Abstract
Objective
The aim of this study was to investigate whether the acute care of frail elderly patients in a comprehensive geriatric assessment (CGA) unit is superior to the care in a conventional acute medical care unit.
Design
This is a clinical, prospective, randomized, controlled, one-center intervention study.
Setting
This study was conducted in a large county hospital in western Sweden.
Participants
The study included 408 frail elderly patients, aged ≥75 years, in need of acute in-hospital treatment. The patients were allocated to the intervention group (n=206) or control group (n=202). Mean age of the patients was 85.7 years, and 56% were female.
Intervention
This organizational form of care is characterized by a structured, systematic interdisciplinary CGA-based care at an acute elderly care unit.
Measurements
The primary outcome was the change in health-related quality of life (HRQoL) 3 months after discharge from hospital, measured by the Health Utilities Index-3 (HUI-3). Secondary outcomes were all-cause mortality, rehospitalizations, and hospital care costs.
Results
After adjustment by regression analysis, patients in the intervention group were less likely to present with decline in HRQoL after 3 months for the following dimensions: vision (odds ratio [OR] =0.33, 95% confidence interval [CI] =0.14–0.79), ambulation (OR =0.19, 95% CI =0.1–0.37), dexterity (OR =0.38, 95% CI =0.19–0.75), emotion (OR =0.43, 95% CI =0.22–0.84), cognition (OR = 0.076, 95% CI =0.033–0.18) and pain (OR =0.28, 95% CI =0.15–0.50). Treatment in a CGA unit was independently associated with lower 3-month mortality adjusted by Cox regression analysis (hazard ratio [HR] =0.55, 95% CI =0.32–0.96), and the two groups did not differ significantly in terms of hospital care costs (P>0.05).
Conclusion
Patients in an acute CGA unit were less likely to present with decline in HRQoL after 3 months, and the care in a CGA unit was also independently associated with lower mortality, at no higher cost.
Keywords: frailty, elderly, acute care, intervention, comprehensive geriatric assessment
Introduction
Background
Frail elderly individuals constitute a high percentage of emergency patients. The current organization of acute care is often poorly adapted to the specific needs of these patients.
Frailty is a biological syndrome reflecting vulnerability to stressors and reduced physiological reserves.1 For the individual, frailty is strongly associated with functional decline, activity limitations, prolonged recovery and a high risk of being institutionalized and dying within a short period of time.2–6
Among community-dwelling older adults, the prevalence of frailty is estimated to be at least 10%.7,8 Several frailty instruments reflect different aspects of the clinical phenotype of frailty, most of them focusing on one or more of the following core domains: slowness, weakness, low physical activity, exhaustion and unintentional weight loss.2,8,9 Other instruments reflect the accumulation of deficits, including a simplified bedside version.3,10,11 Most frail elderly patients have multiple chronic conditions, and recurring acute illness is frequent. A high percentage of health care consumption among these individuals is related to specialized inpatient care.
Frail elderly patients in acute care have complex needs. They would therefore be likely to benefit from a complex intervention including a biopsychosocial approach. A comprehensive geriatric assessment (CGA) aims to meet the needs of these frail patients through an interdisciplinary assessment and intervention with a broad focus on physiological, psychological, and social factors. This includes a person-centered approach focusing on the individual’s needs by validated instruments (Table 1). A few clinical studies have indicated that frail elderly patients could benefit from a CGA, in a general elderly care context, as well as under more specific conditions, eg, ortho-geriatric care.12–17 Two meta-analyses showed no differences in mortality between patients treated in CGA units and in conventional care units.15,17 However, in a recent study, caring for elderly patients in a CGA unit was associated with trends of lower 24-month mortality compared with conventional care units.13 These meta-analyses did not show any difference in rehospitalizations between the types of care. However, they reported that care in a CGA unit is associated with a potential cost reduction and so did a recent study on acute care of elders unit.18
Table 1.
Comparison of the management in the intervention group (CGA) and the control group (conventional acute medical care)
| Characteristics | CGA and care | Conventional acute medical care |
|---|---|---|
| Department and facilities | Two MÄVA (acute elderly care CGA units) wards with a total of 48 beds: one, two, or four bedrooms; Division of Internal Medicine and Emergency Care | Wards of internal and emergency medicine: one, two, or four bedrooms; Division of Internal Medicine and Emergency Care |
| Team members | ||
| Physicians | Yes. Specialists in internal medicine, family medicine and/or geriatrics | Yes. Specialists in internal medicine |
| Licensed practicing nurses | Yes. Including specialized admission and discharge nurses | Yes |
| Occupational therapists | Yes | No. Only counseling |
| Physiotherapists | Yes | No. Only counseling |
| Nutritionists | No. Only counseling | No. Only counseling |
| Treatment | Systematic, structured interdisciplinary CGA and care by validated instruments focusing on the following: somatic and mental health, medication review, functional and activity ability including early rehabilitation, social situation, and early discharge planning | Following routines at departments of internal medicine and emergency care according to guidelines |
| Admission route | Directly to the MÄVA ward via ambulance or primary care | Via the emergency ward |
Notes: For both groups, standard management procedures according to national and international guidelines were followed.
Abbreviation: CGA, comprehensive geriatric assessment.
Importance
In acute care settings, there is a great need to find appropriate organizational forms of CGA. There is also a lack of knowledge regarding the effects of CGA on health-related quality of life (HRQoL) and cost-effectiveness,16,17 particularly for the severely frail patients with poor prognosis who often have not been included in previous acute studies.
In 2008, the NU (NÄL-Uddevalla) hospital group, a large county hospital in the Västra Götaland Region of Sweden, introduced two acute elderly care units (MÄVAs).19 This organizational form of care is characterized by a structured, systematic interdisciplinary CGA and intervention performed on the ward, including an early rehabilitation strategy. To individualize the assessment and treatment, the team has a person-centered approach including care guidance, focusing on the needs of frail elderly patients, including HRQoL. The differences between MÄVA and a conventional care unit are given in Table 1.
Goals of this investigation
The aim was to study whether the acute care of frail elderly patients in a CGA unit is superior to the care in a conventional acute medical care unit when it comes to HRQoL, mortality, rehospitalizations, and hospital care costs. We hypothesized that patients cared for in a CGA unit would be less likely to present with decline in HRQoL dimensions at 3 months after discharge compared with patients treated in a conventional care unit.
Patients and methods
Study design and setting
This is a clinical, prospective, randomized, controlled intervention study with two parallel groups performed at the NU county hospital between March 2013 and July 2015. The total primary population of the NU health care system is 280,000 inhabitants. The study was approved by the independent ethics committee at the Sahlgrenska University Hospital in Gothenburg (8883-12, 20121212) and registered at the Swedish National Database of Research and Development; identifier 113021 (http://www.researchweb.org/is/vgr/project/113021; November 4, 2012).
Selection of participants
The study included patients, aged ≥75 years, being in need of in-hospital treatment and who fulfilled the foundation of frailty according to the recently validated FRESH (FRail Elderly Support research group) screening instrument,12,20,21 ie, two or more of the following criteria: tiredness from a short walk, general fatigue, frequent falls/anticipation of falls, dependence in shopping and three or more visits to the emergency ward during the last 12 months. Patients were excluded if they declined participation in the study, were unable to give informed consent (and it was impossible to obtain informed consent from a relative), were previously defined MÄVA patients (when a patient already had a MÄVA file implying direct access to the MÄVA facilities), or were clearly suited for care at a conventional acute medical care unit due to the severity of his/her acute condition (acute myocardial infarction, acute stroke, sepsis, or other acute life-threatening conditions).
When the staff at a primary care clinic, or the ambulance staff, had identified a patient who fulfilled the inclusion criteria, a MÄVA doctor was contacted via telephone. If he/she agreed, and there was a bed available at MÄVA, the patient was included in the intervention group and admitted directly to MÄVA. If no bed was available on the MÄVA wards, the patient was included in the control group and admitted to a conventional acute medical care unit through the emergency room. This method was considered likely to create a random distribution of patients.
Written informed consent was obtained from the patient or from a member of his/her next of kin when appropriate, as soon as possible after admission, by a study nurse or doctor after oral and written information (repeatedly if necessary). A screening log book, in which all patients identified as being eligible for inclusion were registered, was kept continuously. Data including study instruments were registered by a qualified member of the study team on computerized case record forms. All data were handled according to legislation and good clinical practice.
Interventions
The intervention in this study was the type of hospital unit the patients were treated in. In the intervention group, patients were allocated to MÄVA, characterized by a structured, systematic interdisciplinary CGA and intervention with standardized procedures and validated instruments focusing on somatic and mental health, medication review, social situation, early discharge planning, and functional and activity ability including an early rehabilitation strategy involving physicians, occupational therapists, physiotherapists, and nurses as active team members. The team has a person-centered approach focusing on the individual needs of frail elderly patients (Table 1).
In the control group, patients were allocated to a conventional acute medical care unit, where standard procedures according to national and international guidelines were followed.
Methods and measurements
Clinical characteristics, hospital care consumption, and mortality
The following data were collected mainly from medical records and registers: age, gender, housing, diabetes mellitus, renal function, heart failure, other comorbidities, numbers of in-hospital care days, rehospitalizations, and all-cause mortality.
Health Utilities Index-3 (HUI-3)
HRQoL was measured using the HUI-3 instrument.22 The HUI-3 is a generic HRQoL instrument that includes eight dimensions: vision, hearing, speech, ambulation, dexterity, emotion, cognition, and pain. Each dimension has two to eight questions. From the answers to these questions, an attribute level and a single-attribute utility score can be assigned to each of the dimensions. The HUI version used in the current study was the interview-based, 41-question, 1-week recall version. We used the patient self-assessment version or the proxy-assessment version, when self-assessment was not possible. The HRQoL measurement also included the EuroQoL-visual analog scale (EQ-VAS),23 which is a Visual Analog Scale ranging from 0 (worst imaginable health) to 100 (best imaginable health). Both the HUI-3 and EQ-VAS have been validated and are commonly used for elderly patients. The HUI version used in the current study was not available in Swedish, and a translation–back translation procedure was carried out in collaboration with the HUI Service Centre in Canada.22 As other versions were available in Swedish, the translation for the current study included only minor portions of the instrument, such as recall time.
Charlson Comorbidity Index
The patient’s total burden of morbidity was measured by the Charlson Comorbidity Index.24,25 It contains 19 categories of comorbidity and predicts the 10-year mortality for a patient. Each comorbidity is assigned with a score of 1, 2, 3, or 6 depending on the risk of dying that is associated with this condition.
Costs
The costs of in-hospital and outpatient health care and nursing, including health care staff costs, were extracted at the 3-month follow-up by administrative staff from the hospital database system and the national database on cost per patient,26 and from medical records after due approval by the relevant authorities. Cost per patient is a method used to calculate the cost of each patient and episode of hospital care.
Data concerning clinical and demographic characteristics and HRQoL were collected before discharge from the index hospital care episode when the patient was included in the study. Most patients were assessed by an occupational therapist or a physiotherapist (physical capacity), a dietician (nutritional state), and a nurse (eg, housing). In a few cases, a trained physician made the assessments. Follow-up assessments were made 3 months after discharge, by a physician’s examination, registers, and medical records. These assessments were made at the hospital or in the patient’s home. Patients were the primary informants. Proxy informants were used when patients were unable to participate.
Outcomes
The primary outcome was the change in HRQoL assessed before discharge from the index hospital care episode and at 3 months after discharge from hospital, measured by the HUI-3 instrument, which is given in the following sections. For each of the eight HUI dimensions, a clinically relevant change was defined as a patient-reported decrease to a lower attribute level at the 3-month follow-up compared with the index in-hospital episode.
Secondary outcomes were all-cause mortality, rehospitalizations, and hospital care costs.
Analysis
A sample size calculation was made based on the change in HRQoL after 3 months according to the HUI-3 instrument (significance level, 0.05; power, 80%). As previous studies mostly included patients who were less frail, it was difficult to estimate strict clinically relevant differences. For this purpose, HUI-3 scores from individuals, ≥75 years, were obtained from a previously established database at the Division of Health Care Analysis, Linköping University, Sweden.27 An overall average score of 0.44 (range 0–1) and an SD of 0.25 were calculated for this patient group. A 5% difference between the groups was considered clinically relevant. When using a two-sided test, it was necessary to include 150 patients in each study group. To compensate for the uncertainty, it was estimated that 200 evaluable patients should be included in each study group, ie, 400 patients in total.
The data analysis was based on the intention-to-treat principle, ie, the included patients remained in the study group to which they were allocated. Patients in both groups could, after discharge from the index care episode, be readmitted to the CGA unit or conventional care.
The statistical analyses were performed using SAS 9.3. Categorical data were analyzed using Fisher’s exact test or the χ2 test, and continuous data were compared using Student’s t-test. The association of the intervention with the primary and secondary outcomes was examined by either Cox regression or a multiple logistic regression model adjusted for relevant prognostic variables (age, gender, and relevant comorbidities, ie, the Charlson Comorbidity Index score). All independent variables included in the models were analyzed for possible collinearity with a variance inflation factor test. Variance inflation factor values of >2.5 were considered to indicate collinearity.
Results
Characteristics of study subjects
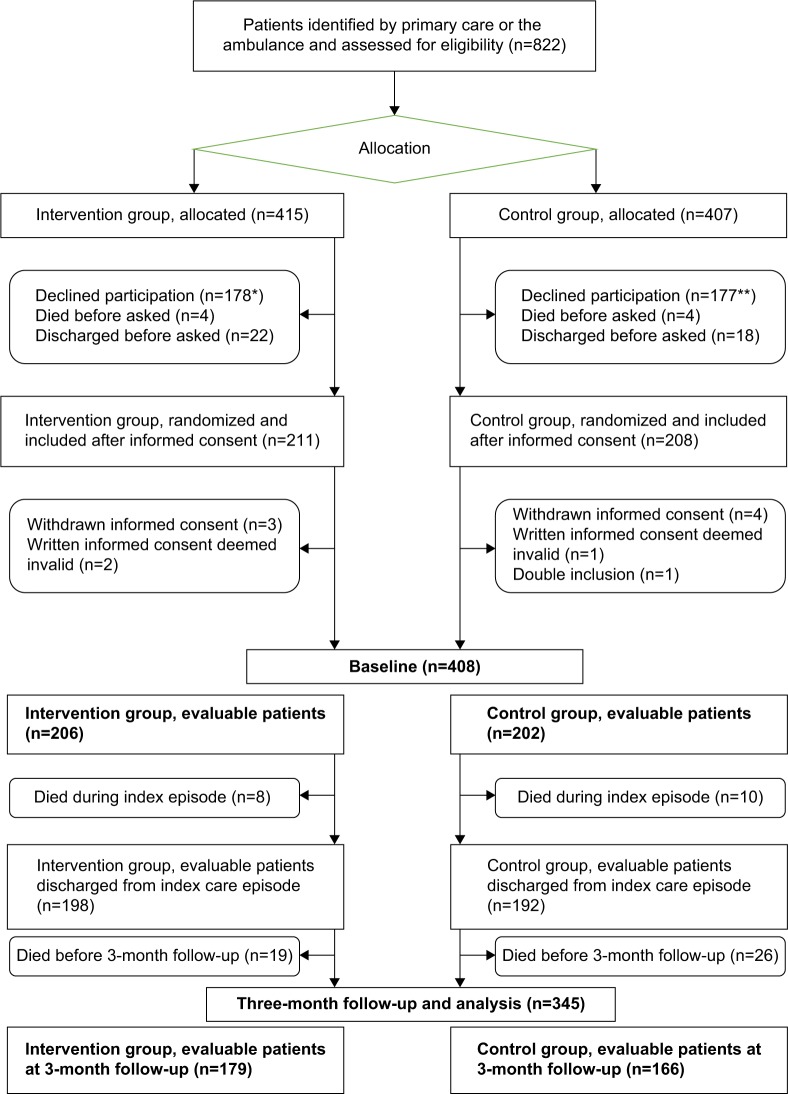
Between March 2013 and July 2015, 822 eligible patients were identified by primary care or the ambulance. Of these, 408 evaluable patients were included. The mean age was 85.7 years, and 56% were female. A total of 63 patients (15.4%) died during the 3-month follow-up study period (Figure 1).
Figure 1.
Flowchart of participant selection and assessment.
Notes: *Including 10 patients unable to give informed consent. **Including six patients unable to give informed consent.
Baseline characteristics are presented in Table 2. The two groups did not differ significantly in terms of age, gender, percentage living alone, and scores of frailty. Patients in both groups were heavily affected by diseases, particularly cardiovascular disease. The participants in the intervention group presented with a significantly higher burden of comorbidity compared with the control group, ie, Charlson Comorbidity Index score was 7.4 versus 6.2 (P<0.001). When it came to the percentage of participants living without home-help services, there was a trend toward a significant difference (intervention group, n=60 [29%]; control group, n=78 [38%]; P=0.054).
Table 2.
Baseline characteristics of the intervention and control groups
| Characteristics | Intervention group (CGA unit) | Control group (conventional care) | P-value |
|---|---|---|---|
| n | 206 | 202 | |
| Age (years), mean (SD) | 85.7 (5.3) | 85.6 (5.4) | 0.850 |
| Gender, female, n (%) | 122 (59) | 108 (53) | 0.241 |
| Living alone, n (%) | 139 (67) | 132 (65) | 0.649 |
| Own living without home-help services, n (%) | 60 (29) | 77 (38) | 0.055 |
| Frailty screening score, mean (SD) | 3.5 (0.9) | 3.4 (0.9) | 0.149 |
| Charlson Comorbidity Index score, mean (SD) | 7.4 (2.1) | 6.2 (1.5) | <0.001 |
| Diabetes mellitus, n (%) | 35 (17) | 37 (18) | 0.725 |
| Ischemic heart disease, n (%) | 57 (28) | 67 (33) | 0.227 |
| Chronic heart failure, n (%) | 90 (44) | 74 (37) | 0.146 |
| Peripheral arterial disease, n (%) | 18 (9) | 8 (4) | 0.048 |
| Dementia, n (%) | 20 (10) | 27 (13) | 0.247 |
| Malignant disease, n (%) | 40 (19) | 27 (13) | 0.099 |
| Malignant disease with metastases, n (%) | 8 (4) | 2 (1) | 0.059 |
| Chronic obstructive pulmonary disease, n (%) | 37 (18) | 40 (20) | 0.635 |
| Renal impairment,* n (%) | 193 (94) | 163 (81) | <0.001 |
| Anemia, n (%) | 104 (50) | 108 (53) | 0.547 |
| Reported reasons for admission, n (%) | |||
| Dyspnea | 67 (32) | 65 (32) | |
| Worsened general condition/tiredness | 48 (23) | 43 (21) | |
| Pain | 29 (14) | 24 (12) | |
| Fever/infection | 28 (14) | 40 (20) | |
| Vertigo/falling | 27 (13) | 30 (15) | |
| Others | 52 (25) | 35 (17) |
Notes:
Defined as glomerular filtration rate <90. In both groups, the five most frequently reported reasons for admission were dyspnea, worsened general condition/tiredness, pain, fever/infection, and vertigo/falling. For some of the patients, more than one reason for admission was reported. No statistical comparisons were performed.
Abbreviation: CGA, comprehensive geriatric assessment.
Main results
For each HUI-3 dimension, the average self-rated single-attribute utility scores at index and follow-up are reported (Table 3). By unadjusted analysis, a significantly lower proportion of patients in the intervention group than in the control group presented with decline in HRQoL from baseline in the following dimensions: vision, ambulation, dexterity, emotions, cognition, and pain (all P<0.05). For hearing and speech, we found no significant difference between the groups.
Table 3.
Unadjusted outcomes
| Outcomes | Index
|
Follow-up (3 months)
|
||||
|---|---|---|---|---|---|---|
| Intervention group | Control group | P-value | Intervention group | Control group | P-value | |
| Mortality, n (%) | 8 (4%) | 10 (5%) | 0.600 | 27 (13%) | 36 (18%) | 0.188 |
| No of hospital days, mean | 11.2 | 9.2 | 0.002 | 16.2 | 16.9 | 0.648 |
| Rehospitalizations, n (%) | 73 (37%) | 88 (46%) | 0.072 | |||
| Hospital care costs (SEK), mean | 55,215 | 48,927 | 0.097 | 83,989 | 96,315 | 0.131 |
| EQ-VAS score, mean | 51.1 (n=173) | 48.9 (n=177) | 0.298 | 56.8 (n=156) | 51.2 (n=133) | 0.003 |
| HUI-3 dimensions, mean | ||||||
| Vision | 0.886 (n=167) | 0.884 (n=170) | 0.937 | 0.873 (n=146) | 0.862 (n=127) | 0.664 |
| Hearing | 0.815 (n=143) | 0.881 (n=137) | 0.013 | 0.818 (n=133) | 0.817 (n=91) | 0.976 |
| Speech | 0.999 (n=170) | 0.975 (n=177) | 0.003 | 0.995 (n=154) | 0.985 (n=131) | 0.036 |
| Ambulation | 0.540 (n=170) | 0.569 (n=173) | 0.388 | 0.584 (n=153) | 0.458 (n=130) | 0.001 |
| Dexterity | 0.871 (n=171) | 0.882 (n=175) | 0.692 | 0.856 (n=152) | 0.804 (n=133) | 0.122 |
| Emotion | 0.823 (n=160) | 0.865 (n=157) | 0.077 | 0.896 (n=149) | 0.896 (n=128) | 0.963 |
| Cognition | 0.896 (n=171) | 0.877 (n=175) | 0.363 | 0.933 (n=155) | 0.834 (n=132) | <0.001 |
| Pain | 0.621 (n=171) | 0.631 (n=175) | 0.830 | 0.766 (n=156) | 0.594 (n=133) | <0.001 |
Notes: Intervention group denotes care in a CGA unit; control group denotes conventional care. For each HUI-3 dimension, the average self-rated single-attribute utility scores at index and 3-month follow-up are reported. Similarly, the average self-rated EQ-VAS scores at index and follow-up are reported. At follow-up, the total mortality, number of hospital days and in-hospital care costs are reported. The analyses excluded missing observations for the hearing dimension. P-values were calculated from means by Student’s t-test, except for mortality and rehospitalizations, for which χ2-test was used. For rehospitalizations, percentages were calculated by the use of 198 (intervention group) and 192 (control group) as denominators.
Abbreviations: CGA, comprehensive geriatric assessment; EQ, EuroQoL; HUI-3, Health Utilities Index-3; VAS, Visual Analog Scale; SEK, Swedish Crowns.
The two groups did not differ significantly in terms of unadjusted mortality, numbers of in-hospital days and costs of in-hospital and outpatient health care and nursing (all P>0.05).
There was no difference with regard to rehospitalizations within 3 months, but, at 1 month, rehospitalizations were more frequent in the control group (56 [28%]) than in the intervention group (40 [19%]; P=0.048).
After adjustment by regression analysis, patients in the intervention group were less likely to present with decline in HUI single scores at 3 months after discharge for the following dimensions: vision (odds ratio [OR] =0.33, 95% confidence interval [CI] =0.14–0.79), ambulation (OR =0.19, 95% CI =0.1–0.37), dexterity (OR =0.38, 95% CI =0.19–0.75), emotion (OR =0.43, 95% CI =0.22–0.84), cognition (OR =0.076, 95% CI =0.033–0.18), and pain (OR =0.28, 95% CI =0.15–0.50). For hearing (OR =0.50, 95% CI =0.22–1.1) and speech (OR =0.45, 95% CI =0.11–1.9), we found no significant difference between the groups (Table 4).
Table 4.
Reduction in HRQoL (HUI dimensions)
| Variables | Vision
|
Hearing
|
Speech
|
Ambulation
|
Dexterity
|
Emotion
|
Cognition
|
Pain
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | P-value | OR | P-value | OR | P-value | OR | P-value | OR | P-value | OR | P-value | OR | P-value | OR | P-value | |
| Clinic | ||||||||||||||||
| CGA unit | 0.33 | 0.013 | 0.45 | 0.057 | 0.45 | 0.274 | 0.20 | <0.001 | 0.39 | 0.007 | 0.43 | 0.014 | 0.08 | <0.001 | 0.27 | <0.001 |
| Conventional care | REF | REF | REF | REF | REF | REF | REF | REF | ||||||||
| Gender | ||||||||||||||||
| Female | 1.21 | 0.655 | 1.23 | 0.620 | 0.17 | 0.035 | 0.80 | 0.467 | 1.84 | 0.087 | 1.43 | 0.307 | 1.79 | 0.095 | 0.62 | 0.102 |
| Male | REF | REF | REF | REF | REF | REF | REF | REF | ||||||||
| Age | 1.08 | 0.052 | 1.05 | 0.161 | 0.94 | 0.343 | 1.07 | 0.019 | 0.98 | 0.553 | 1.02 | 0.598 | 1.01 | 0.772 | 0.99 | 0.572 |
| Charlson Comorbidity | 1.17 | 0.213 | 1.12 | 0.324 | 0.86 | 0.514 | 1.04 | 0.700 | 1.05 | 0.647 | 1.12 | 0.271 | 1.12 | 0.343 | 1.06 | 0.539 |
| Index score | ||||||||||||||||
Notes: Comparison of the proportions of the patients in each group presenting with a reduction in terms of HUI-3 dimensions from baseline to 3-month follow-up, adjusted analyses. Patients who died before follow-up were excluded from the analysis. Gender, age and Charlson Comorbidity Index score are control variables. The independent variables were tested for collinearity with the use of the variance inflation factor. All variables had a variance inflation factor value of <2.5, which does not indicate collinearity.
Abbreviations: CGA, comprehensive geriatric assessment; HRQoL, health-related quality of life; HUI-3, Health Utilities Index-3; OR, odds ratio; REF, reference.
Treatment in the CGA unit was also independently associated with lower mortality than conventional care adjusted for age, gender, and Charlson Comorbidity Index score by Cox regression analysis (hazard ratio [HR] =0.55, 95% CI =0.32–0.96).
The total number of in-hospital days including the index care episode until the follow-up was found to be significantly higher in the control group after adjustment by multiple regression analysis (P=0.0023).
Discussion
The results show that acute care of frail elderly patients in a CGA unit is superior to the care in a conventional acute medical care unit when it comes to several clinical outcomes at 3 months of follow-up. Patients in the CGA unit were less likely to present with decline in HRQoL, and the acute care in a CGA unit was also independently associated with lower mortality and fewer rehospitalizations. There was no significant difference between the study groups regarding the cost of hospital care.
We studied very frail elderly patients with acute illness and a high total morbidity burden. Former studies focusing on the CGA concept have not included such old patients with such a high morbidity burden and poor prognosis. The study adds knowledge relating to a potentially appropriate acute care organization that meets the needs of very frail elderly patients. We have used well-established instruments to evaluate the effects of the intervention in multiple dimensions. This study was integrated in the standard daily clinical context and included a wide spectrum of diagnoses, which enhances the generalizability of the study results.
It might be possible to argue that elderly patients so severely affected by frailty and multi-morbidity as the study patients have a very poor prognosis regardless of the treatment offered. However, patients with similar characteristics generate a large part of everyday hospital care consumption in most western countries, and there is no foreseeable trend in the opposite direction. There is, therefore, a particular need to build evidence relating to the treatment and care of this important patient group.
In the current study, patients treated in a CGA unit were less likely to present with decline in HRQoL dimensions of vision, speech, ambulation, dexterity, cognition, and pain at the 3-month follow-up compared with patients treated in a conventional acute care unit. As far as we know, this is the first study to demonstrate such an advantage in terms of HRQoL from a CGA unit for frail elderly patients. Participants who died before follow-up were excluded. Since a covariation between mortality and low HUI scores can be assumed, the group with higher mortality rate, ie, the control group, is likely to gain from the chosen analysis strategy, which thus favors the control group.
Acute care of frail elderly patients in a CGA unit was associated with a significantly lower 3-month mortality rate than that in a conventional acute medical care unit. In a recent study, caring for elderly patients in an ambulatory CGA unit was associated with lower 36-month mortality compared with conventional care units.13 In this study, the patients were older, in need of acute care, presented with more comorbidities and generally had a poorer prognosis. The findings in these two studies strengthen a suggested association between CGA and lower mortality.
When it comes to hospital care consumption and costs during the first 3 months, there was no significant difference between the study groups. A few previous studies have indicated that care in a CGA unit could be associated with a potential cost reduction,15,18 but some trials have reported results in the opposite direction.28,29
The numbers of in-hospital days during the index care episode were significantly higher in the intervention group. However, the numbers of early rehospitalizations were lower in the CGA-treated group. Consequently, by the 3-month follow-up, there was no difference regarding the total numbers of bed days between the two groups. This explains the lack of difference regarding the total cost of hospital care. It can be hypothesized that the longer index care episode for patients in the intervention group made it possible to optimize the medical treatment, to inform the patients in more detail and to perform a more extensive care planning in cooperation with other caregivers.
There is growing evidence in favor of CGA units for frail elderly patients. The CGA and related care can be considered as a complex intervention. Consequently, there may be several critical differences compared with conventional care, which may interact and benefit frail elderly patients. However, the early rehabilitation perspective including assessment and care should be regarded as crucial.
More research is needed to identify appropriate organizational forms adapted to the different stages of needs the frail elderly patients can manifest, ie, stable chronic disease and acute illnesses. This further research should include evaluations of activities in primary and municipal care, ambulant geriatric care units, and specialized hospital care.
The MÄVA form of care can serve as one example of how acute care of frail elderly people can be organized. This example could be implemented in everyday hospital health care in Sweden and other countries. Frail elderly patients would thereby be offered a health care structure, which is more compatible with their needs and provided through cooperation between several professions and care providers.
The study has some limitations. The fact that the assessments of the patients was not carried out in an examiner-blinded fashion can be regarded as a weakness. However, for practical reasons, it would have been difficult to mask the group to which the patient had been randomized, and few of the outcomes are likely to have been influenced.
There were some practical difficulties in the randomization procedure in this study. Randomization using a lottery was carefully considered, but not deemed feasible with regard to the recurrent shortage of MÄVA beds. A lottery procedure would also have meant that, in practice, several patients would have been allocated to a unit that did not belong to the group selected by the lottery. It was also important to include patients who are representative of the frail elderly population in everyday health care; a significant proportion of them are cognitively impaired, especially in the acute stage of the illness. For these reasons, randomizing representative patients through a lottery, after obtaining informed consent in the ambulance, was considered extremely difficult to implement. Blinding of patients or staff was not possible, as two hospital care forms were evaluated. The randomization was confirmed to a satisfactory extent, as most of the baseline characteristics did not differ between the groups. In cases where any differences were identified, the intervention group patients were slightly more ill, eg, presenting with a higher Charlson Comorbidity Index score with no detectable bias in favor of the intervention group.
Conclusion
The results of the study indicate that well-structured team-based acute care of frail elderly patients in a CGA unit is superior to the care in a conventional acute medical care unit in terms of clinical outcomes after 3 months. Patients in a CGA unit were less likely to present with decline in HRQoL after 3 months, and the care in a CGA unit was also independently associated with lower mortality and fewer rehospitalizations. We identified no significant difference between the study groups regarding the cost of hospital care.
Acknowledgments
This study was funded by grants from the Health Care Subcommittee, Region Västra Götaland; Department of Research and Development, NU Hospital Group and the Fyrbodal Research and Development Council, Region Västra Götaland, Sweden.
Footnotes
Author contributions
NE, JA, SL, and SDI contributed to the overall conception and design of the study. NE wrote the first draft of this manuscript. BWK and NE recruited the patients. BWK, NE, JA, MH, and EH acquired and managed the data. DA and MH did the statistical analyses. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm. Issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62:731–737. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 3.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation. 2011;124(22):2397–2404. doi: 10.1161/CIRCULATIONAHA.111.025452. [DOI] [PubMed] [Google Scholar]
- 5.Shamliyan T, Talley KM, Ramakrishnan R, Kane RL. Association of frailty with survival: a systematic literature review. Ageing Res Rev. 2012;12(2):719–736. doi: 10.1016/j.arr.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Buta BJ, Walston JD, Godino JG, et al. Frailty assessment instruments: systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev. 2016;26:53–61. doi: 10.1016/j.arr.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487–1492. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 8.Afilalo J, Karunananthan S, Eisenberg MJ, et al. Role of frailty in patients with cardiovascular disease. Am J Cardiol. 2009;103:1616–1621. doi: 10.1016/j.amjcard.2009.01.375. [DOI] [PubMed] [Google Scholar]
- 9.de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JS, Olde Rikkert MG, Nijhuis-van der Sanden MW. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10(1):104–114. doi: 10.1016/j.arr.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27(1):17–26. doi: 10.1016/j.cger.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63(8):747–762. doi: 10.1016/j.jacc.2013.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eklund K, Wilhelmson K, Gustafsson H, et al. One-year outcome of frailty indicators and activities of daily living following the randomised controlled trial; “Continuum of care for frail older people”. BMC Geriatr. 2013;13:76. doi: 10.1186/1471-2318-13-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ekdahl AW, Wirehn AB, Alwin J, et al. Costs and effects of an ambulatory geriatric unit (the AGe-FIT study): a randomized controlled trial. J Am Med Dir Assoc. 2015;16(6):497–503. doi: 10.1016/j.jamda.2015.01.074. [DOI] [PubMed] [Google Scholar]
- 14.Prestmo A, Hagen G, Sletvold O, et al. Comprehensive geriatric care for patients with hip fractures: a prospective, randomised, controlled trial. Lancet. 2015;385(9978):1623–1633. doi: 10.1016/S0140-6736(14)62409-0. [DOI] [PubMed] [Google Scholar]
- 15.Ellis G, Whitehead MA, O’Neill D, Langhorne P, Robinson D. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2011;7:CD006211. doi: 10.1002/14651858.CD006211.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.SBU . Comprehensive Geriatric Assessment and Care of Frail Elderly. Stockholm: Swedish Council on Health Technology Assessment (SBU); 2014. 2013. [Accessed May 7, 2016]. (SBU report no 221). (in Swedish). Available from: http://www.sbu.se/upload/Publikationer/Content0/1/Akutvard_aldre.pdf. [Google Scholar]
- 17.Baztán JJ, Suárez-García FM, López-Arrieta J, et al. Effectiveness of geriatric units on functional decline, living at home, and case fatality among older patients admitted to hospital for acute medical disorders: meta-analysis. BMJ. 2009;338:b50. doi: 10.1136/bmj.b50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flood KL, Maclennan PA, McGrew D, Green D, Dodd C, Brown CJ. Effects of an acute care for elders unit on costs and 30-day readmissions. JAMA Intern Med. 2013;173(11):981–987. doi: 10.1001/jamainternmed.2013.524. [DOI] [PubMed] [Google Scholar]
- 19.Johansson M, Johansson P. Multisjuka äldre med upprepade vårdtillfällen bör läggas in direkt [Elderly patients with multimorbidity and re-hospitalizations should be directly admitted] Läkartidningen. 2012;109:1022–1023. Swedish. [PubMed] [Google Scholar]
- 20.Wilhelmson K, Duner A, Eklund K, et al. Design of a randomized controlled study of a multi-professional and multidimensional intervention targeting frail elderly people. BMC Geriatr. 2011;11:24. doi: 10.1186/1471-2318-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eklund K, Wilhelmson K, Landahl S, Dahlin Ivanoff S. Screening for frailty among older emergency department visitors: validation of the new FRESH-screening instrument. BMC Emerg Med. 2016;16(1):27. doi: 10.1186/s12873-016-0087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horsman J, Furlong W, Feeny D, et al. The Health Utilities Index (HUI (R): concepts, measurement properties and applications. Health Qual Life Outcomes. 2003;1:54. doi: 10.1186/1477-7525-1-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.EuroQol Group EuroQol-a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.Frenkel WJ, Jongerius EJ, Mandjes-van Uitert MJ, van Munster BC, de Rooij SE. Validation of the Charlson Comorbidity Index in acutely hospitalized elderly adults: a prospective cohort study. J Am Geriatr Soc. 2014;62(2):342–346. doi: 10.1111/jgs.12635. [DOI] [PubMed] [Google Scholar]
- 26.Swedish Association of Local Authorities and Regions (SALAR) National Cost per Patient-Principles. Version 3. Cost per Patient. Stockholm: Swedish Association of Local Authorities and Regions (SALAR); 2015. In Swedish. [Google Scholar]
- 27.Persson J, Husberg M. Can we rely on QALYs for assistive technologies? Technol Disability. 2012;24:93–100. [Google Scholar]
- 28.Hogan DB, Fox RA, Badley BW, Mann OE. Effect of a geriatric consultation service on management of patients in an acute care hospital. Can Med Assoc J. 1987;136(7):713–717. [PMC free article] [PubMed] [Google Scholar]
- 29.Applegate WB, Miller ST, Graney MJ, Elam JT, Burns R, Akins DE. A randomized, controlled trial of a geriatric assessment unit in a community rehabilitation hospital. N Engl J Med. 1990;322(22):1572–1578. doi: 10.1056/NEJM199005313222205. [DOI] [PubMed] [Google Scholar]