Abstract
Purpose
Cellular immunotherapy has appeared to be a promising modality for the treatment of malignant tumor. The objective of this study was to evaluate the efficacy of cellular immunotherapy combined with minimally invasive therapy.
Methods
We searched PubMed, Web of Science and The Cochrane Library through March 2016 for relevant studies. Short-term efficacy (the disease control rate, the control rate of quality life and the AFP descent rate) and long-term efficacy (overall survival (OS) and progression-free survival (PFS) rate) were compared as the major outcome measures. The meta-analysis was performed using Review Manager 5.3.
Results
A total of 1174 references in 3 databases were found of which 19 individual studies with 1774 HCC patients enrolled in this meta-analysis. Meta-analysis results showed that cellular immunotherapy combined with minimally-invasive treatment significantly improved the measures of short-term response (the disease control rate (OR = 5.91, P = 0.007), the control rate of quality lift (OR = 3.38, P = 0.003) and the AFP descent rate (OR = 4.48, P = 0.02)). Also higher 6-month PFS (OR = 2.78, P = 0.05), ≥12-month PFS (OR = 3.56, P<0.00001) rate and 6-month OS (OR = 2.81, P = 0.0009), 12-month OS (OR = 3.05, P<0.00001) and 24-month OS (OR = 3.52, P<0.0001) rate were observed in patients undergoing cellular immunotherapy.
Conclusions
This meta-analysis suggested that cellular immunotherapy is a feasible adjuvant treatment that could be beneficial for the improvement of the clinical outcomes for hepatocellular carcinoma (HCC) patients after minimally invasive treatment, including short-term response and long-term survival.
Introduction
Hepatocellular carcinoma (HCC) is the most common type of hepatobiliary cancer, the fifth common malignant cancer and the third most cause of cancer-related deaths worldwide [1]. The growth of annual incidence and mortality of HCC is higher in Asia countries accounting for almost 80%, especially in China [2]. Currently, the recurrence rate of HCC was still high after conventional radical resection therapies. Moreover, not all patients may benefit from hepatectomy because of the high incidence of complications due to chronic liver disease or with intermediate-stage HCC [3,4]. Minimally-invasive treatment has been widely used for patients with unresectable HCC. Transcatheter arterial chemoembolization (TACE) has been found to be an effective method to reduce HCC tumor size and improve overall survival (OS) [5,6]. Also, some studies showed that radiofrequency ablation (RFA) and microwave ablation (MWA) could increase tumor necrosis and prolong survival time for HCC [7,8]. However, there were still some limitations existed in long-term prognosis. The recurrence and metastasis after treatment with the TACE-predominant minimally-invasive treatment were still frequent [9]. The prognosis of HCC remained dismal with a low level of survival (5-year survival rate less than 5%) in patients with advanced HCC at diagnosis [10].
In recent decades, cellular immunotherapy has emerged as a promising strategy for cancer treatment [11,12]. It has been reported that cellular immunotherapy could strengthen the immune state and afford a potential value in enhancing the therapeutic outcome [13], although it has not been considered as a standard therapy for HCC. Several studies and meta-analysis had revealed that the combination of cellular immunotherapy of conventional therapies was more effective [14,15]. However, these analysis were about the combination of specific cellular immunotherapy and specific interventional therapy. There were no studies systematically analyzed whether cellular immunotherapy either CIK or DC-CIK was needed after different interventional therapy. The efficacy of cellular immunotherapy still remains controversial, especially in prolonging progression-free and overall survival [16,17].
Therefore, we summarized the trials of cellular immunotherapy, including CIK and DC-CIK, combined with TACE-predominant minimally-invasive treatment for HCC to systematically evaluate the efficacy of cellular immunotherapy.
Materials and Methods
Search strategy for identification of studies
A comprehensive literature search process was conducted in Pubmed, Web of Science and The Cochrane Library, based on combinations of the following keywords: [hepatocellular carcinoma OR liver neoplasms OR liver cancer] AND [(lymphokine-activated killer OR LAK) OR (cytokine induced killer cell OR CIK) OR (tumor infiltrating lymphocyte OR TIL) OR (cytotoxic T lymphocyte OR CTL) OR (dendritic cell OR DC) OR (natural killer cell OR NK)]. We searched these keywords in Title/Abstract of literatures about human between January 2006 and March 2016. Studies were not limited to language.
Inclusion and exclusion criteria for studies in this meta-analysis
In this systematic analysis, literatures were included that met the following criteria: (i) reported clinical outcomes of cellular immunotherapy for HCC; (ii) the case-control study design; (iii) provided enough information to calculate the odds ratio for short term efficacy, PFS or OS.
The exclusion criteria were as follows: (i) reviews, case reports, in vitro experiments, animal models, other diseases or other treatments; (ii) studies without appropriate control groups or enrolled patients less than 15.
Data extraction and quality assessment
All candidate literatures were reviewed and retrieved by two independent authors (Min Ding and Ying Wang) from these 3 databases, discussed and arrived at a consensus with a third author if disagreement occurred, and extracted data. Articles that could not be categorized according to title or abstract alone were retrieved for full-text review.
The following information was extracted from each included article: authors, year of publication, tumor characteristics, case numbers, regimens, immune cell regimens and culture of immune cells. Two independent authors (Min Ding and Ying Wang) evaluated each study for risk of bias and applicability by using The Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool [18]. The QUADAS-2 contained four key domains covering patient selection, index test, reference standard, and flow and timing. The “low risk of bias”, “unclear risk of bias” or “high risk of bias” for each article was attributed in Cochrane reviews of interventions. Any disagreement between two authors regarding quality assessment was resolved through discussion with the third author.
Statistical methods
The analysis was carried out using pair-wise comparison between immunotherapy arms and control arms. Review Manager 5.3 [6] was used to conduct meta-analysis and calculated odds ratio (OR) for the disease control rate, the control rate of quality life and the AFP descent rate to reflect short-term efficacy, and 6-, 12-, 24-month PFS and OS to reflect long-term treatment effects across studies. The number of events evaluated in each arm was utilized to calculate the pooled OR with 95% confidence intervals (CI) combined the Mantel-Haenszel statistical method with random effects. The Cochran’s Q test (chi-square statistic; x2) was applied to evaluate the heterogeneity among studies. We considered inconsistency index (I2) [19] with 25%, 50%, and 75% as representing the evidence of low, moderate, and high heterogeneity, respectively. Publication bias was assessed with Peters test using Meta R package. A p-value of no greater than 0.05 is considered to be statistically significant.
Results
Study characteristics
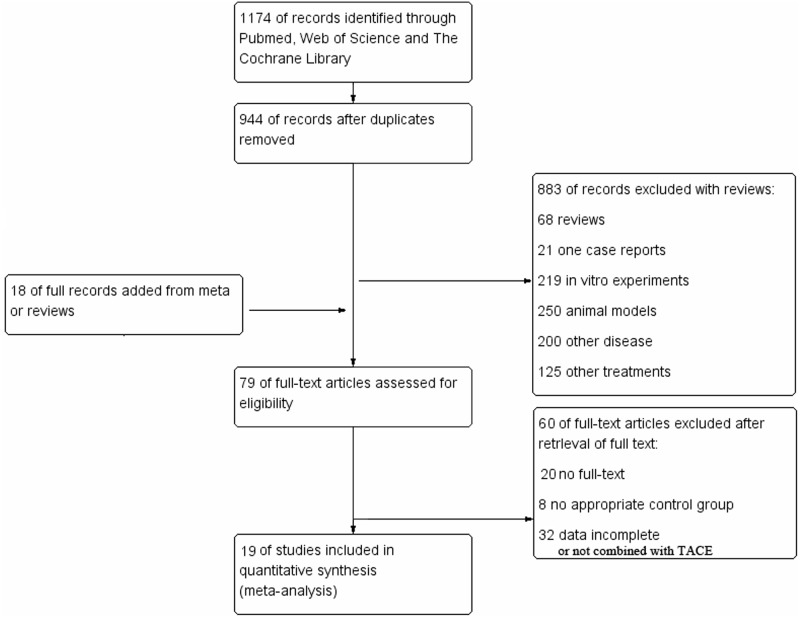
A search strategy in databases of PubMed, Web of science and The Cochrane Library was applied to find a total of 1174 citations, of which 230 were discarded as they were duplicates. After title and abstract screening, 883 articles were excluded for the following reasons: 68 were reviews, 219 were in vitro experiments, 250 used animal models, 21 were a case report, 200 studied other diseases and 125 used other treatments. Additional 18 literatures were supplemented from meta-analysis studies or reviews. In the third step of the eligibility and detailed assessment, 79 full-texts were retrieved, and 60 articles without a control group, full-text, complete clinical data or not combined with TACE-predominant minimally-invasive treatment were excluded (Fig 1). Finally, we included 19 studies with 1774 HCC patients in quantitative synthesis for meta-analysis.
Fig 1. Flow chart of the included studies.
The characteristics of the populations presented in these reports were summarized in Table 1. Overall, 13 studies addressed passive immunotherapeutic approaches including cytokine-induced killer cells (CIKs) [20–32]. Other six studies administrated DC-CIK cells [33–38]. Tumor characteristics and regimens were reported in all studies.
Table 1. Clinical information of the trials for the immunotherapy.
| Reference | Tumor characteristics | Patients No (control) | Regimens (per arm) | Immune cell regimens | Culture of immune cell |
|---|---|---|---|---|---|
| Zhao 2006 | HCC | 33(31) | TACE+RFA+CIK,TACE+RFA | 1.1–1.5×1010/course | CM,CD3MeAb,IL-2,INF-r,IL-1a. |
| Hao 2006 | HCC | 21(46) | TACE+CIK,TACE | 1–5×1010/course | CM, IFN-r, CD3McAb, IL-1a, IL-2. |
| Zhang 2006 | HCC | 52(92) | TACE+CIK/TACE+PEI+CIK,TACE/TACE+PEI | 1.0–1.2×1010/course | CM, IFN-r, CD3McAb, IL-1a, IL-2. |
| Shi 2007 | HCC | 38(214) | TACE+CIK,TACE/TACE+PEI | 1.0–1.2×1010/course | CM, IFN-r, CD3McAb, IL-1a, IL-2. |
| Huang 2007 | Primary HCC | 55(30) | TACE+RFA+CIK,TACE+RFA | ||
| Weng 2008 | HCC | 45(40) | TACE+RFA+CIK,TACE+RFA | 1.0–2.0×1010/course | CM,IFN-r,CD3 McAb,IL-1a,IL-2. |
| Hao 2010 | HCC | 74(72) | TACE+CIK,TACE | 1–5×1010/course | Serum-free culture medium,IFN-r,CD3 McAb,IL-1a,IL-2. |
| Pan 2010 | HCC | 42(39) | TACE+RFA+CIK,TACE+RFA | Once every week, at least 4 infusions, more than 1×1010 cells per course. | |
| Wu 2012 | Primary HCC | 32(38) | TACE+DC-CIK,TACE | ||
| Wang 2012 | HCC | 38(38) | TACE+RFA+CIK,TACE+RFA | Once every twice weeks, at least 3 infusions,1–1.5×1010 cells per course. | IFN-r,CD3 McAb, IL-1a,IL-2. |
| He 2012 | Primary liver cancer | 60(58) | TACE+CIK,TACE | 1 week after TACE | |
| Huang 2013 | HCC | 85(89) | TACE+RFA+CIK,TACE+RFA | 2 weeks after sequential TACE and RFA, the median successive number of CIK cell infusions was 9 (range, 4–25). | IFN-r, CD3-McAb, IL-2, IL-1a. |
| Deng 2013 | HCC | 20(21) | TACE+RFA+CIK,TACE+RFA | ||
| Tong 2013 | Primary liver cancer | 20(18) | TACE+ CIK,TACE | 1–5×1010/course | IFN-r, CD3-McAb, IL-2 |
| Xu 2013 | Large HCC | 40(40) | TACE+PMCT+DC-CIK,TACE+PMCT | ||
| Guo 2014 | Primary liver cancer | 30(38) | TACE+DC-CIK,TACE | 1.0×1010/course | AIM-V serum-free culture medium, IFN-r,rhIL-2,CD3 McAb,IL-1,GM-CSF,rhIL-4,TNF-a. |
| Zhang 2014 | liver cancer | 41(44) | TACE+RFA+DC-CIK,TACE+RFA | 6 times 7 days after TACE and RFA, and the number of DC-CIK cells was above 1.0×1010 | |
| Liu 2014 | Advanced HCC | 23(17) | TACE+DC-CIK,TACE | ||
| Cheng 2014 | liver cancer | 32(28) | TACE+DC-CIK,TACE | 4 infusions of DC-CIK cells | IFN-r, CD3-McAb, IL-2, IL-1a, GM-CSF, IL-4, HSP. |
Quality assessment
The 19 included papers comprised 11 randomized controlled studies [20–22,26,28,29,32,34,35,37,38], 1 studies without describing the randomization method [25] and 7 retrospective studies with a matched-pair control group [23,24,27,30,31,33,36]. It contained eleven (57.89%) with all low risk of bias, one (5.26%) with unclear risk and seven (36.84%) with high risk of bias in random sequence generation, allocation concealment, blinding of participants and personnel and blinding of outcome assessment items, as was shown in S1 Fig.
Short-term efficacy
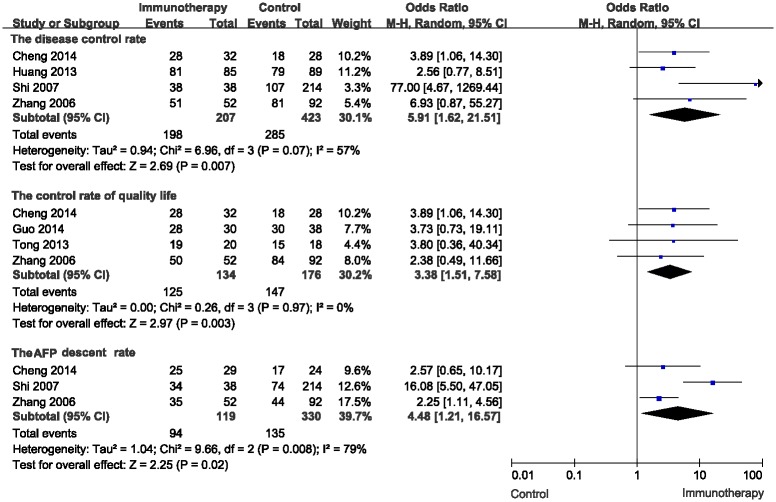
The disease control rate, the control rate of quality life and the AFP descent rate were used to evaluate the short-term efficacy of the cellular immunotherapy. For the disease control rate, the meta-analysis for three CIK studies and one DC-CIK studies showed a significant advantage of the combination therapy (OR = 5.91, 95% CI: 1.62–21.51, P = 0.007). And the heterogeneity was not statistically significant with P value equal to 0.07 (Fig 2A). Four studies, including two CIK studies and two DC-CIK studies were used to evaluate the control rate of quality life in the meta-analysis. The control rate of quality life was significantly higher in patients with cellular immunotherapy compared to without (OR = 3.38, 95% CI: 1.51–7.58, P = 0.003). Also, there was no heterogeneity among the studies (I2 = 0%, P = 0.97) (Fig 2B). For the AFP descent rate, the meta-analysis of three studies (two CIK studies and one DC-CIK studies) showed the combination therapy significantly descent the AFP level (OR = 4.48, 95% CI: 1.21–16.57, P = 0.02) (Fig 2B).
Fig 2. Comparison of short-term efficacy between the patients undergoing cellular immunotherapy or not by using the random effects model (Mantel-Haenszel method).
Long-term efficacy
To evaluate the long-term efficacy of the combination of cellular immunotherapy and TACE-predominant minimally-invasive treatment, we performed subgroup analysis to conduct meta-analysis according to 6-month, 12-month and 24-month progression free survival and overall survival.
Progression free survival
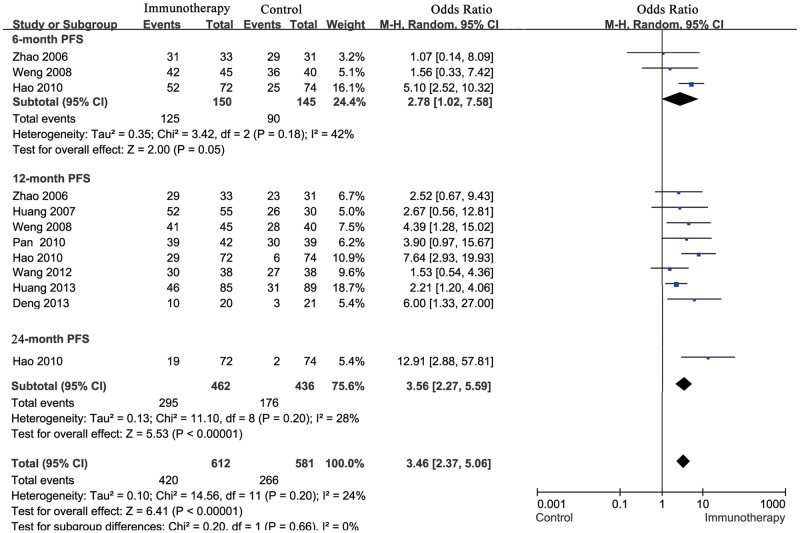
For total effect, progression free survival rate was significantly higher in HCC patients undergoing cellular immunotherapy, compared to patients in control groups (OR = 3.46; 95% CI: 2.37–5.06, P<0.00001). All the Cochran’s Q-test resulted in a P value of 0.20, and the corresponding I2 was 24%, indicating that the degree of variability was basically consistent in each result of study.
In subgroup meta-analysis of studies on 6-, > = 12-month progression free survival, patients in cellular immunotherapy treatment group had significantly higher PFS than those in control group, accordingly (6-month: OR = 2.78; 95% CI: 1.02–7.58, P = 0.05; > = 12-month: OR = 3.56; 95% CI: 2.27–5.59, P<0.00001) (Fig 3).
Fig 3. Comparison of 6, ≥12-month PFS between the patients who received cellular immunotherapy or not by using the random effects model (Mantel-Haenszel method).
Overall survival
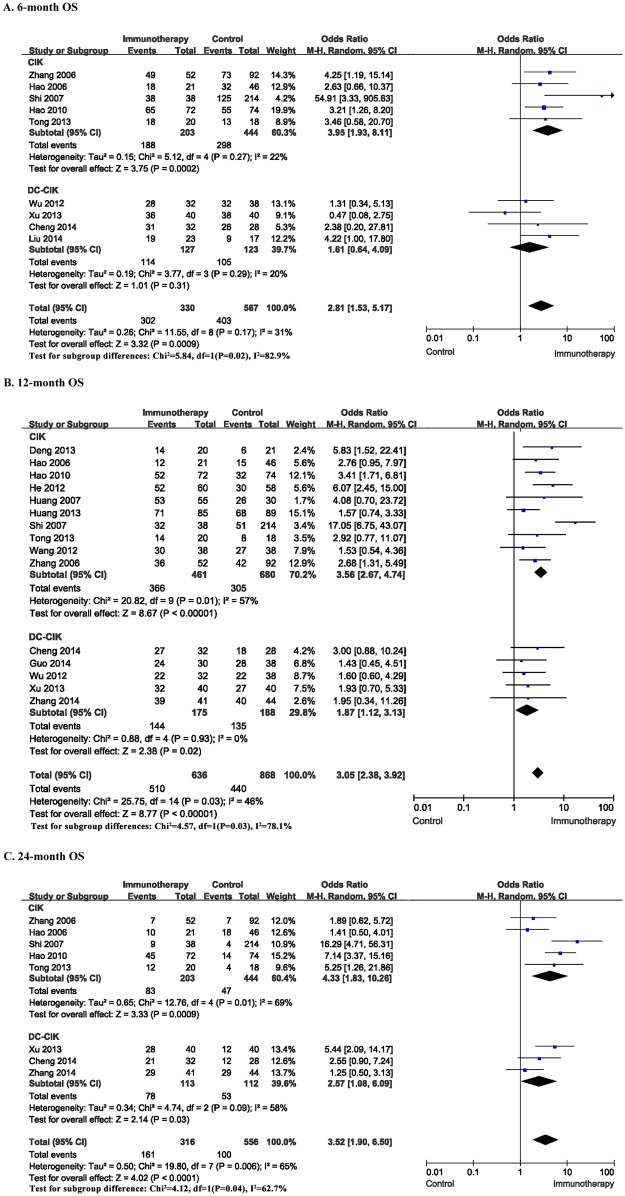
Overall survival rate, including 6-, 12-, and 24-month overall survival rate, was significantly higher in HCC patients undergoing cellular immunotherapy, compared to patients in control groups respectively. For 6-month OS, the meta-analysis for five CIK studies and four DC-CIK studies yielded statistically significant differences (OR = 2.81; 95% CI: 1.53–5.17, P = 0.0009). However, there was no statistically significant differences found in the subgroup of DC-CIK studies (OR = 1.61; 95% CI: 0.64–4.09, P = 0.31). For 12-month OS, the meta analysis for ten CIK studies and five DC-CIK studies showed a higher 12-month rate in the patients with the combination therapy (OR = 3.05; 95% CI: 2.38–3.92, P<0.00001). For 24-month OS, the OR of five CIK studies and three DC-CIK studies was significant (OR = 3.52; 95% CI: 1.90–6.50, P<0.0001), suggesting a higher 24-month OS in HCC patients undergoing cellular immunotherapy. Also, in CIK subgroup and DC-CIK subgroup, the 12-month and 24-month OS were both significantly higher in patients with the combination therapy (P<0.05) (Fig 4).
Fig 4. Comparison of 6, 12, 24-month OS between the patients undergoing cellular immunotherapy or not.
The random effects model (Mantel-Haenszel method) was used.
Assessment of publication bias
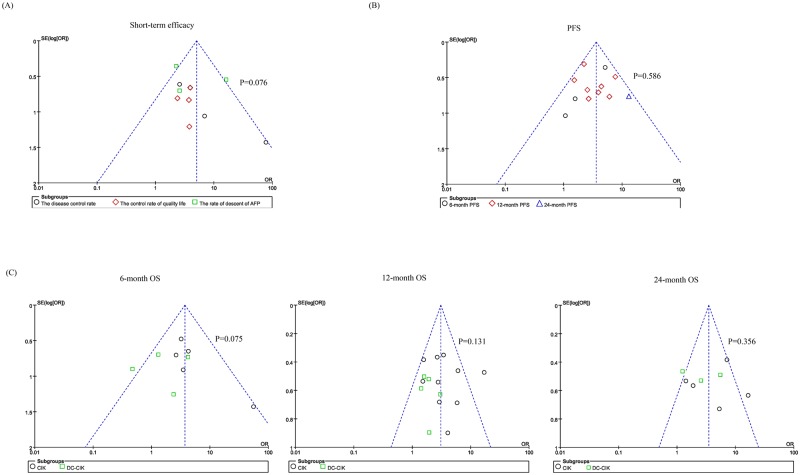
In order to guarantee the validity of the conclusions from the meta-analysis, funnel plots and sensitivity analysis were used in subtypes of the short-term efficacy, PFS, and OS (including 6-month, 12-month and 24-month OS). The relative symmetry of all the funnel plots shape suggested the publication bias was not evident. Also, publication bias was also not suggested by Peters test (P > 0.05) (Fig 5).
Fig 5. Funnel plots to detect any publication bias.
The P value located in this figure indicates the results of Peters test for assessment of publication bias.
Discussion
Our meta-analysis comprehensively analyzed the outcomes of 19 individual studies with 1744 HCC patients from 3 databases, confirming the efficacy of the combination of cellular immunotherapy and TACE-predominant minimally-invasive Treatment on the prognosis of HCC patients.
TACE-predominant minimally-invasive Treatment is one of the main treatments for patients with advanced HCC or patients who are not suitable to surgery [6,39,40]. However, TACE-predominant minimally-invasive Treatment might damage the liver function, reduce the resistance and immunity, and lead to tumor recurrence and residual. So, the combination of TACE-predominant minimally-invasive Treatment and immunotherapy might become an effective treatment [41]. From 18 meta-analysis or reviews in this field, 3 out of 18 performed a meta-analysis on HCC. These systematic reviews focused on the efficacy of the combination of CIK/DC-CIK therapeutic treatment and traditional therapy, respectively. Xie et al [42] studied the efficacy of adoptive immunotherapy in postoperative HCC. Li et al [15] demonstrated that CIK cells transfusion therapy could improve the synergistic effect of HCC patients after TACE or TACE+RFA therapy. Su et al [14] evaluated the efficacy and safety of DC-CIK cell therapy in combination with TACE-predominant minimally-invasive treatment for HCC. However, there was no studies systematically analyzed whether the adjuvant cellular immunotherapy after TACE-predominant minimally-invasive treatment is essential. Notably, it is still controversial that whether CIK or DC-CIK cells immunotherapy was efficacy after different interventional therapy, such as TACE, TACE+RFA, TACE+PEI, and TACE+PMCT for HCC. Our meta-analysis comprehensively analyzed the efficacy of the combination of different cell immunotherapy and different strategy of TACE-predominant minimally-invasive treatment. Through short-term and long-term efficacy analysis, we suggested that the group combined with CIK or DC-CIK cell immunotherapy was associated with better prognosis than minimally invasive treatment alone group.
Our analysis demonstrated that the combination therapy could improve the short-term efficacy, including the disease control rate (P = 0.007), the control rate of quality life (P = 0.003), and the AFP descent rate (P = 0.02). However, the number of included studies for each subgroup was relatively low. And the limitation might lead to bias, even though we found no evidence of publication bias in the meta analysis of the short-term efficacy (P = 0.07) (Fig 5A).
The systematic analysis also showed a significant survival benefit with regard to the PFS and OS when patients undergoing cellular immunotherapy (P≤0.05). There was no heterogeneity across the trails in the meta-analysis for PFS. However, the heterogeneity in 12-month and 24-month OS was statically significant (P<0.05). This demonstrated that the degree of variability was more consistent in each PFS result of study. Notably, for 6-month, 12-month and 24-month OS, the meta analysis was divided into two sub-studies (CIK studies and DC-CIK studies) to reduce the bias caused by different immune cell regimens. For total effect, OS was significantly higher in HCC patients who underwent cellular immunotherapy, compared to patients in control group. However, the heterogeneity in 12-month and 24-month OS in CIK subtypes was statically significant (P<0.05). And the heterogeneity of 6-month, 12-month and 24-month OS between CIK group and DC-CIK group was statically significant (P<0.05) (Fig 4). This result suggested that cellular immunotherapy either CIK or DC-CIK might be needed after different interventional therapy. However, the degree of variability was more consistent in the subtype of DC-CIK studies.
There were some limitations existed in this meta-analysis. First, the number of included studies in each subgroup was relatively low. And the included studies mostly occurred in China lacking multinational larger sample multi-center clinical trials with sufficient statistical power. Second, heterogeneity is a potential issue that may affect the interpretation of the results. The sources of heterogeneity may result from designs and methods, including age distribution, gender, regimens and so on. To address this issue, we employed subgroup analysis and random effects method to perform a meta-analysis on the results of these 19 studies. Third, Publication bias might occurred when the publication of research results depended not just on the quality of the research but on the hypothesis tested, and the significance and direction of effects detected. For meta-analysis, the studies would not be representative when the publication bias presented. In order to guarantee the validity of the conclusions from the meta-analysis, funnel plots and sensitivity analysis (Peters test) were used in the study.
Overall, our systematic analysis demonstrated that the potential clinical value of the combination of cellular immunotherapy with minimally-invasive therapy for prolonging progression free and overall survival. On the basis of the present results, we believe that more advanced and optimized cellular immunotherapy strategies are on their way and may shed a better light on the modality of treatment for HCC.
Supporting Information
(DOC)
(TIF)
(DOC)
Abbreviations
- CI
confidence intervals
- CIK
cytokine-induced killer
- CTL
cytotoxic lymphocyte
- DC
dendritic cell
- HCC
hepatocellular carcinoma
- I2
inconsistency index
- LAK
lymphokine-activated killer
- NK
natural killer cell
- OR
odds ratio
- OS
overall survival
- PFS
progression free survival
- QUADAS-2
quality assessment of diagnostic accuracy studies-2
- TACE
transcatheter arterial chemoembolization
- TIL
tumor infiltrating lymphocyte
Data Availability
The full texts of these 19 studies were downloaded from Pubmed, Web of Science or the Cochrane Library. All data used in our manuscript was collected from these 19 published studies. The detailed information of these 19 studies are shown in Table 1 and reference 20–38.
Funding Statement
This study was funded by The National Natural Science Fund (No. 81472845).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015; 65(1): 5–29. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61(2): 69–90. [DOI] [PubMed] [Google Scholar]
- 3.Tsurusaki M, Murakami T. Surgical and Locoregional Therapy of HCC: TACE. Liver Cancer. 2015; 4(3): 165–175. 10.1159/000367739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003; 362(9399): 1907–1917. 10.1016/S0140-6736(03)14964-1 [DOI] [PubMed] [Google Scholar]
- 5.Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010; 52(2): 762–773. [DOI] [PubMed] [Google Scholar]
- 6.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53(3): 1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poulou LS, Botsa E, Thanou I, Ziakas PD, Thanos L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol. 2015; 7(8): 1054–1063. 10.4254/wjh.v7.i8.1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poggi G, Tosoratti N, Montagna B, Picchi C. Microwave ablation of hepatocellular carcinoma. World J Hepatol. 2015; 7(25): 2578–2589. 10.4254/wjh.v7.i25.2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao M, Wang JP, Pan CC, Li W, Huang ZL, Zhang L, et al. CT-guided radiofrequency ablation after with transarterial chemoembolization in treating unresectable hepatocellular carcinoma with long overall survival improvement. Eur J Radiol. 2012; 81(10): 2717–2725. 10.1016/j.ejrad.2011.10.023 [DOI] [PubMed] [Google Scholar]
- 10.Waghray A, Murali AR, Menon KN. Hepatocellular carcinoma: From diagnosis to treatment. World J Hepatol. 2015; 7(8): 1020–1029. 10.4254/wjh.v7.i8.1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greten TF, Manns MP, Korangy F. Immunotherapy of HCC. Rev Recent Clin Trials. 2008; 3(1): 31–39. [DOI] [PubMed] [Google Scholar]
- 12.Hong YP, Li ZD, Prasoon P, Zhang Q. Immunotherapy for hepatocellular carcinoma: From basic research to clinical use. World J Hepatol. 2015; 7(7): 980–992. 10.4254/wjh.v7.i7.980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aerts M, Benteyn D, Van Vlierberghe H, Thielemans K, Reynaert H. Current status and perspectives of immune-based therapies for hepatocellular carcinoma. World J Gastroenterol. 2016; 22(1): 253–261. 10.3748/wjg.v22.i1.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su Y, Yang Y, Ma Y, Zhang Y, Rao W, Yang G, et al. The Efficacy and Safety of Dendritic Cells Co-Cultured with Cytokine-Induced Killer Cell Therapy in Combination with TACE-Predominant Minimally-Invasive Treatment for Hepatocellular Carcinoma: a Meta-Analysis. Clin Lab. 2016; 62(4): 599–608. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Dai D, Song X, Liu J, Zhu L, Xu W. A meta-analysis of cytokine-induced killer cells therapy in combination with minimally invasive treatment for hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2014; 38(5): 583–591. 10.1016/j.clinre.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 16.Thomas MB, Jaffe D, Choti MM, Belghiti J, Curley S, Fong Y, et al. Hepatocellular carcinoma: consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2010; 28(25): 3994–4005. 10.1200/JCO.2010.28.7805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vauleon E, Avril T, Collet B, Mosser J, Quillien V. Overview of cellular immunotherapy for patients with glioblastoma. Clin Dev Immunol. 2010; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011; 155(8): 529–536. 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327(7414): 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao M W P, Zeng YX, Xia JC, Zhang FJ, Xian J, Zhang YP, Zhou K, Fan WJ, Zhang L, Gao F, Zhou QM. Cytokine-induced killer cell fusion to lower recurrence of hepatocellular carcinoma after transcatheter arterial chemoembolization sequentially combined with radiofrequency ablation: a randomized trial. Zhonghua Yi Xue Za Zhi. 2006; 86: 1823–1828. [PubMed] [Google Scholar]
- 21.Zhang NZ X Y, Chen FX, Gao CJ, Zhang GL, Ma WQ, Xu WD. Clinical study on the treatment of advanced hepatocellular carcinoma by CIK cell. J Southeast Natl Defense Med. 2006; 8(2): 84–87. [Google Scholar]
- 22.Weng DS Z J, Zhou QM, Zhao M, Wang QJ, Huang X, Li YQ, Chen SP, Wu PH, Xia JC. Minimally invasive treatment combined with cytokine-induced killer cells therapy lower the shortterm recurrence rates of hepatocellular carcinomas. J Immunother. 2008; 31: 63–71. 10.1097/CJI.0b013e31815a121b [DOI] [PubMed] [Google Scholar]
- 23.Wang JP L W, Huang ZL, Wu PH, Li XS, Wei YD, Zhou QM, Pan CC, Xia JC, Zhao M. Valueof CIK in the treatment of TACE combined with RFA for HCC inlong-term survival and prognostic analysis. Zhonghua Xi Xue ZaZhi. 2012; 92: 3062–3066. [PubMed] [Google Scholar]
- 24.Tong LQ Z H, You LG, Chen GF, Li XL, Zhang F, Zhou BG, Du G. ransarterial chemoembolization combined with autologouscytokine-induced killer cells therapy for primary liver cancer. Zhong Guo Pu Tong Wai Ke Za Zhi. 2013; 22: 876–879. [Google Scholar]
- 25.Shi Y G C, Dong SL, Chen FX, Xu YM. Cytokine-induced killer cell for interventionla chemotherapy of hepatocellular carcinoma. J Intervent Radiol. 2007; 16(4): 235–239. [Google Scholar]
- 26.Pan CC H Z, Li W, Zhao M, Zhou QM, Xia JC, Wu PH. Serum alpha-fetoprotein measurement in predicting clinical outcome related to autologous cytokine-induced killer cells in patients with hepatocellular carcinoma undergone minimally invasive therapy. Chin J Cancer. 2010; 29: 596–602. [DOI] [PubMed] [Google Scholar]
- 27.Huang ZM L W, Li S, Gao F, Zhou QM, Wu FM, He N, Pan CC, Xia JC, Wu PH, Zhao M. Cytokine-induced killer cells in combination with transcatheter arterial chemoembolization and radiofrequency ablation for hepatocellular carcinoma patients. J Immunother. 2013; 36(5): 287–293. 10.1097/CJI.0b013e3182948452 [DOI] [PubMed] [Google Scholar]
- 28.Huang LX Z Q, Xia JC. Minimally invasive treatmentcombined with autologous CIK cell therapy for primary hep-atocellular carcinoma: safety and efficacy. Guang Dong Yi Xue. 2007; 28: 1466–1468. [Google Scholar]
- 29.He XB W J, Hu JH. CIK cells combined with TACE treatmentof primary liver cancer randomized controlled study. Si ChuanXi Xue. 2012; 33: 1696–1697. [Google Scholar]
- 30.Hao MZ L H, Chen Q, Ye YB, Chen QZ, Chen MS. Efficacy of transcatheter arterial chemoembolization combined with cytokine-induced killer cell therapy on hepatocellular carcinoma: a comparative study. Chin J Cancer Biother. 2010; 29: 172–177. [DOI] [PubMed] [Google Scholar]
- 31.Hao MZ C Q, Ye YB, Xiao JR, Lin HL, Wu H, Yu WC, Zhang KZ, Chen Q, Liu YX, Zheng WS. Transcatheter arterial chemoembolization combined with cytokine induced killers in treatment of hepatocellular carcinoma. Chin J Cancer Biother. 2006; 13(4): 303–305. [Google Scholar]
- 32.Deng WJ C J, Luo YJ, Xiang GY. Efficacy and safety of min-imally invasive treatment combined with autologous CIK cellsinfusion. Ling Nan Xian Dai Ling Chuang Wai Ke. 2013; 13: 29–31. [Google Scholar]
- 33.Guo WW L L, Wu DH. Dendritic cell-cytokine induced killer cell immunotherapy combined with transcatheter arterial chemoembolization for hepatocellular carcinoma: safety and efficacy. J South Med Univ. 2014; 35(5): 674–678. [PubMed] [Google Scholar]
- 34.C W. Clinical research on comprehensive treatment of primary hepatocellular carcinoma. Chinese Journal of Primary Medicine and Pharmacy. 2012; 19(1): 97–98. [Google Scholar]
- 35.Xu YM X D, Zhang NZ, Shi Y, Luan ZY, Liu JQ. TACE combined PMCT sequential own cancer vaccine and immunoeffector cells to treat large hepatocellular carcinoma clinical observation. Chinese Journal of Cancer Prevention and Treatment 2013; 24: 1928–1932. [Google Scholar]
- 36.Zhang QC P G, Li GQ, Yuan QZ, Xu JB, Kong Y, Wang LM. DC-CIK cell therapy combined with transcatheter arterial chemoembolization and radiofrequency ablation for treatment of small hepatocellular carcinoma. World Chinese Journal of Digestology. 2014; 16: 2237–2242. [Google Scholar]
- 37.L L. A randomized controlled study of transcatheter arterial chemoembolization combined with autologous DC-CIK cells in the treatment of advanced hepatocellular carcinoma. Second Military Medical University; 2014. [Google Scholar]
- 38.Cheng XZ P D, Wang MQ, Xie ZZ. Clinical efficacy of autologous dendritic cells and cytokine-induced killer cells combined with transcatheter arterial chemoembolization in the treatment of moderate- and advanced hepatocellular carcinoma. The Journal of Practical Medicine. 2014; 9: 1401–1404. [Google Scholar]
- 39.Yang K, Zhang XM, Yang L, Xu H, Peng J. Advanced imaging techniques in the therapeutic response of transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2016; 22(20): 4835–4847. 10.3748/wjg.v22.i20.4835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012; 56(4): 908–943. 10.1016/j.jhep.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 41.Hao MZ, Lin HL, Chen Q, Ye YB, Chen QZ, Chen MS. Efficacy of transcatheter arterial chemoembolization combined with cytokine-induced killer cell therapy on hepatocellular carcinoma: a comparative study. Chin J Cancer. 2010; 29(2): 172–177. [DOI] [PubMed] [Google Scholar]
- 42.Xie F, Zhang X, Li H, Zheng T, Xu F, Shen R, et al. Adoptive immunotherapy in postoperative hepatocellular carcinoma: a systemic review. PLoS One. 2012; 7(8): e42879 10.1371/journal.pone.0042879 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(TIF)
(DOC)
Data Availability Statement
The full texts of these 19 studies were downloaded from Pubmed, Web of Science or the Cochrane Library. All data used in our manuscript was collected from these 19 published studies. The detailed information of these 19 studies are shown in Table 1 and reference 20–38.