Abstract
Treatment of large, fresh thrombi in the vascular system can be challenging. AngioVac, a cardiopulmonary pump system, has been used to remove large thrombi and even some tumors by a percutaneous route. We report here a case of a 51-year-old man who presented with a large thrombus (7.5 × 1.5 cm) in his inferior vena cava, extending into his right atrium and right ventricle. Because the surgical risk was high, we attempted percutaneous embolectomy via the AngioVac aspiration system. We also review the literature concerning this emerging technique.
Keywords: Thrombectomy/instrumentation/methods, vacuum-assist devices
Large, fresh thrombi in the vascular system are difficult to manage pharmacologically. Even surgical or percutaneous removal might not provide optimal results if the thrombus burden is massive or organized. Recently, the AngioVac® (AngioDynamics; Latham, NY) large-bore catheter system has been used for the removal of large thrombi in various clinical situations, with initial results that have been promising. We report a case in which the AngioVac system was used to remove a large inferior vena cava (IVC) thrombus that had been protruding into the right atrium (RA) and the right ventricle (RV) of a patient at high surgical risk.
Case Report
In June 2014, a 51-year-old black man with a history of diabetes mellitus, nonischemic cardiomyopathy, morbid obesity, obstructive sleep apnea, chronic kidney disease stage 3 (baseline creatinine, 1.3 mg/dL), bronchial asthma, and leukocytoclastic vasculitis presented at the emergency room with severe dyspnea and generalized weakness. He had recently been treated for pneumonia at another hospital.
On admission, the patient was afebrile, normotensive, tachycardic (heart rate, 124 beats/min), and tachypneic (respiratory rate, 26 breaths/min), with oxygen saturation at 98% at a flow rate of 2 L/min by nasal cannula. He was extremely drowsy and showed signs of congestive heart failure, with poor peripheral perfusion.
The patient's laboratory results revealed elevated levels of blood urea nitrogen (51 mg/dL), serum creatinine (2.1 mg/dL), lactic acid (52 mg/dL), troponin I (0.05 ng/mL; normal, <0.03), and brain natriuretic peptide (1,480 pg/mL; normal, 0–100 pg/mL). Other results were alkaline phosphatase (230 U/L), total bilirubin (6.7 mg/dL), direct bilirubin (5 mg/dL), aspartate aminotransferase (49 U/L), alanine aminotransferase (44 U/L), prothrombin time (24.1 s), and international normalized ratio (2.1).
The patient's electrocardiogram showed sinus tachycardia with left-axis deviation and partial left bundle branch block. Chest radiography revealed bilateral pleural effusion, cardiomegaly, and ill-defined opacities in the lower zones. Computed tomograms of the chest showed multiple scattered, bilateral, peripherally situated, wedge-shaped opacities. An emergency transthoracic echocardiogram revealed biventricular dilation with a left ventricular ejection fraction (LVEF) of <0.15, grade III/IV diastolic dysfunction, and severe RV dysfunction. A large echodense mass, likely a thrombus, was seen to enter the RA from the IVC and to extend across the tricuspid valve into the RV. As a consequence, there was severe tricuspid regurgitation and elevated RA pressure, with bowing of the interatrial septum to the left.
The patient was a high-risk candidate for surgery, in view of his severe heart failure and associated metabolic disturbances. Conventional percutaneous devices, such as a basket, would fragment the thrombus, embolizing it distally and removing it incompletely. Hence, we decided to attempt percutaneous embolectomy in the interventional radiology suite, by means of the AngioVac aspiration system and with the patient under general endotracheal anesthesia.
Transesophageal echocardiography (TEE) enabled views of the 7-cm-long, serpentine, mobile thrombus floating in the RA and prolapsing into the RV (Figs. 1 and 2). Intravenous heparin was used to achieve an activated clotted time (ACT) of 300 s. Access to the bilateral common femoral veins was gained with ultrasonographic guidance. We introduced a 24F Gore sheath (W.L. Gore & Associates, Inc.; Flagstaff, Ariz) into the right common femoral vein and a 19F sheath into the left common femoral vein. We inserted the AngioVac catheter into the RA under fluoroscopic guidance, with suction from the right side. Venovenous bypass was established through the left femoral vein and the AngioVac catheter (Fig. 3). Viewing by TEE, we saw that embolectomy had successfully been performed. (See Figure 4 for the excised thrombus.) Bypass time had lasted approximately 30 s. We deployed a filter in the IVC through the right jugular approach. Postprocedural echocardiography revealed no residual thrombus (Fig. 5).
Fig. 1.
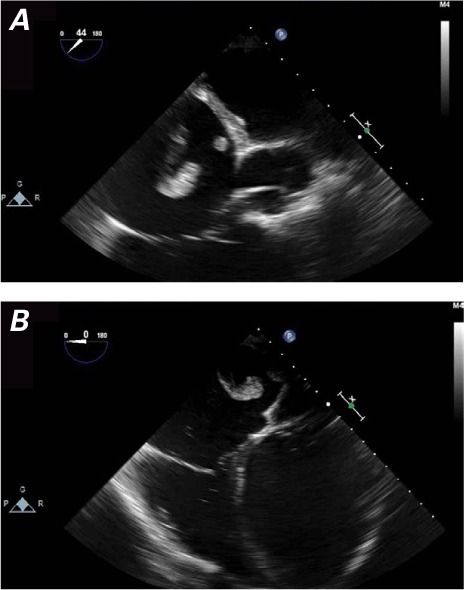
Transesophageal echocardiograms show a large, serpentine right atrial thrombus in A) short-axis and B) 4-chamber views. The dilated left ventricle and the interventricular septum bulge toward the right side.
Fig. 2.
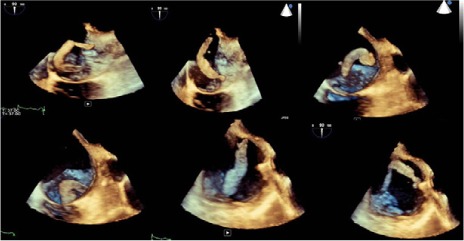
Serial 3-dimensional echocardiographic frames show the anatomy and mobility of the serpentine thrombus. Read the images from left to right in the top panel, followed by left to right in the bottom panel.
Fig. 3.
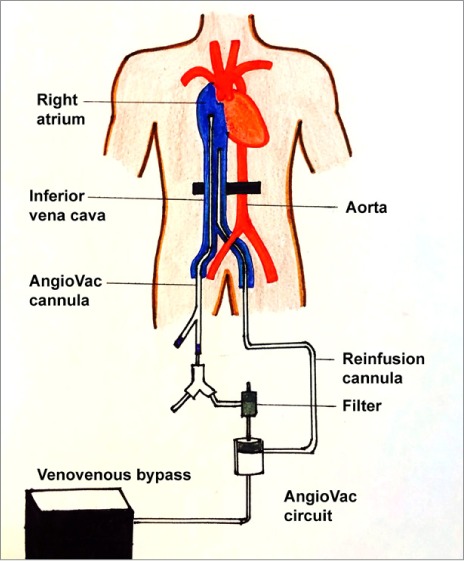
Diagram shows the AngioVac circuit and cannulation technique used in our patient.
Fig. 4.
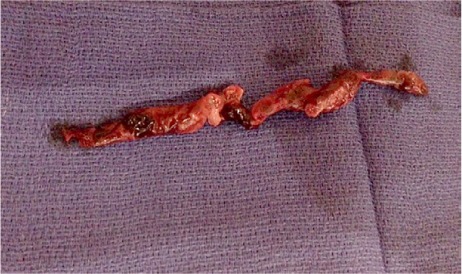
Photograph shows the large extracted thrombus (7.5 × 1.54 cm).
Fig. 5.
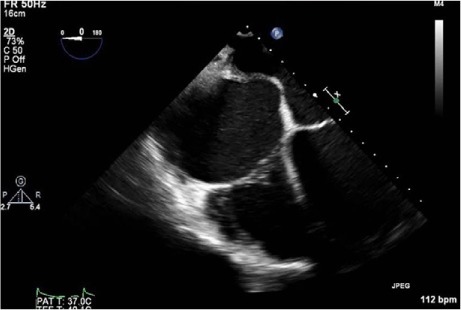
Post-procedural transesophageal echocardiogram shows no thrombus in the right atrium after suctioning.
Our patient's liver function tests were abnormal, with mild transaminitis and hyperbilirubinemia throughout his admission. Ultrasonography of the abdomen showed congestive hepatomegaly and features of portal hypertension. The biochemical abnormalities improved with optimization of the heart-failure therapy, which included milrinone. At an outpatient follow-up appointment 4 months after his hospital discharge, he was noted to be clinically stable and to tolerate exercise better than before.
Discussion
The AngioVac aspiration system consists of a 22F catheter with a balloon-actuated tip, a circuit with a built-in filter, a centrifugal pump head, and a reinfusion cannula. It uses a perfusion circuit to forcefully aspirate clot or intravascular thrombus en bloc.1,2 It was introduced in 2009 for the percutaneous removal of large, fresh thrombi; the initial experience, in 105 patients, was presented at the 2011 Symposium on Vascular and Endothelial Issues (the VEITH symposium).3 Thrombotic material was aspirated successfully in 90% of the cases. Sequelae were rare: RA perforation occurred in only one case. The authors recommended the AngioVac as a safe and effective device for the removal of acute clots (<3 mo), particularly useful in patients who cannot undergo thrombolytic therapy or surgery. However, the use of 2 large-bore cannulas (which increases the risk of vascular complications), the need for general anesthesia and the services of perfusionists, and the technically challenging catheter manipulation are a few of the limitations for the use of this system.3 The AngioVac system is now approved in the United States by the U.S. Food and Drug Administration for removal of unwanted intravascular material. The current version enables a percutaneous approach for distal embolic protection and removal of débris.1
The AngioVac system has been used in a variety of clinical situations, including removal of massive or submassive pulmonary embolism,4 RA masses such as tumors or vegetations,4–7 and IVC or iliac thrombi.8,9 Sengodan and colleagues10 used this device through the internal jugular route to remove a large thrombus from the IVC in a patient with hepatocellular carcinoma and recurrent pulmonary thromboembolism, in whom a prior IVC filter was present. ElMallah and associates11 used this novel technique in 13 patients to extract vegetations, pulmonary artery thrombi, and iliofemoral thrombi after the standard treatment had failed or was contraindicated. The overall procedural success was 76.9%. There were no deaths. All failures were in patients with chronic thrombi. There is a risk of RV, RA, or IVC perforation and the consequent precipitation of acute pulmonary thromboembolism during the procedure. The periprocedural TEE and fluoroscopic guidance can help, largely in avoiding perforations. The administration of heparin before the procedure is recommended for anticoagulation (ACT, >350 s).11 The AngioVac system has also been used in a patient with renal cell carcinoma, to prevent pulmonary embolism during combined radical nephrectomy and IVC thrombectomy.12
Our patient had decompensated biventricular heart failure, poor respiratory reserve, and metabolic abnormalities, yet the AngioVac system was able to remove a large floating thrombus percutaneously from the RA, resulting in remarkable improvement in his condition.
Acknowledgment
We appreciate the suggestions of Nicholas Coldosa, MD, in the preparation of this manuscript.
References
- 1. Toodoran TM, Sobieszczyk PS, Levy MS, Perry TE, Shook DC, Kinlay S, . et al. Percutaneous extraction of right atrial mass using the AngioVac aspiration system. J Vasc Interv Radiol 2011; 22( 9): 1345– 7. [DOI] [PubMed] [Google Scholar]
- 2. Sakhuja R, Gandhi S, Rogers RK, Margey RJP, Jaff MR, Schainfeld R.. A novel endovenous approach for treatment of massive central venous or pulmonary arterial thrombus, mass, or vegetation: the AngioVac suction cannula and circuit [abstract]. J Am Coll Cardiol 2011; 57( 14): E1535 Available from: http://www.sciencedirect.com/science/article/pii/S0735109711615350. [Google Scholar]
- 3. Goodman A. Angiovac system removes large high-risk clots in vasculature [Internet]. Available from: http://www.Medscape.com/viewarticle/754425 [2011 Nov 29]. [Google Scholar]
- 4. Pasha AK, Elder MD, Khurram D, Snyder BA, Movahed MR.. Successful management of acute massive pulmonary embolism using AngioVac suction catheter technique in a hemodynamically unstable patient. Cardiovasc Revasc Med 2014; 15( 4): 240– 3. [DOI] [PubMed] [Google Scholar]
- 5. Patel N, Azemi T, Zaeem F, Underhill D, Gallagher R, Hagberg R, Sadiq I.. Vaccum assisted vegetation extraction for the management of large lead vegetations. J Card Surg 2013; 28( 3): 321– 4. [DOI] [PubMed] [Google Scholar]
- 6. Moriarty J, Patel K, Bradfield J.. Vaccum assisted debulking of a prohibitively large tricuspid valve vegetation prior to laser lead extraction. J Innov Cardiac Rhythm Manag 2014; 5: 1544– 8. Available from: http://www.innovationsincrm.com/cardiac-rhythm-management/2014/march/569-vacuum-assisted-debulking-tricuspid-valve. [Google Scholar]
- 7. Divekar AA, Scholz T, Fernandez JD.. Novel percutaneous transcatheter intervention for refractory active endocarditis as a bridge to surgery-AngioVac aspiration system. Catheter Cardiovasc Interv 2013; 81( 6): 1008– 12. [DOI] [PubMed] [Google Scholar]
- 8. Smith SJ, Behrens G, Sewall LE, Sichlau MJ.. Vaccum-assisted thrombectomy device (Angiovac) in the management of symptomatic iliocaval thrombosis. J Vasc Interv Radiol 2014; 25( 3): 425– 30. [DOI] [PubMed] [Google Scholar]
- 9. Tsai S, Mark F, Patel V, Kwolek CJ.. Initial use of a large-bore suction thrombectomy cannula for the treatment of massive inferior vena cava and iliofemoral deep venous thrombosis. J Vasc Surg 2011; 54( 4): 1226– 7. Available from: http://www.jvascsurg.org/article/S0741-5214(11)01616-8/fulltext. [Google Scholar]
- 10. Sengodan P, Grewal H, Gandhi S.. Invasive hepatocellular carcinoma with recurrent pulmonary embolism: use of AngioVac cannula thrombectomy device for mechanical aspiration. J Invasive Cardiol 2014; 26( 7): E100– 3. [PubMed] [Google Scholar]
- 11. ElMallah W, Kalra N, Wiisanen M, Gurley J.. AngioVac catheter extractions: a novel technique for cardiac mass extraction. Learning curve and predictors of success [abstract]. Catheter Cardiovasc Interv 2014; 83( Suppl S1): S117 Available from: http://onlinelibrary.wiley.com/doi/10.1002/ccd.v83.S1/issuetoc. [Google Scholar]
- 12. Brown RJ, Uhlman MA, Fernandez JD, Collins T, Brown JA.. Novel use of AngioVac system to prevent pulmonary embolism during radical nephrectomy with inferior vena cava thrombectomy. Curr Urol 2013; 7( 1): 34– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]


