Abstract
We retrospectively evaluated early and intermediate outcomes of aortic arch surgery in patients with type A acute aortic dissection (AAD), investigating the effect of arch surgery extension on postoperative results.
From January 2006 through July 2013, 201 patients with type A AAD underwent urgent corrective surgery at our institution. Of the 92 patients chosen for this study, 59 underwent hemiarch replacement (hemiarch group), and 33 underwent total arch replacement (total arch group) in conjunction with ascending aorta replacement.
The operative mortality rate was 22%. Total arch replacement was associated with a 33% risk of operative death, versus 15% for hemiarch (P=0.044). Multivariable analysis found these independent predictors of operative death: age (odds ratio [OR]=1.13/yr; 95% confidence interval [CI], 1.04–1.23; P=0.002), body mass index >30 kg/m2 (OR=9.9; 95% CI, 1.28–19; P=0.028), postoperative low cardiac output (OR=10.6; 95% CI, 1.18–25; P=0.035), and total arch replacement (OR=8.8; 95% CI, 1.39–15; P=0.021) The mean overall 5-year survival rate was 59.3% ± 5.5%, and mean 5-year freedom from distal reintervention was 95.4% ± 3.2% (P=NS).
In type A AAD, aortic arch surgery is still associated with high operative mortality rates; hemiarch replacement can be performed more safely than total arch replacement. Rates of distal aortic reoperation were not different between the 2 surgical strategies.
Keywords: Aneurysm, dissecting/epidemiology/mortality; aortic aneurysm/epidemiology/mortality/surgery; aorta, thoracic/surgery; cardiac surgical procedures/methods; hospital mortality; logistic models; prognosis; retrospective studies; severity of illness index; survival analysis
The surgical mortality rate for type A acute aortic dissection (AAD) still remains high (8%–34%), despite improvements in perioperative and postoperative management during the last decade.1–3 Complete resection of the intimal tear and prosthetic replacement of the ascending aorta are still considered the standard of care for type A dissection surgery. Because the residual dissection flap in the aortic arch and descending aorta carries risks of progressive aortic dilation and rupture and the need for secondary intervention, several groups have suggested immediate extensive surgery that involves the aortic arch, although concomitant distal aortic manipulation has been associated with an increased risk of morbidity and death.4–13 In this situation, 2 different surgical strategies have been proposed: total arch replacement or hemiarch replacement (a more conservative repair limited to the ascending aorta and proximal arch).4,9–12
We evaluated the early and intermediate outcomes of aortic arch surgery in patients with type A AAD, investigating the effect, upon postoperative results, of aortic arch extension.
Patients and Methods
We undertook a retrospective, observational study of prospectively collected data on consecutive patients who had presented with type A AAD at our institution. This cohort study was approved by our local ethics committee, and individual consent was obtained by each patient's physician. From January 2006 through July 2013, 201 patients with type A AAD underwent urgent surgery at our institution. For the purposes of this study, we did not consider 109 AAD patients who underwent isolated replacement of the ascending aorta. We included only the 92 patients who underwent concomitant aortic arch repair: 59 of these underwent hemiarch replacement (the hemiarch group), and the remaining 33 received total arch replacement (the total arch group) in conjunction with ascending aortic replacement.
We confined our study to type A AADs operated on no later than 14 days after symptom onset. We defined visceral ischemia as a reduction of blood flow to the bowel or gastrointestinal system, caused by blood-vessel blockage; we diagnosed it by clinical examination and confirmed it by computed tomography. Low cardiac output syndrome (LCOS) was defined as a severe reduction in cardiac index (<2.2 L/min/m2) or need for prolonged inotropic support (>24 hr). Pulmonary sequelae referred to the need for prolonged ventilation (>48 hr), noninvasive ventilation, or reintubation for respiratory insufficiency or pneumonia. Infections could be associated with any source, including chest, urinary tract, wounds and grafts, and the patient's blood. Finally, we defined recent myocardial infarction as a myocardial infarction (MI) that had occurred within the previous 90 days.
The primary endpoints were operative death, defined as any death occurring within 30 days of operation or before hospital discharge, and midterm (5-year) survival.
The secondary endpoint was distal reintervention for residual aortic pathologic conditions. Distal reintervention was defined as open or endovascular intervention on the aorta distal to the ascending aortic and to the arch prosthesis implanted during the initial surgery.
Surgical Techniques
A standard median sternotomy was the routine surgical approach in all cases. After the administration of heparin, cardiopulmonary bypass (CPB) was established by cannulating the axillary artery (in 56 of the 92 patients), the femoral artery (in 31 patients), or the ascending aorta (in 5 patients), and the venae cavae. Myocardial protection strategies usually included the intermittent antegrade administration of cold blood or a single dose of crystalloid cardioplegic solution. Left ventricular venting was performed by inserting a vent through the superior right pulmonary vein. Hypothermia was always used, and the core temperature was allowed to drift between 21 °C and 33 °C, depending upon the predicted time of circulatory arrest. Once an adequate core temperature had been reached, circulatory arrest was initiated, and surgery on the aortic arch was performed with the aid of hypothermic selective monolateral or bilateral anterograde cerebral perfusion. Monolateral perfusion was always achieved via arterial inflow in the axillary artery, with the brachiocephalic artery clamped at its origin. Bilateral cerebral perfusion was performed via the Kazui technique: both the brachiocephalic and the left common carotid arteries were cannulated and were perfused at a rate of 10 mL/(kg·min) by a single pump. The decision to perform total arch versus hemiarch repair depended on each patient's condition, on the location of the intimal tearing site, or on the diameter of the distal arch: hemiarch replacement was performed in patients whose aortic arch aneurysm (<50 mm) or intimal entry tear was confined to the small curvature. In patients who had a known connective tissue disorder or an intimal entry tear along the greater curvature, total arch replacement was performed.
Follow-Up
Midterm outcomes were determined from the clinical records when available or, when necessary, from direct telephone interviews with the patient or with family members. All follow-up data were collected, and no patient was lost to follow-up. The median and mean durations of follow-up were, respectively, 19.5 months (interquartile range [IQR], 1–59 mo) and 30.5 ± 29.8 months (IQR range, 0–100 mo).
Statistical Analysis
Continuous data were expressed as mean ± SD or as median, with the IQR and categorical data expressed as percentages. For comparison of continuous variables, the Student t test was applied when normal distribution was present, as tested by means of the Kolmogorov-Smirnov test. For abnormally distributed variables, the Mann-Whitney rank sum test was used. Categorical variables were compared by means of the χ2 test. In the case of small group sizes (n <5), the Fisher exact test was used.
Logistic regression analyses were performed to determine risk factors for operative death. Clinically relevant variables with P≤0.1 on univariable analysis were made available in the multivariable model. Results were reported as effect sizes (odds ratios [ORs]) with 95% confidence intervals (CIs). The validity of the regression model was examined by using the Hosmer-Lemeshow goodness-of-fit test. Kaplan-Meier curves were generated in order to provide survival estimates at postoperative points in time. Differences between the 2 groups were determined by log-rank test. These estimates include operative deaths.
All reported P values were 2-sided, and P values <0.05 were considered to indicate statistical significance. All statistical analyses were performed with use of SPSS 15.0 (IBM Corporation; Endicott, NY).
Results
Table I shows the baseline characteristics of the 92 patients. More men (mean age, 64 ± 11 yr) were admitted with type A AAD. Patients in the hemiarch group (in particular) were older, although the difference was not statistically significant (66 ± 10 vs 61 ± 12 yr; P=0.08). No significant differences in other preoperative factors were observed between the study groups. Patients in the total arch group were more likely to have higher percentages of repeat cardiac operation (10% vs 1%; P=0.092).
TABLE I.
Preoperative Data in the 92 Patients
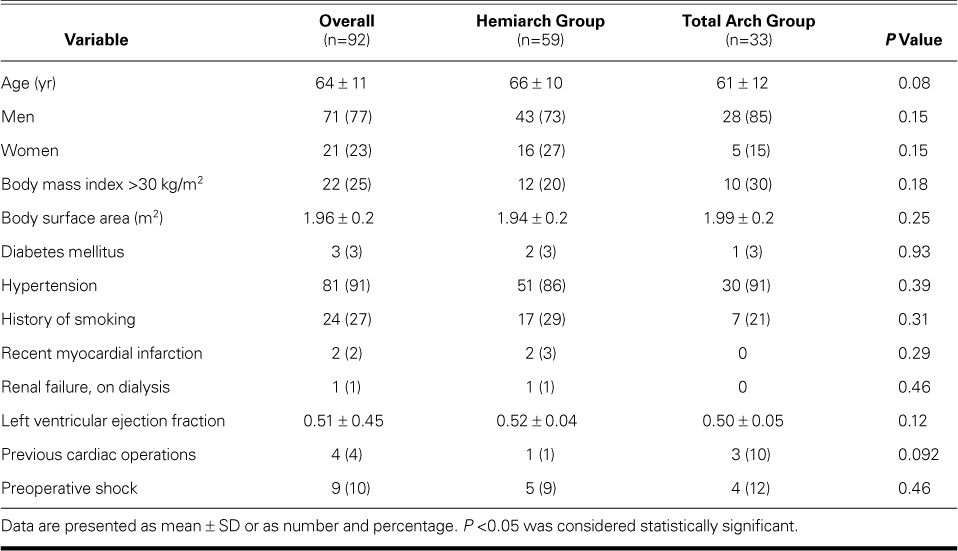
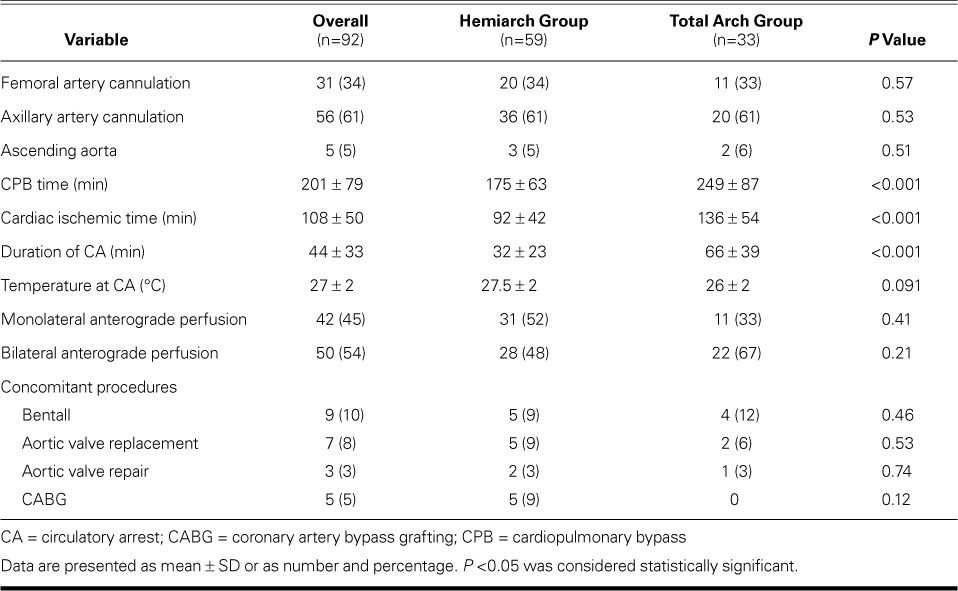
Table II shows the operative variables. Cardiac ischemic, circulatory arrest, and CPB times were significantly longer in the total arch group (P <0.001). Monolateral and bilateral anterograde cerebral perfusion techniques were used, with a higher percentage of bilateral perfusion in the total arch group. No significant differences existed between the groups regarding concomitant surgical procedures.
TABLE II.
Operative Data in the 92 Patients
Operative Morbidity and Death
Table III lists the operative results. The overall operative mortality rate was 22% (20/92 patients): 4 patients died of hemorrhagic shock, 4 of multiorgan failure, 3 of septic shock, 4 of myocardial failure and continuous LCOS, and 5 of respiratory failure. Total arch replacement was associated with an increased risk of operative death: 11 deaths (33%) versus 9 (15%) in the hemiarch group (P=0.044).
TABLE III.
Operative Morbidity and Death
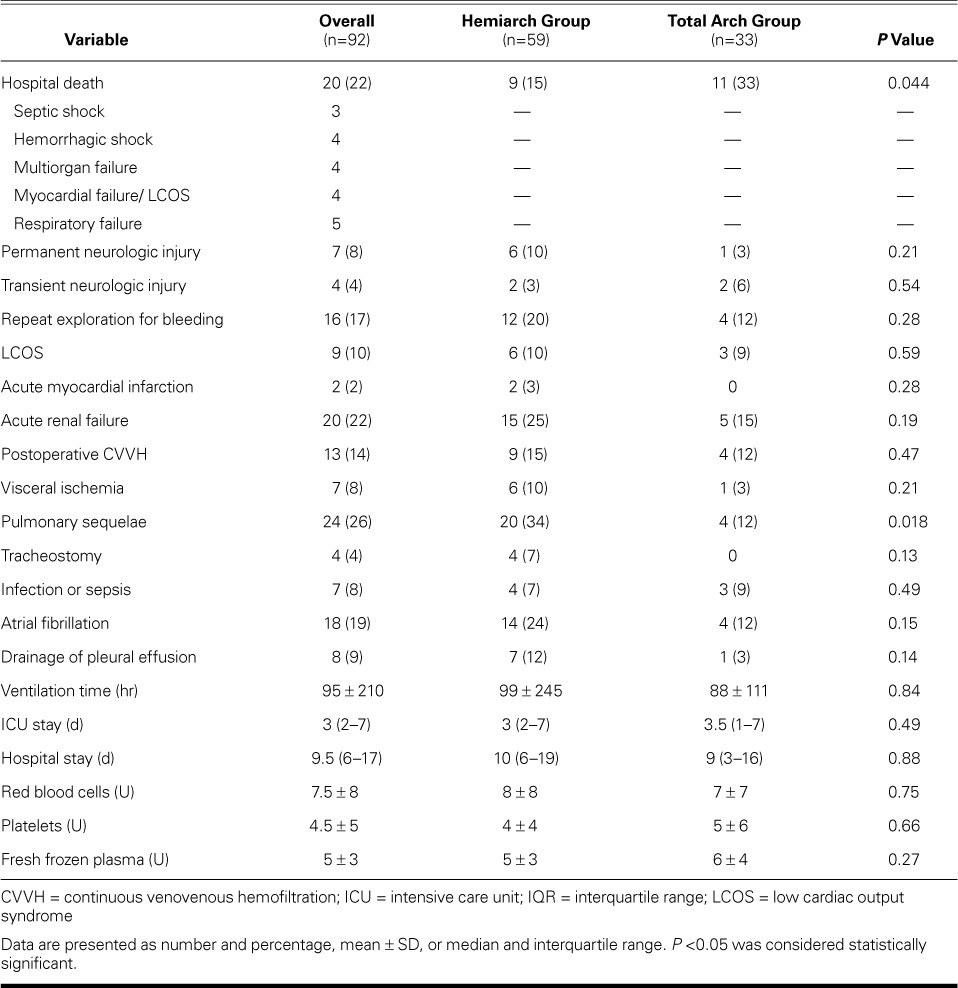
Seven cases (8%) were complicated by the new onset of permanent neurologic injuries, with no significant differences between the 2 study groups (3% in the total arch group vs 10% in the hemiarch group; P=0.21). Sixteen patients (17%) needed reexploration of the mediastinum for bleeding. Acute renal failure occurred in 20 patients (22%), 13 of whom needed postoperative renal replacement therapy with continuous venovenous hemofiltration. The median intensive care unit stay was 3 d (IQR, 2–7) in the hemiarch group versus 3.5 d (IQR, 1–7) in the total arch group (P=0.49). No significant differences existed in ventilation time (P=0.84) or hospital stay (P=0.88).
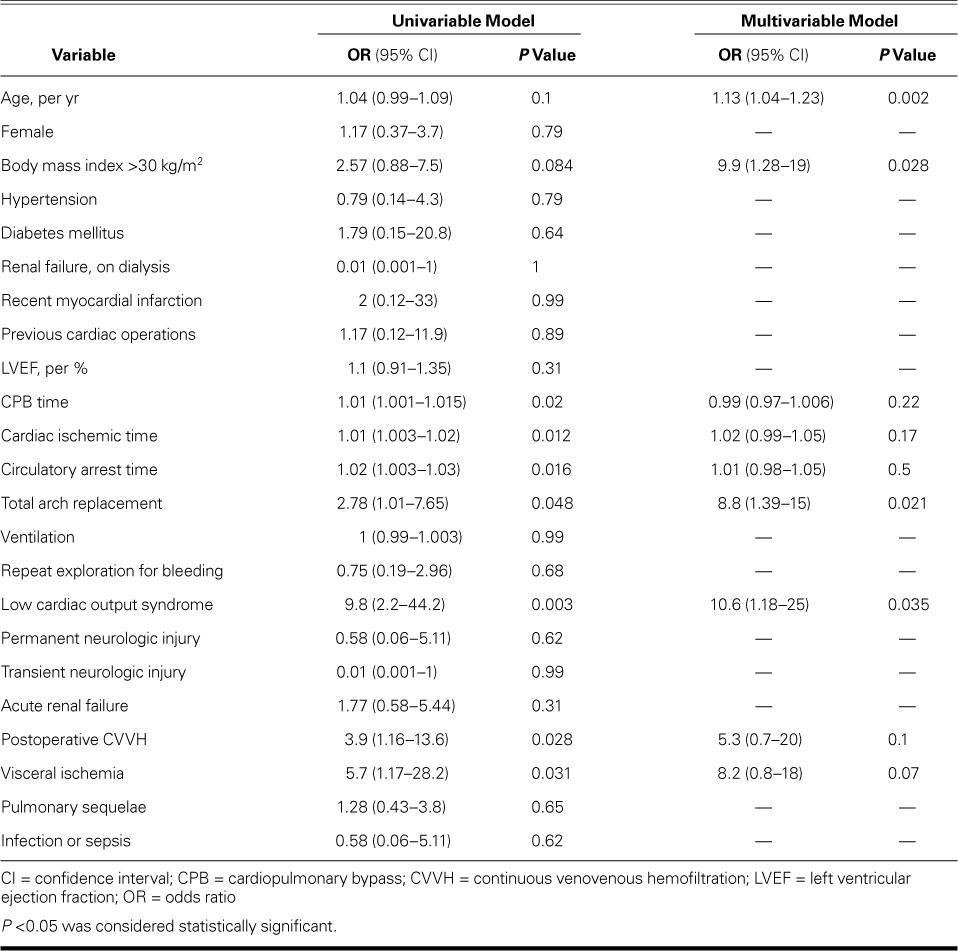
Table IV lists predictors of operative death upon univariable and multivariable analyses. Multivariable analysis revealed, as independent predictors of operative death, age (per yr) (P=0.002), body mass index (BMI) ≥30 kg/m2 (P=0.028), postoperative LCOS (P=0.035), and the type of surgical procedure: in particular, total arch replacement was found to be a predictor of operative death both on univariable analysis (OR=2.78; 95% CI, 1.01–7.65; P=0.048) and on multivariable analysis (OR=8.8; 95% CI, 1.39–15; P=0.021) (Hosmer-Lemeshow test, P=0.1).
TABLE IV.
Univariable and Multivariable Predictors of Operative Death
Late Morbidity and Death
There were 18 late deaths, including 9 cardiovascular-related deaths: 11 in the hemiarch group and 7 in the total arch group (P=0.76). The overall 5-year survival rate was 59.3% ± 5.5% (Fig. 1): 67.5% ± 6.1% in the hemiarch group and 51.5% ± 8.7% in the total arch group, respectively (P=0.12; Fig. 2).
Fig. 1.
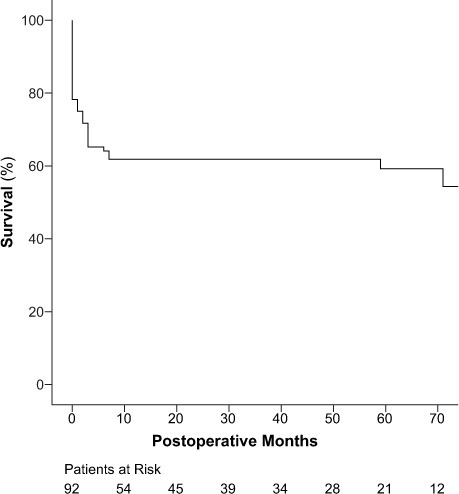
Graph shows Kaplan-Meier curves for overall survival.
Fig. 2.
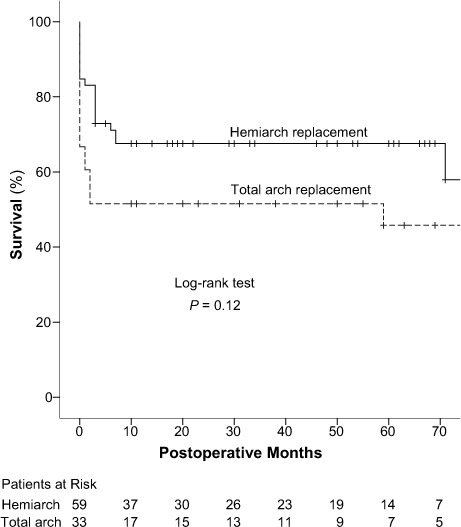
Graph shows Kaplan-Meier curves for survival in the hemiarch and total arch replacement groups.
P <0.05 was considered statistically significant.
There were 3 late stroke cases, all in patients who underwent hemiarch replacement (P=0.23).
Freedom from distal reintervention at 5 years was 95.4% ± 3.2% (Fig. 3): 96.8% ± 3.2% in the hemiarch group and 92.3% ± 7.4% in the total arch group (P=0.99; Fig. 4). Particularly, 3 patients (3.2%)—2 from the hemiarch group and 1 from the total arch group—underwent elective aortic reoperation because of residual or progressive aortic pathologic conditions during follow-up. Of these, 2 were endovascular interventions on the thoracic descending aorta, and 1 was surgical repair of a thoracoabdominal aneurysm. No deaths from distal reintervention were recorded.
Fig. 3.
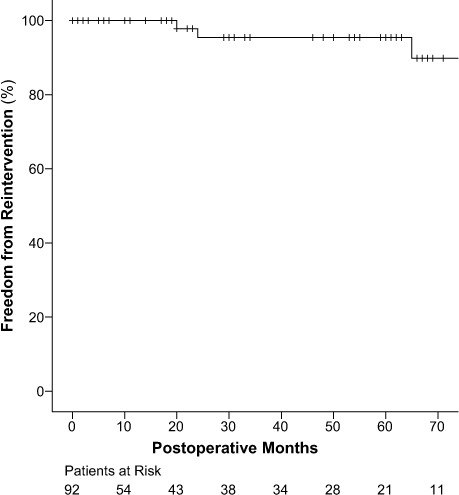
Graph shows Kaplan-Meier curves for overall freedom from aortic reoperation.
Fig. 4.
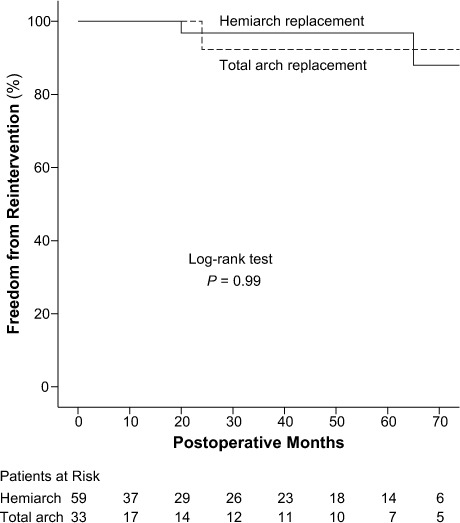
Graph shows Kaplan-Meier curves for freedom from reoperation because of residual or progressive aortic pathologic conditions in the hemiarch and total arch replacement groups.
P <0.05 was considered statistically significant.
Discussion
Despite improvements in perioperative and postoperative management during aortic surgery, the operative mortality rate for patients with type A AAD remains high at 8% to 34%.1–3 Resection of the intimal tear and replacement of the ascending aorta are considered the standard of care in these patients. However, in patients who undergo surgery for type A AAD, a patent false lumen has a well-established prognostic value as the major risk factor for the need to reintervene on the aortic arch or descending aorta.13–17 Halstead and colleagues16 found that initial enlargement (>4 cm) of the thoracic descending aorta and patency of the false lumen are the dominant factors associated with subsequent aortic expansion. In monitoring very closely a cohort of 70 patients after surgical intervention for acute type A dissection, Fattori and associates17 found the annual aortic growth rate to be maximal in the descending aortic segment—and significantly higher in the absence of false luminal thrombosis.
In an effort to maximize the resection of entry tears and to decrease the incidence of residual patent false lumina, several groups4–7,11,12 have advocated a more aggressive approach to aortic arch repair: routine replacement of the total aortic arch regardless of the site of the entry tear, although distal manipulation has been associated with an increased operative mortality rate.
Yet a more conservative approach (including hemiarch replacement) has been shown to be equally effective in the presence of type A AAD. Rylski and colleagues,18 in a series of 534 patients treated with hemiarch repair, found a 12% in-hospital mortality rate and a mean freedom from distal reintervention of 90% ± 2% and 85% ± 3% at 5 and 10 years, respectively. The same lead author, comparing hemiarch and total arch replacement in AAD, found a higher in-hospital mortality rate for the total arch group (29% vs 22%). Moreover, incomplete resection of the dissected aortic arch did not correlate with the need for distal aortic reintervention: instead, Marfan syndrome and dissection of all aortic segments were the only predictors of distal aortic reintervention.19 Kim and colleagues20 reported a reduced survival rate and fewer neurologic sequelae for patients who underwent total arch replacement, with a rate of reoperation that was unaffected by the type of surgery for AAD, and without significant reoperative morbidity or death. Conversely, Uchida and co-authors,6 in comparing hemiarch and total arch replacement in AAD, found similar operative mortality rates (4.5% vs 3.5%, respectively) but a 5-year survival rate that was significantly lower in the hemiarch group (69% vs 95.3%). However, the Uchida series was performed with a concomitant frozen elephant trunk (FET) in all patients who underwent total arch replacement: this could explain the difference in overall survival rates between groups, because FET guaranteed the obliteration of the residual false lumen of the descending aorta.6
Our Findings. In the present study, we have found that concomitant arch surgery is still associated with a high operative mortality rate in patients with type A AAD. Our mortality rate of 22% is in accord with other series.1–3,11,18,19 Total arch replacement is a more complex type of surgery that requires longer CPB, cardiac ischemic, and circulatory arrest times, in comparision with a more conservative approach, such as hemiarch repair. In our experience, patients who underwent total arch replacement had a higher operative mortality rate than did those who underwent hemiarch repair (P=0.044); however, patients in the total arch group had a higher risk profile, with a 10% rate of repeat cardiac interventions.
In attempts to identify independent risk factors for operative death, a type of surgical procedure (in this instance, total arch replacement) was found on multivariable analysis to be associated with an increased mortality rate. Moreover, multivariable analysis revealed other factors to be independent predictors—such as age (per annum), BMI ≥30 kg/m2, and postoperative LCOS. Unfortunately, most of these factors that determine hospital survival are not subject to modification. Consequently, this analysis was useful only in identifying particularly high-risk patients.
Although total arch replacement has shown, in our series, an increased operative mortality rate, we agree that more extensive surgery in type A AAD is necessary in selected situations: in the presence of an intimal entry tear along the greater curvature, for example, or a circumferential dissection around the brachiocephalic vessels. Young patients and those with Marfan syndrome also warrant exception.
The 5-year survival rate of 59.3% ± 5.5% was higher in our hemiarch group (67.5% ± 6.1% vs 51.5% ± 8.7%); however, this estimate included operative deaths, and the difference between the study groups could arise from the higher operative mortality rate of patients who underwent total arch replacement: the incidence of late deaths, indeed, was similar. The rate of distal reoperation for residual aortic disease was low, and it was unaffected by the type of surgery: freedom from distal reintervention at 5 years was 96.8% ± 3.2% in the hemiarch group and 92.3% ± 7.4% in the total arch group.
A low rate of neurologic damage was found in our series, with no differences between the two groups, either in the postoperative or in the follow-up period.
Four patients in our total arch group underwent concomitant FET at the time of surgery. This technique enables the stenting of the proximal descending aorta via an antegrade approach and has been adopted by clinical institutions worldwide, to better obliterate the false lumen and thereby reduce the incidence of late aortic sequelae. However, the operative mortality rate is still high. As suggested by Pochettino and co-authors,21 the combination of a standard hemiarch resection with appropriate anterograde stent-grafting could stabilize the thoracic aorta, while maintaining a low early operative mortality rate.
Study Limitations
This study has several limitations. It is grounded in a retrospective analysis of our institutional, observational, prospectively collected database. Even if the decision to perform total arch rather than hemiarch repair took into consideration the individual patient's condition, the intimal tearing site, and the diameter of the distal arch, the final decision was at the surgeon's discretion.
Furthermore, our study is limited by the small sample size of its study population. Given the median follow-up period of 19 months, larger studies with longer follow-up could provide more insights. In considering the time to reintervention, we must bear in mind that many of our patients might have died before experiencing a sequela that would have prompted reintervention.
Finally, we have not reported postoperative aortic diameters, nor have we evaluated the patency of the residual false lumina at follow-up, with instrumental investigations by contrast computed tomography and the like; however, the objective of our study was to evaluate the early mortality rates, the midterm survival rates, and the rates of distal aortic reintervention in this cohort of patients.
Conclusion
Complex aortic arch surgery in the presence of type A AAD is still associated with high operative mortality rates. Hemiarch replacement can be performed with a lower chance of death than can total arch replacement, and with a rate of distal aortic reoperation that is both low and unaffected by surgical stategy. Therefore, a more aggressive approach that includes total arch replacement might be preferred only in selected situations or in selected patients, such as young or Marfan patients.
References
- 1. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, . et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000; 283( 7): 897– 903. [DOI] [PubMed] [Google Scholar]
- 2. Trimarchi S, Nienaber CA, Rampoldi V, Myrmel T, Suzuki T, Mehta RH, . et al. Contemporary results of surgery in acute type A aortic dissection: the International Registry of Acute Aortic Dissection experience. J Thorac Cardiovasc Surg 2005; 129( 1): 112– 22. [DOI] [PubMed] [Google Scholar]
- 3. Rylski B, Suedkamp M, Beyersdorf F, Nitsch B, Hoffmann I, Blettner M, Weigang E.. Outcome after surgery for acute aortic dissection type A in patients over 70 years: data analysis from the German Registry for Acute Aortic Dissection Type A (GERAADA). Eur J Cardiothorac Surg 2011; 40( 2): 435– 40. [DOI] [PubMed] [Google Scholar]
- 4. Kazui T, Washiyama N, Muhammad BA, Terada H, Yamashita K, Takinami M, Tamiya Y.. Extended total arch replacement for acute type A aortic dissection: experience with seventy patients. J Thorac Cardiovasc Surg 2000; 119( 3): 558– 65. [DOI] [PubMed] [Google Scholar]
- 5. Sun LZ, Qi RD, Chang Q, Zhu JM, Liu YM, Yu CT, . et al. Surgery for acute type A dissection using total arch replacement combined with stented elephant trunk implantation: experience with 107 patients. J Thorac Cardiovasc Surg 2009; 138( 6): 1358– 62. [DOI] [PubMed] [Google Scholar]
- 6. Uchida N, Shibamura H, Katayama A, Shimada N, Sutoh M, Ishihara H.. Operative strategy for acute type A aortic dissection: ascending aortic or hemiarch versus total arch replacement with frozen elephant trunk. Ann Thorac Surg 2009; 87( 3): 773– 7. [DOI] [PubMed] [Google Scholar]
- 7. Zhang H, Lang X, Lu F, Song Z, Wang J, Han L, Xu Z.. Acute type A dissection without intimal tear in arch: proximal or extensive repair? J Thorac Cardiovasc Surg 2014; 147( 4): 1251– 5. [DOI] [PubMed] [Google Scholar]
- 8. Takahara Y, Sudo Y, Mogi K, Nakayama M, Sakurai M.. Total aortic arch grafting for acute type A dissection: analysis of residual false lumen. Ann Thorac Surg 2002; 73( 2): 450– 4. [DOI] [PubMed] [Google Scholar]
- 9. Urbanski PP, Siebel A, Zacher M, Hacker RW.. Is extended aortic replacement in acute type A dissection justifiable? Ann Thorac Surg 2003; 75( 2): 525– 9. [DOI] [PubMed] [Google Scholar]
- 10. Ohtsubo S, Itoh T, Takarabe K, Rikitake K, Furukawa K, Suda H, Okazaki Y.. Surgical results of hemiarch replacement for acute type A dissection. Ann Thorac Surg 2002; 74( 5): S1853– 63. [DOI] [PubMed] [Google Scholar]
- 11. Westaby S, Saito S, Katsumata T.. Acute type A dissection: conservative methods provide consistently low mortality. Ann Thorac Surg 2002; 73( 3): 707– 13. [DOI] [PubMed] [Google Scholar]
- 12. Crawford ES, Kirklin JW, Naftel DC, Svensson LG, Coselli JS, Safi HJ.. Surgery for acute dissection of ascending aorta. Should the arch be included? J Thorac Cardiovasc Surg 1992; 104( 1): 46– 59. [PubMed] [Google Scholar]
- 13. Immer FF, Hagen U, Berdat PA, Eckstein FS, Carrel TP.. Risk factors for secondary dilatation of the aorta after acute type A aortic dissection. Eur J Cardiothorac Surg 2005; 27( 4): 654– 7. [DOI] [PubMed] [Google Scholar]
- 14. Song SW, Chang BC, Cho BK, Yi G, Youn YN, Lee S, Yoo KJ.. Effects of partial thrombosis on distal aorta after repair of acute DeBakey type I aortic dissection. J Thorac Cardiovasc Surg 2010; 139( 4): 841– 7.e1. [DOI] [PubMed] [Google Scholar]
- 15. Yeh CH, Chen MC, Wu YC, Wang YC, Chu JJ, Lin PJ.. Risk factors for descending aortic aneurysm formation in medium-term follow-up of patients with type A aortic dissection. Chest 2003; 124( 3): 989– 95. [DOI] [PubMed] [Google Scholar]
- 16. Halstead JC, Meier M, Etz C, Spielvogel D, Bodian C, Wurm M, . et al. The fate of the distal aorta after repair of acute type A aortic dissection. J Thorac Cardiovasc Surg 2007; 133( 1): 127– 35. [DOI] [PubMed] [Google Scholar]
- 17. Fattori R, Bacchi-Reggiani L, Bertaccini P, Napoli G, Fusco F, Longo M, . et al. Evolution of aortic dissection after surgical repair. Am J Cardiol 2000; 86( 8): 868– 72. [DOI] [PubMed] [Google Scholar]
- 18. Rylski B, Milewski RK, Bavaria JE, Vallabhajosyula P, Moser W, Szeto WY, Desai ND.. Long-term results of aggressive hemiarch replacement in 534 patients with type A aortic dissection. J Thorac Cardiovasc Surg 2014; 148( 6): 2981– 5. [DOI] [PubMed] [Google Scholar]
- 19. Rylski B, Beyersdorf F, Kari FA, Schlosser J, Blanke P, Siepe M.. Acute type A aortic dissection extending beyond ascending aorta: limited or extensive distal repair. J Thorac Cardiovasc Surg 2014; 148( 3): 949– 54. [DOI] [PubMed] [Google Scholar]
- 20. Kim JB, Chung CH, Moon DH, Ha GJ, Lee TY, Jung SH, . et al. Total arch repair versus hemiarch repair in the management of acute DeBakey type I aortic dissection. Eur J Cardiothorac Surg 2011; 40( 4): 881– 7. [DOI] [PubMed] [Google Scholar]
- 21. Pochettino A, Brinkman WT, Moeller P, Szeto WY, Moser W, Cornelius K, . et al. Antegrade thoracic stent grafting during repair of acute DeBakey I dissection prevents development of thoracoabdominal aortic aneurysms. Ann Thorac Surg 2009; 88( 2): 482– 90. [DOI] [PubMed] [Google Scholar]


