Abstract
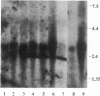
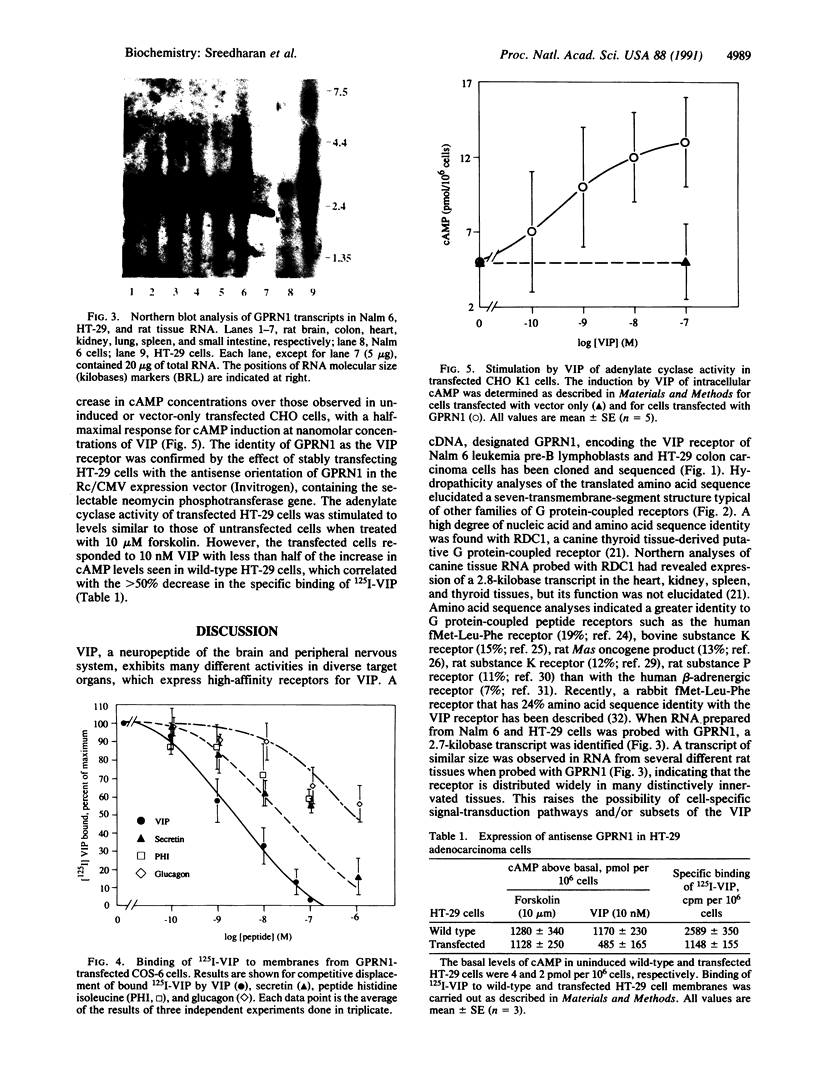
Vasoactive intestinal peptide (VIP) is a neuroendocrine mediator found in the central and peripheral nervous system. Distinct subsets of neural, respiratory, gastrointestinal, and immune cells bear specific high-affinity receptors for VIP, which are associated with a guanine nucleotide-binding (G) protein capable of activating adenylate cyclase. A cDNA clone (GPRN1) encoding the human VIP receptor was identified in libraries prepared from the Nalm 6 line of leukemic pre-B lymphoblasts and the HT-29 line of colon carcinoma cells. The deduced 362-amino acid polypeptide sequence encoded by GPRN1 shares a seven-transmembrane-segment hydropathicity profile with other G protein-coupled receptors. Northern blot analyses identified a 2.7-kilobase transcript of the VIP receptor in Nalm 6 and HT-29 cells as well as in tissues from rat brain, colon, heart, lung, kidney, spleen, and small intestine. COS-6 cells transfected with GPRN1 bound 125I-labeled VIP specifically with a dissociation constant (Kd) of 2.5 nM. VIP--and less effectively secretin, peptide histidine isoleucine (PHI), and glucagon competitively displaced bound 125I-VIP from transfected COS-6 cells, with potencies in the order VIP greater than secretin = PHI much greater than glucagon. VIP stimulated adenylate cyclase activity in stably transfected Chinese hamster ovary K1 cells, inducing a 3-fold increase in the intracellular level of cAMP. When the antisense orientation of the VIP receptor clone was introduced into HT-29 cells, there was a 50% suppression of the specific binding of 125I-VIP and of the VIP-induced increase in cAMP level, relative to untransfected cells. The VIP receptor cloned exhibits less than or equal to 24% homology with other receptors in the same superfamily and thus represents a subset of G protein-coupled receptors for peptide ligands.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beals C. R., Wilson C. B., Perlmutter R. M. A small multigene family encodes Gi signal-transduction proteins. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7886–7890. doi: 10.1073/pnas.84.22.7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulay F., Tardif M., Brouchon L., Vignais P. Synthesis and use of a novel N-formyl peptide derivative to isolate a human N-formyl peptide receptor cDNA. Biochem Biophys Res Commun. 1990 May 16;168(3):1103–1109. doi: 10.1016/0006-291x(90)91143-g. [DOI] [PubMed] [Google Scholar]
- Chatila T., Wong R., Young M., Miller R., Terhorst C., Geha R. S. An immunodeficiency characterized by defective signal transduction in T lymphocytes. N Engl J Med. 1989 Mar 16;320(11):696–702. doi: 10.1056/NEJM198903163201104. [DOI] [PubMed] [Google Scholar]
- Costa M., Furness J. B., Gibbins I. L., Morris J. L., Bornstein J. C., Llewellyn-Smith I. J., Murphy R. Colocalization of VIP with other neuropeptides and neurotransmitters in the autonomic nervous system. Ann N Y Acad Sci. 1988;527:103–109. doi: 10.1111/j.1749-6632.1988.tb26976.x. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Kobilka B. K., Strader D. J., Benovic J. L., Dohlman H. G., Frielle T., Bolanowski M. A., Bennett C. D., Rands E., Diehl R. E. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986 May 1;321(6065):75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch R. J., Sreedharan S. P., Goetzl E. J. High-affinity receptors for vasoactive intestinal peptide on human myeloma cells. J Immunol. 1989 Mar 15;142(6):1977–1981. [PubMed] [Google Scholar]
- Goetzl E. J., Kodama K. T., Turck C. W., Schiogolev S. A., Sreedharan S. P. Unique pattern of cleavage of vasoactive intestinal peptide by human lymphocytes. Immunology. 1989 Apr;66(4):554–558. [PMC free article] [PubMed] [Google Scholar]
- Haegerstrand A., Jonzon B., Dalsgaard C. J., Nilsson J. Vasoactive intestinal polypeptide stimulates cell proliferation and adenylate cyclase activity of cultured human keratinocytes. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5993–5996. doi: 10.1073/pnas.86.15.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus G. G., Vondy K., Holman M. Selective effects of cholera toxin on the activation of mouse B cells by different polyclonal activators. Eur J Immunol. 1987 Dec;17(12):1787–1792. doi: 10.1002/eji.1830171217. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laburthe M., Couvineau A. Molecular analysis of vasoactive intestinal peptide receptors. A comparison with receptors for VIP-related peptides. Ann N Y Acad Sci. 1988;527:296–313. doi: 10.1111/j.1749-6632.1988.tb26988.x. [DOI] [PubMed] [Google Scholar]
- Libert F., Parmentier M., Lefort A., Dinsart C., Van Sande J., Maenhaut C., Simons M. J., Dumont J. E., Vassart G. Selective amplification and cloning of four new members of the G protein-coupled receptor family. Science. 1989 May 5;244(4904):569–572. doi: 10.1126/science.2541503. [DOI] [PubMed] [Google Scholar]
- Magistretti P. J., Dietl M. M., Hof P. R., Martin J. L., Palacios J. M., Schaad N., Schorderet M. Vasoactive intestinal peptide as a mediator of intercellular communication in the cerebral cortex. Release, receptors, actions, and interactions with norepinephrine. Ann N Y Acad Sci. 1988;527:110–129. doi: 10.1111/j.1749-6632.1988.tb26977.x. [DOI] [PubMed] [Google Scholar]
- Marchis-Mouren G., Martin J. M., Luis J., el Battari A., Muller J. M., Marvaldi J., Pichon J. HT 29, a model cell line: stimulation by the vasoactive intestinal peptide (VIP); VIP receptor structure and metabolism. Biochimie. 1988 May;70(5):663–671. doi: 10.1016/0300-9084(88)90251-9. [DOI] [PubMed] [Google Scholar]
- Masu Y., Nakayama K., Tamaki H., Harada Y., Kuno M., Nakanishi S. cDNA cloning of bovine substance-K receptor through oocyte expression system. 1987 Oct 29-Nov 4Nature. 329(6142):836–838. doi: 10.1038/329836a0. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi M., Payan D. G. The mitogenic effects of vasoactive neuropeptides on cultured smooth muscle cell lines. Life Sci. 1987 Mar 2;40(9):853–861. doi: 10.1016/0024-3205(87)90034-8. [DOI] [PubMed] [Google Scholar]
- O'Dorisio M. S., Shannon B. T., Fleshman D. J., Campolito L. B. Identification of high affinity receptors for vasoactive intestinal peptide on human lymphocytes of B cell lineage. J Immunol. 1989 May 15;142(10):3533–3536. [PubMed] [Google Scholar]
- Ottaway C. A., Greenberg G. R. Interaction of vasoactive intestinal peptide with mouse lymphocytes: specific binding and the modulation of mitogen responses. J Immunol. 1984 Jan;132(1):417–423. [PubMed] [Google Scholar]
- Ottaway C. A. Selective effects of vasoactive intestinal peptide on the mitogenic response of murine T cells. Immunology. 1987 Oct;62(2):291–297. [PMC free article] [PubMed] [Google Scholar]
- Pincus D. W., DiCicco-Bloom E. M., Black I. B. Vasoactive intestinal peptide regulates mitosis, differentiation and survival of cultured sympathetic neuroblasts. Nature. 1990 Feb 8;343(6258):564–567. doi: 10.1038/343564a0. [DOI] [PubMed] [Google Scholar]
- Said S. I., Mutt V. Polypeptide with broad biological activity: isolation from small intestine. Science. 1970 Sep 18;169(3951):1217–1218. doi: 10.1126/science.169.3951.1217. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sasai Y., Nakanishi S. Molecular characterization of rat substance K receptor and its mRNAs. Biochem Biophys Res Commun. 1989 Dec 15;165(2):695–702. doi: 10.1016/s0006-291x(89)80022-1. [DOI] [PubMed] [Google Scholar]
- Stanisz A. M., Befus D., Bienenstock J. Differential effects of vasoactive intestinal peptide, substance P, and somatostatin on immunoglobulin synthesis and proliferations by lymphocytes from Peyer's patches, mesenteric lymph nodes, and spleen. J Immunol. 1986 Jan;136(1):152–156. [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Dixon R. A. Structural basis of beta-adrenergic receptor function. FASEB J. 1989 May;3(7):1825–1832. doi: 10.1096/fasebj.3.7.2541037. [DOI] [PubMed] [Google Scholar]
- Sundler F., Ekblad E., Grunditz T., Håkanson R., Uddman R. Vasoactive intestinal peptide in the peripheral nervous system. Ann N Y Acad Sci. 1988;527:143–167. doi: 10.1111/j.1749-6632.1988.tb26979.x. [DOI] [PubMed] [Google Scholar]
- Thomas K. M., Pyun H. Y., Navarro J. Molecular cloning of the fMet-Leu-Phe receptor from neutrophils. J Biol Chem. 1990 Nov 25;265(33):20061–20064. [PubMed] [Google Scholar]
- Yokota Y., Sasai Y., Tanaka K., Fujiwara T., Tsuchida K., Shigemoto R., Kakizuka A., Ohkubo H., Nakanishi S. Molecular characterization of a functional cDNA for rat substance P receptor. J Biol Chem. 1989 Oct 25;264(30):17649–17652. [PubMed] [Google Scholar]
- Young D., O'Neill K., Jessell T., Wigler M. Characterization of the rat mas oncogene and its high-level expression in the hippocampus and cerebral cortex of rat brain. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5339–5342. doi: 10.1073/pnas.85.14.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond R. E., Schwarzschild M. A., Rittenhouse A. R. Acute regulation of tyrosine hydroxylase by nerve activity and by neurotransmitters via phosphorylation. Annu Rev Neurosci. 1989;12:415–461. doi: 10.1146/annurev.ne.12.030189.002215. [DOI] [PubMed] [Google Scholar]