A 69-year-old man with metastatic squamous cell carcinoma of the larynx presented with stable ventricular tachycardia (VT) that necessitated cardioversion. Upon conversion of the heart to sinus rhythm, the resting electrocardiogram showed new, prominent, convex ST-segment elevations in leads I, aVL, V2 through V4, and V6, with 2:1 atrioventricular (AV) block (Fig. 1). The patient's troponin T level peaked at 0.33 ng/mL. Emergency coronary angiography revealed nonobstructive coronary artery disease. A transthoracic echocardiogram showed a left ventricular (LV) ejection fraction of 0.35 and a large LV mass involving the apico-anterolateral and apical septal myocardium (Fig. 2). Cardiac magnetic resonance images delineated a heterogeneously enhancing 6.4 × 3.8-cm infiltrating mass (Fig. 3). A single-chamber permanent pacemaker was placed, and the patient was discharged from the hospital with instructions to take oral amiodarone. He was subsequently readmitted with fatigue and recurrence of hemodynamically stable, monomorphic VT (Fig. 4). Implantable cardioverter-defibrillator (ICD) placement was deferred, given his limited life expectancy and goals of care. Systemic chemotherapy was initiated, but he died of progressive metastatic disease 24 days after VT onset.
Fig. 1.
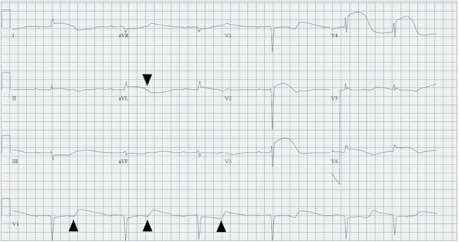
Electrocardiogram shows sinus bradycardia, marked first-degree atrioventricular block with 2:1 conduction (arrowheads indicate nonconducted P waves), and reciprocal ST-segment depressions in leads III and aVF. The QT interval (>600 ms) is prolonged.
Fig. 2.
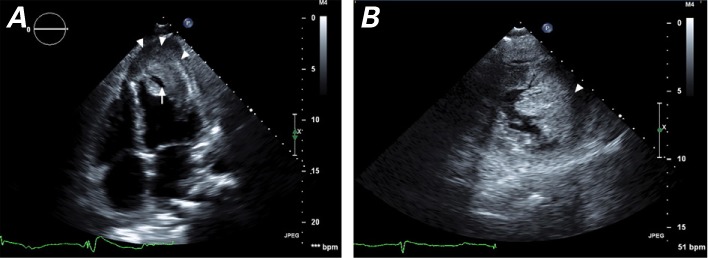
Transthoracic echocardiographic images show a left ventricular mass of heterogeneous echotexture and ill-defined borders. A) Apical 4-chamber view shows the mass occupying the apex and extending into the apical and anterolateral septum (arrowheads). The mass is markedly thicker than the adjacent septal and lateral myocardium, with an echolucent area (arrow) bounded by thick cortex, which might be necrotic tumor mass. B) Short-axis view below the level of the papillary muscles shows the mass (arrowhead) occupying anterior and lateral territories, with 4.6-cm maximal thickness and near-obliteration of the apical cavity. The infiltrated myocardial segments are akinetic.
Supplemental motion image is available for Figure 2A.
Fig. 3.
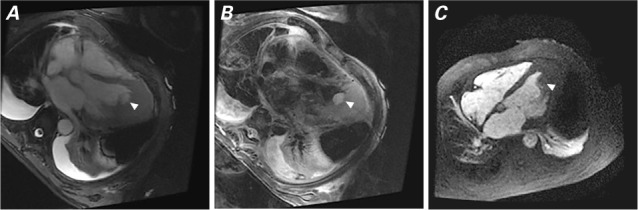
Cardiac magnetic resonance images show the mass (arrowhead). A) Balanced steady-state free-precession image (apical view) shows an isointense mass and normal myocardium. B) T2 weighting shows mass hyperintensity relative to the normal, unaffected myocardium. C) First-pass perfusion image (apical 4-chamber view) shows the mildly hyperintense mass.
Fig. 4.
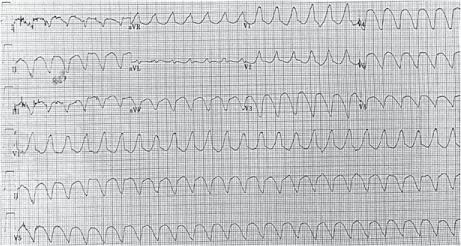
Electrocardiogram shows monomorphic, wide-complex tachycardia (cycle length, 280 ms). Features consistent with ventricular tachycardia include a positive R wave in lead aVR, a superior axis, and a broad QRS width (~200 ms) with right bundle branch morphology.
Comment
Ventricular arrhythmias are rarely caused by intracardiac masses; however, this clinical entity poses management challenges and has a distinctly poor prognosis. Our patient's LV mass was presumed to be a new metastatic focus from his advanced laryngeal carcinoma. Although the electrophysiologic manifestations observed in this case (ventricular arrhythmia, ST-segment elevations, and high-grade AV block) are not entirely specific for tumor-associated cardiac infiltration, multimodal images assisted in distinguishing it from more prevalent cardiomyopathies with arrhythmogenic tendencies (for instance, myocarditis and Chagas disease) and from infiltrative processes such as amyloidosis and sarcoidosis.
Prominent ST-segment elevations strongly suggest myocardial infiltration and might be explained by direct tumor-related local injury or compression of penetrating coronary arteries.1 Monomorphic VT with a right bundle branch morphology and superior axis supports either a posterior or a posteroseptal arrhythmia origin. Possible arrhythmogenic mechanisms include localized ischemia and disrupted myocardial architecture, macro-reentrant circuits, and an inflammatory milieu that triggers electrical activity.2 Our patient's high-grade AV block might have been related to tumor infiltration of the septal conduction system.
The optimal management of tumor-related VT needs to be tailored to the patient's functional status, comorbid diseases, prognosis, and response to conservative management. Aggressive antiarrhythmic therapy often fails to suppress VT, because of ongoing structural triggers. Surgical resection is generally reserved for nonmetastatic primary cardiac tumors. Chemotherapy and radiation to minimize the tumor's footprint might reduce VT recurrence; however, residual disease and tumor necrosis might serve as ongoing foci for VT activity. Typically, ICDs are indicated only for patients whose life expectancy exceeds one year with reasonable functional capacity. Early palliative care might help to optimize near-term quality of life.
Supplementary Material
References
- 1. Cates CU, Virmani R, Vaughn WK, Robertson RM.. Electrocardiographic markers of cardiac metastasis. Am Heart J 1986; 112( 6): 1297– 303. [DOI] [PubMed] [Google Scholar]
- 2. Sheldon R, Isaac D.. Metastatic melanoma to the heart presenting with ventricular tachycardia. Chest 1991; 99( 5): 1296– 8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


