Abstract
Background
Combretastatin A4 (CA4) is a potential therapeutic candidate for a variety of human cancer treatments. However, the inhibitive effects of CA4 on thyroid cancer cells are still not well-clarified. This study aimed to investigate the potential effect of CA4 on thyroid cancer cells, as well as underlying mechanism.
Material/Methods
Human thyroid papillary carcinoma cell line TPC1 was pre-treated with 5 concentrations of CA4 (0, 1, 2, 5, or 10 μM) for 2 h. Cell proliferation was determined by 3-(4, 5-dimethyl-2- thiazolyl)-2, 5-diphenyl -2-H-tetrazolium bromide (MTT) assay. Cell migration and invasion were detected by a modified Boyden chamber assay. Moreover, cell apoptosis was detected by terminal deoxynucleotidyl (TUNEL) staining assay and flow cytometry method. Western blot analysis was performed to determine the expression changes of epithelial-mesenchymal transition (EMT)-related proteins and phosphatidylinositol-3-kinase/serine/threonine kinase (PI3K/Akt) signaling pathway proteins.
Results
CA4 significantly inhibited the cell proliferation, migration, and invasion, and significantly promoted cell apoptosis in a dose-dependent manner compared with the control group. The EMT-related protein levels of N-Cadherin, Vimentin, Snail1, Slug, Twist1, and ZEB1 were significantly decreased by CA4, while E-cadherin had no significant difference compared with the control group. Moreover, PI3K/Akt signaling pathway protein levels of p-PI3K and p-Akt were significantly decreased, whereas PI3K and Akt had no significant differences compared with the control group.
Conclusions
CA4 can inhibit proliferation, migration, and invasion and promote apoptosis of TPC1 cells. These effects might be through the PI3K/Akt signaling pathway. CA4 may be a potential therapeutic target for the treatment of thyroid cancer.
MeSH Keywords: Antineoplastic Agents, Epithelial-Mesenchymal Transition, Signal Transduction, Thyroid Neoplasms
Background
Papillary thyroid carcinoma (PTC) is the most common malignant thyroid tumor, and accounts for ~80% of all thyroid cancers [1]. The incidence of PTC has almost quadrupled over the last 3 decades, from 3.4 to 12.5 per 100 000 people [2]. In many cases, patients with PTC treated with radio- and surgical therapy obtain a good prognosis [3,4]. However, ~50% patients with PTC are prone to metastasis to the regional lymph nodes, which always brings a poor prognosis and causes higher mortality [5,6]. Thus, it is necessary to develop and identify novel therapeutic strategies for treatment of PTC.
Combretastatin A4 (CA4), a natural product isolated from the South African tree Combretum caffrum, is a potential therapeutic candidate for cancer treatment [7,8]. CA4 as an antimitotic agent can strongly cause vascular shutdown and cell death in tumors by binding the colchicine binding site of tubulin to block microtubule assembly [9]. Over the past 2 decades, CA4 and CA4 derivatives have been discovered to confer cytotoxic potency and anti-proliferative activity in a variety of human cancer cells, such as non-small cell lung cancer [10], ovarian cancer [11], and breast cancer cells [12]. In addition, CA4 has demonstrated an anti-metastasis effect in human gastric cancer [13] and bladder cancer cells [14]. However, the potential effects of CA4 on thyroid cancer cells are still not well-clarified.
Therefore, in the present study, the roles of CA4 in the proliferation, migration, invasion, and apoptosis of thyroid cancer cells (TPC1) were investigated in vitro, aiming to reveal the potential effects of CA4 on thyroid cancer. Moreover, the expression of epithelial-mesenchymal transition (EMT)-related proteins (E-cadherin, N-Cadherin, Vimentin, Snail1, Slug, Twist1, and ZEB1) were evaluated to discover whether the process of EMT was involved. We also evaluated the expression of phosphatidylinositol-3-kinase/serine/threonine kinase (PI3K/Akt) signaling pathway proteins (PI3K, p-PI3K, Akt, p-Akt) to determine the associated molecular mechanism. These investigations may facilitate a better understanding of CA4 in thyroid cancer.
Material and Methods
Cell culture
Human thyroid papillary carcinoma cell line TPC1 was obtained from the American Type Culture Collection (ATCC, Manassas, VA). TPC1 cells were cultured in RPMI-1640 (Gibco, Grand Island, NY) medium containing 10% fetal bovine serum (FBS), 100 μg/mL penicillin, and 100 μg/mL streptomycin (Life Technologies, Cergy Pontoise, France), maintained at 37°C in a humidified atmosphere containing 5% CO2 [15].
Proliferation assay
Cell proliferation was assayed by the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Briefly, TPC1 cells were seeded in 96-well plates at a density of 3×103 cells/well, and pre-treated with 5 different concentrations of CA4 (0, 1, 2, 5, or 10 μM) for 2 h. The CA4 with 0 μM acted as a control group. Final concentration of 0.5 mg/mL MTT solution (Sigma Aldrich, St. Louis, MO) was added to each well and incubated for 4 h. After the incubation, formazan crystals were dissolved by addition of 150 μl dimethyl sulfoxide per well. The absorbance was measured at 570 nm using an automated ELISA plate reader (Thermo Scientific®, Rockford, IL) [16].
Migration and invasion assay
Cell migration assay was performed in a modified Boyden chamber (Costar-Corning, New York, USA), with an 8.0-μm pore polycarbonate filter insert in 24-well plates. Briefly, the lower chamber was filled with complete medium. Resuspended TPC1 cells (5×104 cells/well) were placed in the upper chamber, pre-treated with 5 concentrations of CA4 (0, 1, 2, 5, or 10 μM). The CA4 with 0 μM acted as a control group. The 24-well plates were incubated at 37°C for 24 h, after which the non-migrated cells on the upper side of the filter were wiped off and migrated cells were stained with crystal violet (Beyotime, Nantong, China) for 30 min. Stained cells were photographed under an inverted fluorescence microscope (Olympus IX51) equipped with an Olympus Qcolor 3 digital camera. Quantitative cell migration was assessed by counting the stained cells directly [17].
Cell invasion assay was performed as the cell migration assay, except that transwell inserts were matrigel-coated.
Apoptosis detection by TUNEL staining assay
Cell apoptosis was detected by a terminal deoxynucleotidyl (TUNEL) method by using a commercially available kit (ApopTag Peroxidase In Situ Apoptosis Detection Kit S7100; Chemicon International, Billerica, MA). Briefly, the cells were pre-treated with concentrations of CA4 (0, 1, 2, 5, or 10 μM) for 2 h. CA4 with 0 μM acted as a control group, then cells in each sample was washed and stained according to the manufacturer’s instructions. The labeled cells were washed and examined under a fluorescence microscope (Olympus, Germany). The number of the TUNEL-positive cells and TUNEL-negative cells were discriminated by directly counting the green florescent and blue nuclei stains, respectively.
Apoptosis detection by flow cytometry method
The effect of CA4 on TPC1 cell apoptosis was further examined using 2 fluorescent dyes: annexin V-Cy5 and PI. The cells were seeded at 2×105 per 35-mm culture dish. The cells were grown to about 80% confluence and treated with 5 concentrations of CA4 (0, 1, 2, 5, or 10 μM) for 2 h. CA4 with 0 μM acted as a control group. After treatment, the cells in each group were harvested by trypsinization, and then were pelleted and resuspended in annexin V-binding buffer (10 mM HEPES, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, pH 7.4) containing annexin V-Cy5 (1:1000) and 1 mg/mL PI. Each sample was incubated in the dark at room temperature for 5 min, and then analyzed on a FACS Calibur flow cytometer (Becton-Dickinson). The percentage of total apoptotic events was defined by summing the early and late apoptotic cells.
Western blot
The cells were pre-treated with 5 concentrations of CA4 (0, 1, 2, 5, or 10 μM). CA4 with 0 μM acted as a control group. After 2-h treatment, the cells in each sample were lysed with lysis buffer (150 mM NaCl, 50 mM Tris-HCl [pH 8.0], 0.1% SDS, and 1% Triton X-100) for 30 min. The protein concentration was determined by using a Bicinchoninic Acid (BCA) assay kit (Thermo Scientific®, Rockford, IL). Equivalent amounts of whole-cell extracts from each sample were resolved over sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. The membranes were blocked within the blocking buffer (5% non-fat milk) for 2 h at room temperature and then incubated in primary antibody (N-Cadherin, ZEB1, p-PI3K, PI3K, p-Akt, Akt, and GAPDH were purchased from Santa Cruz Biotechnology, Santa Cruz, CA; E-cadherin, Vimentin, Snail1, Slug, and Twist1 were obtained from Abcam, Cambridge, MA) at 4°C overnight. The membranes were then incubated with the secondary antibody horseradish peroxidase (HRP) conjugate for 1 h. Blots were visualized by enhanced chemiluminescence (ECL) method.
Statistical analysis
All data are expressed as means ± standard derivations (SD). The data were analyzed using the t test or one-way analysis of variance (ANOVA). Results with P value of <0.05 were considered statistically significant. GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA) was used for these analyses.
Results
Effects of CA4 on TPC1 cell proliferation
To determine the effects of CA4 on TPC1 cell proliferation, 5 concentrations of CA4 were added to the cell culture, and the cell viability was assessed by using MTT assay. We found that, except for the control group (0 μM), all concentrations of CA4 inhibited cell proliferation. The CA4 with high concentration seemed to possess stronger inhibitive effect on TPC1 cell proliferation. More importantly, CA4 with 5 and 10 μM significantly inhibited cell proliferation compared with the control group (P<0.05). These results reveal that CA4 can effectively inhibit cell proliferation in a dose-dependent manner (Figure 1).
Figure 1.
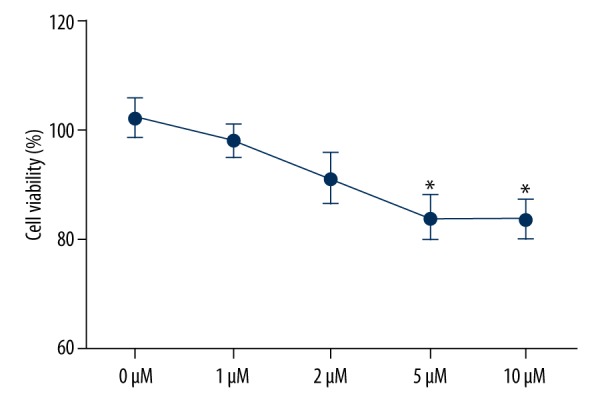
Inhibitive effects of Combretastatin A4 on TPC1 cell proliferation. * P<0.05 compared with control group.
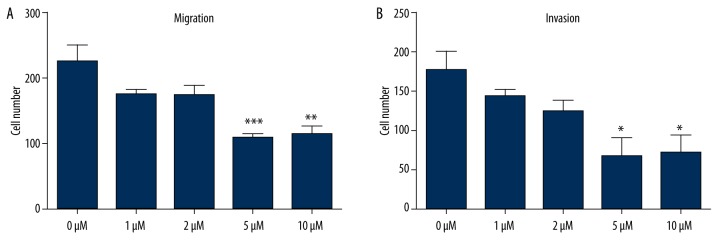
Effect of CA4 on TPC1 cells migration and invasion
To determine the effects of CA4 on TPC1 cell migration and invasion in vitro, 5 concentrations of CA4 were pre-treated with the cells for 2 h. Then the migration and invasion of each sample were assayed. As shown in Figure 2A and 2B, except for the control group, all concentrations of CA4 inhibited migration and invasion. The concentrations of 5 μM and 10 μM remarkably inhibited cell migration and invasion (P<0.05, P<0.01, or P<0.001), showing that CA4 can inhibit cell migration and invasion in a dose-dependent manner.
Figure 2.
Inhibitive effects of Combretastatin A4 on TPC1 cell migration and invasion. (A) cell migration assay; (B) cell invasion assay. * P<0.05 compared with control group; ** P<0.01 compared with control group; *** P<0.001 compared with control group.
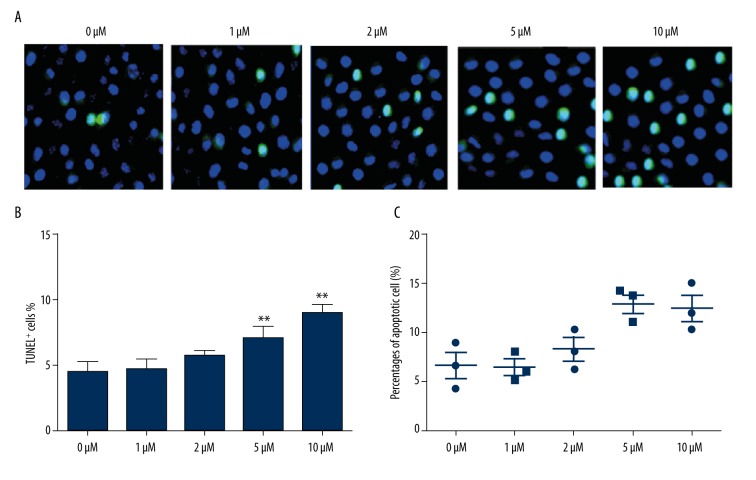
Effect of CA4 on TPC1 cells apoptosis
To reveal the effect of CA4 on TPC1 cell apoptosis, 5 concentrations of CA4 were pre-treated with TPC1 cells, and apoptotic cells were investigated both by TUNEL staining assay (Figure 3A, 3B) and flow cytometry method (Figure 3C). The data shown in Figure 3A and 3B are consistent with the data in Figure 3C, showing that high concentrations of CA4 (5 and 10 μM) can significantly induce apoptosis compared with the control group (P<0.05 or P<0.01). These results indicate that high concentrations of CA4 can promote cell apoptosis.
Figure 3.
Promoting effects of Combretastatin A4 on TPC1 cells apoptosis. (A) and (B) conducted by the TUNEL staining assay; (C) conducted by the flow cytometry method. TUNEL+, terminal deoxynucleotidyl positive cells. * P<0.05 compared with control group; ** P<0.01 compared with control group.
Effect of CA4 on the expression of EMT-related proteins
To reveal whether CA4 can bring about the alterations in EMT-related protein expression, Western blot analysis was conducted to verify the expressions of E-cadherin, N-Cadherin, Vimentin, Snail1, Slug, Twist1, and ZEB1 in TPC1 cells. As shown in Figure 4, CA4 can decrease the expression of N-Cadherin, Vimentin, Snail1, Slug, Twist1, and ZEB1, and it was hardly surprising to see that the cells treated with 5 μM and 10 μM CA4 were more strongly inhibited. No significant differences were found in the levels of E-cadherin among the 5 groups. These results suggest that the effects of CA4 on TPC1 cells might be involved in EMT.
Figure 4.
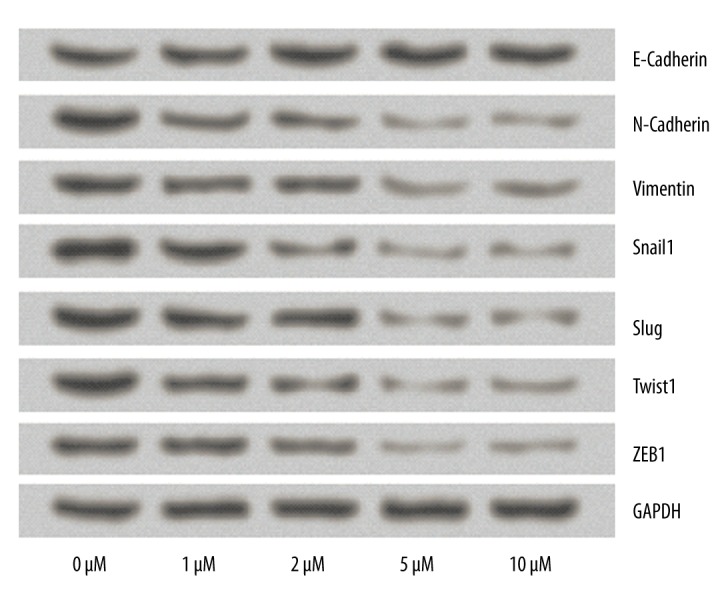
Effects of Combretastatin A4 on the expression of EMT-related proteins. The expression levels of E-Cadherin, N-Cadherin, Vimentin, Snail1, Slug, Twist1, and ZEB1were detected by Western blot analysis. GAPDH acted as an internal control.
Effect of CA4 on the expression of PI3K/Akt signaling pathway proteins
To further investigate the molecular mechanisms by which CA4 affects TPC1 cells, Western blot analysis was conducted again to examine the expression changes of PI3K/Akt signaling pathway proteins. Results in Figure 5 show that no significant differences were found in the levels of PI3K and Akt among the 5 groups, while the 5 μM and 10 μM concentrations of CA4 significantly decreased the expression of p-PI3K and p-Akt proteins. These results indicate that the effects of CA4 on thyroid cancer cells might be through the PI3K/Akt signaling pathway.
Figure 5.
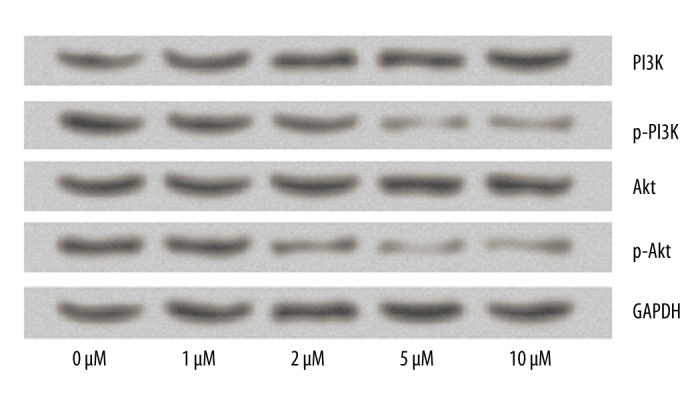
Effects of Combretastatin A4 on the expression of PI3K/Akt signaling pathway proteins. The expression levels of PI3K, p-PI3K, Akt, and p-Akt were detected by Western blot analysis. GAPDH acted as an internal control.
Discussion
PTC is the most common thyroid cancer and the rates of PTC are increasing worldwide. Therefore, there have been many studies on the prevention and treatment of PTC in recent years. However, little is known about the therapeutic effect of CA4 on PTC. In the current study, we provide the first insight into the inhibitive effects of CA4 on TPC1 cells through the PI3K/Akt signaling pathway. We found that CA4 significantly inhibited the proliferation, migration, and invasion of TPC1 cells, and increased the apoptosis rate. Moreover, CA4 decreased the expression levels of EMT-related proteins (N-Cadherin, Vimentin, Snail1, Slug, Twist1, and ZEB1) and PI3K/Akt signaling pathway proteins (p-PI3K and p-Akt).
As an antimitotic agent, CA4 has been demonstrated to display a strong antimitotic effect against cell proliferation [18]. It has been reported that CA4 inhibits proliferation in many cancer cells in vitro, such as Colo-699 and KNS-62 [19]. Iyer et al. suggested that CA4 increases caspase-3 activity and induces human endothelial cells apoptosis [20]. However, the effects of CA4 on thyroid cancer cell proliferation and apoptosis are still unknown. In our study, we found that CA4 inhibited TPC1 cell proliferation and promoted apoptosis in a dose-dependent manner, suggesting that CA4 might be involved in thyroid cancer cell proliferation and apoptosis. We also investigated the effects of CA4 on TPC1 cell migration and invasion. We found that CA4 inhibits migration and invasion of TPC1 cells in a dose-dependent manner, indicating that CA4 might also play an important role in the inhibition of thyroid cancer cell migration and invasion. Similarly, Shen et al. observed CA4 could inhibit cell metastasis in bladder cancer cells [14]. EMT has been found to be involved in the invasive fronts of human tumors, such as papillary thyroid [21], breast [22], and cervical [23] carcinoma. Thus, to better understand the underlying mechanism in migration and invasion, we analyzed the protein expression of EMT. Our results showed that CA4 down-regulates the levels of N-Cadherin, Vimentin, Snail1, Slug, Twist1, and ZEB1, but did not significantly regulate the levels of E-cadherin. Among these proteins, Snail 1, Slug, and ZEB1 directly induce transcription of EMT-activating transcription repressor, whereas Twist 1 is an indirect EMT-activating transcription repressor [24]. N-cadherin and Vimentin are 2 markers positively related to the metastasis of tumor cells, whereas E-cadherin is a negative correlation marker [24,25]. Taken together, these results provided the first in vivo evidence that CA4 inhibits thyroid cancer cell migration and invasion through regulating EMT-related proteins.
PI3K/Akt signaling has been shown to play a central role in cancer cell proliferation, migration, invasion, and apoptosis [26–28]. The PI3K/Akt signaling pathway is also responsible for modulating multiple processes in thyroid cancer cells [29]. For instance, down-regulation of PI3K and Akt phosphorylation inhibits thyroid cancer cell proliferation, invasion, and apoptosis [30,31]. Thus, we detected the PI3K/Akt signaling pathway proteins to reveal the molecular mechanisms of CA4-activated thyroid cancer cells. Our results showed that CA4 decreased the level of p-PI3K and p-Akt, but did not significantly decrease PI3K and Akt. The phosphorylations of PI3K and Akt were inhibited, indicating that CA4 may affect thyroid cancer cells through suppressing the PI3K/Akt signaling pathway. This result is consistent with the findings of Samarin et al. that incubation with CA4 regulated PI3K/Akt signaling in tumor cells, linking CA4 to antitumor strategies [32].
Conclusions
We demonstrated that CA4 inhibited thyroid cancer cell proliferation, migration and invasion, and promoted apoptosis. The effects of CA4 on thyroid cancer cells might be through suppressing the PI3K/Akt signaling pathway. CA4 might be a potential therapeutic target for the treatment of thyroid cancer. However, further investigations should be performed to confirm this conclusion.
Footnotes
Conflicts of interest
There are no conflicts of interest.
Source of support: Cultivation Project for Provincial Key Medical Specialty of Foshan (No. Fspy-2015040)
References
- 1.Zhang J, Yang Y, Liu Y, et al. MicroRNA-21 regulates biological behaviors in papillary thyroid carcinoma by targeting programmed cell death 4. J Surg Res. 2014;189:68–74. doi: 10.1016/j.jss.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 2.Fridman Eran, Gil Z. What is the minimal surgery for papillary thyroid carcinoma? Rambam Maimonides Med J. 2016;7:e0005. doi: 10.5041/RMMJ.10232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 2011;30:569–80. doi: 10.1038/nrendo.2011.142. [DOI] [PubMed] [Google Scholar]
- 4.Williams D. Thyroid growth and cancer. Eur Thyroid J. 2015;4:164–73. doi: 10.1159/000437263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J, Gill A, Atmore B, et al. Upregulation of the signal transducers and activators of transcription 3 (STAT3) pathway in lymphatic metastases of papillary thyroid cancer. Int J Clin Exp Pathol. 2011;4:356–62. [PMC free article] [PubMed] [Google Scholar]
- 6.Sugino K, Kure Y, Iwasaki H, et al. Metastases to the regional lymph nodes, lymph node recurrence, and distant metastases in nonadvanced papillary thyroid carcinoma. Surg Today. 1995;25:324–28. doi: 10.1007/BF00311254. [DOI] [PubMed] [Google Scholar]
- 7.Zhang M, Guo R, Wang Y, et al. Multifunctional dendrimer/combretastatin A4 inclusion complexes enable in vitro targeted cancer therapy. Int J Nanomedicine. 2011;6:2337–49. doi: 10.2147/IJN.S24705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nam NH. Combretastatin A-4 analogues as antimitotic antitumor agents. Curr Med Chem. 2003;10:1697–22. doi: 10.2174/0929867033457151. [DOI] [PubMed] [Google Scholar]
- 9.Zheng S, Zhong Q, Mottamal M, et al. Design, synthesis, and biological evaluation of novel pyridine-bridged analogues of Combretastatin-A4 as anticancer agents. J Med Chem. 2014;57:3369–81. doi: 10.1021/jm500002k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wehbe H, Kearney CM, Pinney KG. Combretastatin A-4 resistance in H460 human lung carcinoma demonstrates distinctive alterations in beta-tubulin isotype expression. Anticancer Res. 2005;25:3865–70. [PubMed] [Google Scholar]
- 11.Zhang C, Zhou SS, Li XR, et al. Enhanced antitumor activity by the combination of dasatinib and combretastatin A-4 in vitro and in vivo. Oncol Rep. 2013;29:2275–82. doi: 10.3892/or.2013.2405. [DOI] [PubMed] [Google Scholar]
- 12.Parihar S, Kumar A, Chaturvedi AK, et al. Synthesis of combretastatin A4 analogues on steroidal framework and their anti-breast cancer activity. J Steroid Biochem Mol Biol. 2013;137:332–44. doi: 10.1016/j.jsbmb.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Lin HL, Chiou SH, Wu CW, et al. Combretastatin A4-induced differential cytotoxicity and reduced metastatic ability by inhibition of AKT function in human gastric cancer cells. J Pharmacol Exp Ther. 2007;323:365–73. doi: 10.1124/jpet.107.124966. [DOI] [PubMed] [Google Scholar]
- 14.Shen CH, Shee JJ, Wu JY, et al. Combretastatin A-4 inhibits cell growth and metastasis in bladder cancer cells and retards tumour growth in a murine orthotopic bladder tumour model. Br J Pharmacol. 2010;160:2008–27. doi: 10.1111/j.1476-5381.2010.00861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li LC, Jayarama S, Pilli T, et al. Down-modulation of expression, or dephosphorylation, of IG20/MADD in tumor necrosis factor-related apoptosis-inducing ligand-resistant thyroid cancer cells makes them susceptible to treatment with this ligand. Thyroid. 2013;23:70–78. doi: 10.1089/thy.2012.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boras E, Slevin M, Alexander MY, et al. Monomeric C-reactive protein and Notch-3 co-operatively increase angiogenesis through PI3K signalling pathway. Cytokine. 2014;69:165–79. doi: 10.1016/j.cyto.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 17.Chiappetta G, Valentino T, Vitiello M, et al. PATZ1 acts as a tumor suppressor in thyroid cancer via targeting p53-dependent genes involved in EMT and cell migration. Oncotarget. 2015;6:5310–23. doi: 10.18632/oncotarget.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z, Ho PC. A nanocapsular combinatorial sequential drug delivery system for antiangiogenesis and anticancer activities. Biomaterials. 2010;31:7115–23. doi: 10.1016/j.biomaterials.2010.05.075. [DOI] [PubMed] [Google Scholar]
- 19.Boehle AS, Sipos B, Kliche U, et al. Combretastatin A-4 prodrug inhibits growth of human non-small cell lung cancer in a murine xenotransplant model. Ann Thorac Surg. 2001;71:1657–65. doi: 10.1016/s0003-4975(01)02408-0. [DOI] [PubMed] [Google Scholar]
- 20.Iyer S, Chaplin DJ, Rosenthal DS, et al. Induction of apoptosis in proliferating human endothelial cells by the tumor-specific antiangiogenesis agent Combretastatin A-4. Cancer Res. 1998;58:4510–14. [PubMed] [Google Scholar]
- 21.Jung CW, Han KH, Seol H, et al. Expression of cancer stem cell markers and epithelial-mesenchymal transition-related factors in anaplastic thyroid carcinoma. Int J Clin Exp Pathol. 2015;8:560–68. [PMC free article] [PubMed] [Google Scholar]
- 22.Chen ZJ, Wei W, Jiang GM, et al. Activation of GPER suppresses epithelial mesenchymal transition of triple negative breast cancer cells via NF-κB signals. Mol Oncol. 2016 doi: 10.1016/j.molonc.2016.01.002. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qureshi R, Arora H, Rizvi MA. EMT in cervical cancer: Its role in tumour progression and response to therapy. Cancer Lett. 2015;356:321–31. doi: 10.1016/j.canlet.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 24.Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:870–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Kimura I, Kitahara H, Ooi K, et al. Loss of epidermal growth factor receptor expression in oral squamous cell carcinoma is associated with invasiveness and epithelial-mesenchymal transition. Oncol Lett. 2016;11:201–7. doi: 10.3892/ol.2015.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma LI, Chang Y, Yu L, et al. Pro-apoptotic and anti-proliferative effects of mitofusin-2 via PI3K/Akt signaling in breast cancer cells. Oncol Lett. 2015;10:3816–22. doi: 10.3892/ol.2015.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang FY, Hu Y, Que ZY, et al. Shikonin inhibits the migration and invasion of human glioblastoma cells by targeting phosphorylated β-catenin and phosphorylated PI3K/Akt: A potential mechanism for the anti-glioma efficacy of a traditional Chinese herbal medicine. Int J Mol Sci. 2015;16:23823–48. doi: 10.3390/ijms161023823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye Y, Tang X, Sun Z, Chen S. Upregulated WDR26 serves as a scaffold to coordinate PI3K/AKT pathway-driven breast cancer cell growth, migration, and invasion. Oncotarget. 2016 doi: 10.18632/oncotarget.7439. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robbins HL, Hague A. The PI3K/Akt pathway in tumors of endocrine tissues. Front Endocrinol (Lausanne) 2015;6:188. doi: 10.3389/fendo.2015.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun D, Zhou R, Liu H, et al. SASH1 inhibits proliferation and invasion of thyroid cancer cells through PI3K/Akt signaling pathway. Int J Clin Exp Pathol. 2015;8:12276–83. [PMC free article] [PubMed] [Google Scholar]
- 31.Gunda V, Bucur O, Varnau J, et al. Blocks to thyroid cancer cell apoptosis can be overcome by inhibition of the MAPK and PI3K/AKT pathways. Cell Death Dis. 2014;5:e1104. doi: 10.1038/cddis.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samarin J, Rehm M, Krueger B, et al. Up-regulation of connective tissue growth factor in endothelial cells by the microtubule-destabilizing agent Combretastatin A-4. Mol Cancer Res. 2009;7:180–88. doi: 10.1158/1541-7786.MCR-08-0292. [DOI] [PubMed] [Google Scholar]