Abstract
Transcatheter device implantation has become an attractive alternative to surgery in the closure of atrial septal defects in selected patients. However, it can lead to early and late sequelae, some of them life threatening. For example, 79 days before her admission to our emergency department with sudden-onset respiratory distress and respiratory arrest (leading to cardiac tamponade and rupture), a 22-year-old woman had undergone percutaneous closure of an atrial septal defect. We describe the damage and its treatment.
Although the adverse effects of transcatheter device implantation are rare, physicians should know that these events can be life threatening. Further data are needed to prevent such sequelae and to design new devices. It is of utmost importance that patients and their family members be informed both of possible sequelae and of life-saving interventions to be administered at early diagnosis.
Keywords: Balloon occlusion/adverse effects/instrumentation/standards; device removal; heart atria/injuries; heart septal defects, atrial/therapy; minimally invasive surgical procedures/adverse effects; prostheses and implants/adverse effects; septal occluder device/adverse effects; treatment outcome; wounds, penetrating/etiology
A trial septal defect (ASD), the most prevalent congenital heart disease in adults, accounts for 5% to 10% of all congenital heart diseases.1 Although most patients are asymptomatic until adulthood, early diagnosis and treatment are crucial to prevent such possible sequelae as right-sided heart failure, pulmonary hypertension, and arrhythmia. Surgery is the gold standard in the treatment of secundum ASD, because its morbidity and mortality rates are very low, while postoperative functional capacity and survival rates are excellent in the long term.1 Although surgery is a low-risk and recommended treatment method, its risks include post-pericardiotomy syndrome, arrhythmia, pleural and pericardial effusion, the need for blood products, and scar formation.1
Percutaneous closure of ASD was first performed in 1976. It has been widely adopted over the subsequent decades because it is a low-risk method, implies a short hospital stay, has no need for blood products, and produces no scars.2 However, it can lead to life-threatening early and late sequelae that require emergency intervention. Early sequelae can include residual shunts, systemic and pulmonary device embolization, thromboembolism, superior vena cava and right-upper pulmonary vein compression, tricuspid or mitral valve compression, and arrhythmia. Late and life-threatening sequelae include free ruptures of the right and left atrial walls that cause cardiac tamponade, erosion of the ascending aorta, and aorta–atrial fistula formation.2
Case Report
In February 2013, a 22-year-old woman was admitted to our emergency department with sudden-onset dyspnea and subsequent syncope. In the initial examination, she was unconscious and in respiratory arrest. After cardiopulmonary resuscitation, she was intubated. Transthoracic echocardiography (TTE) revealed dense pericardial effusion, fibrin, and cardiac tamponade. The patient, in shock, underwent urgent operation.
Intraoperative examination revealed rupture of the left atrial roof and noncoronary aortic sinus, caused by an atrial septal occluder (ASO) (Fig. 1). We placed a temporary pledgeted suture on the ruptured noncoronary sinus of the ascending aorta, to stop the bleeding through a very small perforation. After a standard bicaval cannulation, we initiated cardiopulmonary bypass. The right atrium was opened after aortic cross-clamping and antegrade delivery of blood cardioplegia. The ASO was then removed, and a temporary autologous pericardial patch was constructed for the ostial secundum ASD. Finally, the left atrial and noncoronary aortic sinus walls were primarily repaired.
Fig. 1.
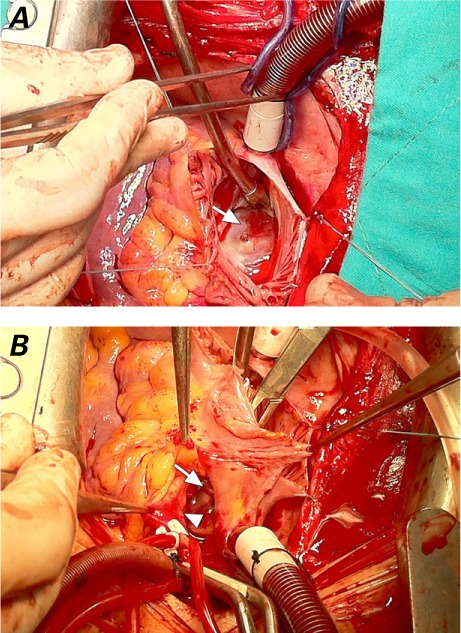
Intraoperative photographs show A) the Amplatzer septal occluder (in situ, arrow) that had been implanted 79 days earlier, and B) the ruptured noncoronary aortic sinus (arrowhead) and the perforated left atrial wall (arrow), before their repair.
The patient was extubated on the first postoperative day, and no neurologic or other major sequelae were seen. She was discharged on the 5th postoperative day in a good overall condition. Review of her records from her previous surgery revealed that an 18-mm Amplatzer® Septal Occluder (St. Jude Medical, Inc.; St. Paul, Minn) had been successfully implanted 79 days before her emergency admission to our hospital, and that she had been discharged on the first postoperative day with no sequelae, as confirmed by TTE.
Discussion
Several devices, in varying types and sizes, have been used in the closure of ASDs, and possible complications have been described. Surgical closure of ASD is associated with low mortality and morbidity rates, together with nearly excellent functional capacity and survival rates in the long term. Transcatheter closure of ASDs, although minimally invasive, does result in morbidity and mortality rates comparable to those of surgery.3
Atrial wall ruptures, aortic erosion, and rupture in association with cardiac tamponade are rare but life-threatening sequelae to the placement of nearly all ASD closure devices, ranging from 0.1% to 0.3%.3 Perforation and erosion usually originate from the atrial wall of the anterosuperior rim, or often from the aortic side. Patients who have a short anterosuperior rim and are given an oversized septal occluder are at higher risk of device erosion. The edge of the device adjacent to the superior rim moves like a seesaw during each cardiac cycle, thereby straining the right or left atrial free wall, or the atrial roof towards the septum. The edge of the device erodes the atrial wall and eventually erodes the adjacent aortic sinus, rupturing the aorta. In addition, aorta–atrial fistulae can develop in certain patients.3–5 Baseline measurements are crucial to prevent such consequences. In particular, an Amplatzer Septal Occluder should not be implanted in patients who have an anterosuperior rim of <4 mm3. All patients should be checked by echocardiography at the time of implantation, one day after implantation, before hospital discharge, and one week after implantation. In addition to this first week of close follow-up, patients should, at minimum, be reevaluated one month, 6 months, and one year after implantation, because adverse events are more frequent in the first 12 months.6,7
In suspect cases, the hospital stay should be prolonged and patients closely monitored. Of note, TTE and cardiac computed tomography are extremely helpful in the diagnosis of sequelae.5
References
- 1. Bialkowski J, Karwot B, Szkutnik M, Banaszak P, Kusa J, Skalski J.. Closure of atrial septal defects in children: surgery versus Amplatzer device implantation. Tex Heart Inst J 2004; 31( 3): 220– 3. [PMC free article] [PubMed] [Google Scholar]
- 2. Preventza O, Sampath-Kumar S, Wasnick J, Gold JP.. Late cardiac perforation following transcatheter atrial septal defect closure. Ann Thorac Surg 2004; 77( 4): 1435– 7. [DOI] [PubMed] [Google Scholar]
- 3. Amin Z, Hijazi ZM, Bass JL, Cheatham JP, Hellenbrand WE, Kleinman CS.. Erosion of Amplatzer septal occluder device after closure of secundum atrial septal defects: review of registry of complications and recommendations to minimize future risk. Catheter Cardiovasc Interv 2004; 63( 4): 496– 502. [DOI] [PubMed] [Google Scholar]
- 4. Divekar A, Gaamangwe T, Shaikh N, Raabe M, Ducas J.. Cardiac perforation after device closure of atrial septal defects with the Amplatzer septal occluder. J Am Coll Cardiol 2005; 45( 8): 1213– 8. [DOI] [PubMed] [Google Scholar]
- 5. Sarris GE, Kirvassilis G, Zavaropoulos P, Belli E, Berggren H, Carrel T, . et al. Surgery for complications of trans-catheter closure of atrial septal defects: a multi-institutional study from the European Congenital Heart Surgeons Association. Eur J Cardiothorac Surg 2010; 37( 6): 1285– 90. [DOI] [PubMed] [Google Scholar]
- 6. Moore J, Hegde S, El-Said H, Beekman R 3rd, Benson L, Bergersen L, . et al. Transcatheter device closure of atrial septal defects: a safety review. JACC Cardiovasc Interv 2013; 6( 5): 433– 42. [DOI] [PubMed] [Google Scholar]
- 7. Rare serious erosion events associated with St. Jude Amplatzer Atrial Septal Occluder (ASO): FDA Safety Communication [Internet]. 2013. Available from: http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm371145.htm [2013 Oct 17; revised 2014 Oct 10]. [Google Scholar]


