Abstract
Background
Percutaneous nephrolithotomy (PNL) is the standard procedure for patients with renal stones over 2 cm in diameter. We analyzed complications after this procedure focusing on two different methods of tract dilation.
Material/Methods
Between August 2008 and April 2016 222 percutaneous nephrolithotomies were performed in a total of 208 patients. The Group I (n=123) comprised patients where Alken dilatators were used, while Group II (n=99) comprised patients where Amplatz dilators were used. Efficacy was examined based on ultrasound and x-ray examination one month after the procedure. Complications were recorded using Clavien Dindo classification.
Results
Efficacy was 85.3% and 86.8% in group I and II, respectively (p=0.77). Grade I complications were present in 14.6% and 3%, grade II were present in 9.7% and 8%, grade IIIa were present in 2.4% and 2%, grade IIIb were present in 1.6% and 2%, grade IVa were present in 1.6% and 7%, grade IVb were present in 3.2% and 1% in Group I and Group II, respectively. These differences were statistically significant (p=0.03).
Conclusions
Efficacy was comparable between Alken dilator and Amplatz dilator groups. In group I, there were more postoperative fevers >38.5 °C and a higher rate of urosepsis. On the other hand, in group II we observed more pleural injuries. All differences resulted from the type of access to the kidney (inter/infracostal), punctured calyx, and utilization (or not) of access sheath rather than type of dilators itself.
MeSH Keywords: Nephrostomy, Percutaneous; Postoperative Complications; Urolithiasis
Background
Percutaneous nephrolithotomy (PNL) is the gold standard for treating patients with kidney stones over 2 cm in diameter [1]. It has replaced open surgeries due to equal efficacy, less morbidity, and higher patient acceptance [2,3]. However, the most common complications are still urine extravasation, infection, and bleeding [4,5]. The classic procedure consists of four major steps: insertion of a ureteral catheter, execution of percutaneous access to the kidney with tract dilation, lithotripsy and stone extraction, and finally protection of the tract with a nephrostomy tube. Tract dilation may be conducted with the aid of metallic Alken dilators, plastic Amplatz dilators, or a pneumatic dilator. One-stage tract dilation is also a well-known technique; however, it is not utilized in our department. It has been previously suggested that blood loss during renal dilation with Amplatz instruments is higher. It has also been suggested that with sequential application of fascial dilators, the hemostatic pressure on the kidney parenchyma is lost, resulting in intermittent bleeding from renal vessels. On the other hand, telescopic Alken dilators exert pressure continuously, hence blood loss may be lower [6]. The rate of other complications seems to be comparable. As there are various methods of tract performance (inter/infracostal approach, various calyx punctures, utilization of access sheath, etc.) we hypothesized that it may not be the dilator type itself but rather the way they are utilized that may influence the type of complications. The aim of our study was to compare these two methods of tract dilation in terms of efficacy and safety, and to find out what may be the source of differences in complication rates.
Material and Methods
Between August 2008 and April 2016, PNL was performed in 283 patients in our department. Complete data were available for 208 patients where 222 PNL procedures were conducted. Patients with a solitary kidney were excluded from the analysis. Two methods of tract dilation were utilized: group I (n=123) comprised patients where Alken dilatator was used; group II (n=99) comprised patients where the Amplatz dilator was used. Both procedures were conducted by two experienced endourologists (one used the Alken dilator and the other used the Amplatz dilator). We retrospectively assessed the efficacy and safety of PNL with both methods. Efficacy was assessed based on ultrasound and x-ray examination one month after the procedures. The presence of residual stone <3 mm in diameter was considered insignificant and patients were classified as stone free. The Clavien-Dindo classification was used to compare complications between groups. Urosepsis was diagnosed if there was positive urine and blood cultures and two out of the following four: temperature >38.5°C or <36°C, white blood cells >12,000 cells/mm3 or <4,000 cells/mm3, respiratory rate >20 breaths per minute or PaCO2 <32 mm Hg, and pulse over 90 beats per minute [7]. Hemoglobin drop and number of transfusions were assessed as additional safety parameters. Other variables were also analyzed including: operating room time, scope time, and hospitalization time. Visual analogue scale of pain was assessed the day after surgery. Failed tract dilations during procedure and tract formation time were also assessed.
Compliance with ethical standard
This study was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki.
Description of procedure
In a supine position, a 6-Fr ureteral catheter was inserted into the appropriate kidney. Pyelography was performed to assess the anatomy of the pyelocalyceal system. The patient was rotated on a table to the prone position. At an angle of 30°, the needle was percutaneously inserted into the most convenient posterior calyx. A hydrophilic guidewire was inserted into the needle and placed in the ureter. Over the wire, the 8-Fr dilator was introduced. The hydrophilic guidewire was exchanged for the stiff Amplatz wire. Subsequently, the Amplatz dilator was introduced to widen the tract up to 28-Fr. The access sheath was positioned within the pyelocalyceal system over the 28-Fr Amplatz dilator. The Amplatz wire was left in the sheath and served as a safety wire. Nephroscope 26-Fr was utilized to perform the stone disintegration with pneumatic or ultrasonic lithotripter. After the lithotripsy, re-entry Malecot catheter was inserted over the Amplatz wire within the kidney and the ureteral catheter was withdrawn. Our Amplatz dilator technique was a standard technique, well-described in the literature where the final stages may be replaced with a tubeless option [2,8,9]. The procedure used for the Alken dilator was similar, up to the point of introduction of the Amplatz wire. Instead of a straight Amplatz wire, a stiff wire with a coiled end was inserted into the pyelocalyceal system. A lumbotomy was used to cut the muscles within the tract to the kidney. Subsequently, the metallic dilator was introduced over the wire to widen the tract up to 26-Fr. The access sheath and safety wire were not used. At the end of the lithotripsy, a drain was left in the pyelocalyceal system and the ureteral catheter was left in place. After the procedure, each patient received 1 g paracetamol intravenously every six hours. In addition, tramadol 100 mg intramuscularly was given on demand. Antibiotic prophylaxis consisted of cephalosporin (second generation) given during induction of general anesthesia.
Statistics
Student t-test for continuous variables with normal distribution was used. For continuous variables without normal distribution, the Mann-Whitney U test was used. For categorical variables, the chi-square test was used. To show dependence between categorical variables we used correspondence analysis. Power analysis was conducted with the assistance of G*Power statistical tool [10,11]. A p value <5% was considered significant. All analyses were conducted using Statistica Statsoft®.
Results
Patients in both groups did not differ in terms of age, sex, BMI, or stone size and affected side (Table 1). All procedures were performed between the years 2008 and 2016. We did not find any differences in total operative time, tract formation time, or fluoroscopy time. Tract dilation failures, although more frequent in group I, were not significantly different between the groups. The hemoglobin drop and transfusion rates were comparable between groups. Most of the procedures in group I were performed with infracostal access while in group II more procedures were conducted with intercostal access. The number of accesses did not differ between groups. In the Amplatz group (group II) most accesses were through middle calyx while in group I (Alken group) most were through the lower calyx. We found significant differences in postoperative complications as shown in Table 2 and Figure 1. Most grade I (postoperative fever over 38.5°C) complications were more common in group I. Similarly grade IVb complications (urosepsis) were more frequently observed in group I. Pleural injury (grade IVa) were more common in group II. Other complications (II – blood transfusions, IIIa – extravasation, IIIb – arterio-venous fistula) and non-complicated course of the disease were similarly distributed between groups. We did not observe grade V (death) complications in our cohort. Power analysis of chi square test for complication rates was 77%.
Table 1.
Preoperative characteristics of analyzed group of patients.
| Group I (n=123) | Group II (n=99) | P value | ||
|---|---|---|---|---|
| Age, years (SD) | 46 (15) | 43 (17) | 0.77 | |
| Sex, no (%) | Males | 70 (56.9) | 57 (57.5) | 0.92 |
| Females | 53 (43%) | 42 (42.4) | ||
| BMI, kg/m2 (SD) | 23.3 (6.3) | 24.8 (5.9) | 0.55 | |
| Stone size, cm (SD)* | 2.5 (0.7) | 2.4 (0.5) | 0.59 | |
| Side, no (%) | Left | 68 (55.2) | 54 (54.5) | 0.9 |
| Right | 55 (44.7) | 45 (45.4) | ||
In case of multiple stones combined diameter is given.
Table 2.
Intraoperative and postoperative characteristics of analyzed group of patients.
| Group I (n=123) | Group II (n=99) | P value | ||
|---|---|---|---|---|
| Total operative time, min (SD) | 114 (46) | 103 (48) | 0.74 | |
| Tract formation time, min (SD) | 6.5 (3.2) | 8.3 (4.1) | 0.58 | |
| Fluoroscopy time, min (SD) | 7.5 (4.2) | 9.4 (3.3) | 0.49 | |
| Tract dilation failure, no (%) | Yes | 12 (9.7) | 4 (4) | 0.1 |
| No | 111 (90.2) | 95 (95.9) | ||
| Residual stones, no (%) | Yes | 17 (13.8) | 15 (15.1) | 0.77 |
| No | 106 (86.1) | 84 (84.8) | ||
| Hemoglobin drop, g/dl (SD) | 2.1 (0.9) | 2.5 (1.3) | 0.48 | |
| Blood transfusion, no (%) | Yes | 4 (3.2) | 5 (5) | 0.49 |
| No | 119 (96.7) | 94 (94.9) | ||
| Punctured calyx | Upper | 6 (4.8) | 36 (36.3) | <0.001 |
| Middle | 25 (30.8) | 56 (56.5) | ||
| Lower | 92 (74.8) | 7 (7.1) | ||
| Access to kidney, no (%) | Intercostal | 40 (32.5) | 91 (91.9) | <0.001 |
| Infracostal | 83 (67.4) | 8 (8) | ||
| Number of accesses, no (%) | One | 109 (88.6) | 82 (82.8) | 0.21 |
| More than one | 14 (11.3) | 17 (17.1) | ||
| Clavien-Dindo complication scale, no (%) | No | 82 (66.6) | 76 (76.8) | 0.03 |
| I | 18 (14.6) | 3 (3) | ||
| II | 12 (9.7) | 8 (8) | ||
| IIIa | 3 (2.4) | 2 (2) | ||
| IIIb | 2 (1.6) | 2 (2) | ||
| IVa | 2 (1.6) | 7 (7) | ||
| IVb | 4 (3.2) | 1 (1) | ||
| Visual analogue scale, median* | 3.5 | 3.2 | 0.31 | |
Assessed one day after procedure.
Figure 1.
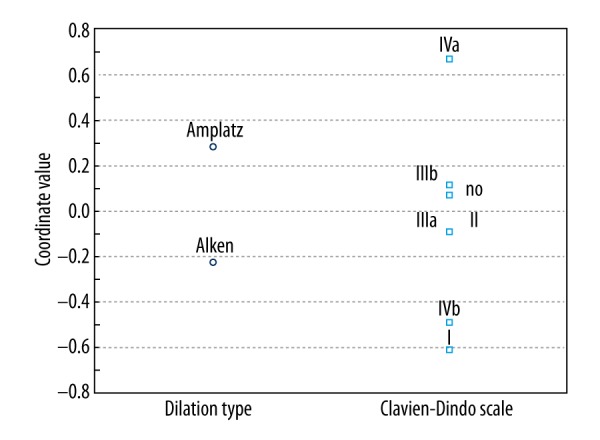
One dimensional plot of correspondence analysis. Position of squares and circles represent the inertia of variables. The higher coordinate value for Clavien-Dindo grade, the higher likelihood it occurred after Amplatz approach. The lower coordinate value for Clavien-Dindo grade, the higher likelihood it occurred after Alken method.
Discussion
PNL is an efficacious procedure with a more than 80% stone-free rate independent of type of access [8,12]. It has replaced open surgeries due to the same efficacy, less morbidity, and higher patient acceptance. Still, one of the most common complications after PNL is infection, sepsis, and bleeding resulting in hemoglobin drop and occasionally the need for blood transfusion. One of the factors contributing to blood loss is tract formation. As stated earlier, the Alken dilators are allegedly safer than the Amplatz dilators due to maintenance of continuous hemostatic pressure on the kidney parenchyma. In our study, both types of tract dilation were comparable in terms of blood loss. Similarly, blood transfusions were not different between groups, supporting the previous reports that both types of tract dilation are equally safe [13]. Other parameters such as total operative time, fluoroscopy time, and tract formation time were not different between the two groups. Similarly, the tract dilation failure rate was comparable between the two groups, which is consistent with other study outcomes [14]. In our cohort, in the Amplatz group there was insignificantly fewer failures. In our experience this may be due to the fact that in the Alken group, the puncture was most often through the lower calyx and the wire was positioned in the renal pelvis. On the other hand, in the Amplatz group, the puncture was usually directed at the middle calyx and the wire was positioned within the ureter. Positioning the wire in the ureter makes it more stable and less prone to fall out of the kidney during tract dilation. Other factors may influence tract dilation failure (e.g., BMI, gender) due to kidney movement during the procedure [15], however, we did not observe differences in distribution of the aforementioned characteristics
As showed in Figure 1, postoperative fever and sepsis in our cohort were more common in the Alken group. Other complications, such as contrast extravasation, arteriovenous fistula or postoperative hematoma, were comparable between groups. We may only speculate why there were differences in rates of grade I and IVb between the two groups, as there was no simple explanation. We hypothesized that the cause lies in the fact that in the Alken group we did not use an access sheath. This may cause higher irrigant pressure inside the kidney during lithotripsy as most of the influent water departs through the ureter instead of the access sheath. A similar situation was described in micro-percutaneous nephrolithotomy, where there was no need for an access sheath and higher irrigant pressures were observed in comparison with classic PNL [16]. As some of the stones were infected, this, with the combination of high irrigant pressure, may cause more septic complications after PNL.
The Clavien-Dindo classification is usually used to evaluate major complications (≥3a) after PNL [17]. However, we showed that even postoperative fever (grade I) may vary between methods. This suggests the source of this difference may be important, and even minor complications should be reported after PNL.
As more procedures in the Amplatz group were conducted through the middle calyx it was not surprising that many of them were intercostal. However, this fact did not have an influence on blood loss, and supports reported evidence that inter/infracostals access is equally safe in this regard. On the other hand, intercostal access results in more frequent pleural injury (Figure 1) [18].
We did not observe any differences in postoperative pain. Both procedures were characterized by mild pain the day after the operation (Table 2).
The way in which PNL may be performed with Alken and Amplatz dilators differs among urological departments. Our approach is to use them as described in the methods section. Indeed our study did not compare two different dilators but rather two different methods of tract dilation where both have their pros and cons.
Based on our study, we cannot determine the superiority of one method of tract dilation over the other as these two approaches were completely different in terms of punctured calyx, inter/infracostal access, and utilization (or not) of a sheath. Therefore, direct comparison cannot be made. However, it is clear that irrespective of the dilator type used, the important differences result from other aforementioned factors.
Conclusions
Both treatment methods were comparable in terms of hemoglobin drop and blood transfusions postoperatively. Total operative time, tract dilation time, fluoroscopy time, and tract dilation failure were comparable between the two groups. Significant differences were observed in complication rates between the two groups. Fever over 38.5°C and urosepsis were more common in the Alken group. On the other hand, pleural injuries were more common after PNL with Amplatz dilators. It is clear that all differences resulted from the punctured calyx, type of access to the kidney (inter/infracostal), and utilization (or not) of an access sheath rather than the type of dilator itself. All three factors directly influenced the type of complications seen postoperatively.
Footnotes
Disclosure statement
Authors declare that they have nothing to disclose
Source of support: Departmental sources
References
- 1.Türk C, Petřík A, Sarica K, et al. EAU guidelines on interventional treatment for urolithiasis. Eur Urol. 2016;69:475–82. doi: 10.1016/j.eururo.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 2.Liatsikos EN, Hom D, Dinlenz CZ, et al. Tail stent versus re-entry tube: A randomized comparison after percutaneous stone extraction. Urology. 2002;59:15–19. doi: 10.1016/s0090-4295(01)01475-3. [DOI] [PubMed] [Google Scholar]
- 3.Basiri A, Ahmadnia H, Moghaddam SM. The efficacy of conventional PCNL and two modifications to standard procedure. J Pak Med Assoc. 2006;56:302–5. [PubMed] [Google Scholar]
- 4.Shah HN, Hegde S, Shah JN, et al. A prospective, randomized trial evaluating the safety and efficacy of fibrin sealant in tubeless percutaneous nephrolithotomy. J Urol. 2006;176:2488–92. doi: 10.1016/j.juro.2006.07.148. [DOI] [PubMed] [Google Scholar]
- 5.Noller MW, Baughman SM, Morey AF, et al. Fibrin sealant enables tubeless percutaneous stone surgery. J Urol. 2004;172:166–69. doi: 10.1097/01.ju.0000129211.71193.28. [DOI] [PubMed] [Google Scholar]
- 6.Yuhico MP, Ko R. The current status of percutaneous nephrolithotomy in the management of kidney stones. Minerva Urol Nefrol. 2008;60:159–75. [PubMed] [Google Scholar]
- 7.Naber KG, Bergman B, Bishop MC, et al. Urinary Tract Infection (UTI) Working Group of the Health Care Office (HCO) of the European Association of Urology (EAU). EAU guidelines for the management of urinary and male genital tract infections. Urinary Tract Infection (UTI) Working Group of the Health Care Office (HCO) of the European Association of Urology (EAU) Eur Urol. 2001;40:576–88. doi: 10.1159/000049840. [DOI] [PubMed] [Google Scholar]
- 8.Bryniarski P, Paradysz A, Zyczkowski M, et al. A randomized controlled study to analyze the safety and efficacy of percutaneous nephrolithotripsy and retrograde intrarenal surgery in the management of renal stones more than 2 cm in diameter. J Endourol. 2012;26:52–57. doi: 10.1089/end.2011.0235. [DOI] [PubMed] [Google Scholar]
- 9.Bryniarski P, Bogacki R, Muskała B, et al. Tubeless percutaneous nephrolithotomy. J Endourol Videourology. 2016 [Google Scholar]
- 10.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 11.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–60. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 12.Assimos D, Krambeck A, Miller NL, et al. Surgical management of stones: American Urological Association/Endourological Society Guideline, PART II. J Urol. 2016;196:1161–69. doi: 10.1016/j.juro.2016.05.091. [DOI] [PubMed] [Google Scholar]
- 13.Ramón de Fata F, Pérez D, Resel-Folkersma L, et al. Analysis of the factors affecting blood loss in percutaneous nephrolithotomy: A registry of the Spanish Association of Urology in the supine position. Actas Urol Esp. 2013;37:527–32. doi: 10.1016/j.acuro.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Ozok HU, Sagnak L, Senturk AB, et al. A comparison of metal telescopic dilators and Amplatz dilators for nephrostomy tract dilation in percutaneous nephrolithotomy. J Endourol. 2012;26:630–34. doi: 10.1089/end.2011.0291. [DOI] [PubMed] [Google Scholar]
- 15.Aminsharifi A, Haghpanah R, Haghpanah S. Predictors of excessive renal displacement during access in percutaneous nephrolithotomy: A randomized clinical trial. Urolithiasis. 2014;42:61–65. doi: 10.1007/s00240-013-0600-9. [DOI] [PubMed] [Google Scholar]
- 16.Tepeler A, Akman T, Silay MS, et al. Comparison of intrarenal pelvic pressure during micro-percutaneous nephrolithotomy and conventional percutaneous nephrolithotomy. Urolithiasis. 2014;42:275–79. doi: 10.1007/s00240-014-0646-3. [DOI] [PubMed] [Google Scholar]
- 17.Karakaş HB, Çiçekbilek İ, Tok A, et al. Comparison of intraoperative and postoperative complications based on ASA risks in patients who underwent percutaneous nephrolithotomy. Turk J Urol. 2016;42:162–67. doi: 10.5152/tud.2016.78545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oner S, Okumus MM, Demirbas M, et al. Factors influencing complications of percutaneous nephrolithotomy: A single-center study. Urol J. 2015;12:2317–23. [PubMed] [Google Scholar]


