Abstract
Constipation is a very common complaint, with slow-transit constipation (STC) accounting for a significant proportion of cases. Old age, female gender, psychiatric illness, and history of sexual abuse are all associated with STC. The exact cause of STC remains elusive; however, multiple immune and cellular changes have been demonstrated. Diagnosis requires evidence of slowed colonic transit which may be achieved via numerous modalities. While a variety of medical therapies exist, these are often met with limited success and a minority of patients ultimately require operative intervention. When evaluating a patient with STC, it is important to determine the presence of concomitant obstructed defecation or other forms of enteric dysmotility, as this may affect treatment decisions. Although a variety of surgical procedures have been reported, subtotal colectomy with ileorectal anastomosis is the most commonly performed and well-studied procedure, with the best track record of success.
Keywords: slow-transit constipation, colonic inertia, surgical management of constipation
Constipation is a common and frustrating clinical entity which affects nearly 63 million individuals in North America with a prevalence of 2 to 28% of the population.1 2 The elderly, women, and those of lower socioeconomic status are particularly affected.3 Women are affected up to 10 times as frequently as men, and the vast majority of individuals who undergo colectomy for constipation are female.4 5 In one series, nearly 74% of nursing home patients reported some degree of difficulty with constipation.6 Constipation can be difficult to define and is by nature a very subjective complaint. In addition to experiencing extended periods between bowel movements, patients may complain of hard stools, feelings of excessive straining, bloating, abdominal pain, or incomplete evacuation. The Rome criteria, which were originally published in 1988, attempt to provide a standardized definition of functional gastrointestinal (GI) disorders including constipation. The most recent iteration7 (Rome III) was produced in 2006 (Table 1). It is important to note that these criteria do not distinguish between different forms of functional constipation. Causes of constipation can be attributed to primary and secondary sources. Primary, or functional, constipation is frequently due to either constipation-predominant irritable bowel syndrome, slow-transit constipation (STC), obstructed defecation (including pudendal neuropathy, paradoxical contraction, or nonrelaxation of the puborectalis or increased perineal descent), or some combination of these.4 8 9 Secondary constipation (Table 2) is more common and causes include dehydration, poor dietary fiber intake, a variety of medications (opiates, iron supplements, etc.), numerous medical conditions, low physical activity levels, and mechanical obstruction such as rectal stricture, rectocele, or colon cancer.4 10
Table 1. Rome III criteria for functional constipation7 .
| A. Must include at least two of the following |
| 1. Straining during greater than 25% of bowel movements 2. Hard or lumpy stools in greater than 25% of bowel movements 3. Sensation of incomplete evacuation in greater than 25% of bowel movements 4. Sensation of anorectal obstruction or blockage in greater than 25% of bowel movements 5. Manual maneuvers are required to facilitate greater than 25% of bowel movements 6. Less than three bowel movements per week |
| B. Without the use of laxatives, loose stools are infrequent |
| C. Insufficient criteria to meet diagnosis of irritable bowel syndrome |
| Prior to diagnosis, the above criteria must be fulfilled for 3 consecutive months with at least 6 months of symptoms in total |
Table 2. Common causes of secondary constipation4 10 .
| Medications |
| Opiates Anticholinergic agents Tricyclic antidepressants Diuretics Nonsteroidal anti-inflammatory drugs Calcium-containing supplements/antacids Calcium channel blockers Iron-containing supplements |
| Medical conditions |
| Hypercalcemia Hyperparathyroidism Hypothyroidism Electrolyte imbalances (i.e., hypomagnesaemia, hypokalemia) Poor dietary fiber intake Dehydration Depression Diabetes mellitus Uremia Parkinson disease Multiple sclerosis Scleroderma Hirschsprung disease |
| Mechanical obstruction |
| Stricture Rectocele/Sigmoidocele/Enterocele Rectal prolapse Colon/Rectal cancer Intussusception |
STC, often used interchangeably with the term colonic inertia, may be defined as functional constipation independent of pelvic floor dysfunction, with the absence of colonic motor activity, with accompanying radiographic evidence of delayed transit and lack of response to pharmacologic augmentation during motility studies.11 Obstructed defecation or dyssynergic defecation involves uncoordinated rectal contractions and anal sphincter function with incomplete internal anal sphincter relaxation or paradoxical sphincter contraction.9 While constipation-predominant irritable bowel syndrome is the most common form of primary constipation, STC is the culprit in 15 to 42% of cases9 10 12 13 and may be accompanied by other forms of enteric dysmotility in a large proportion of individuals. In a series of 212 patients who underwent whole-gut transit scintigraphy, 91 individuals had STC without evidence of obstructed defecation. Of this group, only 48% had normal upper GI transit times with 52% of individuals having delayed gastric function or delayed small bowel transit, or a combination of the two. In the same series, 25% of patients had both STC and obstructed defecation based on anal manometry, defecography, or balloon expulsion tests.9 It is also important to note that multiple series have demonstrated high rates of psychiatric disease and/or histories of sexual abuse in individuals with STC.14 15 Providers should be alert to this fact in the early stages of patient evaluation, especially when surgical options are being considered.
Mechanism of Ordinary Colonic Contraction and Defecation
Under normal circumstances, both long- and short-term rhythmic phase contractions of large bowel circular muscle slowly mix and propel enteric content. Short-phase contractions which occur 3 to 12 times per minute and last up to 3 seconds in duration play little role in propagation, whereas long-phase contractions which occur up to 2 times per minute and last up to 20 seconds in duration provide a moderate amount of forward propulsion. Giant migrating colonic contractions are high amplitude, rapidly propagating contractions which occur approximately 10 times per day and produce mass movements of enteric contents and allow for forceful stool expulsion. Fecal loading of the otherwise empty rectum results in distention of stretch receptors and once a threshold is met, the anal reflex is triggered resulting in propulsion of the fecal load from the sigmoid colon into the rectum/anus with synchronous relaxation of the internal anal sphincter and contraction of the external anal sphincter complex. If defection is desired, the external sphincter is voluntarily relaxed, the puborectalis muscle relaxes, thereby straightening the anorectal angle and the individual Valsalva to increase the intra-abdominal pressure leading to fecal evacuation.8 16
Pathophysiology of Slow-Transit Constipation
The underlying mechanism of colonic dysmotility which leads to STC is ill defined. Numerous studies have demonstrated a variety of physiologic changes which occur in individuals with STC, including reduced sensitivity to cholinergic stimulation and decreased colonic electrical activity resulting in fewer high-amplitude propagated contractions and diminished colonic contractility.17 18 19 Several disturbances of the neuroendocrine system as well as the autonomic and enteric nervous systems have all been implicated in these physiologic disturbances.12 17 20 21 22 23 24 25 26 Various immune, cellular, and histological changes have been demonstrated in individuals with STC, with histologic examination often being notable for intestinal neuronal dysplasia or visceral myopathy.27 Bassotti et al found that colonic mast cells, which are thought to play a role in visceral hypersensitivity and motor activity, are present in much higher concentrations in patients with STC, potentially leading to an inflammatory environment in the muscularis externa and subsequent suppression of normal colonic propulsion.26 28 This inflammatory state hypothesis may be supported by the finding that individuals with STC have significantly higher numbers of colonic macrophages than their peers with normal colonic transit.29 Many series suggest that for currently unknown reasons, patients with STC have an abnormally low number of interstitial cells of Cajal, the colonic pacemaker cells20 30 potentially providing an explanation for known decreased in colonic electrical activity; however, others disagree with this finding.31 Additionally, there are multiple reports documenting the occurrence of STC after pelvic surgery suggesting a relationship between dysfunctional colonic transit and autonomic nerve dysfunction.32 33 It is possible that changes in the level of various GI hormones, neurotransmitters, and their receptors (i.e., progesterone, serotonin, pancreatic polypeptide, vasoactive intestinal peptide, and cholecystokinin) may play a role in the development of STC as well.10 34 35 At this time, it is unclear whether these physiologic changes lead to the development of, or occur as a result of, STC.
Workup and Diagnostic Modalities
In addition to obtaining a thorough medical history including an assessment of neurologic, psychiatric, and endocrine disorders, the clinician should review the patient's current medications and inquire as to details of any prior constipation evaluations. It is important to ask the patient specific and directed questions regarding their bowel habits. A complete interview should include the quality, quantity, and frequency of stools; changes in stooling ability if the stool is hard or soft; feelings of incomplete evacuation, whether straining is required; the ability to defecate if there is an urge to do so; the length of time required to defecate; the use of laxatives; the presence of pain or bleeding with defecation; the need for digital assistance; and the presence of any masses protruding from the anus. Sensations of abdominal pain, cramping, or bloating with associated improvement with defecation may steer the diagnosis toward IBS, although many patients with STC also report these symptoms as well. During the initial evaluation, a comprehensive physical examination should be performed, including an abdominal examination to assess for pain, palpable masses, and the presence of a hernia. An examination of the perineum as well as a digital rectal exam and potentially proctoscopy should be performed in most patients in either the left lateral or the prone position. It is important to assess the patient's anal tone and to rule out perianal sources of obstruction, such as paradoxical puborectalis contraction, rectal or vaginal prolapse, anal stricture, or an obstructing mass.
A reasonable next step at the time of initial evaluation is to obtain appropriate blood work including electrolytes, thyroid stimulating hormone level, and a hematocrit to help rule out potential secondary causes of constipation. While in some practices it is typical for patients to undergo lower endoscopy as part of the initial evaluation,12 in others colonoscopy or contrast enema is considered only if the patient is unresponsive to a trial of medical therapy or if there is a part of the medical history or physical exam which is concerning for mechanical obstruction. Unless the index of suspicion for other conditions leading to constipation is high, it is also very reasonable to start a trial of fiber supplements or mild laxatives, as an initial step (Fig. 1).36 It is important to note that it may be difficult to preform adequate bowel prep for colonoscopy in the chronically constipated patient in the setting of large stool burden. In a large retrospective study of constipated patients who underwent colonoscopy or sigmoidoscopy, Pepin and Ladabaum found that 14.6% of individuals had adenomas and 1.4% had colon cancers.37 The presence of a tumor was thought to have resulted in obstruction in around 1% of cases. Around 58% of patients evaluated had an indication for colonoscopy in addition to constipation. The authors note that the rate of neoplasia discovery in individuals undergoing colonoscopy for constipation was comparable with the rate of neoplasia discovery in those who are evaluated with routine screening colonoscopy. While colonoscopy is the more commonly performed procedure, contrast enemas are useful for providing information about colonic dimensions, external compression, and potential redundancy and are useful in diagnosing certain less common conditions such as Hirschsprung disease.8 Once other potential causes of constipation have been ruled out or once a trial of medical therapy has failed, it may be time to consider physiologic testing. Commonly performed tests used for workup STC include radiopaque marker studies, barium suspension ingestion, wireless capsule endoscopy, and nuclear scintigraphy.8 38 Because the presence of pelvic floor dysfunction in addition to STC affects management decisions as well, patients with STC and suspected pelvic floor dysfunction should undergo anorectal physiology testing as well. Some of the studies used to assess for obstructed defecation include MR defecography, cinedefecography, anorectal manometry, anal sphincter electromyography, pudendal nerve latency, and the balloon expulsion test. The details of these studies are beyond the scope of this article and are reviewed elsewhere.8 12 39 40 41
Fig. 1.
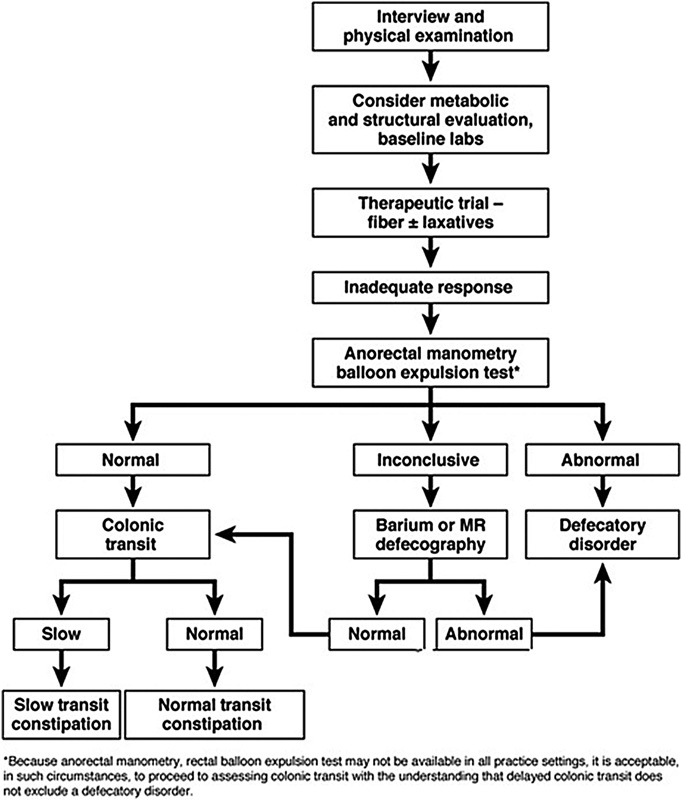
Treatment algorithm for chronic constipation. MR, magnetic resonance (Reprinted with permission from Bharucha et al.36)
Colon transit studies are useful in the differentiation of STC from obstructed defecation, typically using radiopaque marker ingestion (i.e., Sitzmark study), nuclear scintigraphy, or several other methods which are briefly discussed later (Fig. 2).36 There are multiple variations of the radiopaque marker transit study, but typically an individual is started on a high-fiber diet (20–30 g/day) and ingests a capsule containing 24 radiopaque markers (commonly polyvinyl chloride markers or barium-impregnated polyethylene pellets). The patient is instructed to abstain from any stool softeners, laxatives, enemas, motility agents, or any other medication which may affect bowel function for 5 days prior to and during the study. Serial abdominal films are obtained 5 days after marker ingestion. Normal transit is suggested by the presence of fewer than 5 markers remaining in the colon by the fifth day, whereas STC is likely if markers are seen throughout the colon at the 5 day mark. A functional outlet obstruction must be considered if markers simply accumulate in the rectosigmoid colon.4 8 39 42 43 Mean normal colonic transit times in women are 39 versus 31 hours in men.42 More complex tests such as daily abdominal films starting 3 days post–initial ingestion or the ingestion of differently shaped radiopaque markers for 3 consecutive days may be performed to evaluate the transit times of each colonic segment, although these tests are often difficult to interpret and are not always reproducible. An example of such a test was developed by Metcalf and Ross.42 Under this protocol, three different types of radiopaque markers are ingested (O markers and Double-D markers on day 1 and Tri-Chamber markers on day 2). Abdominal plain films are taken on days 3 through 5 and again on day 7 if necessary. This type of study is difficult to perform and interpret; however, it can be used to identify specific colonic segments with delayed transit.
Fig. 2.
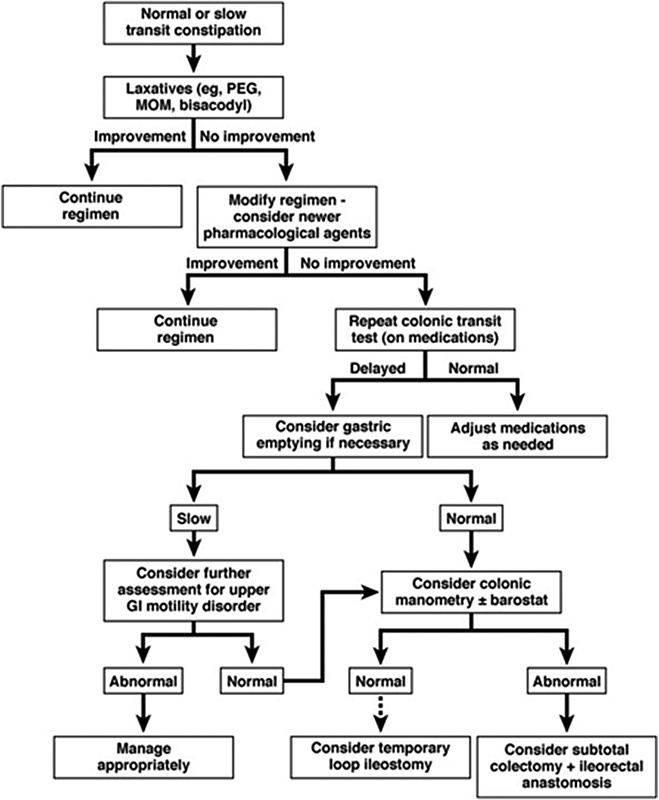
Treatment algorithm for normal transit and slow transit constipation. PEG, polyethylene glycol; MOM, milk of magnesia; GI, gastrointestinal (Reprinted with permission from Bharucha et al.36)
A barium suspension ingestion method of evaluating colonic transit has been reported to be comparable in accuracy to the radiopaque marker test, while also allowing for evaluation of the transit function of different GI segments and may represent a viable alternative diagnostic modality. This test requires the patient to ingest a barium meal and then undergo interval abdominal radiographs in the same manner as during a radiopaque marker ingestion study.44 45 Another alternative to the radiopaque marker test involves ingestion of a wireless motility capsule. This study has been reported to provide accuracy which is equivalent to radiopaque marker studies in the diagnosis of STC, and can be performed in an ambulatory setting and requires less coordination of care as no serial abdominal films need to be obtained. One limitation of this technique, however, is that the patient is required to wear a radiofrequency receiver for 5 days or until the capsule has been defecated.6 46
Nuclear scintigraphy is a less widely available method of studying colonic transit time and is more costly than traditional radiopaque marker ingestion; however, scintigraphy requires only 24 to 48 hours to complete.4 8 Typically the radionucleotide 111-diethylenetriamine pentaacetic acid (111In-DTPA) is given in a resin-coated capsule which dissolves in the distal ileum at a pH of 7.4. Using a gamma probe, the individual undergoes scintigraphic scanning at 24 and 48 hours postingestion to identify signal distribution. This study may be combined with the ingestion of technetium-99 to evaluate small bowel (normal transit time is 90–120 minutes) and stomach motility.4 12 It is important to rule out panenteric slow transit by evaluation of the small bowel and gastric transit time once colonic slow transit has been established, as this may ultimately affect treatment decisions.
Medical Management
While the majority of patients with constipation will respond to medical therapy,4 8 47 STC is often minimally responsive to such measures.48 This is not to say that it is not worth a trial of conservative measures prior to the initiation of more invasive options, and in reality most patients are appropriately trialed on medical therapy prior to transit studies and the diagnosis of STC being obtained. Reasonable initial steps may include increasing dietary fiber intake to the current recommended level of 20 to 35 g/day, increasing water intake, and encouraging physical exercise, though there is little data to support the benefit of increased water intake or exercise when the individual is not dehydrated.2 49 The addition of laxatives is often the next step. Patients are typically started on osmotic laxatives such as polyethylene glycol or magnesium citrate, stimulant laxatives such as senna or bisacodyl, or a combination of the two.47 50 Other agents which have reportedly improved colonic transit times in individuals with STC include erythromycin (a motilin receptor agonist), misoprostol, colchicine, and the prokinetic 5-HT4 receptor agonist prucalopride. While not currently available in the United States, it has been found to increase GI as well as colonic transit without affecting rectal evacuation via increased numbers of colonic high-amplitude, propulsive contractions.3 48 Unlike tegaserod, another member of the 5-HT4 receptor agonist family which initially showed some promise in treating severe constipation but was pulled from the market due to the potential risk of adverse cardiovascular events, prucalopride has a safe cardiac profile.3 12 18 19 50 51 Linaclotide and lubiprostone are two prosecretory agents which have shown promise in the treatment of chronic idiopathic constipation as well as constipation-predominant IBS.52 53 Linaclotide, a synthetic guanylate cyclase-c receptor agonist which increases intraluminal water secretion via ion exchange, leads to increased numbers of spontaneous bowel movements as demonstrated in numerous trials.53 54 55 Lubiprostone is a prostone analog which increases intraluminal chloride secretion with subsequent influx of water. This results in increased frequency of intestinal peristaltic waves, thereby decreasing intestinal transit times.52 56 Aside from a risk of diarrhea, these agents have relatively benign side effect profiles and should be considered as second-line agents for individuals who fail trials of less expensive pharmacologic options. Additional medications which are currently being trialed include Elobixibat, an enterohepatic circulation inhibitor, and the guanylate cyclase-c agonist plecanatide.53 55
Nonpharmacologic treatment options, which have demonstrated success in small series, include biofeedback, rectal irrigation, and acupuncture.57 58 Biofeedback may be of use in cases of STC associated with pelvic floor dysfunction; however, there is no clear evidence as to the efficacy of the biofeedback techniques in the management of isolated STC and reported results are conflicting.59 60 61 62 Brown et al report the successful treatment of four individuals with isolated STC using just four biofeedback sessions. Improved symptoms including less bloating and straining with more frequent bowel movements and decreased laxative use lasted for a median of 9 months.59 In another series, up to 57% of patients at a median follow-up length of 23 months felt that their constipation as well as other abdominal symptoms had improved significantly after undergoing biofeedback therapy.60 Conversely, Battaglia et al found that only 20% of patients reported any sustained improvement in their symptoms 1 year after electromyographic biofeedback and muscle training.61
Surgical Management
Ultimately, some individuals with STC will not improve with conservative measures. Historically, the surgical treatment of choice has been subtotal colectomy with ileodistal sigmoid or ileorectal anastomosis (SC-IRA) which may be performed safely and effectively using either an open or laparoscopic approach.14 15 43 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 Prior to considering a subtotal colectomy, it is very important to rule out synchronous pelvic floor dysfunction and to address any issues with fecal incontinence. Patients should be counseled extensively regarding expected postoperative outcomes and should understand the potential for unwanted frequent bowel movements, loose stools, increased urgency, and possible clustering of bowel movements. In recent years, a variety of surgical approaches including segmental colectomy and subtotal colectomy with cecorectal anastomosis have been described, although the results of these operations have been less positive than those described after SC-IRA.4 43 72 76 81 82 83 84 85 86 87 88 89 90 91 Less invasive options including sacral nerve stimulator placement and antegrade colonic enemas have also been trialed with varying results.24 50 92 93 94 95 96 Alternatively, high-risk patients may benefit from an end ileostomy without undergoing colectomy,43 and end ileostomy represents a potential final surgical option for individuals with STC who do not benefit from subtotal colectomy.90 Ileostomy is also a viable option in individuals with STC and untreatable pelvic floor dysfunction or incontinence.
Prior to considering surgical intervention, several issues should be addressed. As mentioned previously, gastric or small bowel dysmotility as well as pelvic floor dysfunction should be ruled out prior to surgical intervention, as these conditions may warrant changes in the operative plan. Many authors report high rates of continued constipation, postoperative abdominal pain, diarrhea, and small bowel obstruction after SC-IRA in patients with STC accompanied by upper GI tract dysmotility or obstructed defecation.69 81 97 A history of sexual abuse or psychiatric illness should also be ascertained prior to surgical intervention. In a retrospective series, O'Brien et al found that of all patients who underwent SC-IRA over a 15-year period for STC, 85% were undergoing treatment for a psychiatric illness with psychotropic medication.14 Sixty-two percent of individuals reported a history of sexual abuse with most reporting some form of either vaginal or anal abuse. Those with histories of sexual abuse were far more likely to have undergone prior abdominal operations, to have increased numbers of functional diagnoses, and were more likely to seek medical care for continued abdominal complaints postcolectomy than their nonabused peers. As in all cases, it is important for the patient to understand the potential risks and benefits of any surgical intervention and to understand the potential limitations of the proposed procedure. Common risks associated with colectomy which should be discussed include anastomotic stricture or leak, postoperative bowel obstruction (up to 20% after total abdominal colectomy), wound infections, bleeding, and hernia formation. In otherwise healthy patients, surgical mortality should be <1%.43 Postoperatively, patients may experience with frequent bowel movements, urgency, and clustering of bowel movements.
SC-IRA is the most studied surgical method of managing STC. A limited review of the recent literature is provided in Table 3. Most recent series are small, involving anywhere between 4 and 96 patients, which is indicative of the relative infrequency with which colectomy is performed for refractory constipation. Though it is difficult to compare outcomes between series, reported positive outcomes, patient satisfaction scores, and quality-of-life measures ranged from 55 to 100%.14 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 In a review article from 1999 of 32 series published between 1981 and 1998 in which any part of the colon was resected for management of STC, the median “success” rate was 86%.81 Postoperative bowel function and lifestyle satisfaction scores ranged from 39 to 100% and the median number of bowel movements per day was 2.9. The incidence of both postoperative incontinence and diarrhea averaged 14%, and constipation recurrence occurred in 9% with a median reoperative rate of 14%. The authors conclude that while reported surgical outcomes tend to be highly variable, when the performed operation was not selected based on segmental transit studies, superior outcomes were obtained with SC-IRA rather than with segmental colectomy or subtotal colectomy with cecorectal or ileosigmoid anastomosis. Several authors have reported the feasibility of laparoscopic SC-IRA with postoperative outcomes and complication rates that are comparable to the open approach, although the laparoscopic method often results in shorter lengths of hospital stay, less postoperative pain, and earlier return of bowel function.64 70 71 77 78
Table 3. Recent series of SC-IRA for slow-transit constipation.
| Author (year) | Number of patients | Outcomes/Notes of interest |
|---|---|---|
| Ghosh et al73 (1996) | 21 | 71% of patients with SBO at some point postoperatively |
| Platell et al74 (1996) | 96 | SC-IRA or subtotal colectomy with cecorectal anastomosis, mean f/u of 5 y, symptomatic improvement in 81.6%, reoperation in 35.6%, 55% with some degree of persistent abdominal pain |
| Lubowski et al75 (1996) | 59 | Median f/u of 42 mo, 90% “satisfied” with outcome, 52% with some degree of persistent abdominal pain |
| Nyam et al66 (1997) | 74 | Mean f/u of 56 mo, 97% of patients “satisfied,” 90% with improved quality of life, no difference in outcomes in individuals with both STC and pelvic floor dysfunction who received biofeedback preoperatively |
| Ho et al71 (1997) | 17 | Laparoscopic-assisted SC-IRA, 96% satisfaction rates |
| You et al72 (1998) | 40 | SC-IRA or segmental colectomy performed based on transit studies, mean f/u of 2 y, all patients reported “dramatic” improvement of symptoms, 92% with “satisfactory” bowel movements |
| Pikarsky et al63 (2001) | 30 | Mean f/u of 8.9 y, “Excellent” outcome reported by 100% of patients, mean of 2.5 BM per day, 20% with postoperative SBO, 6% with continued constipation, 6% with diarrhea |
| Athanasakis et al70 (2001) | 4 | Laparoscopic SC-IRA, mean of 2–4 BM per day postoperatively at 9 mo f/u |
| Verne et al69 (2002) | 13 | SC-IRA (6 patients) versus subtotal colectomy with ileosigmoid anastomosis (7 patients), no significant difference between groups, average BM increased from 0.5 to 15 per week |
| FitzHarris et al67 (2003) | 75 | Mean f/u of 3.9 y, some patients with end colostomy after subtotal colectomy, 81% “pleased” with outcome, postoperatively 41% with some degree of abdominal pain, 21% with incontinence, 93% would undergo colectomy again if given the chance |
| Thaler et al80 (2005) | 17 | Mean f/u of 58.3 mo, all patients had “some relief” from constipation, 41% with persistent abdominal pain, 47% with occasional incontinence to gas or stool |
| Zutshi et al79 (2007) | 35 | Mean f/u of 10.8 y, 77% felt that surgery was “beneficial” in treating their constipation, 9% had persistent constipation |
| Hsiao et al77 (2008) | 44 | Hand-assisted laparoscopic SC-IRA, 88.6% with “excellent or good” satisfaction scores |
| Jiang et al76 (2008) | 20 | Mean f/u of 4 y, mean GIQLI score of 111, 65% success rate, postoperative average of 3.4 BM per day |
| Riss et al65 (2009) | 12 | Mean f/u 84 mo, postoperative mean GIQLI of 80, 50% with continued constipation |
| O'Brien et al14 (2009) | 13 | Mean f/u of 97 mo, 100% of patients were “highly satisfied” |
| Pinedo et al64 (2009) | 20 | Mean f/u of 25 mo, mean level of satisfaction was 8 out of 10, 95% would recommend surgery to others, 35% with “significant” postoperative complications |
| Sohn68 (2011) | 37 | Mean f/u of 3.4 y, 81.9% of patients “satisfied” with surgical outcome, postoperative ileus in 10.8% |
| Sheng et al78 (2014) | 68 | Hand-assisted laparoscopic SC-IRA (32 patients), open SC-IRA (36 patients), postoperative complication rates similar between groups, laparoscopic group with lower pain scores, earlier return of bowel function, and shorter length of hospital stay |
Abbreviations: BM, bowel movement; f/u, follow-up; GIQLI, gastrointestinal quality-of-life index (control score 126); SBO, small bowel obstruction.
Notes: All patients underwent open subtotal colectomy with ileorectal anastomosis (SC-IRA) unless otherwise noted.
Multiple series describe subtotal colectomy with an antiperistaltic cecorectal anastomosis (Sarli procedure) performed in both the open and laparoscopic fashion as an alternative to ileorectal anastomosis. The proposed advantage of cecorectal anastomosis is that the ileocecal valve is preserved.76 81 83 87 Marchesi et al report that of 43 patients who underwent subtotal colectomy with antiperistaltic cecorectal anastomosis, the mean gastrointestinal quality of life index (GIQLI) scores postoperatively were 115.5 compared with a control score of 125.8.83 Around 88% of individuals stated that they would undergo the procedure again if given the choice. Another series reported postoperative GIQLI scores of 119 in patients who underwent the Sarli procedure.76 These results are comparable to reported outcomes after ileorectal anastomosis. In a separate study, Marchesi et al reported success with laparoscopic subtotal colectomy with antiperistaltic cecorectal anastomosis in the management of STC.98 Compared with patients who underwent an open operation, the laparoscopic group experienced less pain and had earlier return of bowel function with equivalent complication rates. Bowel movements per day at 1 year follow-up in the laparoscopic and open cohorts were 2.8 and 2.4, respectively. Postoperative GIQLI scores at 1 year were equal at 115. Interestingly, there was a higher early fecal incontinence rate in the laparoscopic group, though this equilibrated with time. In a series of 70 patients with STC who underwent subtotal colectomy with either cecorectal or ileosigmoid anastomosis, Feng and Jianjiang found that more patients in the cecorectal group had issues with persistent constipation (27 vs. 7%).88 Additionally, long-term satisfaction score was 93% in the ileosigmoid anastomosis group versus 74% in the cecorectal anastomosis group.
When compared with subtotal colectomy, segmental colectomy is a less extensive procedure and in theory preserves some colonic function; however, in practice it is often difficult to determine which segment is responsible for the delayed transit and thus which segment should be resected. Therefore, when considering a segmental resection, preoperative segmental colonic transit evaluation is critical. As discussed earlier, a variety of methods including radiopaque marker studies, nuclear scintigraphy, barium suspension ingestion tests, and wireless motility capsule studies may be used to diagnose segmental areas of inertia.4 6 43 44 46 Reports concerning outcomes after segmental colectomy in individuals with proven discrete areas of colonic inertia are conflicting.72 81 84 Lundin et al found that 23 out of 28 patients who underwent segmental resection were “pleased with their outcome.”84 Stool frequency increased from one bowel movement per week to one bowel movement per day postoperatively. The authors conclude that segmental colectomy provides similar outcomes to SC-IRA and should be considered in selected patients, although this has not been confirmed by other series. Impaired rectal sensation was noted in patients who failed treatment and was thought to be predictive of poor outcomes after segmental colectomy. Another group found that out of 40 patients who underwent segmental colectomy, most experienced “dramatic improvement of symptoms.” Those individuals with constipation after segmental colectomy typically required reoperation with completion colectomy.72 This suggests that in carefully selected patients, segmental colectomy may be a reasonable starting point with the potential to preserve some degree of colonic function. Improvement in symptoms is achieved in only a proportion of patients when compared with the more extensive subtotal colectomy, while exposing the individual to the same surgical risks; thus, segmental colectomy has only limited use in everyday practice. It is reasonable to consider segmental resection in cases of rectal prolapse and associated constipation (i.e., sigmoid colectomy and rectopexy, otherwise known as the Frykman-Goldberg procedure), but in other cases, a more definitive approach should be undertaken.
The management of patients with STC combined with obstructed defecation, as is the case in up to 25% of cases, represents a particular challenge.9 These patients may benefit from ST-IRA if there is a good response to preoperative biofeedback exercises. Multiple authors have found no difference in functional outcomes postcolectomy in patients with STC combined with pelvic floor dysfunction who received preoperative biofeedback therapy versus patients with STC only.12 15 66 99 Multiple other operative techniques have been described for managing these individuals, but most series involve a limited number of patients and only short-term follow-up. Ding et al describe 42 patients with combined STC and obstructed defecation who underwent subtotal colectomy with ascending colon to rectum anastomosis.89 This technique resulted in improved constipation scores in all patients with a 90% overall “satisfaction” rate at 2 years. Of note, 17 out of 21 patients no longer had evidence of intussusception which was visible on preoperative defecography and only 7 of 29 patients who had a rectocele preoperatively continued to have a detectable rectocele in the follow-up period. In 2014, a group from China reported an alternative procedure for the management of STC combined with obstructed defecation. The so-called Jinling procedure combines a subtotal colectomy with a midposterior ascending colon to rectal anastomosis combined with a side-to-side cecorectal anastomosis; in essence creating a long side-to-side colorectal anastomosis. The authors reported a 24% operative morbidity rate with four anastomotic leaks. In 117 patients who were followed over a 4-year period, the mean GIQLI score was 111, with 93% of patients reporting “adequate” postoperative satisfaction levels.91 Restorative proctocolectomy with ileal pouch anal anastomosis represents another option in individuals with STC and concomitant rectal inertia. In one series, 15 patients underwent ileal pouch-anal anastomosis with a temporary loop ileostomy over a 7-year period. Postoperatively, the mean number of bowel movements per day was five and most individuals reported significant lifestyle improvements. Two individuals did eventually require pouch excision due to pain.86 Overall, little data are available for this approach, and the use of this procedure should be considered on a case-by-case bases.
In some situations, despite extensive workup, it remains unclear which parts of the GI tract are dysfunctional and how much each segment contributes to the patient's constipation. Patients may also be ambivalent about exchanging functional issues related to constipation, for ones related to removal of the colon, or in the cases when stoma creation is considered, potential permanency of one. In these cases, it is reasonable to consider a trial of ileostomy, loop, or divided loop (i.e., functional ileostomy). If the primary problem is due to colonic dysfunction or pelvic floor dysfunction, or a combination of both, stoma creation would eliminate most of the patient's symptoms. If there is impaired transit of the entire GI tract, functional issues will persist even after ileostomy formation. Temporary ostomy placement also allows the patient to experience what it is like to have a stoma while maintaining the ability for a quick conversion to “normal” anatomy, although reversal is associated with surgical risks.
As noted previously, the frequency of high-amplitude, propagated colonic contractions is reduced in individuals with STC. Some authors suggest improvement in STC symptoms via increasingly frequent colonic propagating pressure waves after sacral nerve stimulator placement in both children and adults24 92 93 96; however, other reports are less convincing.100 101 In 2007, Dinning et al showed that electrodes implanted in the S2 and S3 sacral nerve foramina resulted in increased pancolonic antegrade, propagating wave frequency with some improvement in bowel frequency in six out of eight patients who underwent implantation.24 However, a recently reported double-blind randomized controlled trial in which 55 patients with STC underwent permanent sacral nerve stimulator placement found no significant difference in symptom improvement between the treatment and sham arms. The authors concluded that sacral nerve stimulation did not result in any significant improvement in bowel movement frequency.100
Antegrade colonic enema via an appendicostomy or neoappendicostomy is another technique which has been used in the management of STC and may prove successful in improving constipation in up to half of individuals,50 94 95 102 although most of the data supporting the use of this technique are in the pediatric literature. In a group of 12 individuals with STC who underwent laparoscopic creation of an enema access point in the right lower quadrant, the median frequency of defecation was one per day using a variety of enema regimens. Four of 12 patients eventually required a subtotal colectomy. The authors noted that cecal access for initiation of antegrade colonic enemas is a minimally invasive procedure which does not limit the possibility of future colectomy in the event of failure, making this a potentially viable option for trial prior to undergoing colonic resection.94
Conclusion
STC accounts for a significant number of cases of functional constipation. The diagnosis of STC can be made using a variety of modalities and it is important when evaluating these patients to rule out poor gastric and/or small bowel motility as well as obstructed defecation, as the presence of dysmotility outside of the colon has significant therapeutic implications. A trial of medical therapy is often indicated and is typically attempted prior to the acquisition of transit studies; however, conservative measures will frequently fail in individuals with STC. Techniques such as sacral nerve stimulator placement and biofeedback have been met with minimal success, though data at this time are relatively limited. Although there are a variety of different surgical options available, SC-IRA is the best studied and has the best track record with many series reporting significant improvements in quality of life postoperatively. In recent years, this procedure has been performed laparoscopically with at least equivalent functional outcomes. Subtotal colectomy with an antiperistaltic cecorectal anastomosis has demonstrated success in multiple series, and in selected individuals, segmental colectomy may represent a potential option as well, although several patients will ultimately require completion colectomy. Ileostomy is a reasonable option in patients who are of high operative risk, in those who continue to have issues with constipation after subtotal colectomy and in individuals with both STC and pelvic floor dysfunction or issues with fecal incontinence. Individuals with STC accompanied by obstructed defecation may experience improved outcomes if treated with biofeedback prior to undergoing a colectomy; however, these cases should be approached with caution. In all instances, preoperative counseling is vital in order for the patient to have a clear understanding of the potential benefits and limitations of surgical intervention and to establish reasonable postoperative expectations.
References
- 1.Higgins P D, Johanson J F. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004;99(4):750–759. doi: 10.1111/j.1572-0241.2004.04114.x. [DOI] [PubMed] [Google Scholar]
- 2.DiPalma J A. Current treatment options for chronic constipation. Rev Gastroenterol Disord. 2004;4 02:S34–S42. [PubMed] [Google Scholar]
- 3.Dhruva Rao P K, Lewis M, Peiris S P, Shah P R, Haray P N. Long-term outcome of prucalopride for chronic constipation: a single-centre study. Colorectal Dis. 2015;17(12):1079–1084. doi: 10.1111/codi.12993. [DOI] [PubMed] [Google Scholar]
- 4.Alame A M, Bahna H. Evaluation of constipation. Clin Colon Rectal Surg. 2012;25(1):5–11. doi: 10.1055/s-0032-1301753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johanson J F, Sonnenberg A, Koch T R. Clinical epidemiology of chronic constipation. J Clin Gastroenterol. 1989;11(5):525–536. doi: 10.1097/00004836-198910000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Rao S S, Coss-Adame E, Valestin J, Mysore K. Evaluation of constipation in older adults: radioopaque markers (ROMs) versus wireless motility capsule (WMC) Arch Gerontol Geriatr. 2012;55(2):289–294. doi: 10.1016/j.archger.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Longstreth G F, Thompson W G, Chey W D, Houghton L A, Mearin F, Spiller R C. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 8.Denoya P, Sands D R. Anorectal physiologic evaluation of constipation. Clin Colon Rectal Surg. 2008;21(2):114–121. doi: 10.1055/s-2008-1075860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shahid S, Ramzan Z, Maurer A H, Parkman H P, Fisher R S. Chronic idiopathic constipation: more than a simple colonic transit disorder. J Clin Gastroenterol. 2012;46(2):150–154. doi: 10.1097/MCG.0b013e318231fc64. [DOI] [PubMed] [Google Scholar]
- 10.El-Salhy M. Chronic idiopathic slow transit constipation: pathophysiology and management. Colorectal Dis. 2003;5(4):288–296. doi: 10.1046/j.1463-1318.2003.00498.x. [DOI] [PubMed] [Google Scholar]
- 11.Bassotti G, Roberto G D, Sediari L, Morelli A. Toward a definition of colonic inertia. World J Gastroenterol. 2004;10(17):2465–2467. doi: 10.3748/wjg.v10.i17.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frattini J C, Nogueras J J. Slow transit constipation: a review of a colonic functional disorder. Clin Colon Rectal Surg. 2008;21(2):146–152. doi: 10.1055/s-2008-1075864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Surrenti E, Rath D M, Pemberton J H, Camilleri M. Audit of constipation in a tertiary referral gastroenterology practice. Am J Gastroenterol. 1995;90(9):1471–1475. [PubMed] [Google Scholar]
- 14.O'Brien S, Hyman N, Osler T, Rabinowitz T. Sexual abuse: a strong predictor of outcomes after colectomy for slow-transit constipation. Dis Colon Rectum. 2009;52(11):1844–1847. doi: 10.1007/DCR.0b013e3181b13408. [DOI] [PubMed] [Google Scholar]
- 15.Reshef A, Alves-Ferreira P, Zutshi M, Hull T, Gurland B. Colectomy for slow transit constipation: effective for patients with coexistent obstructed defecation. Int J Colorectal Dis. 2013;28(6):841–847. doi: 10.1007/s00384-012-1498-3. [DOI] [PubMed] [Google Scholar]
- 16.Corman M. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. Colon and Rectal Surgery. 5th ed; pp. 457–486. [Google Scholar]
- 17.Bassotti G, Chiarioni G, Imbimbo B P. et al. Impaired colonic motor response to cholinergic stimulation in patients with severe chronic idiopathic (slow transit type) constipation. Dig Dis Sci. 1993;38(6):1040–1045. doi: 10.1007/BF01295719. [DOI] [PubMed] [Google Scholar]
- 18.Bassotti G, Chistolini F, Nzepa F S, Morelli A. Colonic propulsive impairment in intractable slow-transit constipation. Arch Surg. 2003;138(12):1302–1304. doi: 10.1001/archsurg.138.12.1302. [DOI] [PubMed] [Google Scholar]
- 19.Shafik A, Shafik A A, El-Sibai O, Ahmed I. Colonic pacing. A therapeutic option for the treatment of constipation due to total colonic inertia. Arch Surg. 2003;138(7):1007–1011. doi: 10.1001/archsurg.139.7.775. [DOI] [PubMed] [Google Scholar]
- 20.He C-L, Burgart L, Wang L. et al. Decreased interstitial cell of cajal volume in patients with slow-transit constipation. Gastroenterology. 2000;118(1):14–21. doi: 10.1016/s0016-5085(00)70409-4. [DOI] [PubMed] [Google Scholar]
- 21.Bassotti G, Stanghellini V, Chiarioni G. et al. Upper gastrointestinal motor activity in patients with slow-transit constipation. Further evidence for an enteric neuropathy. Dig Dis Sci. 1996;41(10):1999–2005. doi: 10.1007/BF02093603. [DOI] [PubMed] [Google Scholar]
- 22.Camilleri M, Fealey R D. Idiopathic autonomic denervation in eight patients presenting with functional gastrointestinal disease. A causal association? Dig Dis Sci. 1990;35(5):609–616. doi: 10.1007/BF01540409. [DOI] [PubMed] [Google Scholar]
- 23.Knowles C H, Scott S M, Lunniss P J. Slow transit constipation: a disorder of pelvic autonomic nerves? Dig Dis Sci. 2001;46(2):389–401. doi: 10.1023/a:1005665218647. [DOI] [PubMed] [Google Scholar]
- 24.Dinning P G, Fuentealba S E, Kennedy M L, Lubowski D Z, Cook I J. Sacral nerve stimulation induces pan-colonic propagating pressure waves and increases defecation frequency in patients with slow-transit constipation. Colorectal Dis. 2007;9(2):123–132. doi: 10.1111/j.1463-1318.2006.01096.x. [DOI] [PubMed] [Google Scholar]
- 25.Bassotti G, Villanacci V, Rostami Nejad M. Chronic constipation: no more idiopathic, but a true neuropathological entity. Gastroenterol Hepatol Bed Bench. 2011;4(3):109–115. [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H L. Understanding the pathogenesis of slow-transit constipation: one step forward. Dig Dis Sci. 2015;60(8):2216–2218. doi: 10.1007/s10620-015-3754-1. [DOI] [PubMed] [Google Scholar]
- 27.Han E C, Oh H K, Ha H K. et al. Favorable surgical treatment outcomes for chronic constipation with features of colonic pseudo-obstruction. World J Gastroenterol. 2012;18(32):4441–4446. doi: 10.3748/wjg.v18.i32.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bassotti G, Villanacci V, Nascimbeni R. et al. Colonic mast cells in controls and slow transit constipation patients. Aliment Pharmacol Ther. 2011;34(1):92–99. doi: 10.1111/j.1365-2036.2011.04684.x. [DOI] [PubMed] [Google Scholar]
- 29.Liu W, Zhang Q, Li S. et al. The relationship between colonic macrophages and microRNA-128 in the pathogenesis of slow transit constipation. Dig Dis Sci. 2015;60(8):2304–2315. doi: 10.1007/s10620-015-3612-1. [DOI] [PubMed] [Google Scholar]
- 30.Lyford G L, He C-L, Soffer E. et al. Pan-colonic decrease in interstitial cells of Cajal in patients with slow transit constipation. Gut. 2002;51(4):496–501. doi: 10.1136/gut.51.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toman J, Turina M, Ray M, Petras R E, Stromberg A J, Galandiuk S. Slow transit colon constipation is not related to the number of interstitial cells of Cajal. Int J Colorectal Dis. 2006;21(6):527–532. doi: 10.1007/s00384-005-0041-1. [DOI] [PubMed] [Google Scholar]
- 32.Scott S M, Knowles C H, Newell M, Garvie N, Williams N S, Lunniss P J. Scintigraphic assessment of colonic transit in women with slow-transit constipation arising de novo and following pelvic surgery or childbirth. Br J Surg. 2001;88(3):405–411. doi: 10.1046/j.1365-2168.2001.01699.x. [DOI] [PubMed] [Google Scholar]
- 33.Roe A M, Bartolo D C, Mortensen N J. Slow transit constipation. Comparison between patients with or without previous hysterectomy. Dig Dis Sci. 1988;33(9):1159–1163. doi: 10.1007/BF01535794. [DOI] [PubMed] [Google Scholar]
- 34.Zhao R H, Baig M K, Thaler K J. et al. Reduced expression of serotonin receptor(s) in the left colon of patients with colonic inertia. Dis Colon Rectum. 2003;46(1):81–86. doi: 10.1007/s10350-004-6500-x. [DOI] [PubMed] [Google Scholar]
- 35.Guarino M, Cheng L, Cicala M, Ripetti V, Biancani P, Behar J. Progesterone receptors and serotonin levels in colon epithelial cells from females with slow transit constipation. Neurogastroenterol Motil. 2011;23(6):575–e210. doi: 10.1111/j.1365-2982.2011.01705.x. [DOI] [PubMed] [Google Scholar]
- 36.Bharucha A E Dorn S D Lembo A Pressman A; American Gastroenterological Association. American Gastroenterological Association medical position statement on constipation Gastroenterology 20131441211–217. [DOI] [PubMed] [Google Scholar]
- 37.Pepin C, Ladabaum U. The yield of lower endoscopy in patients with constipation: survey of a university hospital, a public county hospital, and a Veterans Administration medical center. Gastrointest Endosc. 2002;56(3):325–332. doi: 10.1016/s0016-5107(02)70033-3. [DOI] [PubMed] [Google Scholar]
- 38.Beck D E, Jagelman D G, Fazio V W. The surgery of idiopathic constipation. Gastroenterol Clin North Am. 1987;16(1):143–156. [PubMed] [Google Scholar]
- 39.Prokesch R W, Breitenseher M J, Kettenbach J. et al. Assessment of chronic constipation: colon transit time versus defecography. Eur J Radiol. 1999;32(3):197–203. doi: 10.1016/s0720-048x(99)00037-6. [DOI] [PubMed] [Google Scholar]
- 40.Rao S S, Singh S. Clinical utility of colonic and anorectal manometry in chronic constipation. J Clin Gastroenterol. 2010;44(9):597–609. doi: 10.1097/MCG.0b013e3181e88532. [DOI] [PubMed] [Google Scholar]
- 41.Bove A, Pucciani F, Bellini M. et al. Consensus statement AIGO/SICCR: diagnosis and treatment of chronic constipation and obstructed defecation (part I: diagnosis) World J Gastroenterol. 2012;18(14):1555–1564. doi: 10.3748/wjg.v18.i14.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Metcalf A, Ross H. New York, NY: Springer; 2007. Constipation; pp. 678–686. [Google Scholar]
- 43.McCoy J A, Beck D E. Surgical management of colonic inertia. Clin Colon Rectal Surg. 2012;25(1):20–23. doi: 10.1055/s-0032-1301755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu H M, Han J G, Na Y, Zhao B, Ma H C, Wang Z J. Colonic transit time in patient with slow-transit constipation: comparison of radiopaque markers and barium suspension method. Eur J Radiol. 2011;79(2):211–213. doi: 10.1016/j.ejrad.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 45.Yuan W, Zhang Z, Liu J. et al. Simplified assessment of segmental gastrointestinal transit time with orally small amount of barium. Eur J Radiol. 2012;81(9):1986–1989. doi: 10.1016/j.ejrad.2011.05.040. [DOI] [PubMed] [Google Scholar]
- 46.Lu L, Yan G, Sun J. A study of human colonic motility in healthy and constipated subjects using the wireless capsule. Comput Biol Med. 2015;65:269–278. doi: 10.1016/j.compbiomed.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 47.Portalatin M, Winstead N. Medical management of constipation. Clin Colon Rectal Surg. 2012;25(1):12–19. doi: 10.1055/s-0032-1301754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Voderholzer W A, Schatke W, Mühldorfer B E, Klauser A G, Birkner B, Müller-Lissner S A. Clinical response to dietary fiber treatment of chronic constipation. Am J Gastroenterol. 1997;92(1):95–98. [PubMed] [Google Scholar]
- 49.Meshkinpour H, Selod S, Movahedi H, Nami N, James N, Wilson A. Effects of regular exercise in management of chronic idiopathic constipation. Dig Dis Sci. 1998;43(11):2379–2383. doi: 10.1023/a:1026609610466. [DOI] [PubMed] [Google Scholar]
- 50.Bassotti G, Blandizzi C. Understanding and treating refractory constipation. World J Gastrointest Pharmacol Ther. 2014;5(2):77–85. doi: 10.4292/wjgpt.v5.i2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spiller R. Serotonergic modulating drugs for functional gastrointestinal diseases. Br J Clin Pharmacol. 2002;54(1):11–20. doi: 10.1046/j.1365-2125.2002.01612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang S B, Marchelletta R R, Penrose H, Docherty M J, McCole D F. A comparison of linaclotide and lubiprostone dosing regimens on ion transport responses in human colonic mucosa. Pharmacol Res Perspect. 2015;3(2):e00128. doi: 10.1002/prp2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas R H, Luthin D R. Current and emerging treatments for irritable bowel syndrome with constipation and chronic idiopathic constipation: focus on prosecretory agents. Pharmacotherapy. 2015;35(6):613–630. doi: 10.1002/phar.1594. [DOI] [PubMed] [Google Scholar]
- 54.Love B L, Johnson A, Smith L S. Linaclotide: a novel agent for chronic constipation and irritable bowel syndrome. Am J Health Syst Pharm. 2014;71(13):1081–1091. doi: 10.2146/ajhp130575. [DOI] [PubMed] [Google Scholar]
- 55.Corsetti M, Tack J. New pharmacological treatment options for chronic constipation. Expert Opin Pharmacother. 2014;15(7):927–941. doi: 10.1517/14656566.2014.900543. [DOI] [PubMed] [Google Scholar]
- 56.Wilson N, Schey R. Lubiprostone in constipation: clinical evidence and place in therapy. Ther Adv Chronic Dis. 2015;6(2):40–50. doi: 10.1177/2040622314567678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang J, Yi Y, Zhan L. Acupuncture: a good choice to patients with intractable slow-transit constipation. Int J Colorectal Dis. 2015;30(5):721–722. doi: 10.1007/s00384-014-2048-y. [DOI] [PubMed] [Google Scholar]
- 58.Chan D S, Saklani A, Shah P R, Lewis M, Haray P N. Rectal irrigation: a useful tool in the armamentarium for functional bowel disorders. Colorectal Dis. 2012;14(6):748–752. doi: 10.1111/j.1463-1318.2011.02797.x. [DOI] [PubMed] [Google Scholar]
- 59.Brown S R Donati D Seow-Choen F Ho Y-H Biofeedback avoids surgery in patients with slow-transit constipation: report of four cases Dis Colon Rectum 2001445737–739., discussion 739–740 [DOI] [PubMed] [Google Scholar]
- 60.Chiotakakou-Faliakou E, Kamm M A, Roy A J, Storrie J B, Turner I C. Biofeedback provides long-term benefit for patients with intractable, slow and normal transit constipation. Gut. 1998;42(4):517–521. doi: 10.1136/gut.42.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Battaglia E, Serra A M, Buonafede G. et al. Long-term study on the effects of visual biofeedback and muscle training as a therapeutic modality in pelvic floor dyssynergia and slow-transit constipation. Dis Colon Rectum. 2004;47(1):90–95. doi: 10.1007/s10350-003-0010-0. [DOI] [PubMed] [Google Scholar]
- 62.Wang J, Luo M-H, Qi Q-H, Dong Z-L. Prospective study of biofeedback retraining in patients with chronic idiopathic functional constipation. World J Gastroenterol. 2003;9(9):2109–2113. doi: 10.3748/wjg.v9.i9.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pikarsky A J, Singh J J, Weiss E G, Nogueras J J, Wexner S D. Long-term follow-up of patients undergoing colectomy for colonic inertia. Dis Colon Rectum. 2001;44(2):179–183. doi: 10.1007/BF02234290. [DOI] [PubMed] [Google Scholar]
- 64.Pinedo G, Zarate A J, Garcia E, Molina M E, Lopez F, Zúñiga A. Laparoscopic total colectomy for colonic inertia: surgical and functional results. Surg Endosc. 2009;23(1):62–65. doi: 10.1007/s00464-008-9901-4. [DOI] [PubMed] [Google Scholar]
- 65.Riss S, Herbst F, Birsan T, Stift A. Postoperative course and long term follow up after colectomy for slow transit constipation—is surgery an appropriate approach? Colorectal Dis. 2009;11(3):302–307. doi: 10.1111/j.1463-1318.2008.01592.x. [DOI] [PubMed] [Google Scholar]
- 66.Nyam D C, Pemberton J H, Ilstrup D M, Rath D M. Long-term results of surgery for chronic constipation. Dis Colon Rectum. 1997;40(3):273–279. doi: 10.1007/BF02050415. [DOI] [PubMed] [Google Scholar]
- 67.FitzHarris G P, Garcia-Aguilar J, Parker S C. et al. Quality of life after subtotal colectomy for slow-transit constipation: both quality and quantity count. Dis Colon Rectum. 2003;46(4):433–440. doi: 10.1007/s10350-004-6576-3. [DOI] [PubMed] [Google Scholar]
- 68.Sohn G, Yu C S, Kim C W. et al. Surgical outcomes after total colectomy with ileorectal anastomosis in patients with medically intractable slow transit constipation. J Korean Soc Coloproctol. 2011;27(4):180–187. doi: 10.3393/jksc.2011.27.4.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Verne G N, Hocking M P, Davis R H. et al. Long-term response to subtotal colectomy in colonic inertia. J Gastrointest Surg. 2002;6(5):738–744. doi: 10.1016/s1091-255x(02)00022-7. [DOI] [PubMed] [Google Scholar]
- 70.Athanasakis H, Tsiaoussis J, Vassilakis J S, Xynos E. Laparoscopically assisted subtotal colectomy for slow-transit constipation. Surg Endosc. 2001;15(10):1090–1092. doi: 10.1007/s004640090046. [DOI] [PubMed] [Google Scholar]
- 71.Ho Y H, Tan M, Eu K W, Leong A, Choen F S. Laparoscopic-assisted compared with open total colectomy in treating slow transit constipation. Aust N Z J Surg. 1997;67(8):562–565. doi: 10.1111/j.1445-2197.1997.tb02039.x. [DOI] [PubMed] [Google Scholar]
- 72.You Y T, Wang J Y, Changchien C R. et al. Segmental colectomy in the management of colonic inertia. Am Surg. 1998;64(8):775–777. [PubMed] [Google Scholar]
- 73.Ghosh S, Papachrysostomou M, Batool M, Eastwood M A. Long-term results of subtotal colectomy and evidence of noncolonic involvement in patients with idiopathic slow-transit constipation. Scand J Gastroenterol. 1996;31(11):1083–1091. doi: 10.3109/00365529609036891. [DOI] [PubMed] [Google Scholar]
- 74.Platell C, Scache D, Mumme G, Stitz R. A long-term follow-up of patients undergoing colectomy for chronic idiopathic constipation. Aust N Z J Surg. 1996;66(8):525–529. doi: 10.1111/j.1445-2197.1996.tb00802.x. [DOI] [PubMed] [Google Scholar]
- 75.Lubowski D Z, Chen F C, Kennedy M L, King D W. Results of colectomy for severe slow transit constipation. Dis Colon Rectum. 1996;39(1):23–29. doi: 10.1007/BF02048263. [DOI] [PubMed] [Google Scholar]
- 76.Jiang C Q, Qian Q, Liu Z S, Bangoura G, Zheng K Y, Wu Y H. Subtotal colectomy with antiperistaltic cecoproctostomy for selected patients with slow transit constipation-from Chinese report. Int J Colorectal Dis. 2008;23(12):1251–1256. doi: 10.1007/s00384-008-0552-7. [DOI] [PubMed] [Google Scholar]
- 77.Hsiao K C, Jao S W, Wu C C, Lee T Y, Lai H J, Kang J C. Hand-assisted laparoscopic total colectomy for slow transit constipation. Int J Colorectal Dis. 2008;23(4):419–424. doi: 10.1007/s00384-007-0431-7. [DOI] [PubMed] [Google Scholar]
- 78.Sheng Q S, Lin J J, Chen W B. et al. Comparison of hand-assisted laparoscopy with open total colectomy for slow transit constipation: a retrospective study. J Dig Dis. 2014;15(8):419–424. doi: 10.1111/1751-2980.12156. [DOI] [PubMed] [Google Scholar]
- 79.Zutshi M, Hull T L, Trzcinski R, Arvelakis A, Xu M. Surgery for slow transit constipation: are we helping patients? Int J Colorectal Dis. 2007;22(3):265–269. doi: 10.1007/s00384-006-0189-3. [DOI] [PubMed] [Google Scholar]
- 80.Thaler K, Dinnewitzer A, Oberwalder M. et al. Quality of life after colectomy for colonic inertia. Tech Coloproctol. 2005;9(2):133–137. doi: 10.1007/s10151-005-0211-8. [DOI] [PubMed] [Google Scholar]
- 81.Knowles C H, Scott M, Lunniss P J. Outcome of colectomy for slow transit constipation. Ann Surg. 1999;230(5):627–638. doi: 10.1097/00000658-199911000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Iannelli A, Fabiani P, Mouiel J, Gugenheim J. Laparoscopic subtotal colectomy with cecorectal anastomosis for slow-transit constipation. Surg Endosc. 2006;20(1):171–173. doi: 10.1007/s00464-005-0099-4. [DOI] [PubMed] [Google Scholar]
- 83.Marchesi F, Sarli L, Percalli L. et al. Subtotal colectomy with antiperistaltic cecorectal anastomosis in the treatment of slow-transit constipation: long-term impact on quality of life. World J Surg. 2007;31(8):1658–1664. doi: 10.1007/s00268-007-9111-6. [DOI] [PubMed] [Google Scholar]
- 84.Lundin E, Karlbom U, Påhlman L, Graf W. Outcome of segmental colonic resection for slow-transit constipation. Br J Surg. 2002;89(10):1270–1274. doi: 10.1046/j.1365-2168.2002.02213.x. [DOI] [PubMed] [Google Scholar]
- 85.Akervall S, Fasth S, Nordgren S, Oresland T, Hultén L. The functional results after colectomy and ileorectal anastomosis for severe constipation (Arbuthnot Lane's disease) as related to rectal sensory function. Int J Colorectal Dis. 1988;3(2):96–101. doi: 10.1007/BF01645313. [DOI] [PubMed] [Google Scholar]
- 86.Kalbassi M R, Winter D C, Deasy J M. Quality-of-life assessment of patients after ileal pouch-anal anastomosis for slow-transit constipation with rectal inertia. Dis Colon Rectum. 2003;46(11):1508–1512. doi: 10.1007/s10350-004-6804-x. [DOI] [PubMed] [Google Scholar]
- 87.Sarli L, Costi R, Sarli D, Roncoroni L. Pilot study of subtotal colectomy with antiperistaltic cecoproctostomy for the treatment of chronic slow-transit constipation. Dis Colon Rectum. 2001;44(10):1514–1520. doi: 10.1007/BF02234608. [DOI] [PubMed] [Google Scholar]
- 88.Feng Y, Jianjiang L. Functional outcomes of two types of subtotal colectomy for slow-transit constipation: ileosigmoidal anastomosis and cecorectal anastomosis. Am J Surg. 2008;195(1):73–77. doi: 10.1016/j.amjsurg.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 89.Ding W, Jiang J, Feng X, Ni L, Li J, Li N. Clinical and pelvic morphologic correlation after subtotal colectomy with colorectal anastomosis for combined slow-transit constipation and obstructive defecation. Dis Colon Rectum. 2015;58(1):91–96. doi: 10.1097/DCR.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 90.Ghellal A, Trilling B, Izard G, Faucheron J L. Permanent ileostomy as the last surgical option for severe slow-transit constipation. Tech Coloproctol. 2015;19(6):373–374. doi: 10.1007/s10151-015-1316-3. [DOI] [PubMed] [Google Scholar]
- 91.Li N, Jiang J, Feng X, Ding W, Liu J, Li J. Long-term follow-up of the Jinling procedure for combined slow-transit constipation and obstructive defecation. Dis Colon Rectum. 2013;56(1):103–112. doi: 10.1097/DCR.0b013e318273a182. [DOI] [PubMed] [Google Scholar]
- 92.Kenefick N J, Vaizey C J, Cohen C R, Nicholls R J, Kamm M A. Double-blind placebo-controlled crossover study of sacral nerve stimulation for idiopathic constipation. Br J Surg. 2002;89(12):1570–1571. doi: 10.1046/j.1365-2168.2002.02278.x. [DOI] [PubMed] [Google Scholar]
- 93.Malouf A J, Wiesel P H, Nicholls T, Nicholls R J, Kamm M A. Short-term effects of sacral nerve stimulation for idiopathic slow transit constipation. World J Surg. 2002;26(2):166–170. doi: 10.1007/s00268-001-0202-5. [DOI] [PubMed] [Google Scholar]
- 94.Rongen M J, van der Hoop A G, Baeten C G. Cecal access for antegrade colon enemas in medically refractory slow-transit constipation: a prospective study. Dis Colon Rectum. 2001;44(11):1644–1649. doi: 10.1007/BF02234385. [DOI] [PubMed] [Google Scholar]
- 95.Worsøe J, Christensen P, Krogh K, Buntzen S, Laurberg S. Long-term results of antegrade colonic enema in adult patients: assessment of functional results. Dis Colon Rectum. 2008;51(10):1523–1528. doi: 10.1007/s10350-008-9401-6. [DOI] [PubMed] [Google Scholar]
- 96.Lu M L, He J, Lu S. Electrical stimulation therapy for slow transit constipation in children: a systematic review. Int J Colorectal Dis. 2015;30(5):697–702. doi: 10.1007/s00384-015-2180-3. [DOI] [PubMed] [Google Scholar]
- 97.Glia A, Akerlund J E, Lindberg G. Outcome of colectomy for slow-transit constipation in relation to presence of small-bowel dysmotility. Dis Colon Rectum. 2004;47(1):96–102. doi: 10.1007/s10350-003-0016-7. [DOI] [PubMed] [Google Scholar]
- 98.Marchesi F, Percalli L, Pinna F, Cecchini S, Ricco' M, Roncoroni L. Laparoscopic subtotal colectomy with antiperistaltic cecorectal anastomosis: a new step in the treatment of slow-transit constipation. Surg Endosc. 2012;26(6):1528–1533. doi: 10.1007/s00464-011-2092-4. [DOI] [PubMed] [Google Scholar]
- 99.Pemberton J H Rath D M Ilstrup D M Evaluation and surgical treatment of severe chronic constipation Ann Surg 19912144403–411., discussion 411–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dinning P G, Hunt L, Patton V. et al. Treatment efficacy of sacral nerve stimulation in slow transit constipation: a two-phase, double-blind randomized controlled crossover study. Am J Gastroenterol. 2015;110(5):733–740. doi: 10.1038/ajg.2015.101. [DOI] [PubMed] [Google Scholar]
- 101.Ratto C Ganio E Naldini G; GINS. Long-term results following sacral nerve stimulation for chronic constipation Colorectal Dis 2015174320–328. [DOI] [PubMed] [Google Scholar]
- 102.Meurette G, Lehur P A, Coron E, Regenet N. Long-term results of Malone's procedure with antegrade irrigation for severe chronic constipation. Gastroenterol Clin Biol. 2010;34(3):209–212. doi: 10.1016/j.gcb.2009.12.009. [DOI] [PubMed] [Google Scholar]


