Abstract
Superior vena cava syndrome is a well-known disease entity that carries substantial rates of morbidity and mortality. Although most cases of superior vena cava syndrome are secondary to a malignant process, additional causes (such as mediastinal fibrosis, pacemaker lead implantation, or central venous catheter placement) have been reported. Multiple treatment options include percutaneous transluminal angioplasty, stent implantation, thrombolysis, mechanical thrombectomy, and venous grafting. We present a case of superior vena cava syndrome in a symptomatic 30-year-old woman who obtained complete relief of obstruction and marked symptomatic improvement through venoplasty and stenting, aided by our use of a balloon-in-balloon catheter system.
Keywords: Angioplasty, balloon/methods; blood vessel prosthesis implantation/instrumentation; pacemaker, artificial/adverse effects; stents; superior vena cava syndrome/etiology/therapy; treatment outcome; vena cava, superior/pathology
Superior vena cava (SVC) syndrome is a well-known disease entity that carries substantial rates of morbidity and mortality. Although most cases of SVC syndrome are secondary to a malignant process,1 benign causes, such as pacemaker lead implantation, have been reported.2 We present a case of SVC syndrome in a young woman after pacemaker implantation, whom we treated with venoplasty and stenting, aided by a balloon-in-balloon (BIB) catheter system.
Case Report
A 30-year-old woman with a known history of SVC stenosis presented to us for further evaluation and management of positional syncope. The patient had a history of sick sinus syndrome and of multiple radiofrequency ablations of the sinus node—hence the eventual need for a dual-chamber permanent pacemaker. She eventually underwent multiple revisions for lead malfunction and generator-site infection. She was next diagnosed, by means of computed tomography (CT), with SVC obstruction, after which she had a 5-cm saphenous vein bypass graft implanted from the right brachiocephalic vein to the right atrial appendage, through which the pacemaker leads were rerouted.
When the patient presented to us, she reported chronic fatigue, shortness of breath, and palpitations that had progressively worsened. She also reported facial and neck swelling that was worse in the morning, along with positional syncope that occurred whenever she bent forward. On physical examination, she had slightly distended jugular veins, with only minor bilateral arm swelling at rest—and positional syncope was reproduced. Results of repeat chest CT scanning with contrast medium were consistent with SVC obstruction, as was near-occlusion of her SVC bypass graft. Venography then confirmed that the pacemaker leads were in an occluded saphenous vein graft and that she had longstanding SVC stenosis, with a large azygos vein “pop-off” to the inferior vena cava. There was no gradient across the SVC stenosis, given that the azygos vein was offloading flow from the SVC into the inferior vena cava. Both brachiocephalic veins were patent (Fig. 1). The native SVC was estimated to be 18 mm in diameter.
Fig. 1.
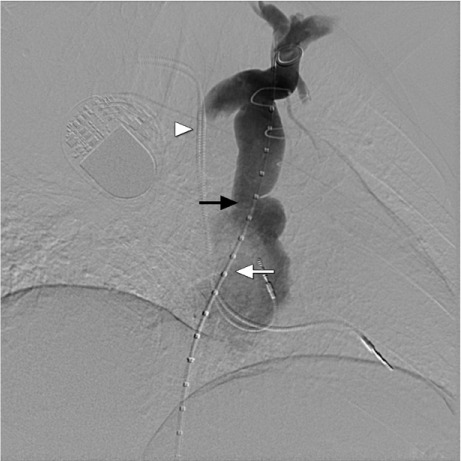
Diagnostic venogram with pigtail catheter (white arrow) shows the superior vena cava stenosis (black arrow) with pacemaker leads (arrowhead) in the saphenous vein graft. Note that both brachiocephalic veins are patent.
At a later date, we proceeded with angioplasty and stent implantation at the SVC stenosis. Access was attained through the right common femoral vein with use of an 8F sheath. Heparin was used as an anticoagulant to maintain an activated clotting time of >200 s. An intravascular ultrasound catheter (Volcano Corporation; San Diego, Calif) was advanced through the right common femoral vein for imaging of the site of SVC obstruction. The normal segment of the SVC was 16 × 18 mm, with a minimal stricture diameter of less than 4 mm. The femoral sheath was upgraded to a 12F × 75-cm Mullins transseptal sheath (Cook Medical Inc.; Bloomington, Ind) in preparation for stent deployment. Over a 0.035-in Amplatz super-stiff wire (Boston Scientific/Scimed; Maple Grove, Minn), balloon angioplasty was performed with use of a 14-mm × 2-cm XXL Balloon Dilatation Catheter (Boston Scientific/Scimed) at a pressure of 8 atm.
Given the significant venous recoil, we decided to implant a stent. We hand-crimped a 10 × 50-mm Palmaz® XL® stent (Cordis, a Johnson & Johnson company; Miami Lakes, Fla) on a 20 × 45-mm NuMED BIB (balloon-in-balloon) catheter (NuMED, Inc.; Hopkinton, NY). The stent was then advanced through the transseptal sheath and was positioned across the stenotic region (Fig. 2). Before stent delivery, a hand injection of contrast medium was administered through the sheath, to show the proper stent position across the SVC stenosis and to ensure that the azygos vein was not obstructed. The stent was then partially deployed by inflating the inner balloon to a pressure of 5 atm (Fig. 3). Using fluoroscopy, we confirmed the persistence of proper stent position and finalized stent deployment by inflating the outer balloon to a pressure of 6 atm (Fig. 4). After stent implantation, repeat angiography revealed proper stent positioning, with substantial improvement in the flow of contrast medium across the SVC (Fig. 5).
Fig. 2.
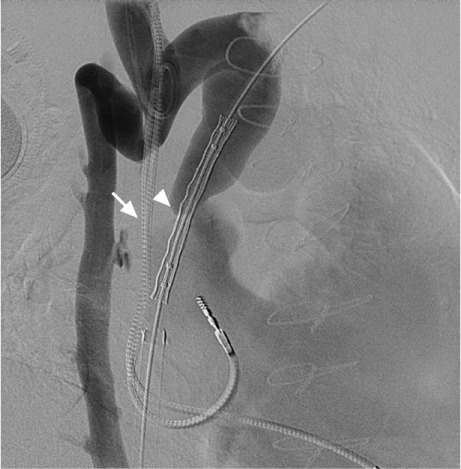
Fluoroscopic image shows stent advancement and positioning across the superior vena cava stenosis (arrowhead). Care was taken to prevent obstruction of the azygos vein pop-off. Pacemaker leads (arrow) are also shown.
Fig. 3.
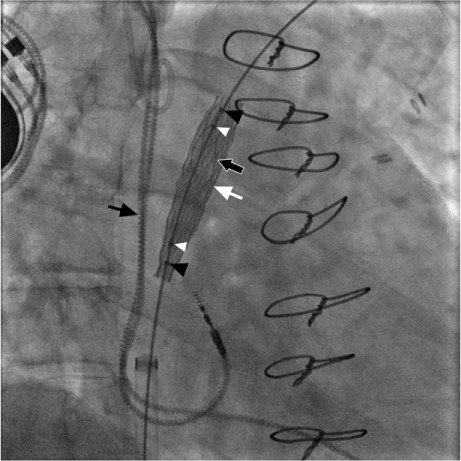
Fluoroscopic image shows inner balloon inflation (black arrow at right), the first step in stent deployment. Also seen are the partially deployed stent (white arrow), inner balloon markers (white arrowheads), outer balloon markers (black arrowheads) and pacemaker leads (black arrow at left).
Fig. 4.
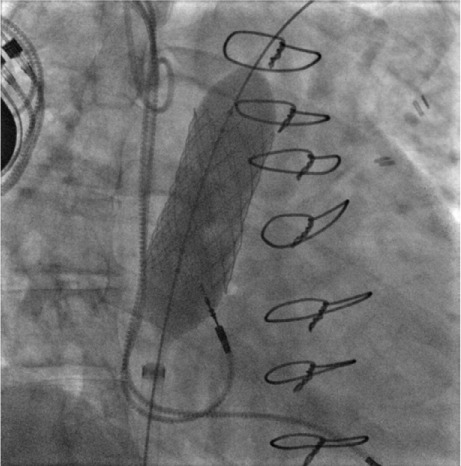
Fluoroscopic image shows outer balloon inflation as the final step in stent deployment.
Fig. 5.
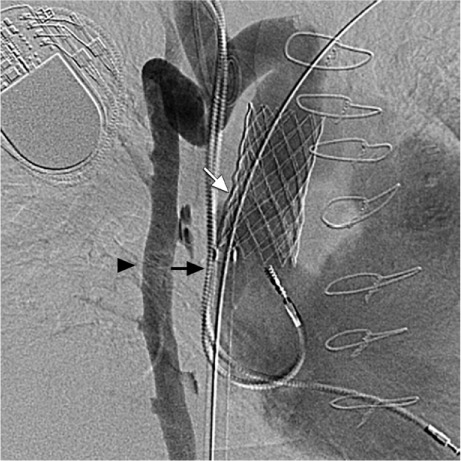
Venogram shows the deployed stent (white arrow), the azygos vein (black arrowhead), and the pacemaker leads (black arrow).
The patient had no postoperative complications and was discharged from the hospital the next morning, with instructions to take dual antiplatelet agents (aspirin and prasugrel). Clinical follow-up after one month revealed marked improvement in her symptoms, including resolution of her positional syncope. Follow-up CT angiograms 8 months later showed a patent stent with no evidence of in-stent stenosis.
Discussion
Although most cases of SVC syndrome are secondary to a malignant process,1 there are a few benign causes such as mediastinal fibrosis, pacemaker lead implantation, or central venous catheter placement.2 Superior vena cava syndrome secondary to pacemaker lead placement is characteristic of patients in whom there is concern about the compromise (and functioning) of pacemaker leads when those leads are adversely affected by stent placement.3,4 Currently, only a few case reports describe the use of uncovered stents as a treatment option.3,5 The theory is that the pacemaker wires will be incorporated into the venous wall, thereby obviating any contact with the stent or balloon.6 In our patient, the pacemaker wires were in a saphenous vein graft and thus had no contact with the stent.
Surgical correction of SVC syndrome is a risky procedure, so emphasis has been placed over the last few decades on percutaneous approaches. In the United States, uncovered balloon-expandable stents such as the Z stent, Palmaz stent, and Wallstent® (Boston Scientific) are the main stents in use. Typical sequelae of SVC-stent placement include thrombosis, stent migration, hemopericardium, and transient chest pain.6 The use of uncovered stents or covered stents with uncovered edges has helped decrease the incidence of stent migration.7 Outside the U.S., there have been reported cases of the use of balloon-expandable covered stents because of their lower risk of thrombosis and their higher patency rate.7 Yet covered stents still bear a higher risk of stent migration and unilateral brachiocephalic or azygos vein obstruction, if the SVC stenosis is at the confluence of those 2 veins.7
In our patient, we implanted the Palmaz stent by means of the NuMed BIB catheter technique, which is a novel approach in adult patients with SVC syndrome. The NuMed BIB catheter has an inner balloon half the size of the outer balloon. We advocate the use of large-diameter balloon-expandable stenting (with the aid of a BIB catheter) as an alternative to mounting the stent on a single large-diameter balloon. This enables controlled inflation, more-even stent expansion, fine-tuned repositioning, and (after inner balloon inflation) stent stabilization. The outer balloon is then inflated, thereby securing the stent against the vascular wall.
Currently, there is no clear consensus about which pharmacologic agent is best to use after stenting, and various agents such as warfarin, aspirin, and clopidogrel have been tried for durations ranging from 6 months to a lifetime.8 In most cases, the interventionalist adjusts the decision to the patient.8 In our case, we placed the patient on prasugrel for 6 to 12 months, to prevent stent thrombosis.
References
- 1. Bierdrager E, Lampmann LE, Lohle PN, Schoemaker CM, Schijen JH, Palmen FM, van der Heul C. . Endovascular stenting in neoplastic superior vena cava syndrome prior to chemotherapy or radiotherapy. Neth J Med 2005; 63( 1): 20– 3. [PubMed] [Google Scholar]
- 2. de Gregorio Ariza MA, Gamboa P, Gimeno MJ, Alfonso E, Mainar A, Medrano J, . et al. Percutaneous treatment of superior vena cava syndrome using metallic stents. Eur Radiol 2003; 13( 4): 853– 62. [DOI] [PubMed] [Google Scholar]
- 3. Teo N, Sabharwal T, Rowland E, Curry P, Adam A.. Treatment of superior vena cava obstruction secondary to pacemaker wires with balloon venoplasty and insertion of metallic stents. Eur Heart J 2002; 23( 18): 1465– 70. [DOI] [PubMed] [Google Scholar]
- 4. Klop B, Scheffer MG, McFadden E, Bracke F, van Gelder B. . Treatment of pacemaker-induced superior vena cava syndrome by balloon angioplasty and stenting. Neth Heart J 2011; 19( 1): 41– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lanciego C, Rodriguez M, Rodriguez A, Carbonell MA, Garcia LG.. Permanent pacemaker-induced superior vena cava syndrome: successful treatment by endovascular stent. Cardiovasc Intervent Radiol 2003; 26( 6): 576– 9. [DOI] [PubMed] [Google Scholar]
- 6. Anand G, Lewanski CR, Cowman SA, Jackson JE.. Superior vena cava stent migration into the pulmonary artery causing fatal pulmonary infarction. Cardiovasc Intervent Radiol 2011; 34 Suppl 2: S198– 201. [DOI] [PubMed] [Google Scholar]
- 7. Cho Y, Gwon DI, Ko GY, Ko HK, Kim JH, Shin JH, . et al. Covered stent placement for the treatment of malignant superior vena cava syndrome: is unilateral covered stenting safe and effective? Korean J Radiol 2014; 15( 1): 87– 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sheikh MA, Fernandez BB Jr, Gray BH, Graham LM, Carman TL.. Endovascular stenting of nonmalignant superior vena cava syndrome. Catheter Cardiovasc Interv 2005; 65( 3): 405– 11. [DOI] [PubMed] [Google Scholar]


