Abstract
We used fMRI to examine the neural substrates of sublexical phoneme-grapheme conversion during spelling in a group of healthy young adults. Participants performed a writing-to-dictation task involving irregular words (e.g., choir), plausible nonwords (e.g., kroid), and a control task of drawing familiar geometric shapes (e.g., squares). Written production of both irregular words and nonwords engaged a left-hemisphere perisylvian network associated with reading/spelling and phonological processing skills. Effects of lexicality, manifested by increased activation during nonword relative to irregular word spelling, were noted in anterior perisylvian regions (posterior inferior frontal gyrus/operculum/precentral gyrus/insula), and in left ventral occipito-temporal cortex. In addition to enhanced neural responses within domain-specific components of the language network, the increased cognitive demands associated with spelling nonwords engaged domain-general frontoparietal cortical networks involved in selective attention and executive control. These results elucidate the neural substrates of sublexical processing during written language production and complement lesion-deficit correlation studies of phonological agraphia.
Keywords: writing, spelling, phonological agraphia, phonological processing, sublexical processing, fMRI
1. Introduction
Cognitive models of written language postulate two distinct mechanisms that support reading and spelling: lexical-semantic and sublexical. Lexical-semantic processing relies on interactions between conceptual knowledge of word meanings and word-specific phonological and orthographic representations. The lexical-semantic procedure is typically used when reading/spelling familiar words, and is especially important for generating correct pronunciations or spellings of irregular words that contain atypical sound-letter (phoneme-grapheme) correspondences (e.g., choir). By contrast, sublexical processing relies on the systematic application of letter-to-sound or sound-to-letter conversion rules critical for reading/spelling unfamiliar words or novel nonwords that are not represented in lexical-semantic memory. Initial evidence regarding the neural underpinnings of lexical-semantic and sublexical processing came from lesion-deficit correlation studies of individuals with acquired surface and phonological alexia/agraphia (Beauvois & Derouesne, J., 1981; Rapcsak et al., 2009; Rapcsak & Beeson, 2004, 2015; Rapp, Purcell, Hillis, Capasso, & Miceli, 2016; Roeltgen & Heilman, 1984; Shallice, 1981). Surface alexia/agraphia reflects the breakdown of lexical-semantic procedures and is manifested as a disproportionate deficit in reading/spelling irregular words relative to regular words and nonwords that contain predictable phoneme-grapheme mappings. Surface alexia/agraphia have been associated with lesions involving left ventral occipito-temporal (lvOT) cortex encompassing the visual word-form area (VWFA) implicated in lexical orthographic processing, but the syndrome can also be produced by damage to a distributed network of extrasylvian cortical regions involved in semantic processing, including left anterior temporal lobe structures and posterior temporo-parietal cortex (middle temporal gyrus/angular gyrus) (Binder et al., 2016; Graham, Patterson, & Hodges, 2000; Rapcsak & Beeson, 2004; Rapcsak & Beeson, 2015; Wilson et al., 2009). By contrast, phonological alexia/agraphia is characterized by disproportionate impairment in nonword reading/spelling due to dysfunction of sublexical procedures, and has been associated with damage to a network of perisylvian cortical regions implicated in phonological processing, including posterior inferior frontal gyrus/operculum, precentral gyrus, insula, superior temporal gyrus/sulcus, and supramarginal gyrus (Alexander, Friedman, Loverso, & Fischer, 1992; Henry, Beeson, Stark, & Rapcsak, 2007; Rapcsak et al., 2009; Roeltgen, Sevush, & Heilman, 1983). Collectively, these functionally linked perisylvian regions constitute the dorsal language pathway that plays a critical role in mapping phonological representations onto articulatory networks during speech production and also provides the neural substrate of phonological short-term memory and phonological awareness (Hickok & Poeppel, 2007).
More recently, functional imaging studies have been used to isolate the neural systems that support lexical-semantic and sublexical processing during reading and spelling in healthy individuals. Regarding the lexical-semantic pathway, these investigations have confirmed the critical role of the VWFA in gaining access to word-specific orthographic representations during reading and the recruitment of perisylvian phonological and extrasylvian semantic networks when reading familiar words (Binder, Medler, Desai, Conant, & Liebenthal, 2005; Graves, Desai, Humphries, Seidenberg, & Binder, 2010; Jobard, Crivello, & Tzourio-Mazoyer, 2003; Taylor, Rastle, & Davis, 2012). Functional imaging studies of reading nonwords relative to real words show greater activation in left perisylvian cortical areas involved in phonological processing (IFG/operculum, PCG, insula, STG/STS, and SMG) (Graves et al., 2010; Jobard et al., 2003; Mechelli, Gorno-Tempini, & Price, 2003; Taylor et al., 2012), overlapping with regions recruited during speech production, phonological short-term memory, and phonological awareness (Acheson, Hamidi, Binder, & Postle, 2011; Buchsbaum et al., 2011; Burton, Locasto, Krebs-Noble, & Gullapalli, 2005; Jobard et al., 2003; Katzir, Misra, & Poldrack, 2005; Price, 2012; Vigneau et al., 2006). Reading nonwords also produced greater activation in the VWFA relative to real words, presumably reflecting the increased processing demands associated with mapping unfamiliar combinations of letters onto the corresponding phonological representations (Price & Mechelli, 2005; Taylor et al., 2012). In addition to increased activation within domain-specific components of the language network implicated in phonological and orthographic processing, the greater task difficulty and cognitive effort associated with reading novel nonwords is also reflected by the engagement of domain-general frontoparietal networks involved in selective attention and executive control (Binder et al., 2005; Graves et al., 2010; Ihnen, Petersen, & Schlaggar, 2015). Components of this (bilateral) multi-demand frontoparietal system include regions within dorsal and ventrolateral prefrontal cortex (e.g., inferior frontal junction), intraparietal sulcus (IPS), and anterior cingulate gyrus (Fedorenko, 2014; Fedorenko, Duncan, & Kanwisher, 2013; Vincent, Kahn, Snyder, Raichle, & Buckner, 2008).
The vast majority of imaging studies of written language processing have focused on reading, and empirical data regarding the neural substrates of spelling is relatively modest. Nevertheless, recent meta-analyses of functional imaging studies of written language production have revealed that the cortical regions involved in spelling show considerable overlap with those implicated in reading (Planton, Jucla, Roux, & Démonet, 2013; Purcell, Turkeltaub, Eden, & Rapp, 2011). Specifically, these studies have confirmed the central role of lvOT/VWFA for gaining access to orthographic lexical representations during both reading and spelling (Planton et al., 2013; Purcell et al., 2011; Tsapkini & Rapp, 2010). In addition, similar to reading, written language production has been associated with activation in several perisylvian cortical areas implicated in phonological processing, including IFG/operculum, PCG, insula, STG/STS, and SMG (Beeson et al., 2003; Planton et al., 2013; Purcell et al., 2011; Rapcsak & Beeson, 2015). It is important to note, however, that although these imaging studies have provided important information about the neural correlates of lexical-semantic processing associated with spelling familiar words, conclusions about the sublexical spelling pathway were limited by the fact that these studies did not specifically investigate spelling nonwords. An exception is the recent study by Ludersdorfer, Kronbichler, & Wimmer (2015) that attempted to identify the neural systems that support lexical-semantic versus sublexical processing by directly contrasting real word and nonword spelling in German speakers. These investigators reported that the lvOT/VWFA, left IFG (pars triangularis, pars opercularis), and superior frontal gyrus/paracingulate gyrus were activated to a greater extent during real word than nonword spelling, whereas the superior temporal gyrus (STG) showed the opposite response pattern. As acknowledged by the authors, these results were somewhat surprising because studies of reading have consistently demonstrated increased activation to novel nonwords relative to familiar real words in cortical regions implicated in orthographic and phonological processing, including the VWFA and posterior IFG/operculum.
The aim of the present investigation was to elucidate the cortical regions recruited during sublexical spelling using fMRI data collected in healthy English speakers while they spelled irregular words and nonwords to dictation1. A control task of drawing geometric shapes to dictation was employed to enable us to remove peripheral components of the experimental task relating to motor planning and implementation. Based on the results of neuroimaging studies of reading, we hypothesized that spelling irregular words and nonwords would produce overlapping patterns of activation in left-hemisphere regions specialized for phonological and orthographic processing, including perisylvian cortical areas comprising the dorsal language pathway and the lvOT/VWFA. Given the greater computational difficulty/cognitive effort associated with spelling novel nonwords compared to familiar real words, we anticipated that the nonword/irregular word contrast would reveal evidence of increased neural activation within components of the language network critical for sublexical phonology-to-orthography translations as well as the recruitment of domain-general frontoparietal networks involved in selective attention and executive control.
2. Material and methods
2.1 Participants
Thirteen healthy right-handed English-speaking adults (5 male, 8 female) participated in this study. The mean age for the group was 29.5 years (20–53 years) with an average of 15 years of education (12–18 years). Right handedness was confirmed in all participants using the Edinburgh Handedness Inventory (Oldfield, 1971), yielding a mean laterality quotient of 83.5 (64–100). The participants had no history of neurological impairment or learning disability. The study was approved by the University of Arizona Human Subjects Protection Program and informed consent was obtained from each individual prior to participating.
2.2 Design and materials
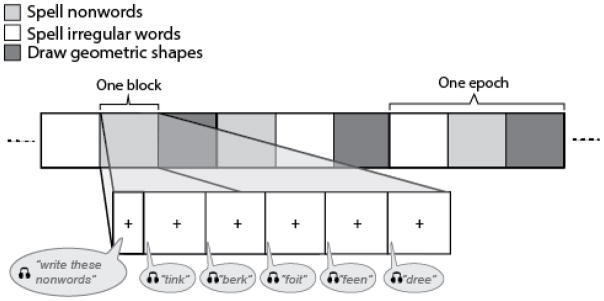
A functional MRI experiment was implemented to examine and isolate the relevant processes that support sublexical spelling using a blocked design with the following conditions: a) writing real words with irregular spellings, b) writing nonwords and c) drawing common geometric shapes. Participants were instructed to write or draw each item on a pad of paper that rested on their lap during scanning. The stimuli were presented auditorily as a writing-to dictation or drawing-to-dictation task, as appropriate, via MR compatible headphones (Resonance Technologies) during 30-second blocks. Each block was initiated by a 3-second spoken instruction, followed by spoken presentation of five items presented at 6-second intervals over the course of the 30-second block (see Figure 1). For the nonword condition, the participant heard, “Write this nonword, “followed by a verbal prompt for each item and six seconds to respond, for example, “‘boke,’ … ‘herm,’ … ‘feen,’ … ‘dewt,’ … ‘sume.’” The irregular word condition was similarly prompted with the command, “Write this word,” followed by five items at six-second intervals. For the shape condition, the participant heard: “Draw this shape: ‘circle,’… ‘rectangle,’ … ‘oval,’ … ‘square,’ … ‘diamond.’” Each condition was administered five times within a run, using one of three condition orders counterbalanced across participants. In total, each run lasted 495 seconds (33 seconds × 3 conditions × 5 epochs), or 8:15 minutes. This paradigm was administered twice to each participant.
Figure 1.
Schematic of the experimental task. Each run consisted of five epochs of three pseudorandomized conditions: a) spelling irregular words (e.g., choir), b) spelling nonwords (e.g., kroid), and c) drawing common geometric shapes as a control for motor planning and implementation (see text for detail).
Stimuli included 50 real words with irregular spellings, 50 pronounceable nonwords, and 5 geometric shapes (see Appendix). The irregularly spelled words had at least one grapheme that did not follow conventional rules for sound-letter correspondences. Nonwords were selected from existing sources (e.g., Psycholinguistic Assessment of Language Processing in Aphasia, Kay, Coltheart, & Lesser, 1992) or generated so that the list roughly matched the irregular words in length and number of syllables. As is typical, most nonwords differed from a real word by a single sound or consonant cluster, such as “boke” being similar to “bake” or “bike,” and some items, like “murnee” did not have an obvious lexical analogue. The words and nonwords were 4 or 5 letters in length, containing two to seven phonemes, and were predominantly one syllable. Length in letters was not significantly different for words versus nonwords, Mann-Whitney U = 1101, p = 0.153, r = 0.103 (see Table 2). For a complete list of word and nonword stimuli, see Appendix 1. The same set of five geometric shapes (circle, square, triangle, oval, and rectangle) was used throughout the experiment presented in pseudorandomized order within each block.
Table 2.
Stimulus summary statistics
| Letters | Phonemes | Syllables | ||||
|---|---|---|---|---|---|---|
| Mdn | Range | Mdn | Range | Mdn | Range | |
| Irregular words | 5 | 4–5 | 2 | 2–5 | 1 | 1–2 |
| Pseudowords | 5 | 4–5 | 3 | 3–7 | 1 | 1–3 |
Prior to scanning, participants were familiarized with the protocol using practice trials in a reclining chair outside of the scanning room. A short pencil was grasped in the right hand, and the left hand held in place a pad of paper was placed on the individual’s lap. Participants were instructed to overtly write responses on the paper. Practice was conducted outside the scanner with eyes closed to simulate the lack of visual feedback experienced in the scanner. Written words, nonwords, and shapes were overwritten on the same sheet of paper, so that individual responses were not scored. Participants were instructed that some groups of stimuli would be real words and some would be “nonwords.” They were also told that if, for some reason, they were uncertain regarding a dictated item, to simply write what they thought to be correct. To roughly match the amount of time spent writing/drawing on each trial, they were instructed to draw several representations of the dictated geometric shape. So, for example, in response to “Draw this shape … ‘circle,’” the individual would draw several circles in sequence. The precise number of shapes was not indicated in the task instructions to avoid subvocal counting. During scanning, each participant’s head was stabilized using foam padding placed under the neck and around the head as needed to pack the space between the head and inner surface of the coil. A Velcro strap was secured just above each participant’s elbow to minimize potential arm movement during writing/drawing.
Online behavioral data were not collected regarding responses during the scanning session, but the pad of paper was examined to confirm that participants had been writing during the scanning session. After the functional imaging was completed, participants were administered the writing/drawing task outside of the scanner at a table with paper and pencil. As in the scanner, items were presented auditorily with no repetition of the items and no feedback given.
2.3 MRI acquisition
Whole brain images were acquired on a 1.5 T Signa whole-body MRI system equipped with a standard quadrature head coil (General Electric, Milwaukee, WI). Each participant completed two functional runs consisting of 165 T2*-weighted spiral echo-planar images (EPI) acquired with the following parameters: 19 sequential AC/PC-aligned axial slices; slice thickness = 6 mm with no gap; field-of-view = 22 × 22 cm2; matrix 64 × 64; TR = 3 s, TE = 40 ms, flip angle = 90°. For registration, high-resolution T1-weighted anatomical reference images were obtained (124 sagittal slices slice thickness = 1.5 mm; matrix 256 × 256; field-of-view = 24 × 24 cm2; TR = 24 ms; TE = 5 ms; flip angle = 45°). Each functional run was preceded by the acquisition of 2 discarded baseline images (6 seconds) to allow the MR signal to reach equilibrium, followed by the first 3-second audio instruction and 30-second response interval.
2.4 fMRI data analysis
2.4.1 Preprocessing
The functional imaging data were pre-processed with AFNI (Cox, 1996). Data were corrected for slice timing, realigned to account for minor head motion, smoothed with an 8-mm Gaussian kernel (FWHM), high-pass filtered at 0.006 Hz and detrended. Each individual’s functional data were linearly aligned to their anatomical image with SPM5’s automated coregistration tool (Friston, Ashburner, Kiebel, Nichols, & Penny, 2007). Then each anatomical T1 image was warped to the Montreal Neurological Institute (MNI) average of 152 brains using SPM5’s unified segmentation. These two transformations were later used for warping first-level effect size maps to MNI space.
2.4.2 First-level analysis
For each functional run, a general linear model was fit voxel-by-voxel using the fmrilm function from FMRISTAT (Worsley et al., 2002). A boxcar design was constructed containing one explanatory variable (EV) for each of the three experimental conditions (irregular word spelling, nonword spelling, and shape drawing) each with a duration of 30 seconds. Each EV in the design matrix was convolved with a canonical hemodynamic response function (HRF) modeled as the difference of two gamma density functions (Glover, 1999). Motion artifact was reduced by including in the model the six translation and rotation parameters previously estimated during motion correction as covariates in the model, unconvolved with the HRF.
Four contrasts were constructed for statistical analysis. The first contrast compared writing irregular words to drawing shapes. The second contrast compared writing nonwords to drawing shapes. These two contrasts were intended to isolate cortical regions supporting the central components of spelling by controlling for peripheral processes common to the auditory processing and graphomotor control required to draw shapes to dictation. The third contrast compared nonword spelling to irregular word spelling. This contrast excluded the control condition from the model, but was restricted to voxels (via a search mask) with positive beta values estimated for nonword spelling versus the graphomotor control task. The fourth and final contrast compared irregular word spelling to nonword spelling. This contrast also excluded the control condition from the model, and was restricted to voxels with positive beta values estimated for irregular word spelling versus the graphomotor control task. Prior to group analysis, the pairs of contrast images from each participant’s two functional runs were combined in a fixed-effects model using the multistat function in FMRISTAT.
2.4.3 Group analysis
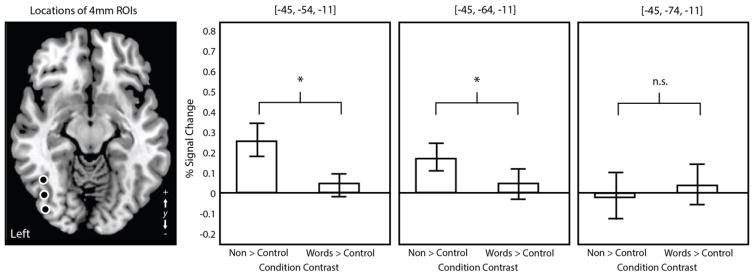
Second level analysis was conducted in SPM5 (Friston et al., 2007). Each participant’s effect size images were warped to MNI space using the parameters from their high resolution anatomical image. Brain activation common to the group was modeled using a single sample t-test with 12 degrees of freedom conducted on the combined first-level effect size maps. Resulting SPMs were thresholded voxelwise at p < 0.005, and then corrected for multiple comparisons (p < 0.05, family-wise error; FWE) for spatial extent by applying a minimum cluster size determined by Gaussian random field theory (Worsley et al., 1996) implemented in SPM5. A region of interest (ROI) analysis was also conducted to allow the direct comparison of the present findings against patterns of activation reported by Ludersdorfer et al. (2015) in their study of nonword versus word spelling. Following these investigators, three spheres with radii of 4mm were placed along the anterior-posterior axis of the fusiform gyrus (MNI coordinates = −45, −54, −11, and −45, −64, −11, and −45, −74, −11) and contrast estimates for the two spelling conditions (irregular words and nonwords) versus rest were extracted using a custom MATLAB script.
3. Results
3.1 Behavioral performance
The writing-to-dictation task performed outside of the scanner indicated the average spelling accuracy on irregular words was 96% (SD = 5%) and 89.1% (SD = 5.2%) for nonwords. The few errors produced for irregular words consisted primarily of common misspellings, such as theif for thief. For nonwords, 78.7% of errors appeared to be auditory misperceptions, some of which were lexicalizations (e.g., brute for bruth), and the rest (21.3%) were phonologically implausible spelling errors (e.g., donsit for donsept).
3.2 Whole Brain Analysis
The results of each of the four contrasts show the neural activation unique to each experimental condition.
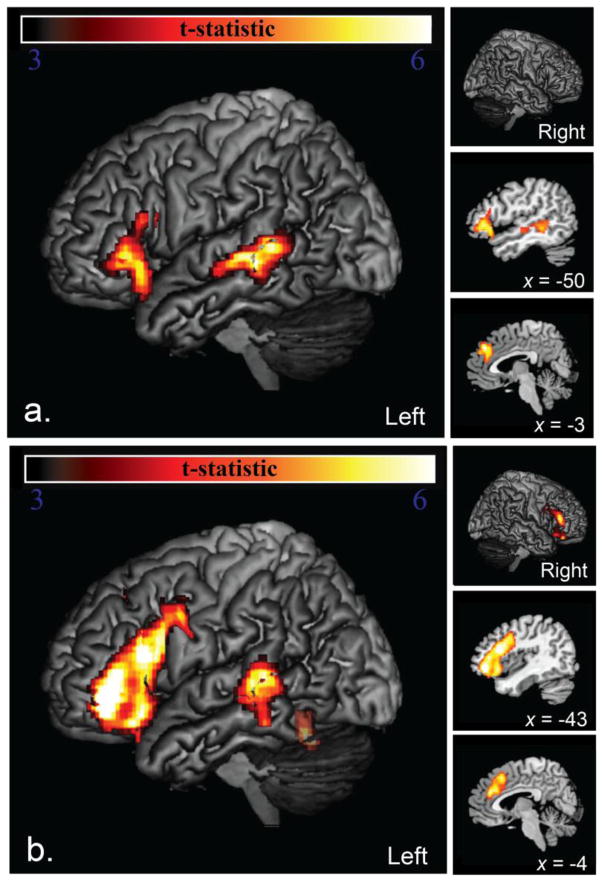
Brain regions that were significantly more active during spelling of irregular words than the graphomotor control included three clusters encompassing the left posterior IFG (pars, triangularis/opercularis) and adjacent insula, left mid-posterior superior temporal sulcus (STS), and the anterior cingulate/supplementary motor area (SMA) (Figure 2, top).
Figure 2.
Areas of significantly greater activation for (a) irregular word spelling than a graphomotor control (top) included three clusters encompassing the left IFG and adjacent insula, left STS, and the anterior cingulate/SMA. Areas of significantly greater activation for (b) nonword spelling than a graphomotor control (bottom). Areas activated greater for nonword spelling included six clusters encompassing most of the left IFG and adjacent PCG and insula, a less extensive region in the homotopic right IFG, left STS almost entirely overlapping with that region activated for irregular words, the anterior cingulate/SMA, and lvOT/VWFA (translucency indicates that that this region did not meet full significance for FWE cluster extent).
Brain regions significantly more active during nonword spelling than the graphomotor control task included five clusters encompassing left IFG (pars triangularis/opercularis) and adjacent PCG and insula, a less extensive region in the homotopic right IFG, left STS overlapping with the region activated for irregular words, and the anterior cingulate/SMA (Figure 2, bottom). A sixth cluster of activation was also observed in lvOT/VWFA that did not quite meet the minimum cluster extent (MNI = −46, −58, −22, maximum t = 7.32, p = 0.483 corrected, 0.011 a prior; indicated by translucency in Figure 2, bottom).
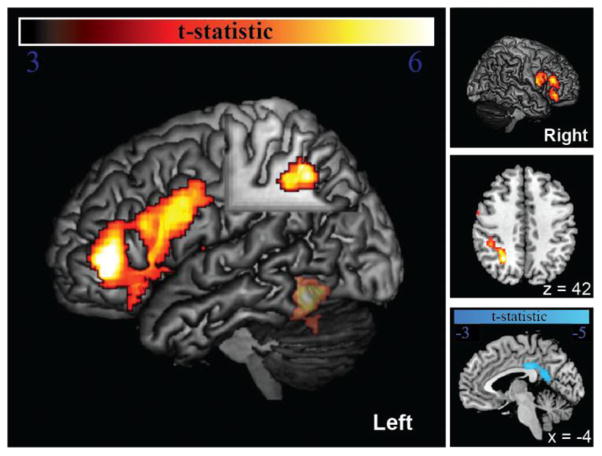
Brain regions significantly more activated during spelling of nonwords than spelling irregular words included three large clusters in 1) left IFG with three separate peaks in pars triangularis, pars opercularis, and insula/PCG; 2) left intraparietal sulcus (IPS), spanning from the caudal end (y = −58) antero-laterally (y = −33); and 3) a less extensive set of homotopic regions in right IFG with separate peaks in the pars opercularis, pars triangularis, and pars orbitalis (Figure 3, left large pane and top and middle small panes). We also observed an activated region in lvOT/VWFA that did not quite meet the minimum cluster extent (MNI = −46, −58, −16, maximum t = 6.74, p = 0.067 corrected, 0.002 a prior; indicated by translucency in Figure 3). Brain regions significantly more activated during spelling irregular words than spelling nonwords included a single cluster encompassing posterior cingulate gyrus/retrosplenial cortex (Figure 3, bottom right, cool colors).
Figure 3.
Areas of significantly greater activation for nonword spelling than irregular word spelling (hot) and the reverse contrast (cool). Areas with greater activation during nonword spelling included left IFG (pars triangularis, opercularis) and adjacent PCG and insula, less extensive homotopic regions in right IFG, left intraparietal sulcus (IPS), and lvOT/VWFA (translucency indicates that that this region did not meet full significance for FWE cluster extent). Areas activated greater for irregular word spelling were limited to posterior cingulate gyrus/retrosplenial cortex at midline (bottom right).
3.3 Region of interest analysis
A region of interest analysis was conducted to examine differences in activation during real word versus nonword spelling within the lvOT/VWFA, to enable a direct comparison of the results with those of Ludersdorfer et al. (2015). Contrast estimates were significantly greater for nonword spelling compared to the control task than for real word spelling compared to the control task (drawing shapes) in the ROIs placed in the anterior lvOT (MNI = −45, −54, −11; t(11) = 4.837, p < 0.001) and mid-lvOT (MNI = −45, −64, −11; t(11) = 3.256, p = 0.007). There was no significant difference between contrast estimates in the ROI placed in the posterior lvOT (MNI = −45, −74, −11; t(11) = −1.025, p = 0.326). In summary, similar to Ludersdorfer et al. (2015), effects of lexicality during spelling were observed in the anterior and middle lvOT ROIs corresponding to the VWFA, but the direction of this effect was the exact opposite of that observed by those investigators, with greater activation to nonwords compared to real words.
4. Discussion
In this study we used fMRI to identify the neural substrates of sublexical phoneme-grapheme conversion during written spelling. In order to isolate cortical regions preferentially engaged during sublexical compared to lexical-semantic processing, we manipulated the lexicality of the stimuli and contrasted patterns of activation associated with spelling nonwords and irregular words to dictation. Because written language production and comprehension rely on shared cognitive representations within the central domains of phonology, orthography, and semantics, we expected that our spelling tasks would produce activation within a set of brain regions that are also recruited during reading. Furthermore, based on neuroimaging studies of reading that have documented common patterns of activation during orthographic-to-phonological translations involving nonwords and real words, we hypothesized that spelling by sublexical phoneme-grapheme conversion would engage a network of left-lateralized cortical areas specialized for phonological and orthographic processing, overlapping with regions activated during real word spelling. In addition, given the greater task difficulty/cognitive effort associated with spelling novel nonwords compared to familiar real words, we anticipated stronger activation within these domain-specific components of the language network dedicated to phonological-to-orthographic transcoding, as well as the recruitment of domain-general cortical systems involved in attention and executive control.
Consistent with these expectations, our results demonstrate that spelling nonwords and irregular words to dictation produced overlapping patterns of activation within a network of perisylvian cortical regions implicated in phonological processing. In particular, we found that both stimulus types engaged mid-posterior STS, a region that is considered to play a central role in representing phonological information during speech production and perception tasks (Hickok & Poeppel, 2007; Turkeltaub & Coslett, 2010). It has been proposed that pSTS contains lexical phonological networks that are also activated by nonwords via sublexical features (phonemes, syllables) that are shared with real words (Hickok & Poeppel, 2007). Thus, this cortical region constitutes a critical neural substrate of both lexical and sublexical phonological processing. Our spelling tasks also produced activation in anterior perisylvian language areas that are functionally integrated with pSTS via the dorsal language pathway, including posterior IFG/operculum (Broca’s area), precentral gyrus, and insula. Furthermore, in these frontal regions, we observed a lexicality effect characterized by greater neural response during nonword relative to real word spelling.
According to contemporary models of speech processing, the dorsal language pathway is involved in mapping phonological representations activated in pSTS onto frontal lobe articulatory networks during speech production, and this distributed system also constitutes the neural substrate of the phonological storage and articulatory rehearsal components of phonological short-term memory (Hickok & Poeppel, 2007; Saur et al., 2008). Phonological awareness tasks that require explicit access to sublexical phonological information (e.g., phoneme discrimination, segmentation, rhyme judgments) also depend on the dorsal language pathway (Hickok & Poeppel, 2004). Specifically, it has been demonstrated that such phonological tasks, particularly those involving segmentation, produce robust activation in left pIFG/operculum and neural responses in this region show a lexicality effect with greater activation to nonwords compared to real words (Burton et al., 2005; Katzir et al., 2005). The present results suggest that the maintenance and manipulation of sublexical phonological information necessary to write unfamiliar nonwords places greater demands on articulatory rehearsal and working memory resources supported by pIFG/operculum than processing phonological representations for familiar words. The segmentation of phonological representations into their constituent sounds is an important component of spelling by a sublexical strategy and the increased task demands associated with performing these operations on nonwords that contain unfamiliar combinations of phonological elements may account for the lexicality effect (nonwords > irregular words) observed in the pIFG in our study. We note that a similar effect in favor of greater activation for nonwords in pIFG/operculum is also a highly reliable finding in neuroimaging studies of reading (Binder et al., 2005; Jobard et al., 2003; Mechelli et al., 2003; Taylor et al., 2012), confirming the critical contribution of this region to sublexical phonological processing during both spoken and written language tasks.
Taken together, our results suggest that the lexical and sublexical phonological codes used in spelling are generated, maintained, and manipulated by perisylvian phonological and articulatory networks that collectively constitute the dorsal language pathway (Rapcsak & Beeson, 2015). Specifically, during written language production phonological representations of familiar words are retrieved from pSTS and the same region is involved in constructing novel sound-based representations for unfamiliar nonword targets in spelling-to-dictation tasks. During the spelling process, phonological representations computed within pSTS are maintained in an active state and refreshed by articulatory rehearsal mechanisms mediated via the dorsal language pathway that constitutes a common neural substrate of speech production, phonological short-term memory, and phonological awareness (Hickok & Poeppel, 2007). Processing of information within the phonological network and the efficiency of the phonological-to-orthographic mapping procedure is influenced by stimulus familiarity with an advantage for high-frequency over low-frequency lexical items (Rapp & Dufor, 2011; Rapp & Lipka, 2011). Nonwords contain unfamiliar combinations of phonological elements and spelling these novel items places greater demands on phonological processing resources, including phonological short-term memory/articulatory recoding, and phonological awareness than spelling familiar real words. This is reflected in the greater activation within the perisylvian networks that support these functions.
The results of our imaging study are consistent with lesion-deficit correlation studies that have demonstrated a highly reliable association between phonological agraphia/alexia, characterized by an increased lexicality effect in spelling and reading (words > nonwords), and left perisylvian lesions involving various components of the dorsal language pathway (Alexander et al., 1992; Henry et al., 2007; Rapcsak et al., 2009; Rapcsak & Beeson, 2015). Damage to this pathway, which is critical for processing sublexical phonological information in both spoken and written language tasks, also explains the strong correlation between performance on non-orthographic tests of phonological awareness and nonword spelling/reading accuracy in patients with phonological agraphia/alexia (Rapcsak et al., 2009). Thus, converging evidence from lesion-deficit and functional imaging studies provides compelling empirical support for the notion that phonological agraphia/alexia are manifestations of a central phonological impairment attributable to damage to perisylvian phonological/articulatory networks common to both spoken and written language production (Rapcsak & Beeson, 2015).
Nonword spelling in our study was also associated with activation of the VWFA, providing evidence for the participation of this cortical region in sublexical phoneme-grapheme conversion in addition to its well-established role in retrieving orthographic lexical representations during spelling familiar words (Beeson et al., 2003; Planton et al., 2013; Purcell et al., 2011; Tsapkini & Rapp, 2010). Collectively, these results suggest that during spelling to dictation the VWFA is involved in mapping lexical and sublexical phonological representations computed within pSTS/dorsal language pathway onto the corresponding orthographic units. Furthermore, consistent with interactive models of spelling (Folk, Rapp, & Goldrick, 2002; Rapp, Epstein, & Tainturier, 2002; Tainturier, Bosse, Roberts, Valdois, & Rapp, 2013), we propose that the VWFA is the critical neural site for integrating the output of the lexical-semantic and sublexical spelling pathways (Rapcsak & Beeson, 2015). According to this view, lexical and sublexical procedures for spelling operate in parallel rather than in isolation and both pathways are engaged in processing/generating orthographic representations for both real words and nonwords. At the behavioral level, evidence for lexical-sublexical integration is provided by demonstrations of lexical influences on nonword spelling (Tainturier et al., 2013). In a similar vein, VWFA activation during nonword spelling in our study may have reflected not only sublexical phoneme-grapheme conversion but also lexical influences associated with the automatic activation of orthographic lexical representations for real words similar in sound to the nonword targets. The availability of word-specific orthographic information may have resulted in the use of a lexical analogy procedure that involved incorporating lexical spelling knowledge derived from real words into the nonword responses. For example, to spell the nonword/ne s/as n-a-c-e one might retrieve orthographic information shared with lexical neighbors such as face, lace, race, pace, grace, place, trace, etc. Although this process involves mapping between larger units (rime-body conversion), phoneme-grapheme conversion is still necessary to compute the appropriate grapheme for the first sound.
Given the central role of the VWFA in orthographic processing, it may seem surprising that we did not observe robust activation in this region during our spelling tasks. This finding most likely reflects active engagement of the VWFA during our control task of drawing geometrical forms to dictation. Whereas repetitive circle-drawing was a good control task in previous studies of written spelling (e.g., Beeson et al. 2003; Rapp & Dufor, 2011), the control task in our current study required retrieval of stored information for a set of unique visual shapes and the neural responses were comparable to the activation of word-specific orthographic representations. Thus, the drawing of specific geometrical shapes engaged VWFA to the same extent as irregular word spelling. This is consistent with other research showing that writing and drawing shapes from memory produce overlapping patterns of activation within the VWFA (Harrington, Farias, Davis, & Buonocore, 2007). These results, together with demonstrations of similar neural responses to written words, pictures of objects, and faces indicate that functional specialization for orthographic processing in the VWFA cannot be considered absolute and suggest that this cortical area may play a more general role in representing information about visual shapes (Dehaene & Cohen, 2011; Price & Devlin, 2011). Despite the unanticipated limitations of our control task, we obtained evidence of VWFA activation in the nonword spelling condition and the ROI analyses demonstrated lexicality effect in this region with greater responses to nonwords relative to real words.
Consistent with its involvement in word-level phonological-to-orthographic translations, neural responses within the VWFA show sensitivity to whole-word frequency during both reading and spelling, with greater activation when processing low- compared to high-frequency words (Rapp & Dufor, 2011; Rapp & Lipka, 2011). These word frequency effects are attributable to the fact that the mapping process for relatively unfamiliar lexical items is less efficient and therefore requires greater cognitive effort. Previous imaging studies have also shown VWFA activation during nonword reading, indicating that this region also contributes to sublexical grapheme-phoneme conversion (Binder, Swanson, Hammeke, & Sabsevitz, 2008; Graves et al., 2010; Jobard et al., 2003; Mechelli et al., 2003; Taylor et al., 2012). Furthermore, the VWFA shows a stronger neural responses to reading novel nonwords compared to real words, presumably reflecting the greater task difficulty/computational load associated with mapping unfamiliar combinations of sublexical orthographic units onto their phonological equivalents (Mechelli et al., 2003; Taylor et al., 2012). Our results are consistent with these observations and demonstrate a similar lexicality effect in the VWFA during spelling. Collectively, these findings provide evidence that the VWFA is sensitive to the familiarity of whole-word and subword-level phonological-to-orthographic translations during both reading and spelling.
When comparing real word spelling to nonwords, the only region that showed greater activation to words was localized to posterior cingulate gyrus/retrosplenial cortex. This region is considered part of the semantic network (Binder, Desai, Graves, & Conant, 2009) and greater activation in this region to real words compared to nonwords has been documented in functional imaging studies of reading (Binder et al., 2005). These results indicate the activation of semantic representations during familiar word reading that is considered especially important for computing correct pronunciations for irregular words that cannot be processed accurately by relying on a sublexical phoneme-grapheme conversion strategy. Thus, the engagement of posterior cingulate/retrosplenial cortex in our study may signify the activation of semantic representation during irregular word spelling.
We also predicted that the greater cognitive demands associated with spelling novel nonwords would result in the engagement of domain-general cortical networks implicated in selective attention and executive control. Neuroimaging studies have identified a “multi-demand system” comprised of bilateral frontoparietal networks that are recruited across a wide range of language and non-language cognitive tasks with levels of activation modulated by task difficulty (hard > easy) (Duncan, 2010, 2013; Fedorenko et al., 2013, 2014). Key components of these domain-general frontoparietal networks include regions within dorsolateral and ventrolateral prefrontal cortex, posterior parietal cortex/intraparietal sulcus (IPS), and anterior cingulate/insula (Duncan, 2010, 2013; Fedorenko, 2014; Fedorenko et al., 2013; Vincent et al., 2008). Functional imaging studies of reading have shown that the increased computational demands associated with reading nonwords compared to real words were associated with bilateral activation of frontoparietal networks assumed to support attention, decision, and response selection/monitoring functions (Binder et al., 2005). Importantly, levels of activation within these frontoparietal regions showed a positive correlation with processing speed (RT) or “time on task” regardless of reading condition (i.e., independent of the lexical status of the stimuli), providing evidence that neural responses in these brain areas are modulated in a non-specific manner by task difficulty. Similarly, Ihnen et al. (2013) documented the recruitment of frontoparietal attention and cognitive control networks in a difficult reading task that required normal subjects to read irregular words, regular words, and nonwords by a sublexical “sounding out” strategy, thus forcing them to “regularize” items with atypical letter-sound correspondences. This experimental paradigm placed high demands on top-down executive control mechanisms that included setting up an attentional bias to process all stimuli by the sublexical route and suppress the lexical-semantic route, and effortful error monitoring/verification to ensure that the appropriate response is selected from competing alternatives. In our study, we also observed greater activation during nonword compared to real word spelling in cortical regions that are considered components of this multi-demand attention and executive control network, including bilateral anterior ventrolateral prefrontal cortex (left IFG/pars orbitalis/triangularis and homologous regions within right IFG), left IPS, and bilateral anterior cingulate/insula. We suggest that the recruitment of these “demand-sensitive” brain regions during nonword spelling reflects the engagement of executive processes important for focusing and maintaining attention on the complex sequence of cognitive operations involved in spelling by a sublexical strategy, exerting strategic top-down control over the division of labor between spelling pathways (e.g., enhancing processing via the sublexical pathway, suppressing competing information generated by the lexical-semantic pathway that could result in lexicalization errors), selecting between multiple potential phoneme-grapheme mapping options, and error monitoring to ensure that the written response is consistent with the target stimulus held in working memory. Thus, our results indicate that spelling nonwords by a sublexical strategy depends on functional integration between domain-specific language areas representing phonology and orthography and domain-general frontoparietal networks involved in selective attention and executive control. An important goal for future research is to explore the nature of the interaction between domain-specific components of the language network and domain-general regions involved in cognitive control during reading and spelling and how these network dynamics change as a result of brain damage in patients with acquired alexia/agraphia (cf. Fedorenko & Thompson-Schill, 2014).
There are a number of similarities, as well as some important differences, between our results and the study by Ludersdorfer et al. (2015), who used fMRI to explore the neural correlates of real word and nonword spelling in German speakers. In terms of similarities, both studies documented coactivation/engagement of perisylvian phonological/articulatory networks and the VWFA during spelling. Ludersdorfer et al. (2015) reported that spelling words and nonwords produced overlapping patterns of activation within the VWFA, providing support for the notion that this region contributes to both whole-word and subword-level mappings between phonology and orthography. Our results and Ludersdorfer et al. (2015) provide converging evidence that, in addition to its role in orthographic lexical processing, the VWFA also contributes to sublexical phoneme-grapheme conversion during spelling. However, it is unclear why the direction of the effect of lexicality in our study (nonwords > words) was the exact opposite of Ludersdorfer et al. (2015) who found greater activation for words versus nonwords both within the VWFA and in pIFG/frontal operculum.
In considering the discrepancies between our results and those of Ludersdorfer et al. (2015), we speculate that they may be related to differences in the experimental paradigms and/or stimulus materials used, or they may be attributable to language-specific differences in the alphabetic writing systems involved (English vs. German) that differ substantially in terms of “orthographic depth” or the predictability of sublexical sound-letter mappings (Aro & Wimmer, 2003). It has been shown that orthographic depth influences the degree of reliance on the lexical-semantic versus sublexical pathways during reading (Ischebeck et al., 2004; Paulesu et al., 2000) and it is likely that language-specific differences in the division of labor between pathways are also evident during written language production. Specifically, German is characterized by a shallow orthography with predictable and consistent mappings between letters and sounds, so, in principle, a sublexical strategy could be used to spell both words and nonwords. Because German speakers are used to relying on sublexical letter-sound conversion, spelling nonwords may be an easier task than it is for English speakers who predominantly rely on a lexical-sematic strategy in spelling words, whereas the sublexical strategy is used primarily when processing unfamiliar nonwords. In addition, English is a deep orthography characterized by less predictable/consistent phoneme-grapheme mappings. These differences may influence the relative activation of the VWFA in the direction of the response to nonwords being greater in English speakers compared to German speakers. However, this would not explain the finding that familiar words in German produced greater activation than unfamiliar nonwords as observed by Ludersdorfer et al. (2015).
With regard to task demands, our study employed the typical clinical assessment task of writing to dictation which engages spelling in a top-down manner from phonological and/or semantic networks. Ludersdorfer and colleagues implemented a cross-modal judgement task wherein participants were to determine whether a visually presented letter was contained in an auditorily presented word or nonword after Rapp and Lipka (2011) who used this task to examine neural activation for real word spelling. As described by Ludersdorfer et al. (2015), the participant heard a nonword (e.g., tiska) and then saw a grapheme (e.g., m) and was expected to respond “no,” indicating that there is not an/m/sound in “tiska.” This phonologically weighted task differed from the current study which required generation of a complete orthographic response. Finally, levels of activation in the VWFA are likely to be influenced by the psycholinguistic properties of the word and nonword items used in the experiment (e.g., length, bigram frequency, whole-word frequency, lexical neighborhood size) and differences in these stimulus attributes may have contributed to the discrepancy between our results and those obtained by Ludersdorfer et al. (2015).
Our findings are consistent with the majority of functional imaging investigations of reading that examined effects of lexicality which found greater activation to nonwords compared to real words in the VWFA (Jobard et al., 2003; Mechelli et al., 2003; Taylor et al., 2012). It should be noted, however, that damage to this cortical region has not been consistently associated with the clinical profile of phonological alexia/agraphia (Rapcsak et al., 2009). Specifically, while there are some reports of patients showing an increased lexicality effect (words > nonwords) in reading following damage to the VWFA (Friedman, 1995; Rapcsak, Rothi, & Heilman, 1987; Rapcsak et al., 2009) lesions involving this cortical region are typically associated with a profile of surface agraphia, characterized by an increased regularity effect reflecting disproportionate difficulty in spelling irregular words with relative preservation of regular word and nonword spelling (Rapcsak & Beeson, 2004; Tsapkini & Rapp, 2010). The observation that VWFA lesions do not typically produce a profile of phonological agraphia suggests that the top-down activation of orthographic codes from speech sounds documented in our study and also by Ludersdorfer et al. (2015) is typical, but not required, for correct spelling of nonwords to dictation. Additional behavioral and imaging studies of patients with damage to the VWFA are needed to better understand the contribution of this region to sublexical reading and spelling.
In conclusion, our investigation of the functional neuroanatomy of written language production revealed common patterns of activation during word and nonword spelling in cortical regions dedicated to phonological and orthographic processing. Previous functional imaging studies have demonstrated the recruitment of the same cortical regions during word and nonword reading, providing neuroanatomical support for shared component models of written language processing. Furthermore, consistent with studies of reading, we demonstrated an effect of lexicality within these domain-specific components of the language network manifested by greater activation during nonword than real word spelling, presumably reflecting the greater cognitive demands associated with phonological-to-orthographic mappings involving unfamiliar stimuli. Increasing processing demands during nonword spelling were also associated with the recruitment of domain-general frontoparietal networks involved in attention and executive control.
Figure 4.
Results of three ROI analyses with the lvOT. Error bars are ±1 standard error of the mean.
Table 1.
Brain regions with significant activation in each fMRI contrast
| Brain Region | MNI Coordinates
|
Volume (mm3) | Max t | P | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Irregular word spelling > drawing shapes | ||||||
|
| ||||||
| Left IFG, pars orbitalis | −20 | 38 | 10 | 71680 | 7.96 | <0.001 |
| pars triangularis | −50 | 30 | 0 | 5.52 | ||
| pars opercularis/triangularis | −40 | 20 | 18 | 5.31 | ||
| Left mid-posterior STS | −54 | −34 | −2 | 52352 | 6.08 | <0.001 |
| −56 | −42 | 6 | 5.95 | |||
| −54 | −16 | −6 | 4.47 | |||
| Anterior cingulate/SMA | −2 | 42 | 38 | 45120 | 5.23 | <0.001 |
| 0 | 48 | 28 | 5.93 | |||
| −8 | 34 | 28 | 5.17 | |||
| Nonword spelling > drawing shapes | ||||||
|
| ||||||
| Left IFG, Pars orbitalis | −44 | 40 | −6 | 263360 | 8.80 | <.001 |
| Pars opercularis/PCG | −40 | 20 | 20 | 7.38 | ||
| Pars triangularis | −46 | 34 | 8 | 7.37 | ||
| Anterior insula | −39 | 22 | −10 | 5.72 | ||
| Left mid-posterior STS | −54 | −38 | 6 | 56064 | 5.97 | <.001 |
| −64 | −32 | 4 | 5.85 | |||
| −50 | −36 | −2 | 5.27 | |||
| Anterior cingulate/SMA | −4 | 38 | 26 | 64960 | 5.89 | <.001 |
| −4 | 30 | 30 | 5.58 | |||
| −4 | 32 | 44 | 5.53 | |||
| lvOTC/VWFA* | −46 | −60 | −22 | 1280 | 7.32 | .483 (corr) |
| .011 (unc) | ||||||
| Right IFG, pars triangularis | 48 | 30 | 14 | 34880 | 5.74 | <.001 |
| Pars opercularis/PCG | 42 | 12 | 22 | 4.10 | ||
| Right IFG, pars orbitalis | 32 | 34 | −16 | 27392 | 6.79 | .001 |
| Pars opercularis | 44 | 24 | −12 | 4.26 | ||
| Nonword spelling > Irregular word spelling | ||||||
|
| ||||||
| Left IFG, pars triangularis | −46 | 44 | 0 | 25912 | 11.51 | <.001 |
| Pars opercularis | −36 | 18 | 18 | 5.31 | ||
| PCG | −48 | 4 | 30 | 5.20 | ||
| Anterior insula | −31 | 23 | 4 | 3.65 | ||
| Left IPS | −26 | −58 | 46 | 3240 | 7.82 | 0.025 |
| −36 | −36 | 32 | 5.14 | |||
| −30 | −48 | 42 | 5.08 | |||
| lvOTC/VWFA* | 2576 | 6.74 | .067 (corr) | |||
| −46 | −58 | −16 | .002 (unc) | |||
| −46 | −62 | −30 | 4.05 | |||
| −50 | −54 | −24 | 3.82 | |||
| Right IFG, pars triangularis | 48 | 28 | 14 | 10488 | 5.27 | <.001 |
| Pars opercularis/PCG | 40 | −4 | 20 | 5.12 | ||
| Pars orbitalis | 38 | 32 | −6 | 5.05 | ||
| Irregular word spelling > Nonword spelling | ||||||
|
| ||||||
| Posterior cingulate | 6 | −30 | 32 | 11008 | 6.08 | <.001 |
| Retrosplenial cortex | −12 | −50 | 14 | 5.83 | ||
| −8 | −42 | 26 | 5.22 | |||
This area was specified as an a priori ROI.
Table 3.
Stimulus summary statistics
| Letters | Phonemes | Syllables | ||||
|---|---|---|---|---|---|---|
| Mdn | Range | Mdn | Range | Mdn | Range | |
| Irregular words | 5 | 4–5 | 2 | 2–5 | 1 | 1–2 |
| Pseudowords | 5 | 4–5 | 3 | 3–7 | 1 | 1–3 |
Highlights.
Nonword spelling recruits left-lateralized language and domain-general networks for attention
FMRI findings for nonword spelling are generally consistent with studies of nonword reading
Results support the idea that sublexical spelling depends on dorsal language pathway
Acknowledgments
This work was supported by the National Institutes of Health (DC007646 to P.M.B., DC010878 to S.M.W, DC008286 to S.Z.R. and P.M.B, and DC014389 to A.T.D) and the University of Arizona. We thank Scott Squire and Sarah Andersen for assistance with imaging data collection and analysis, and the individuals who participated in our study. The authors would also like to thank two anonymous reviewers for their helpful comments.
Appendix 1. List of pseudowords and irregular words employed in this study
| Irregular Words | Pronounceable Pseudowords | ||
|---|---|---|---|
| give | gone | boke | troe |
| move | dead | herm | snoy |
| talk | grew | feen | foys |
| type | have | dewt | tuddy |
| shoe | group | sume | lorn |
| blood | voice | bruth | kwine |
| chief | book | kroid | hannee |
| yacht | budge | pites | sarcle |
| choir | shove | foit | sheem |
| fight | thief | reesh | thalk |
| fruit | floor | skart | berk |
| knife | learn | chench | kittle |
| learn | field | merber | remmon |
| noise | cross | floke | gort |
| share | fence | wessel | doncept |
| debt | dumb | feve | gand |
| lamb | beak | leng | murnee |
| myth | germ | nuck | veece |
| yawn | worm | pesh | plen |
| cloak | glove | tink | wundoe |
| moose | pulse | sheen | sloser |
| rinse | phase | thalk | dree |
| shove | knock | jenior | trin |
| thief | sauce | resords | thell |
| vague | tread | suntry | skart |
Footnotes
The data for this study were previously presented in abstract form (Beeson & Rapcsak, 2003).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acheson DJ, Hamidi M, Binder JR, Postle BR. A common neural substrate for language production and verbal working memory. Journal of Cognitive Neuroscience. 2011;23(6):1358–1367. doi: 10.1162/jocn.2010.21519. http://doi.org/10.1162/jocn.2010.21519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander MP, Friedman RB, Loverso F, Fischer RS. Lesion localization of phonological agraphia. Brain and Language. 1992;43(1):83–95. doi: 10.1016/0093-934x(92)90022-7. [DOI] [PubMed] [Google Scholar]
- Aro M, Wimmer H. Learning to read: English in comparison to six more regular orthographies. Applied Psycholinguistics. 2003;24(4):621–635. http://doi.org/10.1017/S0142716403000316. [Google Scholar]
- Beauvois MF, Derouesne J. Lexical or orthographic agraphia. Brain. 1981;104:21–49. doi: 10.1093/brain/104.1.21. [DOI] [PubMed] [Google Scholar]
- Beeson P, Rapcsak S, Plante E, Chargualaf J, Chung A, Johnson S, Trouard T. The neural substrates of writing: A functional magnetic resonance imaging study. Aphasiology. 2003;17(6–7):647–665. [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex (New York, NY: 1991) 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. http://doi.org/10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Desai R, Conant LL, Liebenthal E. Some neurophysiological constraints on models of word naming. NeuroImage. 2005;27(3):677–693. doi: 10.1016/j.neuroimage.2005.04.029. http://doi.org/10.1016/j.neuroimage.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Binder JR, Pillay SB, Humphries CJ, Gross WL, Graves WW, Book DS. Surface errors without semantic impairment in acquired dyslexia: a voxel-based lesion–symptom mapping study. Brain. 2016;139(5):1517–1526. doi: 10.1093/brain/aww029. http://doi.org/10.1093/brain/aww029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Swanson SJ, Hammeke TA, Sabsevitz DS. A comparison of five fMRI protocols for mapping speech comprehension systems. Epilepsia. 2008;49(12):1980–1997. doi: 10.1111/j.1528-1167.2008.01683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum BR, Baldo J, Okada K, Berman KF, Dronkers N, D’Esposito M, Hickok G. Conduction aphasia, sensory-motor integration, and phonological short-term memory - an aggregate analysis of lesion and fMRI data. Brain and Language. 2011;119(3):119–128. doi: 10.1016/j.bandl.2010.12.001. http://doi.org/10.1016/j.bandl.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton MW, Locasto PC, Krebs-Noble D, Gullapalli RP. A systematic investigation of the functional neuroanatomy of auditory and visual phonological processing. NeuroImage. 2005;26(3):647–661. doi: 10.1016/j.neuroimage.2005.02.024. http://doi.org/10.1016/j.neuroimage.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, an International Journal. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L. The unique role of the visual word form area in reading. Trends in Cognitive Sciences. 2011;15(6):254–262. doi: 10.1016/j.tics.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends in Cognitive Sciences. 2010;14(4):172–179. doi: 10.1016/j.tics.2010.01.004. http://doi.org/10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Duncan J. The structure of cognition: attentional episodes in mind and brain. Neuron. 2013;80(1):35–50. doi: 10.1016/j.neuron.2013.09.015. http://doi.org/10.1016/j.neuron.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E. The role of domain-general cognitive control in language comprehension. Language Sciences. 2014;5:335. doi: 10.3389/fpsyg.2014.00335. http://doi.org/10.3389/fpsyg.2014.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, Kanwisher N. Broad domain generality in focal regions of frontal and parietal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(41):16616–16621. doi: 10.1073/pnas.1315235110. http://doi.org/10.1073/pnas.1315235110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Thompson-Schill SL. Reworking the language network. Trends in Cognitive Sciences. 2014;18(3):120–126. doi: 10.1016/j.tics.2013.12.006. http://doi.org/10.1016/j.tics.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk JR, Rapp B, Goldrick M. The interaction of lexical and sublexical information in spelling: What’s the point? Cognitive Neuropsychology. 2002;19(7):653–671. doi: 10.1080/02643290244000184. http://doi.org/10.1080/02643290244000184. [DOI] [PubMed] [Google Scholar]
- Friedman RB. Two types of phonological alexia. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 1995;31(2):397–403. doi: 10.1016/s0010-9452(13)80372-3. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Kiebel S, Nichols T, Penny WD. Statistical parametric mapping the analysis of functional brain images. Amsterdam; Boston: Elsevier/Academic Press; 2007. [Google Scholar]
- Glover GH. Deconvolution of impulse response in event-related BOLD fMRI. NeuroImage. 1999;9(4):416–429. doi: 10.1006/nimg.1998.0419. http://doi.org/10.1006/nimg.1998.0419. [DOI] [PubMed] [Google Scholar]
- Graham NL, Patterson K, Hodges JR. The impact of semantic memory impairment on spelling: evidence from semantic dementia. Neuropsychologia. 2000;38(2):143–163. doi: 10.1016/s0028-3932(99)00060-3. [DOI] [PubMed] [Google Scholar]
- Graves WW, Desai R, Humphries C, Seidenberg MS, Binder JR. Neural systems for reading aloud: a multiparametric approach. Cerebral Cortex. 2010;20(8):1799–1815. doi: 10.1093/cercor/bhp245. http://doi.org/10.1093/cercor/bhp245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington GS, Farias D, Davis CH, Buonocore MH. Comparison of the neural basis for imagined writing and drawing. Human Brain Mapping. 2007;28(5):450–459. doi: 10.1002/hbm.20286. http://doi.org/10.1002/hbm.20286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry ML, Beeson PM, Stark AJ, Rapcsak SZ. The role of left perisylvian cortical regions in spelling. Brain and Language. 2007;100(1):44–52. doi: 10.1016/j.bandl.2006.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92(1–2):67–99. doi: 10.1016/j.cognition.2003.10.011. http://doi.org/10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nature Reviews Neuroscience. 2007;8(5):393–402. doi: 10.1038/nrn2113. http://doi.org/10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Ihnen SKZ, Petersen SE, Schlaggar BL. Separable roles for attentional control sub-systems in reading tasks: a combined behavioral and fMRI study. Cerebral Cortex. 2015;25(5):1198–1218. doi: 10.1093/cercor/bht313. http://doi.org/10.1093/cercor/bht313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischebeck A, Indefrey P, Usui N, Nose I, Hellwig F, Taira M. Reading in a regular orthography: an fMRI study investigating the role of visual familiarity. Journal of Cognitive Neuroscience. 2004;16(5):727–741. doi: 10.1162/089892904970708. http://doi.org/10.1162/089892904970708. [DOI] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. Neuroimage. 2003;20(2):693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Katzir T, Misra M, Poldrack RA. Imaging phonology without print: assessing the neural correlates of phonemic awareness using fMRI. NeuroImage. 2005;27(1):106–115. doi: 10.1016/j.neuroimage.2005.04.013. http://doi.org/10.1016/j.neuroimage.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Ludersdorfer P, Kronbichler M, Wimmer H. Accessing orthographic representations from speech: The role of left ventral occipitotemporal cortex in spelling. Human Brain Mapping. 2015;36(4):1393–1406. doi: 10.1002/hbm.22709. http://doi.org/10.1002/hbm.22709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Gorno-Tempini ML, Price CJ. Neuroimaging studies of word and pseudoword reading: consistencies, inconsistencies, and limitations. Journal of Cognitive Neuroscience. 2003;15(2):260–271. doi: 10.1162/089892903321208196. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. http://doi.org/10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paulesu E, McCrory E, Fazio F, Menoncello L, Brunswick N, Cappa SF, Cotelli M, Cossu G, Corte F, Lorussa M. A cultural effect on brain function. Nature Neuroscience. 2000;3(1):91–96. doi: 10.1038/71163. [DOI] [PubMed] [Google Scholar]
- Planton S, Jucla M, Roux FE, Démonet JF. The “handwriting brain”: A meta-analysis of neuroimaging studies of motor versus orthographic processes. Cortex. 2013;49(10):2772–2787. doi: 10.1016/j.cortex.2013.05.011. http://doi.org/10.1016/j.cortex.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Price CJ. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage. 2012;62(2):816–847. doi: 10.1016/j.neuroimage.2012.04.062. http://doi.org/10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The interactive account of ventral occipitotemporal contributions to reading. Trends in Cognitive Sciences. 2011;15(6):246–253. doi: 10.1016/j.tics.2011.04.001. http://doi.org/10.1016/j.tics.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Mechelli A. Reading and reading disturbance. Current Opinion in Neurobiology. 2005;15(2):231–238. doi: 10.1016/j.conb.2005.03.003. http://doi.org/10.1016/j.conb.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Purcell JJ, Turkeltaub PE, Eden GF, Rapp B. Examining the central and peripheral processes of written word production through meta-analysis. Frontiers in Psychology. 2011;2(239) doi: 10.3389/fpsyg.2011.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapcsak SZ, Beeson PM. The role of left posterior inferior temporal cortex in spelling. Neurology. 2004;62(12):2221–2229. doi: 10.1212/01.wnl.0000130169.60752.c5. [DOI] [PubMed] [Google Scholar]
- Rapcsak SZ, Beeson PM. The Handbook of Adult Language Disorders. 2. Psychology Press; 2015. Neuroanatomical correlates of spelling and writing; pp. 87–116. [Google Scholar]
- Rapcsak SZ, Beeson PM, Henry ML, Leyden A, Kim E, Rising K, Anderson S, Cho HS. Phonological dyslexia and dysgraphia: Cognitive mechanisms and neural substrates. Cortex. 2009;45(5):575–591. doi: 10.1016/j.cortex.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapcsak SZ, Rothi LJG, Heilman KM. Phonological alexia with optic and tactile anomia: A neuropsychological and anatomical study. Brain and Language. 1987;31(1):109–121. doi: 10.1016/0093-934x(87)90063-0. http://doi.org/10.1016/0093-934X(87)90063-0. [DOI] [PubMed] [Google Scholar]
- Rapp B, Dufor O. The neurotopography of written word production: an fMRI investigation of the distribution of sensitivity to length and frequency. Journal of Cognitive Neuroscience. 2011;23(12):4067–4081. doi: 10.1162/jocn_a_00109. [DOI] [PubMed] [Google Scholar]
- Rapp B, Epstein C, Tainturier MJ. The integration of information across lexical and sublexical processes in spelling. Cognitive Neuropsychology. 2002;19(1):1–29. doi: 10.1080/0264329014300060. http://doi.org/10.1080/0264329014300060. [DOI] [PubMed] [Google Scholar]
- Rapp B, Lipka K. The literate brain: The relationship between spelling and reading. Journal of Cognitive Neuroscience. 2011;23(5):1180–1197. doi: 10.1162/jocn.2010.21507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp B, Purcell J, Hillis AE, Capasso R, Miceli G. Neural bases of orthographic long-term memory and working memory in dysgraphia. Brain. 2016;139(2):588–604. doi: 10.1093/brain/awv348. http://doi.org/10.1093/brain/awv348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeltgen DP, Heilman KM. Lexical agraphia. Brain. 1984;107(3):811–827. doi: 10.1093/brain/107.3.811. [DOI] [PubMed] [Google Scholar]
- Roeltgen DP, Sevush S, Heilman KM. Phonological agraphia: writing by the lexical-semantic route. Neurology. 1983;33(6):755–765. doi: 10.1212/wnl.33.6.755. [DOI] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kümmerer D, Kellmeyer P, Vry MS, Umarova R, Musso M, Glauche V, Abel S. Ventral and dorsal pathways for language. Proceedings of the National Academy of Sciences. 2008;105(46):18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T. Phonological agraphia and the lexical route in writing. Brain. 1981;104(3):413–429. doi: 10.1093/brain/104.3.413. [DOI] [PubMed] [Google Scholar]
- Tainturier M-J, Bosse M-L, Roberts DJ, Valdois S, Rapp B. Lexical neighborhood effects in pseudoword spelling. Frontiers in Psychology. 2013;4(862) doi: 10.3389/fpsyg.2013.00862. http://doi.org/10.3389/fpsyg.2013.00862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JSH, Rastle K, Davis MH. Can cognitive models explain brain activation during word and pseudoword reading? A meta-analysis of 36 neuroimaging studies. Psychological Bulletin. 2013;139(4):766–791. doi: 10.1037/a0030266. http://doi.org/10.1037/a0030266. [DOI] [PubMed] [Google Scholar]
- Tsapkini K, Rapp B. The orthography-specific functions of the left fusiform gyrus: Evidence of modality and category specificity. Cortex. 2010;46(2):185–205. doi: 10.1016/j.cortex.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Coslett HB. Localization of sublexical speech perception components. Brain and Language. 2010;114(1):1–15. doi: 10.1016/j.bandl.2010.03.008. http://doi.org/10.1016/j.bandl.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Hervé PY, Duffau H, Crivello F, Houdé O, Mazoyer B, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. NeuroImage. 2006;30(4):1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. http://doi.org/10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology. 2008;100(6):3328–3342. doi: 10.1152/jn.90355.2008. http://doi.org/10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Brambati SM, Henry RG, Handwerker DA, Agosta F, Miller BL, Wilkins DP, Ogar JM, Gorno-Tempini ML. The neural basis of surface dyslexia in semantic dementia. Brain. 2009;132(1):71–86. doi: 10.1093/brain/awn300. http://doi.org/10.1093/brain/awn300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, Evans AC. A general statistical analysis for fMRI data. Neuroimage. 2002;15(1):1–15. doi: 10.1006/nimg.2001.0933. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4(1):58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]