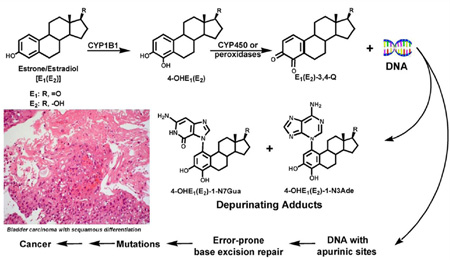
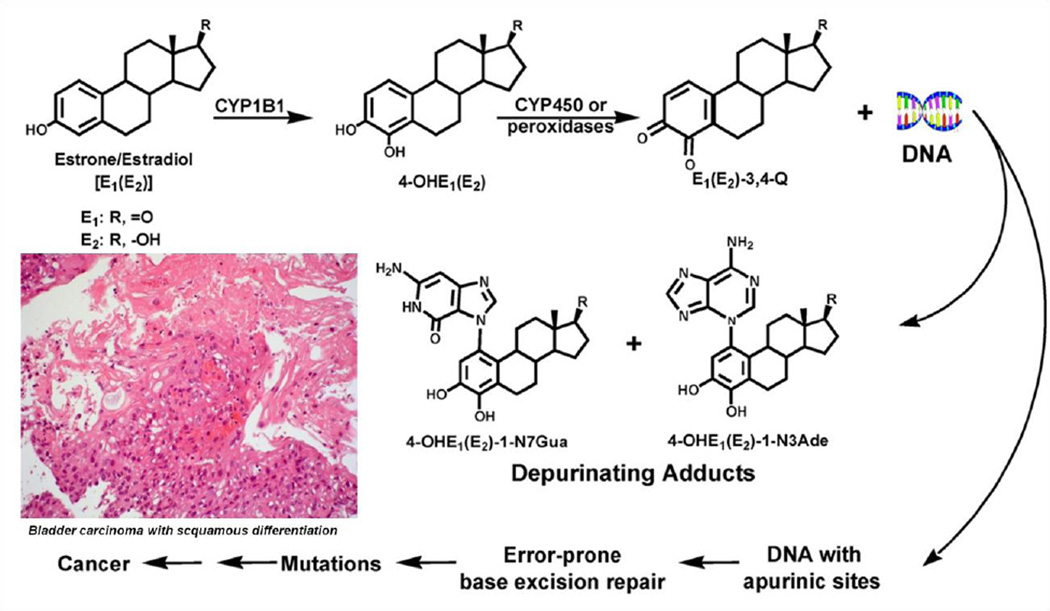
The molecules named catechol-estrogens are synthesised by a reaction of hydroxylation on the steroid aromatic ring A component of the steroid molecule. This hydroxylation of the atoms of carbon #2 and #3 of the steroid molecule led to a further reaction of oxidation creating another molecule called estradiol-2,3-quinone. We have speculated that reactive catechol-estrogens from schistosome eggs play a decisive act in urogenital schistosomiasis associated bladder cancer (Figure 1) [4,10] and infertility [3,11].
Figure 1.
Major metabolic pathways in cancer initiation by estrogens [8].
Molecular mechanism underlying schistosome estrogen catechols in cancer and infertility
Given the context of the unarguable link between S. haematobium infection and bladder cancer, the presence of putative carcinogenic molecules in S. haematobium eggs hopefully may have practical consequences for new approaches to disease control [5,10]. Metabolism of estrogens and the production of depurinating estrogen–DNA adducts can be implicated in a pathway underlying S. haematobium-promoted host cell DNA damage, leading eventually to cell transformation. The carcinogenic effect of this estrogen–DNA adduct mediated pathway could explain the link between chronic schistosomiasis haematobia and SCC of the bladder [10]. We anticipate that these findings will contribute to understanding how schistosomiasis haematobia leads to SCC of the bladder.
Hormonal disturbances in women with FGS may be linked to infertility and suboptimal fecundity [3,11]. Recently, estrogen-like metabolites were detected by LC–MS in urine of S. haematobium-infected women. These metabolites are similar to those identified previously in the adult worm and egg stages of S. haematobium [10]. The presence of estrogen-like metabolites during FGS was statistically associated with self-reported infertility [3]. These electrophilic compounds can react with DNA to form depurinating adducts. It is not inconceivable that apurinic sites in chromosomal DNA that result from this reaction generate mutations that might underlie infertility [3,11].
The impact of Mass Spectrometry as a diagnostic and prognostic tool in schistosomiasis associated cancer and infertility
Only a few years ago, the bladder cancer and female infertility observed in schistosomiais infected individuals were assumed to result exclusively from the tissue apoptosis caused by the immune response against the entrapped eggs.
We have been working for more than 10 years with S. haematobium and felt that a shift in this paradigm was needed. This shift happened after discovering and identifying estrogen-like molecules circulating in patients infected with schistosomiasis that potentially cause cancer [5,10]. Recently, we observed these molecules not only circulating in the blood of infected patients but also in the urine of these patients. We observed in the urine of infected women (but not on healthy individuals) catechol-estrogens. These molecules were further associated with infertility [3].
Catechol- and quinone-estrogens have been linked before to cancer (thyroid, breast and prostate) [12] and to auto-immunity [13]. In schistosomiasis it has to be seen, if and to what extent the catechols produced by S. haematobium induce carcinogenesis and infertility by changes in the hormonal environment (hormones are known to affect the immune response also), and/or by the increase of the probability of mutations, The LC-MS-MS for the search of estrogen metabolites in infected patients is a non-invasive test, suitable especially for female genital schistosomiasis. With its potential to detect biomarkers of cancer and infertility associated to schistosomiasis, it could improve the public health in under-resourced and under-served populations.
Potential Future Impact of catechol estrogens detection in schistosomiasis
Potentially, the possibility to detect catechols in the urine of infected patients offers new perspectives for controlling schistosomiasis-induced morbidity. An automated diagnostic method or point-of-care testing based on analysis of catechol estrogens in urine of S. haematobium infected persons could provide a novel, non-invasive tool to complement current approaches. The use of the recent high resolution mass spectrometry could be used without the need of standards and with higher throughput.
Major drawbacks of the use of LC-MS-MS as a diagnostic tool in schistosomiasis
The cost of new LC-MS instruments ranges from € 100,000 to € 600,000. In addition to initial purchase cost, machines require annual maintenance work. This type of contract currently cost approximately € 10,000 per year. It is estimated that analysis of one analyte per urine costs an average of €15. Therefore, the use of LC-MS for diagnostic and prognostic testing in schistosomiasis would be only cost-efficient when it is used for routine analysis [14].
The level of training and expertise varies among personnel working with LC-MS. Interpretation of data and the development of methods requires in-depth knowledge of both organic and inorganic chemistry. Maintenance of the instruments and troubleshooting requires the technical, mechanical and electronic skills of a scientist or engineer [14]. On the other hand. for the system operation and data analysis of the analytes in the urine of schistosome infected patients can be carried by a technician trained after analytical validation.
In future, LC-MS equipment would be a valuable tool for proper diagnosis of schistosomiasis. These are cheaper routine analyzers facilitating their application in endemic countries and may thus be applied at least in a context of an emerging economy country.
Concluding remarks
Further studies are necessary to identify and characterize production of these estradiol-like molecules in schistosomes and define their hormonal function(s) in the developmental cycle of this blood fluke.
Importance will be given to evaluate the specific effects of the estrogenic molecules identified in the lysates of schistosomes and to assess activities of specific catechol-estrogens identified in the schistosome eggs. It will be informative to use either catechol-estrogens purified from eggs of S. haematobium and/or synthetic versions of these putative carcinogens.
Acknowledgments
This work was financed by FEDER - Fundo Europeu de Desenvolvimento Regional funds through the COMPETE 2020 - Operacional Programme for Competitiveness and Internationalisation (POCI), Portugal 2020, and by Portuguese funds through
FCT - Fundação para a Ciência e a Tecnologia/ Ministério da Ciência, Tecnologia e Inovação in the framework of the project "Institute for Research and Innovation in Health Sciences" (POCI-01-0145-FEDER-007274).
References
- 1.Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014;383(9936):2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO, World Health Organization. Schistosomiasis. Progress Report 2001–2011 and Strategic Plan 2012–2020. WHO; Geneva: 2014. http://www.who.int. [Google Scholar]
- 3.Santos J, Gouveia MJ, Vale N, Delgado ML, Gonçalves A, Silva JM, Oliveira C, Xavier P, Gomes P, Santos LL, Lopes C, Barros A, Rinaldi G, Brindley PJ, da Costa JM, Sousa M, Botelho MC. Urinary estrogen metabolites and self-reported infertility in women infected with Schistosoma haematobium. PLoS One. 2014;9:e96774. doi: 10.1371/journal.pone.0096774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Botelho MC, Machado JC, Brindley PJ, Correia da Costa JM. Targeting molecular signaling pathways of Schistosoma haemotobium infection in bladder cancer. Virulence. 2011;2:267–279. doi: 10.4161/viru.2.4.16734. [DOI] [PubMed] [Google Scholar]
- 5.Botelho MC, Soares R, Vale N, Ribeiro R, Camilo V, Almeida R, Medeiros R, Gomes P, Machado JC, Correia da Costa JM. Schistosoma haematobium: identification of new estrogenic molecules with estradiol antagonistic activity and ability to inactivate estrogen receptor in mammalian cells. Exp Parasitol. 2010;126:526–535. doi: 10.1016/j.exppara.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Botelho MC, Ribeiro R, Vale N, Oliveira P, Medeiros R, Lopes C, Machado JC, Correia da Costa JM. Inactivation of estrogen receptor by Schistosoma haematobium total antigen in bladder urothelial cells. Oncol Rep. 2012;27:356–362. doi: 10.3892/or.2011.1552. [DOI] [PubMed] [Google Scholar]
- 7.Kjetland EF, Leutscher PD, Ndhlovu PD. A review of female genital schistosomiasis. Trends Parasitol. 2012;28(2):58–65. doi: 10.1016/j.pt.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Botelho MC, Alves H, Barros A, Rinaldi G, Brindley PJ, Sousa M. The role of estrogens and estrogen receptor signaling pathways in cancer and infertility: the case of schistosomes. Trends Parasitol. 2015;31(6):246–250. doi: 10.1016/j.pt.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Botelho MC, Crespo M, Almeida A, Vieira P, Delgado ML, Araujo L, Machado JC, Correia da Costa JM. Schistosoma haematobium and Schistosomiasis mansoni: production of an estradiol-related compound detected by ELISA. Exp Parasitol. 2009;122:250–253. doi: 10.1016/j.exppara.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Botelho MC, Vale N, Gouveia MJ, Rinaldi G, Santos J, Santos LL, Gomes P, Brindley PJ, Correia da Costa JM. Tumour-like phenotypes in urothelial cells after exposure to antigens from eggs of Schistosoma haematobium: an oestrogen-DNA adducts mediated pathway? Int J Parasitol. 2013;43:17–26. doi: 10.1016/j.ijpara.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 11.Botelho MC, Sousa M. New biomarkers to fight urogenital schistosomiasis: a major neglected tropical disease. Biomark Med. 2014;8(9):1061–1063. doi: 10.2217/bmm.14.68. [DOI] [PubMed] [Google Scholar]
- 12.Cavalieri EL, Rogan EG. Unbalanced metabolism of endogenous estrogens in the etiology and prevention of human cancer. J Steroid Biochem Mol Biol. 2011;125:169–180. doi: 10.1016/j.jsbmb.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cutolo M, Sulli A, Straub RH. Estrogen metabolism and autoimmunity. Autoimmun Rev. 2012;11:4460–4644. doi: 10.1016/j.autrev.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Murtagh R, Behringer V, Deschner T. LC-MS as a method for non-invasive measurement of steroid hormones and their metabolites in urine and faeces of animals. Wien Tierärztl Monat - Vet Med Austria. 2013;100:247–254. [Google Scholar]