Abstract
Thermal tolerance is improved in burn survivors following 7 days of exercise heat acclimation. It is unknown whether post-junctional sudomotor and/or cutaneous vascular adaptations in non-injured skin contribute to this improvement. Thirty-three burn survivors were stratified into moderately (17-40% BSA grafted, n = 19) and highly (>40% BSA grafted, n = 14) skin grafted groups. Nine non-burned subjects served as controls. All subjects underwent a 7 day heat acclimation protocol, which improved thermal tolerance in all groups. Before and after this heat acclimation protocol, post-junctional cutaneous vascular responses were assessed by administering increasing doses of sodium nitroprusside (SNP) and methacholine (MCh) using intradermal microdialysis in non-injured skin. MCh infusion was also used to assess post-junctional responses in sudomotor function in non-injured skin. Cutaneous vascular responses to SNP and MCh were not different between pre and post heat acclimation in either group of burn survivors (both P > 0.05). The maximal sweating rate to MCh increased post acclimation in the control group (0.41±0.20 to 0.54±0.21 mg.min−1.cm−1; P = 0.016) but was unchanged in both groups of burn survivors (both P > 0.05). The number of sweat glands activated during the highest dose of MCh was elevated in the >40% BSA grafted group (49±16 to 56±18 glands.cm2; P = 0.005) but was unchanged in control subjects and the <40% BSA grafted group (both P > 0.05). Given that post-junctional administration of MCh and SNP did not alter sweating or skin blood flow from non-injured skin of burn survivors, improved thermal tolerance in these individuals following heat acclimation is more likely a result of either an increased sweating efficiency and/or an increased neural drive for sweating.
Introduction
In the last 10 years, approximately 200,000 civilians in the United States were hospitalized for burn related injuries 1 and approximately 16% of burn survivors experience burns covering at least 20% of their body surface area (BSA) 2. Burn survivors display evidence of heat intolerance 3 and an elevated risk of hyperthermia and thus related illnesses 4. This is evident by a greater increase in internal body temperature during exercise in a hot environment in burn survivors with grafted skin relative to unburned individuals 5-7. Split thickness grafting is commonly used to treat areas of burned skin. This procedure results in an initial physical disruption between the nerves governing skin blood flow and the vasculature in the grafted tissue, while sweat glands are typically absent in grafted skin 8-10. It is notable that in these areas of grafted skin the capacity to increase skin blood flow and sweating is significantly reduced and often absent in response to a thermal provocation such as whole-body heat stress 11-13. This reduced/absent skin blood flow and sweating responsiveness likely contributes to the impaired heat dissipation in burn survivors 14.
In uninjured subjects, heat acclimation is associated with an improved heat tolerance 15,16 owing to greater levels of skin blood flow 17-22 and sweating 17,19,20,22-24 which ultimately translate into greater whole-body heat loss 25. Up-regulation of these two thermoregulatory pathways occurs through both central and peripheral (i.e. post-junctional) cutaneous and sudomotor adaptations. In particular, a number of studies have shown that the post-junctional sensitivity of the sweat glands and cutaneous vasculature is increased in uninjured subjects following various heat acclimation regimens17,23,26-31. That said, nothing is known about the effects of heat acclimation on post-junctional sensitivity of the sweat glands and cutaneous vasculature in the non-injured, non-donor skin of burn survivors.
It is noteworthy that a 7 day exercise heat acclimation regimen can improve heat tolerance and dissipation in burn survivors 32. Whether this observation is due to central and/or peripheral thermoregulatory adaptations remains unknown. Therefore, the aim of this study was to examine if peripheral adaptations in the sweat glands and cutaneous vasculature of non-injured skin contribute to the increased thermal tolerance observed in burn victims following heat acclimation. Specifically, we tested the hypothesis that post-junctional sudomotor and cutaneous vascular responsiveness to a 7 day exercise heat acclimation regimen would be improved in the non-injured (non-donor) skin of burn survivors.
Methods
Thirty-three otherwise healthy burn survivors with grafted skin covering at least 17% of their BSA and nine non-burned control subjects completed this study. The burn survivors were stratified into two groups based upon the relative amount of BSA grafted: 17-40% (n=19) and >40% (n=14). The subject characteristics are listed in Table 1. Total BSA grafted was calculated by using the rule of nines. All subjects were free of any known cardiovascular, metabolic, neurological, or psychological diseases. Subjects taking cardiovascular acting medications, or other medications influencing heat dissipation mechanisms were excluded. Each subject was fully informed of the experimental procedures and possible risks before giving informed, written consent. This protocol and informed consent were approved by the Institutional Review Boards at the University of Texas Southwestern Medical Center at Dallas and Texas Health Presbyterian Hospital of Dallas. The present data were collected simultaneously with data previously published that addressed unique hypotheses 7,32.
Table 1.
Subject characteristics (mean ± SD).
| Burn survivors with grafted skin |
|||
|---|---|---|---|
| 17-40% BSA Grafted | >40% BSA Grafted | Control | |
| Number of subjects (male/female) | 19 (12/7) | 14 (8/6) | 9 (4/5) |
| Years post burn injury | 20.8 ± 15.8 | 12.2 ± 9.4‡ | n/a |
| Percentage of BSA Grafted (%) | 30 ± 7 | 53 ± 9‡ | n/a |
| Absolute BSA Grafted (m2) | 0.59 ± 0.17 | 1.00 ± 0.20‡ | n/a |
| Absolute BSA Ungrafted (m2) | 1.36 ± 0.21* | 0.92 ± 0.22‡* | 1.87 ± 0.16 |
| Weight (kg) | 82.9 ± 14.6 | 78.7 ± 15.5 | 75.0 ± 12.1 |
| Height (cm) | 170 ± 13 | 172 ± 8 | 172 ± 7 |
| Age (y) | 40 ± 12 | 34 ± 11 | 32 ± 10 |
BSA, Body surface area
different from 17-40% group (P≤0.059)
different from control (P<0.001
Instrumentation
Subjects visited the laboratory on two separate occasions, both before and after a 7 day heat acclimation regimen as described previously in detail 7,32. Briefly the heat acclimation regimen involved 7 days of exercise in a 39.6 ± 1.1°C temperature, 31 ± 3% relative humidity environment. Within 2 days both before and after the heat acclimation regimen, subjects arrived at the laboratory having refrained from strenuous exercise (24 hours), alcohol and caffeine (12 hours). Testing was completed in the northern hemisphere (Dallas, Texas, USA) during the fall, winter, and spring months.
The post-junctional sensitivity of sweat glands to a cholinergic agonist, and of the cutaneous vasculature to vasoactive substances, were assessed before and after the heat acclimation regimen using intradermal microdialysis. Four microdialysis membranes were inserted into unanesthetized non-injured/non-grafted skin by advancing a 25-guage needle approximately 15 to 20mm through the dermal layer, followed by threading the microdialysis probe through the lumen of the needle and finally withdrawing the needle. In all subjects the microdialysis membranes were positioned in the same location between pre and post heat acclimation trials. In non-injured controls the forearm was used to assess sweating rate while in burn survivors this was not always possible due to the site of the burn injury; in these cases non-forearm sites were used. Each microdialysis membrane consisted of two reinforced sections of polyimide tubing connected to a 1-cm dialysis membrane (Bio-analytical Systems, West Lafayette, IN). Initially, microdialysis probes were perfused with lactated ringers solution (Baxter, Deerfield, IL) at a rate of 2 μl/min as controlled by an infusion pump (Harvard Apparatus, Holliston, MA) for a minimum of 90 minutes to allow for the hyperemia associated with the insertion of microdialysis membranes to subside.
Experimental Protocol
In all groups post-junctional sweating responses were assessed by infusing methacholine (Sigma-Aldrich, MO, USA) in progressively increasing doses (MCh; 1 × 10−7 M to 1 M in 10 fold increments) through two microdialysis membranes inserted into unanesthetized non-injured/non-grafted skin. Methacholine is a non-selective muscarinic receptor agonist and an analog of acetylcholine, which is a neurotransmitter released from sympathetic cholinergic nerves. Stimulation of muscarinic receptors induces eccrine sweat gland stimulation and endothelial dependent cutaneous vasodilation. Unlike acetylcholine though, MCh is not degraded by acetylcholinesterase. Each dose of MCh was initially perfused through at a rate of 102 μl/min for 1 minute, to accelerate the time for the new dose to be presented at the membrane, and then 2 μl/min for an additional 5 minutes.
Two custom built capsules (2.54 cm2 surface area on the skin) were attached to the skin directly above each microdialysis membrane using adhesive rings (3M, Saint Paul, MN). Local sweat rate (SR; mg.min−1.cm2) was measured via capacitance hygrometry (Vaisala, Woburn, WA, USA) with the capsules perfused with 100% nitrogen at a flow rate of 300 ml/min. Each capsule also housed a laser Doppler probe (Moor Instruments, Devon, UK) allowing simultaneous measurement of local skin blood flow and sweat rate over each microdialysis membrane. Skin blood flow, sweating rate and blood pressure (Brachial artery auscultation; Tango, SunTech Medical Instruments, Raleigh, NC) were assessed in the final 30 seconds of the 5 minute perfusion period of each dose of MCh. The duration of MCh administration for the final dose persisted until a plateau in skin blood flow and sweating were observed, after which the capsules were removed and the areas were completely dried. This was followed by application of iodine permeated paper as previously reported 33,34 to obtain a measure of the number of activated sweat glands to the highest dose of MCh. Uniform exposure was ensured by attaching the iodine paper to a hard and smooth plastic surface prior to application on the skin. The duration of contact between the skin and iodine paper was adjusted to obtain the clearest image of activated sweat glands on the iodine paper. After removal of the iodine paper, the paper was scanned at high resolution and stored for subsequent analysis using the commercially available Image J program for image processing and analysis 35 as previously described 34. The number of active sweat glands was counted using the Image J software.
Post-junctional endothelial independent cutaneous vasodilation was assessed at the remaining 2 microdialysis membranes through intradermal administration of increasing doses of sodium nitroprusside (Hospira, IL, USA) (SNP; 5 × 10−8 to 5 × 10−2 M at 10 fold increments). Each dose of SNP was initially perfused through the microdialysis membrane at a rate of 102 μl /min for 1 minute and then 2 μl /min for an additional 5 minutes. Skin blood flow was assessed directly above each microdialysis membrane as previously described throughout the administration of SNP. Blood pressure measurements were likewise obtained during the final 30 seconds of each dose of SNP. The highest dose of SNP continued until a plateau in skin blood flow was observed.
Data and Statistical Analysis
Skin blood flow and sweat rate were collected continuously via a data-acquisition system, (Biopac System, Santa Barbara, CA). Responses from paired membranes were averaged at baseline and during each dose of SNP and MCh. Skin blood flow is expressed as cutaneous vascular conductance (CVC; arbitrary units (AU)·mmHg) which is calculated as laser Doppler flux divided by mean arterial pressure. Maximal cutaneous vascular conductance was calculated from maximal skin blood flow during MCh or SNP infusion. Maximal sweat rate was recorded after a plateau in sweat rate was observed during the largest dose of MCh administered. This value was subsequently divided by the number of active sweat glands to provide peak sweat output per gland (μl.min−1 per gland). The dose of MCh and SNP that resulted in half of the maximal response (i.e., EC50) was calculated using commercially available software (Prism version 6, GraphPad Software, Inc., La Jolla, CA, USA) and taken as a measure of post-junctional responsiveness.
Subject characteristics were analyzed using one-way analysis of variance (ANOVA). Post-junctional sweating (SR) and cutaneous vascular (CVC) responsiveness to SNP and MCh were compared between subject groups from pre- to post- heat acclimation using a two way mixed model ANOVA (main effects: group × heat acclimation). To examine whether the maximal sweating and skin blood flow responses were different within each group after heat acclimation, the maximal SR and CVC in response to MCh and SNP respectively were compared within groups from pre- to post- heat acclimation using a paired t-test. With the exception of EC50 identification, all data were analyzed using a commercially available statistical package (SPSS v20, IBM, NY, USA).
Results
Post-junctional sweating responses to MCh infusion
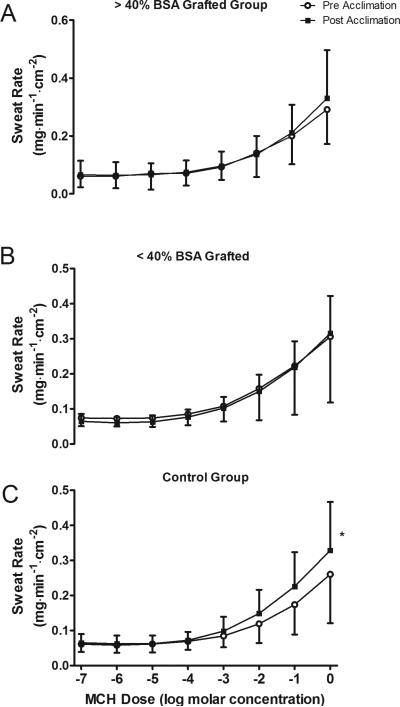
Sweat rate increased with MCh infusion in all groups for both pre and post heat acclimation trials (Main effect for drug dose; P < 0.001; Fig. 1). The increase in sweat rate was not different between pre and post heat acclimation trials in either grafted group (both P > 0.05; Fig. 1A and 1B). However, in the control group increases in sweat rate during MCh infusion were greater post heat acclimation (Main Effect P = 0.05; Fig. 1C).
Figure 1. Sweat rate responsiveness to heat acclimation.
Sudomotor responsiveness, expressed as sweating rate, to methacholine (MCh) infusion for >40% BSA grafted (A), <40% body surface area (BSA) grafted (B) and control (C) groups before and after the 7 day heat acclimation regimen. * Main effect of heat acclimation within group (P = 0.05). Data are mean ± SD
Post-junctional cutaneous vascular response to SNP and MCh infusion
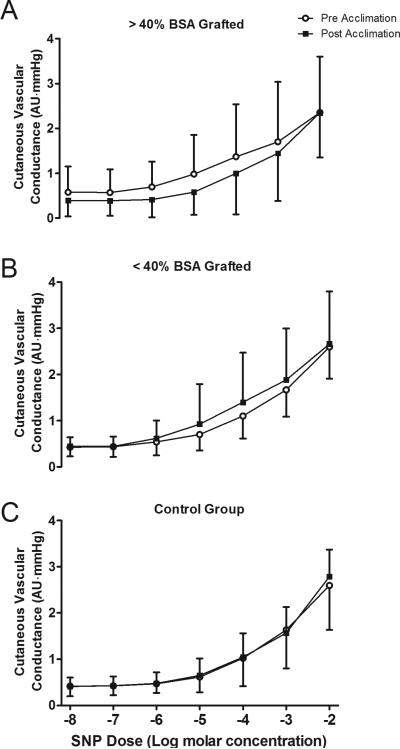
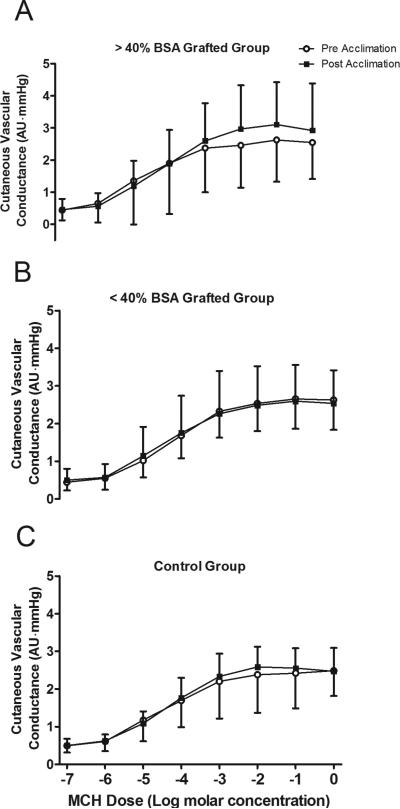
Cutaneous vascular conductance increased with SNP and MCh infusions in all groups, both pre and post heat acclimation (Main Effect for drug dose; P < 0.001 for both SNP and MCh infusion; Figs. 2 and 3). However, the increase in cutaneous vascular conductance to these drugs was not different between pre and post heat acclimation in any group (all P > 0.05; Figs. 2 and 3).
Figure 2. Endothelial independent skin blood flow responsiveness before and after heat acclimation.
Cutaneous vascular responsiveness to sodium nitroprusside (SNP) infusion for >40% body surface area (BSA) grafted (A), <40% BSA grafted (B) and control (C) groups before and after the 7 day heat acclimation regimen. Data are mean ± SD.
Figure 3. Endothelial dependent skin blood flow responsiveness before and after heat acclimation.
Cutaneous vascular responsiveness to methacholine (MCh) infusion for >40% body surface area (BSA) grafted (A), <40% BSA grafted (B) and control (C) groups before and after the 7 day heat acclimation regimen. Data are mean ± SD
Maximal post-junctional sweating rate and skin blood flow
Maximal sweat rate was not different from pre to post heat acclimation after collapsing all groups together, although it approached significance (Main effect P = 0.062). Maximal sweat rate was higher post heat acclimation in the control group (P = 0.016; Table 2). This was accompanied by a greater sweat output per gland after acclimation (P = 0.017), while the number of active sweat glands was unchanged (P = 0.378). However, in both grafted groups, the maximal sweat rate and sweat output per gland were not different from pre to post heat acclimation (all P > 0.05). There was an increase in the number of activated sweat glands post heat acclimation for the >40% BSA grafted group (P = 0.005) but not in the < 40% BSA grafted group (P = 0.090). Maximal cutaneous vascular conductance was not different between pre and post heat acclimation within any group (Table 3; all group P > 0.05), although it approached significance with MCh infusion in the > 40% BSA grafted group (P = 0.077).
Table 2.
Sweating before and after heat acclimation.
| Burn survivors with grafted skin |
||||||
|---|---|---|---|---|---|---|
| 17-40% BSA Grafted | >40% BSA Grafted | Control | ||||
| Pre Acclimation | Post Acclimation | Pre Acclimation | Post Acclimation | Pre Acclimation | Post Acclimation | |
| Number of sweat glands activated (glands·cm−2) | 50±17 | 55±16 | 49±16 | 56±18# | 65±15* | 69±16 |
| Maximal sweat rate (mg·min−1·cm−2) | 0.46±0.23 | 0.51±0.17 | 0.43±0.19 | 0.50±0.24 | 0.41 ± 0.20 | 0.54 ± 0.21# |
| Maximal sweat output per gland (μl·min−1per gland) | 8.64±4.50 | 9.33±4.19 | 9.06±3.73 | 10.27±3.61 | 6.70±2.77 | 8.41±2.28# |
| MCh sweat rate EC50 (log molar concentration) | −1.40±0.47 | −1.49±0.36 | −1.39±0.49 | −1.18±0.30 | −1.12±0.13 | −1.34±0.30 |
Different from <40% BSA grafted and >40% BSA grafted at pre acclimation (P < 0.05).
Different from pre acclimation within that respective group (P < 0.05). EC50: Concentration of the indicated drug that resulted in half the peak response.
Table 3.
Skin blood flow responses before and after heat acclimation.
| Burn survivors with grafted skin |
||||||
|---|---|---|---|---|---|---|
| 17-40% BSA Grafted | >40% BSA Grafted | Control | ||||
| Pre Acclimation | Post Acclimation | Pre Acclimation | Post Acclimation | Pre Acclimation | Post Acclimation | |
| SNP: Maximal cutaneous vascular conductance (AU·mmHg) | 2.63±0.47 | 2.68±0.79 | 2.38±1.38 | 2.36±1.19 | 2.67±0.83 | 2.79±0.58 |
| MCh: Maximal cutaneous vascular conductance (AU·mmHg) | 2.84±0.87 | 2.69±1.10 | 2.73±1.18 | 3.12±1.22 | 2.70±0.77 | 2.74±0.77 |
| SNP cutaneous vascular conductance EC50 (log molar concentration) | −3.64±0.76 | −3.63±0.70 | −3.85±1.09 | −3.48±0.86 | −3.68±0.78 | −3.69±0.50 |
| MCh cutaneous vascular conductance EC50 (log molar concentration) | −4.30±0.45 | −4.17±0.81 | −4.29±1.67 | −4.18±0.92 | −4.12±1.14 | −4.29±0.68 |
EC50: Concentration of the indicated drug that resulted in half the peak response. SNP: sodium nitroprusside. MCh: methacholine.
Post-junctional responsiveness (EC50)
The dosage of MCh necessary to elicit the EC50 sweating response was not different between pre and post heat acclimation in any group (Table 2; all P > 0.05), although it did approach significance in the control group (Pre: −1.12±0.13 vs. Post: −1.34±0.30 log molar concentration; P = 0.060). Furthermore for both SNP and MCh, the dose required to elicit half of the maximal cutaneous vascular response (EC50) was not different between pre and post heat acclimation in any group (Table 3; all P > 0.05).
Discussion
We recently showed that burn survivors improve heat tolerance following a heat acclimation regimen 32. The objective of the present project was to identify whether post-junctional sudomotor and cutaneous vascular adaptations in the non-injured, non-donor skin of these individuals contribute to this improvement. In contrast to our hypothesis, post-junctional adaptations to heat acclimation in non-injured/non-donor skin of burn survivors were generally small or absent.
Sudomotor adaptations
An improved thermal tolerance is associated with various adaptations in sudomotor function, including both central and peripheral (i.e. post-junctional) components. Post-junctional sudomotor adaptations proposed to contribute to an improved thermal tolerance, include increased post-junctional sensitivity of the sweat glands to cholinergic agonists 17,23,26, increased number of active sweat glands 27, an increased efficiency of sweat evaporation 36, sweat gland hypertrophy 23,29 and/or an increased output of sweat per active gland 28,30.
While we did not observe a statistical difference in maximal sweating rate following heat acclimation in either group of burn survivors, this response approached significance when collapsed across all groups (P = 0.062), a finding that is similar with previous studies showing increased sweating rates 17,19,20,22-24 and sweat sensitivity 32 to heat acclimation. Overall though, the observed findings indicate that heat acclimation was associated with minimal post-junctional sudomotor adaptations, at best, in the non-injured skin of burn survivors. That is, the sweat rate at the maximal dose of MCh, the volume of sweat output per gland, and the post-junctional sensitivity of the sweat glands were not increased post heat acclimation in burn survivors. However, the number of active sweat glands at the highest dose of MCh was increased after heat acclimation only in the larger BSA grafted group (i.e., > 40% BSA grafted group). Heat acclimation did not change the number of activated sweat glands at the highest dose of MCh yet maximal sweating rate increased in this group likely due to an increased sweat output per gland (Table. 2).
An increase in the number of active sweat glands in non-injured skin may provide burn survivors with an increased potential to control internal body temperatures during exercise and/or exposure to a hot environment. Prior to heat acclimation, both burn survivor groups displayed evidence of a reduced number of active sweat glands, but a similar sweating rate, relative to control subjects, at the highest dose of MCh. Logically, this is suggestive of an increased sweat output per active gland in burn survivors. In burn survivors, the reliance on fewer active sweat glands in non-injured skin prior to heat acclimation may be a contributing factor predisposing these individuals to impaired heat dissipation and thermal intolerance 5-7, although such a maladaptation would pale in comparison to the reduced amount of skin available for sweating 14. Following heat acclimation, the increased number of active sweat glands in non-injured skin may help improve thermal tolerance on the basis that a greater number of active skin glands may result in a larger wetted area of the skin and therefore a larger surface area able to participate in evaporative cooling. This may be more frequently observed during exercise, for example, where sweating rates are higher than in the present study. Greater increases in sweat rate during exercise are due to greater increases in internal temperatures and accompanying increased neural drive for sweating, coupled with the release of co-transmitters from sudomotor nerves that are known to sensitize sweat glands 37,38. Differing responses between stimulating sweat glands exogenously with drugs, relative to that which occurs during either a passive or exercise heat stress, may explain why there was an increased sweat sensitivity in the burn survivors during exercise post heat acclimation, as previously reported 32, but not when post-junctional sudomotor sensitivity was assessed via intradermal infusion of MCh.
Cutaneous vascular adaptations
Post-junctional alterations in cutaneous vascular function following a heat acclimation regimen include an increased sensitivity of the cutaneous vasculature to vasoactive stimuli 17,23,26,31 and structural changes enabling an increased maximal cutaneous vasodilation 31. Herein, we assessed the sensitivity of the cutaneous vasculature to the endothelial independent (SNP) and dependent (MCh) vasodilators. Despite the aforementioned finding in non-burned individuals 17,23,26,31, we did not observe any changes in cutaneous vascular responsiveness to progressively increasing or maximal doses of SNP and MCh in burn survivors after heat acclimation (Figs. 2 and 3), though some differences in control subjects were observed (Table 3). While not statistically significant, the > 40% BSA grafted group displayed a slightly higher absolute cutaneous vascular conductance with SNP infusion after heat acclimation (main effect: P = 0.057; Fig. 2a). We cannot rule out the possibility that a high level of variability in the skin blood flow responses prevented statistical differences between both burn survivors and healthy controls, as well as from pre to post heat acclimation within groups.
Limitations
Mechanisms responsible for cutaneous vascular and sudomotor responses to stimuli, such as increased environmental temperatures and exercise, are multifactorial involving integrated responses between central and peripheral effectors. Here, we used intradermal microdialysis to assess changes in post-junctional sweating and skin blood flow responsiveness following a 7 day heat acclimation protocol. While microdialysis is a powerful methodological tool, it does not allow the examination of any adaptations resulting from altered central drive and/or integrated responses to perturbations such as exercise and heat stress. Moreover, exogenous administration of such agents does not simultaneously release co-transmitter neuropeptides that augment sweating responsiveness 37,38. Using the microdialysis method, therefore, we are unable to replicate the larger increase in sweating rates that are associated with an exercise heat stress. Thus, interpretation of the data is limited to changes in post-junctional sudomotor and cutaneous vascular responsiveness with heat acclimation, which is likely a different local environment from what occurs during exercise or heat stress. While the complete list of the neurotransmitters which modulate cutaneous vascular responses is unknown, the interpretation of cutaneous vascular responses to MCh and SNP must be viewed with the understanding that the responsiveness of these drugs may be different relative to the actual neurotransmitters responsible for active cutaneous vasodilation during exercise heat stress. That said, these drugs can be beneficial to evaluate possible post-junctional adaptations to conditions such as heat acclimation.
Often the location of skin blood flow and sweating assessment were different between non-injured controls and burn survivors. In non-injured controls, the forearm was always used to assess these responses, while in burn survivors this was not possible as forearm skin was frequently burned; in these cases non-forearm sites were used. While the measurement site was always at the same location between pre and post heat acclimation trials, it is possible that post junctional adaptations to heat acclimation are not uniform between the limbs and the trunk. For example, after heat acclimation sweating from the limbs increases more than other body regions and thereby limb sweating accounts for a greater amount of the total body sweating39-43. Such a variable response may have contributed to the absence of an overall increase in sweating, and possibly cutaneous vascular, responsiveness post-acclimation in burn survivors. Similarly, the heterogeneity of the cutaneous vasculature in uninjured skin for all groups is also of consideration given that there were likely small variations in the exact placement of the microdialysis membranes within an anatomical region between trials. Skin blood flow can vary appreciably between measurement sites in close proximity 44 owing to the spatial distribution of the microvascular network 45,46 Consequently, the release of MCh and SNP from the microdialysis membranes may have influenced a different number of blood vessels between pre and post heat acclimation trials. Therefore factors associated with subtle variations in the positioning of the microdialysis membranes may have contributed to the absence in the cutaneous vascular response to heat acclimation.
Perspectives and significance
Both control subjects and burn survivors displayed increased thermal tolerance and heat dissipation following heat acclimation 32. This is the first study to examine whether such responses are due, in part, to improvements in post-junctional sweating and cutaneous vascular responses from non-injured, non-donor skin in burn survivors. Interestingly, the increase in core temperature during exercise, and therefore the stimulus for adaptation to heat acclimation, was greater in burn survivors relative to healthy uninjured subjects 7,32. Given this, it was reasonable to hypothesize that the heat acclimation regimen would provide a sufficient stimulus for post-junctional adaptations in the healthy non-injured/non-donor skin of burn survivors. However, despite burn survivors showing improved thermal tolerance and heat dissipation after heat acclimation 32, minimal to no improvements in post-junctional sudomotor/cutaneous vasodilator responsiveness were observed in the current study. This observation suggests that improvements in thermal tolerance of burn survivors during exercise may, in part, be due to an increased neural activation of sweat glands. This adaptation would be important for burn survivors as it would provide an increased potential for heat dissipation, especially during exercise when the neural drive is greatly elevated and sweat rate is appreciably higher than that achieved using the intradermal microdialysis approach. Together, these data are important as they suggest that improvements in heat dissipation and thermal tolerance in both burn survivors and healthy individuals alike, may be due to an increased neural drive for sweating, increased sweating efficiency (i.e., an increased evaporative heat loss per mg of sweat) 36 and/or an increased sensitivity to the neural component of sweating (inclusive of co-transmitters) 20. Regardless of the mechanism(s), improved heat tolerance will reduce the risk of hyperthermia and heat related injuries in burn survivors, a group more susceptible to heat intolerance and related illness 4,7,14.
Conclusion
The current study evaluated changes in the post-junctional sensitivity of sweat glands and the cutaneous vasculature in non-injured, non-donor skin following a 7 day heat acclimation regimen in healthy burn survivors. The main findings indicate that post junctional cutaneous vascular and sudomotor adaptations to the applied heat acclimation regimen in burn survivors are small to non-existent. Therefore, we propose that increased heat tolerance following heat acclimation in these individuals is more likely due to an increased neural drive for sweating, increased sweating efficiency and/or an increased sensitivity to the neural component of sweating (inclusive of co-transmitters).
Acknowledgements
We would like to thank the subjects for participating in our study. We would also like to thank Kim Guilliotti, M.S., Jena Kern, R.N. and Naomi Kennedy, R.N. for their technical assistance. This study was supported by awards from the National Institutes of Health (R01GM068865, F32AG04328).
Footnotes
disclosures
There are no conflicts of interest to report.
References
- 1.Association AB. National Burn Repository (2013 report) Chicago: 2013. [Google Scholar]
- 2.Association AB. National Burn Repository (2006 report) Chicago: 2007. [Google Scholar]
- 3.McEntire SJ, Chinkes DL, Herndon DN, Suman OE. Temperature responses in severely burned children during exercise in a hot environment. Journal of burn care & research : official publication of the American Burn Association. 2010;31(4):624–630. doi: 10.1097/BCR.0b013e3181e4ca14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roskind JL, Petrofsky J, Lind AR, Paletta FX. Quantitation of thermoregulatory impairment in patients with healed burns. Annals of plastic surgery. 1978;1(2):172–176. doi: 10.1097/00000637-197803000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Simchon C, Tsur H, Keren G, Epstein Y, Shapiro Y. Heat tolerance in patients with extensive healed burns. Plastic and Reconstructive Surgery. 1981;67(4):499–504. doi: 10.1097/00006534-198104000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro Y, Epstein Y, Ben-Simchon C, Tsur H. Thermoregulatory responses of patients with extensive healed burns. Journal of applied physiology: respiratory, environmental and exercise physiology. 1982;53(4):1019–1022. doi: 10.1152/jappl.1982.53.4.1019. [DOI] [PubMed] [Google Scholar]
- 7.Ganio MS, Schlader ZJ, Pearson J, et al. Nongrafted Skin Area Best Predicts Exercise Core Temperature Responses in Burned Humans. Medicine & Science in Sports & Exercise. 2015 doi: 10.1249/MSS.0000000000000655. Publish Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freund PR, Brengelmann GL, Rowell LB, Engrav L, Heimbach DM. Vasomotor control in healed grafted skin in humans. Journal of applied physiology: respiratory, environmental and exercise physiology. 1981;51(1):168–171. doi: 10.1152/jappl.1981.51.1.168. [DOI] [PubMed] [Google Scholar]
- 9.McGibbon B, Beaumont WV, Strand J, Paletta FX. Thermal regulation in patients after the healing of large deep burns. Plastic and Reconstructive Surgery. 1973;52(2):164–170. doi: 10.1097/00006534-197308000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Ponten B. Grafted skin. Acta chirurgica Scandinavica. Supplementum. 1960;(Suppl 257):1–78. [PubMed] [Google Scholar]
- 11.Davis SL, Shibasaki M, Low DA, et al. Cutaneous vasoconstriction during whole-body and local cooling in grafted skin five to nine months postsurgery. J Burn Care Res. 2008;29(1):36–41. doi: 10.1097/BCR.0b013e31815f2b63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis SL, Shibasaki M, Low DA, et al. Sustained impairments in cutaneous vasodilation and sweating in grafted skin following long-term recovery. J Burn Care Res. 2009;30(4):675–685. doi: 10.1097/BCR.0b013e3181abfd43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis SL, Shibasaki M, Low DA, et al. Impaired cutaneous vasodilation and sweating in grafted skin during whole-body heating. J Burn Care Res. 2007;28(3):427–434. doi: 10.1097/BCR.0B013E318053D312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganio MS, Gagnon D, Stapleton J, Crandall CG, Kenny GP. Effect of human skin grafts on whole- body heat loss during exercise heat stress: a case report. Journal of burn care & research : official publication of the American Burn Association. 2013;34(4):e263–270. doi: 10.1097/BCR.0b013e31826c32c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor NA. Human heat adaptation. Compr Physiol. 2014;4(1):325–365. doi: 10.1002/cphy.c130022. [DOI] [PubMed] [Google Scholar]
- 16.Sawka MN, Leon LR, Montain SJ, Sonna LA. Integrated physiological mechanisms of exercise performance, adaptation, and maladaptation to heat stress. Compr Physiol. 2011;1(4):1883–1928. doi: 10.1002/cphy.c100082. [DOI] [PubMed] [Google Scholar]
- 17.Lorenzo S, Minson CT. Heat Acclimation Improves Cutaneous Vascular Function and Sweating in Trained Cyclists. J Appl Physiol. 2010:japplphysiol.00725.02010. doi: 10.1152/japplphysiol.00725.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielsen B, Hales JR, Strange S, Christensen NJ, Warberg J, Saltin B. Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. J Physiol. 1993;460:467–485. doi: 10.1113/jphysiol.1993.sp019482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nielsen B, Strange S, Christensen NJ, Warberg J, Saltin B. Acute and adaptive responses in humans to exercise in a warm, humid environment. Pflugers Arch. 1997;434(1):49–56. doi: 10.1007/s004240050361. [DOI] [PubMed] [Google Scholar]
- 20.Nadel ER, Pandolf KB, Roberts MF, Stolwijk JA. Mechanisms of thermal acclimation to exercise and heat. Journal of Applied Physiology. 1974;37(4):515–520. doi: 10.1152/jappl.1974.37.4.515. [DOI] [PubMed] [Google Scholar]
- 21.Roberts MF, Wenger CB, Stolwijk JA, Nadel ER. Skin blood flow and sweating changes following exercise training and heat acclimation. J Appl Physiol. 1977;43(1):133–137. doi: 10.1152/jappl.1977.43.1.133. [DOI] [PubMed] [Google Scholar]
- 22.Fox RH, Goldsmith R, Kidd DJ, Lewis HE. Blood flow and other thermoregulatory changes with acclimatization to heat. The Journal of Physiology. 1963;166:548–562. doi: 10.1113/jphysiol.1963.sp007122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buono MJ, Numan TR, Claros RM, Brodine SK, Kolkhorst FW. Is active sweating during heat acclimation required for improvements in peripheral sweat gland function? American journal of physiology. Regulatory, integrative and comparative physiology. 2009;297(4):R1082–1085. doi: 10.1152/ajpregu.00253.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wyndham CH. Effect of acclimatization on the sweat rate-rectal temperature relationship. Journal of Applied Physiology. 1967;22(1):27–30. doi: 10.1152/jappl.1967.22.1.27. [DOI] [PubMed] [Google Scholar]
- 25.Poirier MP, Gagnon D, Friesen BJ, Hardcastle SG, Kenny GP. Whole-Body Heat Exchange during Heat Acclimation and Its Decay. Medicine and science in sports and exercise. 2015;47(2):390–400. doi: 10.1249/MSS.0000000000000401. [DOI] [PubMed] [Google Scholar]
- 26.Inoue Y, Havenith G, Kenney WL, Loomis JL, Buskirk ER. Exercise- and methylcholine-induced sweating responses in older and younger men: effect of heat acclimation and aerobic fitness. International journal of biometeorology. 1999;42(4):210–216. doi: 10.1007/s004840050107. [DOI] [PubMed] [Google Scholar]
- 27.Frye AJ, Kamon E. Responses to dry heat of men and women with similar aerobic capacities. J Appl Physiol. 1981;50(1):65–70. doi: 10.1152/jappl.1981.50.1.65. [DOI] [PubMed] [Google Scholar]
- 28.Chen WY, Elizondo RS. Peripheral modification of thermoregulatory function during heat acclimation. Journal of Applied Physiology. 1974;37(3):367–373. doi: 10.1152/jappl.1974.37.3.367. [DOI] [PubMed] [Google Scholar]
- 29.Sato K, Sato F. Individual variations in structure and function of human eccrine sweat gland. The American journal of physiology. 1983;245(2):R203–208. doi: 10.1152/ajpregu.1983.245.2.R203. [DOI] [PubMed] [Google Scholar]
- 30.Sato F, Owen M, Matthes R, Sato K, Gisolfi CV. Functional and morphological changes in the eccrine sweat gland with heat acclimation. Journal of Applied Physiology. 1990;69(1):232–236. doi: 10.1152/jappl.1990.69.1.232. [DOI] [PubMed] [Google Scholar]
- 31.Green DJ, Carter HH, Fitzsimons MG, Cable NT, Thijssen DH, Naylor LH. Obligatory role of hyperaemia and shear stress in microvascular adaptation to repeated heating in humans. J Physiol. 2010;588(Pt 9):1571–1577. doi: 10.1113/jphysiol.2010.186965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlader ZJ, Ganio MS, Pearson J, et al. Heat acclimation improves heat exercise tolerance and heat dissipation in individuals with extensive skin grafts. Journal of Applied Physiology. 2015;119(1):69–76. doi: 10.1152/japplphysiol.00176.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis SL, Wilson TE, Vener JM, Crandall CG, Petajan JH, White AT. Pilocarpine-induced sweat gland function in individuals with multiple sclerosis. Journal of Applied Physiology. 2005;98(5):1740–1744. doi: 10.1152/japplphysiol.00860.2004. [DOI] [PubMed] [Google Scholar]
- 34.Gagnon D, Ganio MS, Lucas RA, Pearson J, Crandall CG, Kenny GP. Modified iodine-paper technique for the standardized determination of sweat gland activation. Journal of Applied Physiology. 2012;112(8):1419–1425. doi: 10.1152/japplphysiol.01508.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abràmoff MD, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophotonics international. 2004;11(7):36–43. [Google Scholar]
- 36.Gagnon D, Jay O, Kenny GP. The evaporative requirement for heat balance determines whole- body sweat rate during exercise under conditions permitting full evaporation. The Journal of Physiology. 2013;591(Pt 11):2925–2935. doi: 10.1113/jphysiol.2012.248823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlereth T, Dittmar JO, Seewald B, Birklein F. Peripheral amplification of sweating--a role for calcitonin gene-related peptide. The Journal of Physiology. 2006;576(Pt 3):823–832. doi: 10.1113/jphysiol.2006.116111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumazawa K, Sobue G, Mitsuma T, Ogawa T. Modulatory effects of calcitonin gene-related peptide and substance P on human cholinergic sweat secretion. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 1994;4(6):319–322. doi: 10.1007/BF01821532. [DOI] [PubMed] [Google Scholar]
- 39.Hofler W. Changes in regional distribution of sweating during acclimatization to heat. Journal of Applied Physiology. 1968;25(5):503–506. doi: 10.1152/jappl.1968.25.5.503. [DOI] [PubMed] [Google Scholar]
- 40.Sawka MN, Wenger CB, Pandolf KB. Comprehensive Physiology. John Wiley & Sons, Inc.; 2010. Thermoregulatory Responses to Acute Exercise-Heat Stress and Heat Acclimation. [Google Scholar]
- 41.Fox RH, Goldsmith R, Hampton IF, Lewis HE. The Nature of the Increase in Sweating Capacity Produced by Heat Acclimatization. The Journal of Physiology. 1964;171:368–376. doi: 10.1113/jphysiol.1964.sp007382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laaser U. [Physiologic reactions during 5 weeks of continuous residence i an artificial humid and hot climate]. Internationale Zeitschrift fur angewandte Physiologie, einschliesslich Arbeitsphysiologie. 1968;25(4):279–302. [PubMed] [Google Scholar]
- 43.Shvartz E, Bhattacharya A, Sperinde SJ, Brock PJ, Sciaraffa D, Van Beaumont W. Sweating responses during heat acclimation and moderate conditioning. Journal of applied physiology: respiratory, environmental and exercise physiology. 1979;46(4):675–680. doi: 10.1152/jappl.1979.46.4.675. [DOI] [PubMed] [Google Scholar]
- 44.Tenland T, Salerud EG, Nilsson GE, Oberg PA. Spatial and temporal variations in human skin blood flow. International journal of microcirculation, clinical and experimental / sponsored by the European Society for Microcirculation. 1983;2(2):81–90. [PubMed] [Google Scholar]
- 45.Mack GW. Assessment of cutaneous blood flow by using topographical perfusion mapping techniques. Journal of Applied Physiology. 1998;85(1):353–359. doi: 10.1152/jappl.1998.85.1.353. [DOI] [PubMed] [Google Scholar]
- 46.Braverman IM, Keh A, Goldminz D. Correlation of laser Doppler wave patterns with underlying microvascular anatomy. The Journal of investigative dermatology. 1990;95(3):283–286. doi: 10.1111/1523-1747.ep12484917. [DOI] [PubMed] [Google Scholar]