Abstract
Nobiletin, a bioactive polymethoxylated flavone, has been described to possess a diversity of biological effects through its antioxidant and anti-inflammatory properties. Vasodilator-stimulated phosphoprotein (VASP) is a common substrate for cyclic AMP and cyclic GMP-regulated protein kinases [i.e., cyclic AMP-dependent protein kinase (PKA; also known as protein kinase A) and cyclic GMP-dependent protein kinase (PKG; also known as protein kinase G)] and it has been shown to be directly phosphorylated by protein kinase C (PKC). In the present study, we demonstrate that VASP is phosphorylated by nobiletin in human platelets via a non-cyclic nucleotide-related mechanism. This was confirmed by the use of inhibitors of adenylate cyclase (SQ22536) and guanylate cyclase [1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ)], since they prevented VASP phosphorylation induced by nobiletin. Furthormore, this event was also not affected by specific inhibitors of PKA (H-89), PKG (KT5823) and PKC (Ro318220), representing cyclic nucleotide-dependent pathways upon nobiletin-induced VASP phosphorylation. Similarly, inhibitors of p38 mitogen-activated protein kinase (MAPK; SB203580), extracellular signal-regulated kinase 2 (ERK2; PD98059), c-Jun N-terminal kinase 1 (JNK1; SP600125), Akt (LY294002) and nuclear factor-κB (NF-κB; Bay11-7082) did not affect nobiletin-induced VASP phosphorylation. Moreover, electron spin resonance, dichlorofluorescein fluorescence and western blotting techniques revealed that nobiletin did not affect hydroxyl radicals (OH•), intracellular reactive oxygen species (ROS) and on protein carbonylation, respectively. Furthermore, the nobiletin-induced VASP phosphorylation was surprisingly reversed by the intracellular antioxidant, N-acetylcysteine (NAC), but not by the inhibitor of NADPH oxidase, diphenyleneiodonium chloride (DPI). It was surprising to observe the differential effects of nobiletin and NAC on VASP phosphorylation in human platelets, since they both have been reported to have antioxidant properties. The likely explanation for this discrepancy is that NAC may bind to allosteric sites on the receptor different from those that nobiletin binds to in human platelets. Taken together, our findings suggest that nobiletin induces VASP phosphorylation in human platelets through non-cyclic nucleotide-related mechanisms. Nevertheless, the exact mechanisms responsible for these effects need to be further confirmed in future studies.
Keywords: nobiletin, vasodilator-stimulated phosphoprotein, cyclic AMP-dependent protein kinase/cyclic GMP-dependent protein kinase, mitogen-activated protein kinases, reactive oxygen species, nuclear factor-κB, N-acetylcysteine, protein carbonylation
Introduction
Nobiletin, a major flavonoid polymethexylated flavone (5,6,7,8,3′,4′-hexamethoxyflavone; PMF) found in citrus, has been found in very high concentrations in immature citrus peels (1). It can been extracted from different citrus species, such as Citrus reticulata Blanco (mandarin orange), Citrus unshiu Markovich, Citrus depressa (shiikuwasa) and Citrus limon (lemon) (2–4). Nobiletin has been reported to be an encouraging antioxidant and anti-inflammatory agent in the treatment of asthma, colitis and Alzheimer's disease (2,5,6). Nobiletin has been reported to protect PC12 cells from hydrogen peroxide (H2O2)-induced cytotoxicity (7), and to atttenuate ethanol-induced liver injury by augmenting the phosphorylation of AMP-activated protein kinase (AMPK) (8). This compound possesses potent anti-neuroinflammatory abilities by suppressing the activation of the extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (p38 MAPK) pathways, as well as the translocation of nuclear factor-κB (NF-κB) and the subsequent gene expression of inducible nitric oxide synthase (iNOS), tumor necrosis factor-α (TNF-α) and interleukin (IL)-1β (9). A previous study demonstrated that nobiletin was the most potent inhibitor of neuroinflammation among 8 common tangerine flavonoids (10). Another study revealed that the administration of nobiletin protected rat brains from ischemic damage by activating the Akt/cyclic AMP response element-binding protein (CREB) signaling pathway (11).
Vasodilator-stimulated phosphoprotein (VASP) is a regulator of actin reorganization in platelets. As VASP is a common downstream target of various signaling pathways, an increasing attention to this molecule has been paid in platelet studies (12–14). Cyclic AMP-dependent protein kinase (PKA; also known as protein kinase A) is considered the main mediator of the numerous effects associated with increased cyclic AMP levels. In platelets, PKA activation has been shown to be involved in the phosphorylation of VASP (15). It is also noteworthy that cyclic AMP may cross-activate the cyclic GMP-dependent protein kinase (PKG; also known as protein kinase G) in some vascular tissues (16). Ii has also been shown that PKA and PKG are not the only kinases able to phosphorylate VASP, but that protein kinase C (PKC) may also have this ability (17). VASP phosphorylation in response to cyclic AMP/cyclic GMP in platelets correlates with fibrinogen receptor inhibition (18). In addition, platelets from VASP-knockout mice exhibit enhanced thrombin-induced platelet activation and impaired cyclic AMP-dependent inhibition (19). These studies suggest an important role of VASP in signal transduction pathways in platelets, its phosphorylation closely correlating with adenylate cyclase stimulation, platelet cyclic AMP/cyclic GMP increase, and the inhibition of platelet aggregation.
In our previous study, we found that nobiletin (10–30 µM) inhibited collagen and arachidonic acid-induced platelet aggregation in a concentration-dependent manner through the inhibition of the activation of the phospholipase C (PLC) γ2-PKC cascade and Akt/MAPK signaling pathways, and also found that nobiletin prolonged closure time in human whole blood ex vivo and increased the occlusion time of thrombotic platelet plug formation in mice (20). Moreover, at a maximum concentration of 30 µM, nobiletin had no effects on the intracellular levels of cyclic AMP or cyclic GMP in human platelets, which is consistent with the recent observation by Vaiyapuri et al (21). Of note, our preliminary experiments revealed that nobiletin (30 µM) markedly increased VASP phosphorylation in a similar manner to prostaglandin E1 (PGE1) (Fig. 1A). Therefore, it is of considerable interest to understand what the novel mechanisms responsible for nobiletin-induced VASP phosphorylation, since cyclic nucleotides do not play a role in this process.
Figure 1.
Effects of nobiletin and cyclic nucleotide inhibitors on vasodilator-stimulated phosphoprotein (VASP) phosphorylation in washed human platelets. (A) Human platelets (1.2×109 cells/ml) were pre-incubated with or without SQ22536 (100 µM) or ODQ (10 µM), followed by the addition of 1 nM prostaglandin E1 (PGE1), 10 µM nitroglycerin (NTG), 30 µM nobiletin, or 0.5% dimethyl sulfoxide (DMSO) (control) for 3 min; (B) human platelets were pre-incubated with SQ22536 (100 µM) or ODQ (10 µM) in the absence or presence of 30 µM nobiletin, or 0.5% DMSO (control) for 3 min. The platelets were collected, and subcellular extracts were analyzed to determine VASP phosphorylation. Values are presented as the means ± SEM (A) n=7; (B) n=4. *P<0.05, and **P<0.001, compared with the resting platelets; #P<0.05, compared with the PGE1- or ODQ-treated platelets.
Materials and methods
Chemicals and reagents
Nobiletin (≥97%), collagen (type I), 5,5-dimethyl-1-pyrroline N-oxide (DMPO), SQ22536 (inhibitor of adenylate cyclase), 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; inhibitor of guanylate cyclase), heparin, PGE1, nitroglycerin (NTG), H89 (inhibitor of PKA), KT583 (PKG inhibitor), LY294002 (Akt inhibitor), Ro318220 (PKC inhibitor), DPI (NOX inhibitor), Bay11-7082 (NF-κB inhibitor), PD98059 (ERK2 inhibitor), SB203580 (p38 MAPK inhibitor, SP600125 (JNK inhibitor) and N-acetylcysteine (NAC) were all purchased from Sigma-Aldrich (St. Louis, MO, USA). Anti-VASP (Cat. no. GTX132176) and anti-VASP (phospho Ser157; Cat. no. GTX32362) polyclonal antibodies (pAbs) were purchased both from GeneTex, Inc. (Irvine, CA, USA). The anti-α-tubulin monoclonal antibody (mAb; Cat. no. MS-581-P0) was purchased from NeoMarkers (Fremont, CA, USA). Hybond-P polyvinylidene difluoride (PVDF) membranes, an enhanced chemiluminescence western blotting detection reagent, the horseradish peroxidase (HRP)-conjugated donkey anti-rabbit immunoglobulin G (IgG; Cat. no. RPN4301), and the sheep anti-mouse IgG (Cat. no. RPN4201) were all purchased from GE Healthcare UK Ltd. (Buckinghamshire, UK). Nobiletin was dissolved in 0.5% dimethyl sulfoxide (DMSO) and stored at 4°C.
Platelets
Our study was approved by the Institutional Review Board of Taipei Medical University, Taipei, Taiwan and conformed to the directives of the Helsinki Declaration. Human platelet suspensions were prepared as previously described (22). Blood was collected from 20 healthy human volunteers (who had given informed consent and had taken no medication during the 2 weeks preceding collection) and was mixed with an acid-citrate-dextrose solution. Following centrifugation at 120 × g for 37°C, the supernatant (platelet-rich plasma) was supplemented with 0.5 µM PGE1 and 6.4 IU/ml of heparin. The washed platelets were finally suspended in Tyrode's solution containing 3.5 mg/ml of bovine serum albumin (BSA). The final concentration of Ca2+ in Tyrode's solution was 1 mM.
Immunoblotting
The washed platelets (1.2×109 cells/ml) were pre-incubated with 30 µM nobiletin or 0.5% DMSO for 3 min. The reaction was terminated by using 10 mM ethylenediaminetetraacetic acid (EDTA), and the platelets were immediately re-suspended in 200 µl of a lysis buffer. Samples containing 80 µg of protein were separated on a 12% acrylamide gel using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the proteins were electrotransferred onto the PVDF membranes by using a Bio-Rad semidry transfer unit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The blots were blocked with TBST (10 mM Tris-base, 100 mM NaCl and 0.01% Tween-20) containing 5% BSA for 1 h and probed with various primary antibodies. The membranes were incubated with the HRP-linked anti-mouse IgG or anti-rabbit IgG (diluted 1:3,000 in TBST) for 1 h. Immunoreactive bands were detected using an enhanced chemiluminescence system. Ratios of the semi-quantitative results were obtained by scanning the reactive bands and quantifying the optical density using a video densitometer and Bio-profil Biolight software, version V2000.01 (Vilber Lourmat, Marne-la-Vallée, France).
Measurement of hydroxyl radicals (OH•) using electron spin resonance (ESR) spectrometry
ESR spectrometry was performed using a Bruker EMX ESR spectrometer (Bruker, Billerica, MA, USA) as previously described (23). The platelet suspensions (3.6×108 cells/ml) were treated with 1 µg/ml of collagen, 500 µM nobiletin or 0.5% DMSO for 3 min in a separate vial. The suspensions were incubated for 5 min, and 100 µM DMPO were added before ESR was conducted. The ESR spectrometer was operated at a power of 20 mW and 9.78 GHz, and a scan range of 100 G and a receiver gain of 5×104 was applied. Moreover, a Fenton reaction solution (50 µM FeSO4 + 2 mM H2O2) was also pre-treated with a solvent control (0.5% DMSO) or nobiletin (500 µM) for 3 min. The rate of free-radical generation is expressed in the following equation: inhibition rate = 1 − [signal height (nobiletin)/signal height (solvent control)], as previously described (23).
Measurement of intracellular reactive oxygen species (ROS) by fluorometric assay
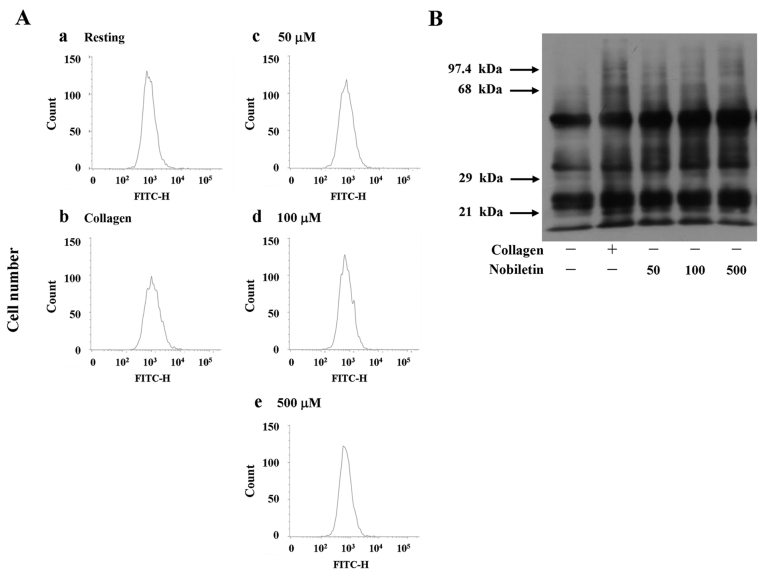
The method, based on a fluorometric assay, was adapted to measure the intracellular ROS. Briefly, the platelet suspensions (3.6×108 cells/ml) were incubated (20 min, 37°C in the dark) in 24-well microtiter plates with 20 µM of dichlorofluorescein fluorescence (DCF) stock in ethanol. The content of each well was then transferred into a 5 ml tube for centrifugation (335 × g, 10 min, 37°C). After removing the supernatant, the suspensions were subsequently resuspended in Hank's Balanced Salt Solution (HBSS) buffer and transferred to the wells. Following treatment with various concentrations (50, 100, and 500 µM) of nobiletin and 1 µg/ml of collagen, the generation of the fluorescent product, DCF, was followed in an automated plate fluorescence reader (Beckman Coulter, Miami, FL, USA) using an excitation wavelength of 485 nm and emission wavelength of 555 nm. The effect of nobiletin and collagen on ROS production was compared to the resting platelets set as 100% of the ROS-induced fluorescence.
Detection of protein carbonyls
The expression of protein carbonyl was monitored in 2,4-dinitrophenylhydrazine (DNPH)-derivatized proteins using an OxyBlot Protein Oxidation Detection kit (Millipore, Billerica, MA, USA). Briefly, the washed platelets (1.2×109 cells/ml) were incubated with collagen (1 µg/ml) and nobiletin (50–500 µM) or 0.5% DMSO for 3 min. The reaction was terminated by using EDTA, and the platelets were immediately re-suspended in 200 µl of a lysis buffer. Samples containing 30 µg of protein were separated by electrophoresis on 12% SDS-PAGE, and western blotting procedures were followed as described in immunoblotting assay. A primary rabbit monoclonal antibody (1:150) against DNP (Cat. no. S7150; Millipore) and a secondary anti-rabbit IgG antibody (1:300) were used to detect protein carbonyl.
Statistical analysis
The experimental results are expressed as the means ± SEM and are accompanied by the number of observations (n). Values of 'n' refer to the number of experiments, and each experiment was conducted using different blood donors. Differences between groups in the experiments were assessed using an analysis of variance (ANOVA). When this analysis indicated significant differences among group means, the groups were compared using the Student-Newman-Keuls method. A value of P<0.05 was considered to indicated a statistically significant difference. Statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Effects of cyclic nucleotides on nobiletin-induced VASP phosphorylation in washed human platelets
It has been demonstrated that cyclic nucleotides can induce VASP Ser157 phosphorylation in human platelets (24). Thus, in the present study, we investigated whether the nobiletin-induced VASP phosphorylation is dependent on cyclic nucleotides. The phosphorylation level of VASP in unstimulated platelets was at lower levels; however, the addition of 1 nM PGE1 and 10 µM NTG and 30 µM nobiletin significantly increased this phosphorylation compared with the resting group (Fig. 1A). Moreover, VASP phosphorylation was absolutely abolished by pre-treatment with SQ22536 (100 µM), an inhibitor of adenylate cyclase or ODQ (10 µM), an inhibitor of guanylate cyclase. Moreover, pre-treatment with SQ22536 or ODQ did not significantly reverse the nobiletin (30 µM)-induced VASP phosphorylation (Fig. 1B). These results indicate that nobiletin-induced VASP phosphorylation in human platelets is not dependent on cyclic nucleotide-related pathways.
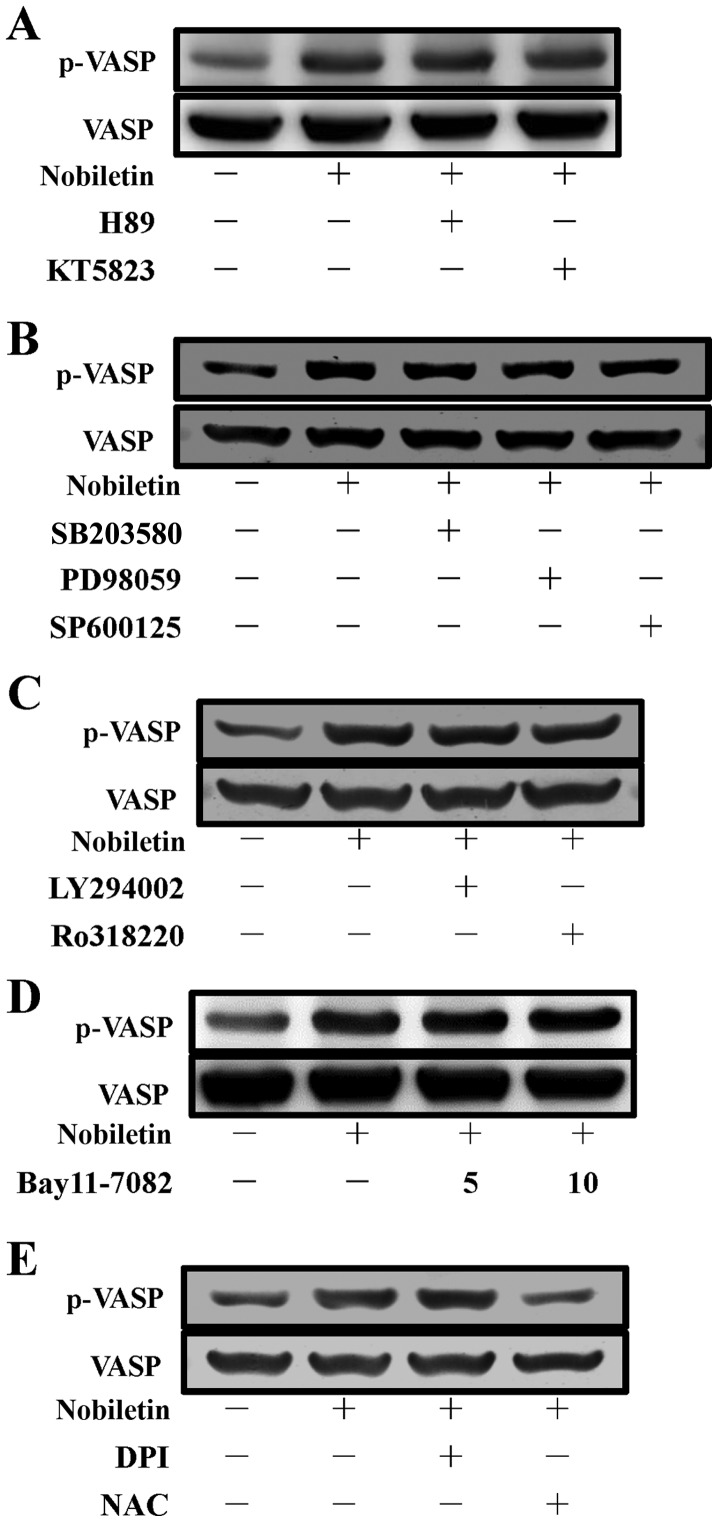
These results were further confirmed by using PKA and PKG inhibitors, since it has previously been described that VASP is a substrate of cyclic nucleotide (cyclic AMP/cyclic GMP)-dependent PKA-PKG (25), which phosphorylate it at 3 sites: Ser157, Ser239 and Thr278. These sites are phosphorylated, with differing kinetics, both in vitro and in intact human platelets (26). In the present study, as shown in Fig. 2A, the inhibitors of PKA (H89, 5 µM) and PKG (KT5823, 2 µM) were not essentially effective in reversing the nobiletin-induced VASP phosphorylation in platelets. These data indicate that PKA and PKG are not involved in the regulation of nobiletin-induced VASP phosphorylation.
Figure 2.
Effects of nobiletin on vasodilator-stimulated phosphoprotein (VASP) phosphorylation in washed human platelets. Human platelets (1.2×109 cells/ml) were pre-incubated with (A) 5 µM H89 [cyclic AMP-dependent protein kinase (PKA) inhibitor] or 2 µM KT5823 [cyclic GMP-dependent protein kinase (PKG) inhibitor]; (B) 10 µM SB203580 [p38 mitogen-activated protein kinase (MAPK) inhibitor], 20 µM PD98059 [extracellular signal-regulated kinase (ERK) inhibitor], or SP600125 [c-Jun N-terminal kinase (JNK) inhibitor]; (C) 10 µM LY294002 (Akt inhibitor) or 2 µM Ro318220 [protein kinase C (PKC) inhibitor]; (D) 5 and 10 µM Bay11-7082 [nuclear factor-κB (NF-κB) inhibitor] and (E) 10 µM diphenyleneiodonium chloride (DPI) and 10 mM N-acetylcysteine (NAC) for 3 min, followed by the addition of 30 µM nobiletin or 0.5% dimethyl sulfoxide (DMSO) (control). VASP phosphorylation was analyzed by immunoblotting using an anti-phospho-VASP antibody. Profiles are representative of 4 independent experiments.
MAPK, Akt and PKC have no essential function in nobiletin-induced VASP phosphorylation
To investigate whether MAPKs, including p38 MAPK, ERK and JNK, Akt and PKC, play a role in the stimulatory mechanisms of nobiletin on VASP phosphorylation in human platelets, we used the well-established inhibitors of p38 MAPK (SB203580, 10 µM), ERK (PD98059, 20 µM), JNK (SP600125, 10 µM), Akt (LY294002, 10 µM) and PKC (Ro318220, 2 µM) to examine their effects on VASP phosphorylation induced by nobiletin. The results revealed that all the tested inhibitors of p38 MAPK, ERK and JNK, Akt and PKC did not alter the nobiletin-induced VASP phosphorylation (Fig. 2B and C), which indicate that the MAPK, Akt and PKC signaling pathways do not play a role in nobiletin-induced of VASP phosphorylation.
NF-κB is not responsible for nobiletin-induced VASP phosphorylation in human platelets
NF-κB has been reported to function independently of gene regulation in platelets, and follwoing platelet activation, it is phosphorylated and degraded (27). NF-κB induction can be abrogated using its inhibitor, Bay11-7082 [(E)3-[(4-methylphenyl)-sulfonyl]-2-propenenitrile], which is an irreversible inhibitor of IκBα phosphorylation, resulting in the downregulation of the induced NF-κB activation. Given the significance of NF-κB and platelets in atherosclerosis and inflammation, we investigated whether NF-κB plays a role in nobiletin-induced VASP phosphorylation by using its inhibitor, Bay11-7082. The data indicated that Bay11-7082 had no visible effect on nobiletin-induced VASP phosphorylation (Fig. 2D), and consequently suggest that NF-κB is not involved in the process of nobiletin-induced VASP phosphorylation.
Involvement of NADPH oxidase (NOX) and ROS in nobiletin-induced VASP phosphorylation
Since it has been previously demonstrated that NOX and ROS regulate intracellular transduction pathways and many of the structural properties of NOX have been found in platelets (28), in the present study, the effects of NOX inhibitor, diphenyleneiodonium chloride (DPI), and the cell-permeable antioxidant, NAC, on nobiletin-induced VASP phosphorylation in platelets were examined. We found that DPI (10 µM) did not affect nobiletin-induced VASP phosphorylation, whereas, NAC (10 mM) significantly antagonized this phosphorylation (Fig. 2E). These findings indicate that the stimulatory effect of nobiletin on VASP phosphorylation is also independent of NADPH oxidase, but that it is dependent on NAC-mediated inhibitory mechanisms.
Effect of nobiletin on •OH and ROS formation
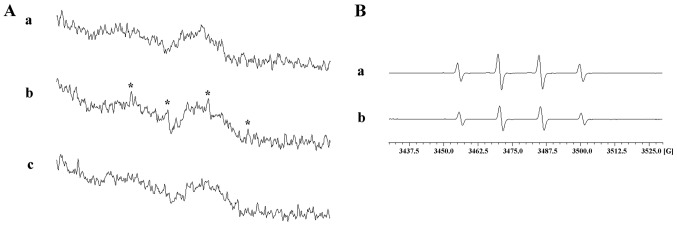
The antioxidant properties of plants or their active principles, mainly their radical scavenging activities, have been intensively investigated over the years (29–31). In this study as shown in Fig. 2E, NAC, a cell-permeable antioxidant, significantly reversed nobiletin-induced VASP phosphorylation. Thus, we examined whether the process of nobiletin-induced VASP phosphorylation is dependent on persuading •OH radicals or ROS formation. ESR spectrometry and the fluorescence-based method using DCF as a fluorescent probe revealed that nobiletin, even at a higher concentration of 500 µM, did not significantly stimulate •OH radical signals, as that stimulated by either collagen (1 µg/ml; positive control) (Fig. 3A) in washed human platelets or by Fenton reaction (Fig. 3B). Furthermore, our results also demonstrated that nobiletin (50 and 100 µM) did not reduce the fluorescence intensity of DCF even at the highest concentration of 500 µM (Fig. 4A), which clearly indicated that nobiletin does not have a stimulatory effect on •OH radicals as well as on intracellular ROS.
Figure 3.
Effect of nobiletin on hydroxyl radical (•OH) formation by electron spin resonance (ESR) spectrometry. (A) Human platelet suspensions (3.6×108 cells/ml) were treated with (a) 0.5% dimethyl sulfoxide (DMSO), (b) 1 µg/ml of collagen or (c) 500 µM nobiletin for 3 min to induce •OH formation. The suspensions were incubated for 5 min, and 100 µM 5,5-dimethyl-1-pyrroline N-oxide (DMPO) were added before the analysis of ESR; (B) a Fenton reaction solution (50 µM FeSO4 + 2 mM H2O2) was pretreated with (a) solvent control (0.5% DMSO) or (b) nobiletin (500 µM) for 3 min to induce •OH formation, and the ESR spectrometry was conducted. (A and B) Profiles are representative of 4 independent experiments; (A-b) asterisk (*) indicates the formation of •OH.
Figure 4.
Effects of nobiletin on intracellular reactive oxygen species (ROS) formation and protein carbonyl expression in human platelets. (A) Platelet suspensions (3.6×108 cells/ml) were incubated in 24-well microtiter plates with 20 µM of dichlorofluorescein fluorescence (DCF) stock in ethanol. The content of each well was then transferred into a 5 ml tube for centrifugation (335 × g, 10 min, 37°C). After removing the supernatant, suspensions were subsequently re-suspended in Hank's Balanced Salt Solution (HBSS) buffer and transferred to the wells. Following treatment with various concentrations (50, 100 and 500 µM) of nobiletin and 1 µg/ml of collagen, the generation of the fluorescent product DCF was followed in an automated plate fluorescence reader using an excitation wavelength of 485 nm and emission wavelength of 555 nm; (B) the expression of protein carbonyl was monitored in 2,4-dinitrophenylhydrazine (DNPH)-derivatized proteins using an OxyBlot Protein Oxidation Detection kit. (A and B) Profiles are representative of 4 independent experiments.
Effects of nobiletin on protein carbonyls
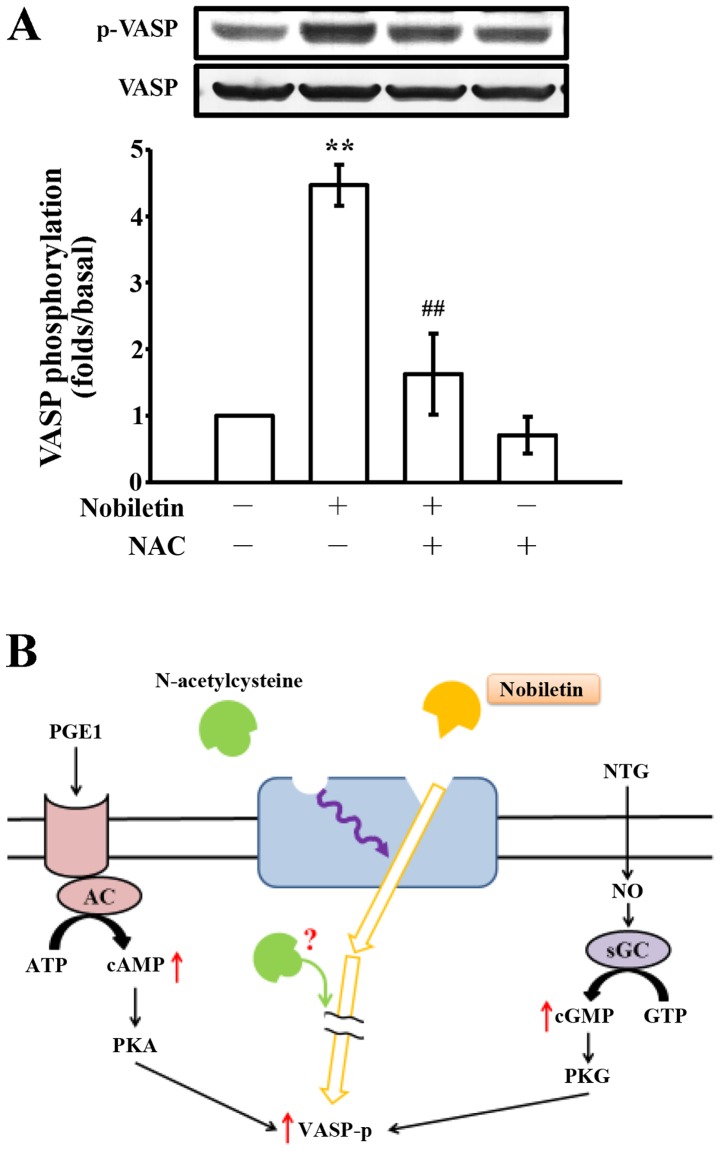
At present, much attention is focused on the protein carbonyl content as the most widely used marker of the oxidative modification of proteins in correlation with a variety of pathologies (32). Since we noted that nobiletin did not affect •OH radicals or ROS production, this event was further confirmed by detecting carbonylated proteins in nobiletin (50–500 µM)-treated platelets, which was compared with collagen (positive control)-induced protein carbonylation. When the platelets were activated by 1 µg/ml collagen, protein carbonylation was markedly increased compared to the resting platelets (Fig. 4B). This augmentation is due to the oxidative stress generated by the cells themselves when activated by the exogenous exposure of the platelets to collagen. Furthermore, treatment with nobiletin at various concentrations (50–500 µM) did not significantly induce or reduce protein carbonylation, which was evidenced by observing the unlettered intensity of the carbonylated bands, as compared to the collagen-stimulated cells (Fig. 4B). Moreover, pre-treatment with NAC (10 mM) markedly reversed the nobiletin-induced VASP phosphorylation; however, treatment with NAC alone did not significantly induce VASP phosphorylation (Fig. 5A).
Figure 5.
Effects of nobiletin and N-acetylcysteine (NAC) on vasodilator-stimulated phosphoprotein (VASP) phosphorylation in human platelets. (A) Human platelets (1.2×109 cells/ml) were pre-incubated with solvent control [0.5% dimethyl sulfoxide (DMSO)], with or without NAC (10 mM) and 30 µM nobiletin for 3 min. VASP phosphorylation was then analyzed by immunoblotting using an anti-phospho-VASP antibody. Values are presented as the means ± SEM (n=4). **P<0.001, compared with the resting platelets; ##P<0.05, compared with the nobiletin-treated platelets; (B) schematic diagram illustrating the hypothesis of the participation of NAC and nobiletin on VASP phosphorylation in human platelets. VASP phosphorylation is induced by prostaglandin E1 (PGE1) and nitroglycerin (NTG) via stimulating cyclic AMP and cyclic GMP-regulated protein kinases [i.e., cyclic AMP-dependent protein kinase (PKA) and cyclic GMP-dependent protein kinase (PKG)], respectively. NAC interrupts nobiletin-triggered VASP phosphorylation may through binding to the allosteric sites on the receptor distinct from that nobiletin binds; or NAC directly interrupts intracellular signaling cascades which are responsible for nobiletin-triggered VASP phosphorylation through an unidentified mechanism. AC, adenylyl cyclase; NO, nitric oxide; sGC, soluble guanylate cyclase.
Discussion
It has been considered that increased cyclic AMP/cyclic GMP in platelets activates cyclic AMP-dependent PKA and cyclic GMP-dependent PKG, and thus regulates the platelet activation response by phosphorylating intracellular protein substrates, such as VASP. Therefore, VASP phosphorylation may act as a negative regulator of platelet activation. In this study, we demonstrated that cyclic nucleotide-related pathways were not responsible for nobiletin-induced VASP phosphorylation in washed human platelets. Considering the fact that platelets are anuclear cells, the projected hypothesis was thus confirmed by using various well established cyclic nucleotide-related inhibitors, such as adenylate cyclase inhibitor (SQ22536), guanylate cyclase inhibitor (ODQ), PKA inhibitor (H89) and PKG inhibitor (KT5823) instead of using their respective siRNAs. Our results indicated that nobiletin-induced VASP phosphorylation was not affected by these inhibitors. In addition, it was also demonstrated that this process was independent of NF-κB, MAPKs, Akt, PLCγ2/PKC, NOX and ROS, since the respective inhibitors of these molecules did not affect VASP phosphorylation induced by nobiletin. Hence, it was important to determine the actual mechanisms underlying the phosphorylation of VASP by nobiletin.
VASP is a critical protein that plays a role in regulating adhesive events that are involved in platelet aggregation (15,33). Cyclic nucleotides activate cyclic nucleotide-dependent kinases which inhibit all steps of this cascade and simultaneously phosphorylate a number of proteins, including VASP. Moreover, considering the results of a previous study that suggested that VASP plays an important role in the signaling of cyclic GMP- and cyclic AMP-dependent kinases (34), it was surprising that in this study, nobiletin stimulated VASP phosphorylation in washed human platelets without increasing the cyclic nucleotide levels. This process was examined by observing whether the adenylate cyclase inhibitor, SQ22536, and the guanylate cyclase inhibitor, ODQ, modulate nobiletin-induced VASP phosphorylation in platelets. The results confirmed that neither SQ22536 nor ODQ affected nobiletin induced VASP phosphorylation as shown in Fig. 1B. A likely explanation for this unexpected finding is that the absence of cyclic nucleotide function in VASP phosphorylation may be substituted by other members of proteins.
It is well-established that PKA is an essential negative modulator of platelet function through the inhibition of multiple biochemical events which all converge in the inhibition of platelet function. It is also noteworthy that cyclic AMP may cross-activate PKG in some vascular tissues. PKG has been identified as the intracellular mediator of the effects of cyclic GMP-elevating agents, such as nitric oxide or natriuretic peptides (35). Therefore, we wished to confirm whether nobiletin-induced VASP phosphorylation is dependent on cyclic AMP/cyclic GMP and is linked to PKA/PKG activity. In order to examine this hypothesis, the PKA inhibitor, H89, and the PKG inhibitor, KT5823, were added to the nobiletin pre-treated platelets. KT5823, an in vitro inhibitor of PKG, has been extensively used to demonstrate or rule out an involvement of PKG in signaling processes, and the PKA inhibitor, H89, is a chemical compound which inhibits PKA in a competitive manner (36). In this study, we found that the elevation of nobiletin-induced VASP phosphorylation in platelets was not modulated by either H89 or KT5823, and thus, it appears that VASP phosphorylation stimulated by nobiletin may be independent of cyclic AMP/PKA and cyclic GMP/PKG pathways.
A previous study suggested that PKA and PKG are not the only kinases able to phosphorylate VASP, but that PKC may also have this ability (17). Chitaley et al (17) reported that, in cultured rat aortic smooth muscle cells, VASP becomes phosphorylated in a PKG- and PKA-independent manner. They established that VASP is also a substrate of PKC and provide evidence for the involvement of a classical PKC isoform in VASP phosphorylation (17). Moreover, in our previous study, we reported that Akt acts as an upstream regulator of PKC (37), and this pathway was found to be responsible for phosphorylation of VASP in activated platelets (38). Hence, it is interesting to speculate whether PKC and Akt play a crucial role in regulating nobiletin-induced phosphorylation of VASP in platelets. Our results clearly demonstrated that this phosphorylation was independent of the PKC and Akt pathways, since their specific inhibitors, Ro318220 (PKC) and LY294004 (Akt), did not alter the phosphorylation status of VASP, as its level was similar in nobiletin alone-treated platelets (Fig. 2C).
MAPKs control cell proliferation, differentiation, mitosis, survival and apoptosis. Among these MAPKs, p38 MAPK, JNK1 and ERK2 have been reported to exist in platelets and are activated by various stimuli (39). ERK2 and JNK1 phosphorylation stimulated by collagen has been proven to be involved in platelet aggregation (40). In addition, p38 MAPK activation has also been shown in collagen-induced platelet activation and secretion, which was restored by p38 MAPK inhibitors (41). However, our findings demonstrated that the phosphorylation of VASP-stimulated by nobiletin was not modulated by the p38 MAPK inhibitor SB203580, ERK inhibitor, PD98059, and also by the JNK inhibitor, SP600125, indicating that MAPK signaling pathways do not account for the stimulatory effects of nobiletin on VASP phosphorylation. In addition, NF-κB induction can be abrogated using its inhibitor, Bay11-7082, which is an irreversible inhibitor of IκBα phosphorylation, resulting in the downregulation of the cytokine-induced NF-κB activation (42). A previous study indicated that Bay11-7082 potently inhibited collagen- and thrombin-induced platelet aggregation, and it induced VASP phosphorylation through cyclic AMP elevation and PKA activation (43). Following this, it was important to determine whether Bay11-7082 can modulate nobiletin-induced phosphorylation of VASP, and the data showed that this inhibitor did not account for the increased phosphorylation of VASP in response to nobiletin, indicating that NF-κB is also not involved in this process.
ROS derived from platelet activation may augment platelet reactivity during in vivo thrombus formation. Our previous study suggested that free radical species act as secondary messengers that increase cytosolic Ca2+ during the initial phase of platelet activation processes, and PKC is involved in receptor-mediated free radical production in platelets (44). That study also showed H2O2 derived from platelets is converted into •OH, and platelet aggregation can be inhibited by •OH scavengers (44). A previous study found that platelet-induced ROS production was inhibited by the radical scavenger, NAC, and the NOX inhibitor, DPI (45). We found that NAC potently reversed the stimulatory effects of nobiletin on VASP phosphorylation, but DPI did not alter this phosphorylation, which indicated that NADPH is also not involved in this process.
Protein carbonyl groups serve as a biomarker of oxidative stress, since protein oxidation typically results in increased carbonyl contents. It has been reported that H2O2 produces a concentration-dependent increase in the carbonylation of platelet proteins, and it can be quenched by antioxidant catalase, suggesting that carbonylation is induced by the oxidative stress generated by activated platelets (32). Several ROS, including •OH, oxidize amino acid residues in proteins to form products with carbonyl groups that can be measured after reaction with DNPH. In this study, the protein carbonyl concentrations in platelets treated with collagen, as well as with nobiletin correlated with changes in the intensity of DNPH reactive protein bands on immunoblots. In addition, an active role of ROS in VASP phosphorylation stimulated by nobiletin was monitored by DCF fluorescence as a marker for the intracellular generation of radicals. From these experiments, it was found that nobiletin-induced VASP phosphorylation is independent of ROS formation. Moreover, it has been demonstrated that NAC decreases human platelet aggregation and increases the intracellular levels of cyclic GMP by interplaying with endogenous NO (46), which is constitutively synthesized by platelets (47) and inhibits aggregation by activating the soluble guanylate cyclase (48). In this study, as shown in Fig. 2E, NAC alone did not trigger VASP phosphorylation as nobiletin did. The possible explanations for this finding may be due to the following reasons: first, NAC may bind to allosteric sites on the receptor distinct from that nobiletin binds in the human platelets to block the signals of nobiletin-triggered VASP phosphorylation; second, since NAC is a cell-permeable antioxidant, it may interrupt the intracellular signaling cascade which is responsible for VASP phosphorylation through an unidentified mechanism (Fig. 5B).
In conclusion, our previous study suggested that nobiletin represents a potential therapeutic agent for the prevention or treatment of thromboembolic disorders. In the present study, and to the best of our knowledge, we found for the first time that nobiletin stimulates VASP phosphorylation via a novel non-cyclic nucleotide-dependent mechanisms in washed human platelets. In addition, an unexpected result was observed in that NAC reversed the nobiletin-induced VASP phosphorylation. However, the exact inhibitory mechanisms of action of NAC warrant further investigation.
Acknowledgments
This study was supported by grants (MOST103-2320-B-038-017, MOST104-2622-B-038-003 and MOST104-2320-B-038-045-MY2) from the Ministry of Science and Technology of Taiwan and Chi Mei Medical Center, Taipei Medical University (104CM-TMU-04).
References
- 1.Manthey JA, Grohmann K. Phenols in citrus peel byproducts. Concentrations of hydroxycinnamates and polymethoxylated flavones in citrus peel molasses. J Agric Food Chem. 2001;49:3268–3273. doi: 10.1021/jf010011r. [DOI] [PubMed] [Google Scholar]
- 2.Nakajima A, Aoyama Y, Shin EJ, Nam Y, Kim HC, Nagai T, Yokosuka A, Mimaki Y, Yokoi T, Ohizumi Y, Yamada K. Nobiletin, a citrus flavonoid, improves cognitive impairment and educes soluble Aβ levels in a triple transgenic mouse model of Alzheimer's disease (3XTg-AD) Behav Brain Res. 2015;289:69–77. doi: 10.1016/j.bbr.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 3.Kawahata I, Yoshida M, Sun W, Nakajima A, Lai Y, Osaka N, Matsuzaki K, Yokosuka A, Mimaki Y, Naganuma A, et al. Potent activity of nobiletin-rich Citrus reticulata peel extract to facilitate cAMP/PKA/ERK/CREB signaling associated with learning and memory in cultured hippocampal neurons: identification of the substances responsible for the pharmacological action. J Neural Transm (Vienna) 2013;120:1397–1409. doi: 10.1007/s00702-013-1025-x. [DOI] [PubMed] [Google Scholar]
- 4.Kawaii S, Tomono Y, Katase E, Ogawa K, Yano M. Quantitation of flavonoid constituents in citrus fruits. J Agric Food Chem. 1999;47:3565–3571. doi: 10.1021/jf990153+. [DOI] [PubMed] [Google Scholar]
- 5.Xiong Y, Chen D, Yu C, Lv B, Peng J, Wang J, Lin Y. Citrus nobiletin ameliorates experimental colitis by reducing inflammation and restoring impaired intestinal barrier function. Mol Nutr Food Res. 2015;59:829–842. doi: 10.1002/mnfr.201400614. [DOI] [PubMed] [Google Scholar]
- 6.Wu YQ, Zhou CH, Tao J, Li SN. Antagonistic effects of nobiletin, a polymethoxyflavonoid, on eosinophilic airway inflammation of asthmatic rats and relevant mechanisms. Life Sci. 2006;178:2689–2696. doi: 10.1016/j.lfs.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 7.Lu YH, Su MY, Huang HY, Lin-Li, Yuan CG. Protective effects of the citrus flavanones to PC12 cells against cytotoxicity induced by hydrogen peroxide. Neurosci Lett. 2010;484:6–11. doi: 10.1016/j.neulet.2010.07.078. [DOI] [PubMed] [Google Scholar]
- 8.Choi BK, Kim TW, Lee DR, Jung WH, Lim JH, Jung JY, Yang SH, Suh JW. A polymethoxy flavonoids-rich Citrus aurantium extract ameliorates ethanol-induced liver injury through modulation of AMPK and Nrf2-related signals in a binge drinking mouse model. Phytother Res. 2015;29:1577–1584. doi: 10.1002/ptr.5415. [DOI] [PubMed] [Google Scholar]
- 9.Cui Y, Wu J, Jung SC, Park DB, Maeng YH, Hong JY, Kim SJ, Lee SR, Kim SJ, Kim SJ, et al. Anti-neuroinflammatory activity of nobiletin on suppression of microglial activation. Biol Pharm Bull. 2010;33:1814–1821. doi: 10.1248/bpb.33.1814. [DOI] [PubMed] [Google Scholar]
- 10.Ho SC, Kuo CT. Hesperidin, nobiletin, and tangeretin are collectively responsible for the anti-neuroinflammatory capacity of tangerine peel (Citri reticulatae pericarpium) Food Chem Toxicol. 2014;71:176–182. doi: 10.1016/j.fct.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Zhao H, Zhang X, Chen L, Zhao X, Bai X, Zhang J. Nobiletin protects against cerebral ischemia via activating the p-Akt, p-CREB, BDNF and Bcl-2 pathway and ameliorating BBB permeability in rat. Brain Res Bull. 2013;96:45–53. doi: 10.1016/j.brainresbull.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Benz PM, Blume C, Seifert S, Wilhelm S, Waschke J, Schuh K, Gertler F, Münzel T, Renné T. Differential VASP phosphorylation controls remodeling of the actin cytoskeleton. J Cell Sci. 2009;122:3954–3965. doi: 10.1242/jcs.044537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Major TC, Handa H, Brisbois EJ, Reynolds MM, Annich GM, Meyerhoff ME, Bartlett RH. The mediation of platelet quiescence by NO-releasing polymers via cGMP-induced serine 239 phosphorylation of vasodilator-stimulated phosphoprotein. Biomaterials. 2013;34:8086–8096. doi: 10.1016/j.biomaterials.2013.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee DH, Cho HJ, Kim HH, Rhee MH, Ryu JH, Park HJ. Inhibitory effects of total saponin from Korean red ginseng via vasodilator-stimulated phosphoprotein-Ser(157) phosphorylation on thrombin-induced platelet aggregation. J Ginseng Res. 2013;37:176–186. doi: 10.5142/jgr.2013.37.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aszódi A, Pfeifer A, Ahmad M, Glauner M, Zhou XH, Ny L, Andersson KE, Kehrel B, Offermanns S, Fässler R. The vasodilator-stimulated phosphoprotein (VASP) is involved in cGMP- and cAMP-mediated inhibition of agonist-induced platelet aggregation, but is dispensable for smooth muscle function. EMBO J. 1999;18:37–48. doi: 10.1093/emboj/18.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckly-Michel A, Martin V, Lugnier C. Involvement of cyclic nucleotide-dependent protein kinases in cyclic AMP-mediated vasorelaxation. Br J Pharmacol. 1997;122:158–164. doi: 10.1038/sj.bjp.0701339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chitaley K, Chen L, Galler A, Walter U, Daum G, Clowes AW. Vasodilator-stimulated phosphoprotein is a substrate for protein kinase C. FEBS Lett. 2004;556:211–215. doi: 10.1016/S0014-5793(03)01435-2. [DOI] [PubMed] [Google Scholar]
- 18.Horstrup K, Jablonka B, Hönig-Liedl P, Just M, Kochsiek K, Walter U. Phosphorylation of focal adhesion vasodilator-stimulated phosphoprotein at Ser157 in intact human platelets correlates with fibrinogen receptor inhibition. Eur J Biochem. 1994;225:21–27. doi: 10.1111/j.1432-1033.1994.00021.x. [DOI] [PubMed] [Google Scholar]
- 19.Hauser W, Knobeloch KP, Eigenthaler M, Gambaryan S, Krenn V, Geiger J, Glazova M, Rohde E, Horak I, Walter U, Zimmer M. Megakaryocyte hyperplasia and enhanced agonist-induced platelet activation in vasodilator-stimulated phosphoprotein knockout mice. Proc Natl Acad Sci USA. 1999;96:8120–8125. doi: 10.1073/pnas.96.14.8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu WJ, Lin KC, Liu CP, Lin CY, Wu HC, Chou DS, Geraldine P, Huang SY, Hsieh CY, Sheu JR. Prevention of arterial thro mbosis by nobiletin: in vitro and in vivo studies. J Nutr Biochem. 2016;28:1–8. doi: 10.1016/j.jnutbio.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 21.Vaiyapuri S, Roweth H, Ali MS, Unsworth AJ, Stainer AR, Flora GD, Crescente M, Jones CI, Moraes LA, Gibbins JM. Pharmacological actions of nobiletin in the modulation of platelet function. Br J Pharmacol. 2015;172:4133–4145. doi: 10.1111/bph.13191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheu JR, Lee CR, Lin CH, Hsiao G, Ko WC, Chen YC, Yen MH. Mechanisms involved in the antiplatelet activity of Staphylococcus aureus lipoteichoic acid in human platelets. Thromb Haemost. 2000;83:777–784. [PubMed] [Google Scholar]
- 23.Chou DS, Hsiao G, Shen MY, Tsai YJ, Chen TF, Sheu JR. ESR spin trapping of a carbon-centered free radical from agonist-stimulated human platelets. Free Radic Biol Med. 2005;39:237–248. doi: 10.1016/j.freeradbiomed.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Smolenski A, Bachmann C, Reinhard K, Hönig-Liedl P, Jarchau T, Hoschuetzky H, Walter U. Analysis and regulation of vasodilator-stimulated phosphoprotein serine 239 phosphorylation in vitro and in intact cells using a phospho-specific monoclonal antibody. J Biol Chem. 1998;273:20029–20035. doi: 10.1074/jbc.273.32.20029. [DOI] [PubMed] [Google Scholar]
- 25.Pula G, Schuh K, Nakayama K, Nakayama KI, Walter U, Poole AW. PKCdelta regulates collagen-induced platelet aggregation through inhibition of VASP-mediated filopodia formation. Blood. 2006;108:4035–4044. doi: 10.1182/blood-2006-05-023739. [DOI] [PubMed] [Google Scholar]
- 26.Butt E, Abel K, Krieger M, Palm D, Hoppe V, Hoppe J, Walter U. cAMP- and cGMP-dependent protein kinase phosphorylation sites of the focal adhesion vasodilator-stimulated phosphoprotein (VASP) in vitro and in intact human platelets. J Biol Chem. 1994;269:14509–14517. [PubMed] [Google Scholar]
- 27.Liu F, Morris S, Epps J, Carroll R. Demonstration of an activation regulated NF-kappaB/I-kappaBalpha complex in human platelets. Thromb Res. 2002;106:199–203. doi: 10.1016/S0049-3848(02)00130-5. [DOI] [PubMed] [Google Scholar]
- 28.Seno T, Inoue N, Gao D, Okuda M, Sumi Y, Matsui K, Yamada S, Hirata KI, Kawashima S, Tawa R, et al. Involvement of NADH/NADPH oxidase in human platelet ROS production. Thromb Res. 2001;103:399–409. doi: 10.1016/S0049-3848(01)00341-3. [DOI] [PubMed] [Google Scholar]
- 29.Gülçin I, Elmastaş M, Aboul-Enein HY. Determination of antioxidant and radical scavenging activity of Basil (Ocimum basilicum L. Family Lamiaceae) assayed by different methodologies. Phytother Res. 2007;21:354–361. doi: 10.1002/ptr.2069. [DOI] [PubMed] [Google Scholar]
- 30.Kuczmannová A, Gál P, Varinská L, Treml J, Kováč I, Novotný M, Vasilenko T, Dall'Acqua S, agy M, Mučaji P. Agrimonia eupatoria L. and Cynara cardunculus L. Water Infusions: Phenolic profile and comparison of antioxidant activities. Molecules. 2015;20:20538–20550. doi: 10.3390/molecules201119715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Timmers MA, Guerrero-Medina JL, Esposito D, Grace MH, Paredes-López O, García-Saucedo PA, Lila MA. Characterization of phenolic compounds and antioxidant and anti-inflammatory activities from mamuyo (Styrax ramirezii Greenm.) fruit. J Agric Food Chem. 2015;63:10459–10465. doi: 10.1021/acs.jafc.5b04781. [DOI] [PubMed] [Google Scholar]
- 32.Alexandru N, Constantin A, Popov D. Carbonylation of platelet proteins occurs as consequence of oxidative stress and thrombin activation, and is stimulated by ageing and type 2 diabetes. Clin Chem Lab Med. 2008;46:528–536. doi: 10.1515/CCLM.2008.104. [DOI] [PubMed] [Google Scholar]
- 33.Massberg S, Grüner S, Konrad I, Garcia Arguinzonis MI, Eigenthaler M, Hemler K, Kersting J, Schulz C, Muller I, Besta F, et al. Enhanced in vivo platelet adhesion in vasodilator-stimulated phosphoprotein (VASP)-deficient mice. Blood. 2004;103:136–142. doi: 10.1182/blood-2002-11-3417. [DOI] [PubMed] [Google Scholar]
- 34.Halbrügge M, Friedrich C, Eigenthaler M, Schanzenbächer P, Walter U. Stoichiometric and reversible phosphorylation of a 46-kDa protein in human platelets in response to cGMP- and cAMP-elevating vasodilators. J Biol Chem. 1990;265:3088–3093. [PubMed] [Google Scholar]
- 35.Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev. 2000;52:375–414. [PubMed] [Google Scholar]
- 36.Butt E, van Bemmelen M, Fischer L, Walter U, Jastorff B. Inhibition of cGMP-dependent protein kinase by (Rp)-guanosine 3′,5′-monophosphorothioates. FEBS Lett. 1990;263:47–50. doi: 10.1016/0014-5793(90)80702-K. [DOI] [PubMed] [Google Scholar]
- 37.Lu WJ, Lee JJ, Chou DS, Jayakumar T, Fong TH, Hsiao G, Sheu JR. A novel role of andrographolide, an NF-kappa B inhibitor, on inhibition of platelet activation: the pivotal mechanisms of endothelial nitric oxide synthase/cyclic GMP. J Mol Med (Berl) 2011;89:1261–1273. doi: 10.1007/s00109-011-0800-0. [DOI] [PubMed] [Google Scholar]
- 38.Dorsam RT, Kim S, Jin J, Kunapuli SP. Coordinated signaling through both G12/13 and G(i) pathways is sufficient to activate GPIIb/IIIa in human platelets. J Biol Chem. 2002;277:47588–47595. doi: 10.1074/jbc.M208778200. [DOI] [PubMed] [Google Scholar]
- 39.Adam F, Kauskot A, Rosa JP, Bryckaert M. Mitogen-activated protein kinases in hemostasis and thrombosis. J Thromb Haemost. 2008;6:2007–2016. doi: 10.1111/j.1538-7836.2008.03169.x. [DOI] [PubMed] [Google Scholar]
- 40.Kauskot A, Adam F, Mazharian A, Ajzenberg N, Berrou E, Bonnefoy A, Rosa JP, Hoylaerts MF, Bryckaert M. Involvement of the mitogen-activated protein kinase c-Jun NH2-terminal kinase 1 in thrombus formation. J Biol Chem. 2007;282:31990–31999. doi: 10.1074/jbc.M701596200. [DOI] [PubMed] [Google Scholar]
- 41.Kuliopulos A, Mohanlal R, Covic L. Effect of selective inhibition of the p38 MAP kinase pathway on platelet aggregation. Thromb Haemost. 2004;92:1387–1393. doi: 10.1160/TH04-03-0187. [DOI] [PubMed] [Google Scholar]
- 42.Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, Gerritsen ME. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 1997;272:21096–21103. doi: 10.1074/jbc.272.34.21096. [DOI] [PubMed] [Google Scholar]
- 43.Lee HS, Kim SD, Lee WM, Endale M, Kamruzzaman SM, Oh WJ, Cho JY, Kim SK, Cho HJ, Park HJ, et al. A noble function of BAY 11-7082: inhibition of platelet aggregation mediated by an elevated cAMP-induced VASP, and decreased ERK2/JNK1 phosphorylations. Eur J Pharmacol. 2010;627:85–91. doi: 10.1016/j.ejphar.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Sheu JR, Hung WC, Wu CH, Ma MC, Kan YC, Lin CH, Lin MS, Luk HN, Yen MH. Reduction in lipopolysaccharide-induced thrombocytopenia by triflavin in a rat model of septicemia. Circulation. 1999;99:3056–3062. doi: 10.1161/01.CIR.99.23.3056. [DOI] [PubMed] [Google Scholar]
- 45.Berg C, Trofast C, Bengtsson T. Platelets induce reactive oxygen species-dependent growth of human skin fibroblasts. Eur J Cell Biol. 2003;82:565–571. doi: 10.1078/0171-9335-00344. [DOI] [PubMed] [Google Scholar]
- 46.Anfossi G, Russo I, Massucco P, Mattiello L, Cavalot F, Trovati M. N-acetyl-L-cysteine exerts direct anti-aggregating effect on human platelets. Eur J Clin Invest. 2001;31:452–461. doi: 10.1046/j.1365-2362.2001.00815.x. [DOI] [PubMed] [Google Scholar]
- 47.Radomski MW, Palmer RMJ, Moncada S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc Natl Acad Sci USA. 1990;87:5193–5197. doi: 10.1073/pnas.87.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moro MA, Russel RJ, Cellek S, Lizasoain I, Su Y, Darley-Usmar VM, Radomski MW, Moncada S. cGMP mediates the vascular and platelet actions of nitric oxide: confirmation using an inhibitor of the soluble guanylyl cyclase. Proc Natl Acad Sci USA. 1996;93:1480–1485. doi: 10.1073/pnas.93.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]