Abstract
Objective
This study investigated microRNA and target gene profiles under different conditions of burn, bed rest, and exercise training.
Methods
Male Sprague-Dawley rats (n = 48) were assigned to sham ambulatory, sham hindlimb unloading, burn ambulatory, or burn plus hindlimb unloading groups. Rats received a 40% total body surface area scald burn or sham treatments and were ambulatory or hindlimb unloaded. Rats were further assigned to exercise or no exercise. Plantaris tissues were harvested on day 14 and pooled to analyze for microRNA and gene expression profiles.
Results
Compared to the sham ambulatory-no exercise group, 73, 79, and 80 microRNAs were altered two-fold in the burn ambulatory, sham hindlimb unloading, and burn hindlimb unloading groups, all with no exercise, respectively. Over 70% of microRNAs were upregulated in response to burn and hindlimb unloading, while 60% microRNA of the profile decreased in hindlimb unloaded burn rats with exercise training. MiR-182 was the most affected in rat muscle. GO biological process and pathway analysis showed that the oxidative stress pathway was most stimulated in the hindlimb unloaded burn rats; while in response to exercise training, all genes in related pathways such as hypermetabolic, inflammation and blood coagulation were alleviated.
Conclusions
MicroRNAs and transcript gene profiles were altered in burn and hindlimb unloading groups, with additive effects on hindlimb unloaded burn rats. The altered genes' signal pathways were associated with muscle mass loss and function impairment. Muscle improvement with exercise training was observed in gene levels with microRNA alterations as well.
Keywords: epigenetics, transcriptome, thermal injury, bed rest, exercise
Introduction
Following a severe burn, patients suffer a hypercatabolic state in response to cytokine and stress hormone challenges. These stress signals, such as cortisol, catecholamine, and TNF-α, induce a catabolic/anabolic imbalance.1 The body's flood of catabolic signals increases muscle breakdown and metabolism in muscle to call upon its role as an energy reservoir. Patients with severe burn showed 83% muscle protein degradation and a 50% increase in protein synthesis. Alongside protein degradation, skeletal muscles demonstrate a net loss of amino acids, with a 50% increased transport into the blood and a 40% decreased transport from the blood.2
Scientists explored a robust bi-phase genomic profile in response to burn and sepsis from animals and patients.3 In this multicenter study, they found that circulating leukocytes increase production of these inflammatory signals via a “genomic storm” of transcriptional changes. Genomic networks constructed for several major pathways such as inflammation and proteolysis are precisely demonstrated in burn patients.
Epigenetic changes are caused by external environmental stimulations which regulate the transcriptome but not the DNA sequence directly. Mechanisms of epigenetics include DNA methylation, histone modification microRNA (miRNA), etc. The miRNA is a conserved class of small (20-25 bases), abundant RNA-interfering (RNAi) molecules that inhibit gene expression at the translational level. It acts by transiently binding to the 3′ UTR of messenger RNA (mRNA) with partial complementarity and by blocking their translation. At least 20%-30% of protein-encoding genes in human are regulated by miRNAs, and these genes are often targeted by multiple miRNAs.4 Clinically, miRNAs have been studied as markers for cancer progression, which are related to tumor cell growth or death through tumor-suppressor gene silencing. miRNAs are also involved in other disease states, such as type 2 diabetes, and PTSD neural trauma injury.5 The alteration of miRNA profiles were related to muscle disuse and atrophy,6 and further affected with exercise.7 Particularly in the area of burn, Liang et al. previously studied the miRNA profile of cells in burned dermis and found 66 miRNAs that were significantly up- or downregulated.8 However, there is little information regarding the mechanism of miRNAs mediating skeletal muscle atrophy after burn.
Due to the injury, burn patients are often in bed for extended periods of time.9 Wu demonstrated that rat muscle function decreased after burn and hindlimb unloading in an animal model.10 We speculated that miRNA changes in skeletal muscle after burn and bed rest contribute to muscle atrophy due to its abundance and sweeping range of actions on different genes.
Exercise studies have shown to positively mitigate muscle atrophy.11 In pediatric burn patients, beneficial improvement of muscle mass was achieved with a combination of aerobic and resistance exercise training.12,13 We recently studied the effects of resistance exercise on muscle function recovery in burn rats with hindlimb unloading.14 There was the question of whether gene expression alteration with exercise training is correlated with miRNA regulation. Therefore, we further hypothesized that exercise improves muscle pathophysiological change that is associated with epigenetics-regulated gene expression after burn and muscle disuse. The purpose of the current study was to characterize the miRNA and genomic profile in burn and hindlimb unloaded animals with exercise training.
Methods
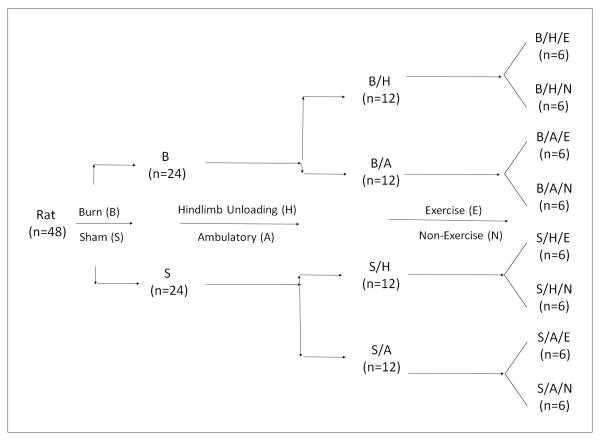
Forty-eight adult male Sprague-Dawley rats (Envigo [Harlan Labs], Indianapolis, IN) were used in this study. The animals' protocol was approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at Houston in accordance with NIH guidelines. All animal procedures were carried out at UT Houston and fully described previously.14 The experiment flow chart is presented in Figure 1 and briefly addressed below.
Figure 1.
Flow chart of animal experiment that outlines the size of animal and the order of operation
Burn procedure: All animals received a full-thickness scald burn of 40% total body surface area under 2%-4% isoflurane anesthesia. The burned animals (B) were resuscitated with 20 ml of intraperitoneal lactated Ringer's with buprenorphine for analgesia treatment. Sham animals (S) received the same procedure except for the scald burn.
Hindlimb unloading: Animals were placed in a hindlimb unloading system described by Morey-Holton and Globus after burn or sham injury.15 Rats were able to freely access regular chow (Harlan Teklad #2018) and water without the hindlimbs contacting the walls of the cage. Animals in the ambulatory groups (A) were housed in similar cages but without hindlimb unloading.
Resistance exercise: On the day of injury, animals were trained (E) to climb 1 meter at an 80-degree incline with tail weights five times twice daily. Weights were calculated as percent body mass of each individual rat and gradually increased in increments of 10% every few days as tolerated with a maximum weight of 50% body mass. All animals including the non-exercised group (N) were pre-trained 10 days prior to injury.
Total RNA extraction and genomic analysis
On day 14 after injury, all animals were euthanized, and the hindlimb muscles on the right were harvested and weighed. Half the plantaris tissue was immersed in RNA stabilization reagent (Qiagen, Hilden, Germany) and stored at -80°C. Tissue samples from 3 animals in each group were pooled, and total RNA was extracted using Qiagen miRNeasy Mini Kit (Qiagen, Hilden, Germany). RNA purity was over 99%, and 1 μg of RNA sample was processed at the UT Southwestern microarray core facility for the following miRNA and gene expression measurements.
The Affymetrix miRNA 4.0 Arrays chip (Santa Clara, CA) was applied for miRNA detection. The chips' reproducibility (intra and inter-lot) is greater than 0.95. A total of 36,333 small non-coding RNA probes, including varied species and controls coated in one chip for each sample. Rat gene expression from each pooled sample was detected in triplicate using the Affymetrix Rat Gene 2.0 chip (Santa Clara, CA). The chips contains 30,429 rat gene probes. Raw signal intensity data was normalized with robust multi-array average (RMA) from the Affymetrix data bank. The raw intensity values ratio of signal intensity were background corrected, log 2 transformed, and then quantile normalized. A linear model was then fitted to the normalized data to obtain an expression measure for each probe set on each array. The linear fold change of signal data was analyzed with Affymetrix Transcriptome Analysis Console 3.0 software. Threshold filters were set as the default value for both miRNA and gene expression data analysis. The absolute linear fold change value was greater than 2. For triplicated genomic sample data analysis, a one-way between-subject ANOVA (unpaired) was further applied with significant acceptance of p < 0.05. The interaction of miRNA and target genes, gene ontology (GO) biological processing, and related pathways were also analyzed.
Results
General description of miRNA and gene expressions
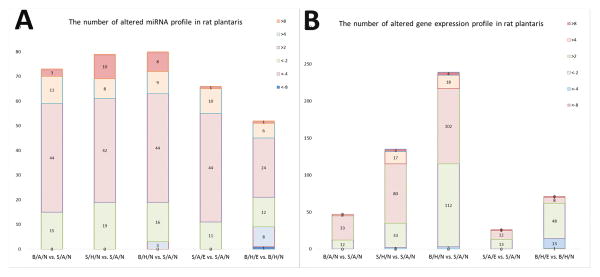
There were 1218 rat species miRNAs in a total of 36,333 miRNAs detected in rat muscle samples, including 728 rat mature miRNA probe sets and 490 pre-miRNA probes. Most signal intensities were lower than 2 in all groups. The highest probe signal intensity (binary log ratio) was 15.4 for miRNA-206-3p in all groups. We observed 74.5% of transcripts in the S/A/N group and 73.9% of the B/A/N group with a signal intensity of less than 2. There were 73, 79 and 80 miRNAs altered in the B/A/N, S/H/N, and B/H/N groups, respectively, compared to S/A/N group. Over 70% of the miRNAs were upregulated in response to burn and hindlimb unloading, while about 60% of miRNAs were upregulated in B/H rats with exercise training (Figure 2 A).
Figure 2.
Stacked bar figures showing the number of miRNA (2A) and genes (2B) in rat plantaris altered in response to burn (B/A/N vs. S/A/N), hindlimb unloading (S/H/N vs. S/A/N), combination of burn and hindlimb unloading (B/H/N vs. S/A/N), exercise in normal rats (S/A/E vs. S/A/N), and exercise in burn and hindlimb unloaded rats (B/H/E vs. B/H/N).
There were 30,429 rat genes detected using the Rat Gene 2.0 chip. GAPDH (transcript cluster ID 17799923) demonstrated the greatest signal intensity (13.5 binary log ratio). Filtered by default threshold values, there were 47 and 135 genes altered in rat muscle with burn or hindlimb unloading, respectively, while 239 genes were disturbed in combined burn and hindlimb unloading rats. There were 2, 20, and 22 genes that increased greater than fourfold in the B/A, S/H, and B/H groups separately. In contrast, 62 out of 71 genes decreased in the B/H/E versus B/H/N groups (Figure 2 B).
MiRNA and gene expression profiles are distinguished in response to burn, hindlimb unloading, and exercise and described separately as follows:
The miRNA and gene expression profile in response to burn (B/A/N vs. S/A/N)
In all, 79.4% of 73 miRNAs were upregulated in burn animals. The amplitude of upregulated miRNAs was higher than that of downregulated ones. There were 14 miRNAs upregulated more than fourfold, including the 3 most upregulated miRNAs, miR-182 (12.81), miR-184 (10.50) and miR-155-5p (8.82). Fold changes were less than 3 in all 15 downregulated miRNAs. The miR-409a-3p was the most decreased (-2.95) in burn animals (Supplemental Table 1-1).
There were 47 genes changed, 12 down- and 35 upregulated, in the B/A/N group compared to the S/A/N group (Supplemental Table 1-2). Thirty-two changed genes were associated with multiple GO biological processes, and 6 signal pathways were altered. The ketone body metabolism, inflammatory response, and striated muscle contraction pathways were most activated (Supplemental Table 1-3).
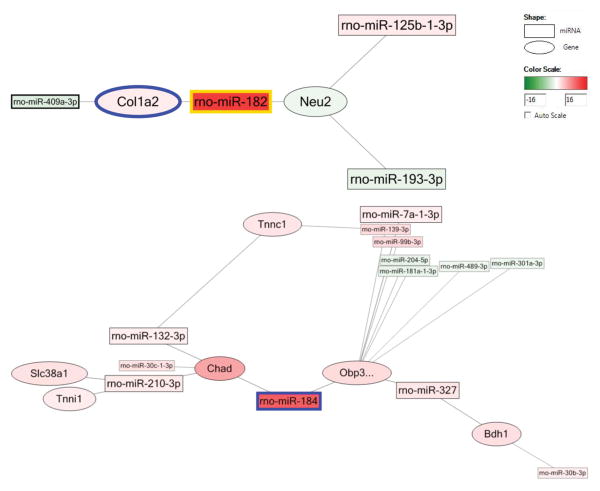
In viewing the interaction network, the most upregulated miRNA, miR-182, decreased the col1a2 gene, which collaborates with downregulated miR-409-3p. MiR-182 also works with miR-193-3p and 125-b-1p to downregulate the neu2 gene, which participates in muscle cell differentiation. The second most upregulated miRNA, miR-184, was associated with the decreased obp3 and chad genes (which are associated with GO biological processes of small molecular transportation and cartilage condensation, respectively), and also collaborates with other miRNAs (Figure 3).
Figure 3.
The network of miRNA and related genes after burn (B/A/N vs. S/A/N). Square shape stands for miRNA, oval shape stands for gene. Pseudo-color from green to red stands for fold change from -16 to 16.
The miRNA and gene expression profile in rats with hindlimb unloading (S/H/N vs. S/A/N)
There were 79 total altered miRNAs, including 19 downregulated and 60 upregulated ones. The amplitude of upregulated miRNAs was greater than that of the downregulated. Eighteen of the miRNAs' linear fold changes were upregulated over fourfold, and 10 miRNAs even changed over eight-fold; in contrast, downregulated miRNAs were all changed less than fourfold. MiR-182 (23.83), miR-184 (17.73), miR-183-5p (16.61), and miR-122-5p (14.19) were the most upregulated in the S/H/N group, and the most downregulated miRNAs included miR-489-3p (-3.54), miR-665 (-3.21), and miR-675-5p (-3.19) (Supplemental Table 2-1).
There were 135 genes, 100 up- and 35 downregulated, in rat muscle in response to hindlimb unloading. In all, 20 genes increased more than fourfold; only 2 genes decreased more than fourfold (Supplemental Table 2-2). Seventy-nine genes had varied biological process functions involving 9 pathways, with the 3 most prominent pathways being the MAPK cascade, the blood-clotting cascade, and fatty acid synthesis (Supplemental Table 2-3).
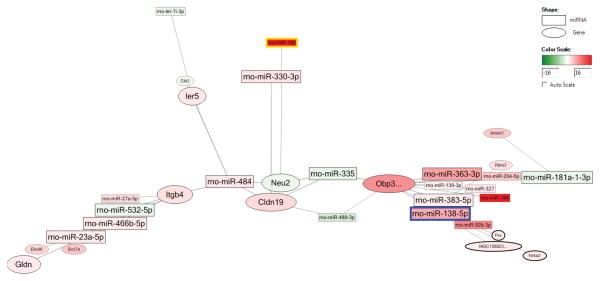
In viewing the interaction network, a large complex network was constructed between miRNAs and genes demonstrating altered expression. The most increased miRNA, miR-182, upregulated the clnd19 gene (for neuronal action potential propagation) in collaboration with decreased miR-489-3p. MiR-182 also worked with other miRNAs such as miR-335 and -484 to decrease the neu2 gene (for myotube differentiation positive regulation). The second most upregulated miRNA, miR-184, worked with miR-342 and miR-484 to increase obp3 and decrease cib2 and plcd4 genes, which are both for calcium ion binding (Figure 4).
Figure 4.
The network of miRNA and related genes after hindlimb unloading (S/H/N vs. S/A/N). Square shape stands for miRNA, oval shape stands for gene. Pseudo-color from green to red stands for fold change from -16 to 16.
The miRNA and gene expression profile in rats with combined burn and hindlimb unloading (B/H/N vs. S/A/N)
We found 80 miRNAs, including 61 upregulated miRNAs and 19 downregulated miRNAs, with only 3 downregulated over fourfold. There were 17 miRNAs upregulated over fourfold, and miR-182 even increased 35-fold (Supplemental Table 3-1).
There were 239 genes altered between the B/H/N versus S/A/N group. Only 3 of 115 downregulated genes changed more than fourfold, whereas there were 22 out of 124 genes upregulated more than fourfold (Supplemental Table 3-2). Overall, 165 genes related to multiple GO biological processes were involved in 14 pathways. Oxidative stress, the MAPK cascade, fatty acid synthesis, and the blood-clotting pathway were activated, and cardiovascular signaling and the p53 signal pathway were inhibited (Supplemental Table 3-3).
In viewing the interaction network, there were independent and additive effects in miRNA and gene expression levels when comparing the effects of burn and hindlimb unloading factors. While miR-409a-3p decreased 2.95-fold in B/A/N without detection in S/H/N mice, in B/H/N, miR-409a-5p decreased 2.03-fold. MiR-92b-3p only demonstrated an 8.04-fold upregulation in the S/H/N group but increased to a 9.46-fold upregulation in the B/H/N group. Most strikingly, miR-182 has an additive increased effect with combined burn and hindlimb unloading (35.35) compared to burn (12.81) and hindlimb unloading (23.82) individually. At the transcriptional level, Fmod only increased in burn rats but not in rats with hindlimb unloading; the Mbp gene increased in an opposite manner; while Nr4a3 had an additive effect in response to the combination of burn and hindlimb unloading (Supplemental Table 4).
The miRNA and gene expression profile in rats with burn and hindlimb unloading response to exercise training (B/H/E vs. B/H/N)
There were 52 miRNAs altered, including 21 down- and 31 upregulated, in burn and hindlimb unloading rats with exercise training. There were 9 miRNAs downregulated over fourfold, while 7 were upregulated over fourfold. The most upregulated miRNAs include miR-1843-3p (8.52), miR-495 (5.92), and miR-6324 (5.78) (Supplemental Table 5-1).
In viewing the interaction network, there were 71 gene changes, with 62 downregulated genes and only 9 genes upregulated less than fourfold in transcriptional level (Supplemental Table 5-2). Overall, 54 genes partook in different biological processes and 7 pathways. The MAPK cascade, fatty acid synthesis, and the inflammation response pathway were the top 3 alleviated pathways (Supplemental Table 5-3).
We further estimated the different effect of exercise between burn rats with hindlimb unloading and sham controlled rats. In S/A/E rats, there were 66 miRNAs altered, with 11 down- and 55 upregulated. We found that miR-182 increased 10.06-fold in S/A/E. There were only 12 genes altered, with 7 upregulated and 5 downregulated. Though 9 of these genes had functions in various biological processes, no pathway change was observed in normal rats with exercise training.
Discussion
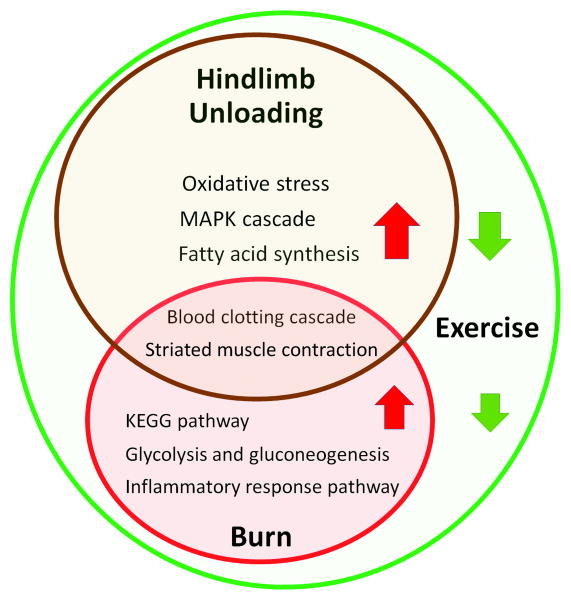
In this study, we characterized the miRNA and gene profiles in rat plantaris under conditions of burn, hindlimb unloading, and resistance exercise. The complexity of this regulation's network is displayed at both the epigenetic and transcriptome levels. Those consequent changes of biological processing and involved signal pathways reflect muscle pathophysiological changes in response to burn and hindlimb unloading. Hyper-metabolic response ketone bodies KEGG and glucose, and inflammation response were activated after burn injury; oxidative stress, MAPK cascade, and fat acid synthesis pathways were stimulated by hindlimb unloading; and striated muscle contraction and blood clotting pathways were stimulated by both burn and hindlimb unloading. Resistance exercise altered the transcriptome profile associated with muscle structure and function improvement (Figure 5).
Figure 5.
The scheme of affected signal pathways in response to burn, hindlimb unloading and exercise training. Red arrows indicated activated pathways. Green arrows indicates inhibited pathways.
Cells respond to the body's stress signals by making coordinated changes in gene expression. Padfield et al. reported the genomic profile in mice mouse muscle by 3 days after burn, including muscle development and function, inflammation and acute-phase immune response, amino acid and protein synthesis, and energy metabolism pathways.16 Vemula et al. verified this, linking 28% of the changed genes to metabolism. These included genes responsible for triglyceride utilization, fatty acid import, and acute phase proteins.17 Merritt reported that the inflammatory response activated stat/NFκβ to calcium-mediated proteolysis and ubiquitin-proteasome with absence of protein synthesis inhibition.18 In addition, we found that muscle mass loss is associated with insufficient myogenesis in response to burn and that TNF-α as a pro-inflammatory cytokine plays an important role in inhibiting muscle myogenesis 19. Though samples were collected 14 days after burn in the current study, we still observed increased numbers and amplitudes of gene and miRNA profile changes. Furthermore, we found those affected genes were mainly related to the metabolic and inflammatory response signal pathways. We are therefore not surprised by Jeschke's previous report that the hypermetabolic status can even last for years in burn patients.20
In human patients with bed rest, a gene profile reveals changes in energy pathways: oxidative phosphorylation, TCA cycle, organic compound usage, and carbohydrate metabolism.21 Bonaldo and Sandri revealed the intracellular mechanism by which hindlimb unloading activates cell apoptosis in an NF-KB dependent manner.22 In the current study, we found that burn mainly affects inflammation and metabolic pathways in rats, such as ketone bodies synthesis and degradation, inflammatory response, striated muscle contraction, and glucose metabolism; hindlimb unloading affects muscle signal pathways, including oxidative stress, MAPK cascade, fatty acid synthesis, and blood clotting cascade pathways; and Mal, Hmox1 and Btg2 genes are directly related to GO biological process of cell apoptosis. Severe burn and disuse have independent roles in body composition change.23 Therefore, it is logically believed that burn and hindlimb unloading might activate different major pathways with distinguishable characteristic profiles.
Muscle disuse amplified muscle function impairment in severely burned rats. In plantaris, the tissue's wet weight significantly decreased in response to burn and hindlimb unloading, respectively. Normalized to body mass, the tissue weight still decreased between the ambulatory and hindlimb unloading with decreased twitch and tetanic forces.10 In our current study, we observed that muscle disuse affects more genes in rat muscle than burn, so hindlimb unloading could be more closely associated with muscle impairment and function loss than burn. Furthermore, an overlap of miRNA and gene changes were observed in burn rats with hindlimb unloading. The double factors of burn and hindlimb unloading could amplify the signal strength and extend the pathophysiological phenotype in muscle.
The current study is the first to investigate miRNA profiles related to their target genes in muscle atrophy after burn. The miRNA differs from a similar class of RNAi, short interfering RNA (siRNA), in that it does not usually cleave the complement mRNA or affect gene transcription. The importance of miRNAs has been observed in regulating skeletal myogenesis. MiRNAs are highly-enriched in skeletal muscle and participate in skeletal myogenesis and muscle regeneration.24 In addition, miRNA-1 improves myogenic differentiation by inhibiting histone deacetylate 4, and miR-133a increases myoblast proliferation by repressing serum response factor.25 Cardiomyocyte hypertrophy can be induced by miR-195, and miR-195 with other 4 miRNAs increased in human heart failure and upregulated during cardiac hypertrophy in vivo.26
Exercise affects gene expression through miRNA changes. Following 90 minutes of exhaustive endurance exercise (forced treadmill running) in mice, miR-1 and miR-181, both thought to increase muscle differentiation and development, and miR-107, were increased.7 Resistance exercise training reduced anabolic signaling with gene alteration, including hypertrophic growth, protein degradation, and angiogenesis.27 In another clinical study, investigators distinguished an miRNA profile from human vastus lateralis with a 5 day/week resistance exercise for 12 weeks, and speculated those miRNAs served as compensatory mechanisms.28 We observed that there was a complicated network of epigenetic regulation in the current study. Not just one but several miRNAs control one single gene, and a single miRNA is also involved in multiple genes' regulation. It is therefore better to examine the whole profiles of miRNA and the related genes, and furthermore understand the protein structure and function change.
Previous studies showed that miR-182 has multiple-functions as a regulator of apoptosis, growth, and differentiation programs. Kouri reported that the injection of synthesized miR-182-based spherical nucleic acids suppressed tumor glioma burden and increased animal survival.29 More interestingly, miR-182 was shown to prevent skeletal muscle atrophy by interfering with forkhead box O3 (FoxO3) mRNA. The miR-182 decreased Foxo3 expression in C2C12 with further inhibition of atrohgin-1 and ATG12.30 Overall, miR-182 was the most phenomenally affected microRNA within all treatment groups in the current study. It increased 12.8- and 23.8-fold in burn and hindlimb unloading, respectively, and additively 35.5-fold in B/H/N rats. Exercise training decreased its expression 7.8-fold afterwards.
Burn causes a hypermetabolic status with a hyper-inflammation response, and muscle is a key participant in the systemic metabolic response. The current study could provide novel insight into potential target treatment at the epigenetic level. For instance, we have shown insulin resistance in animal models and burn patients.31 One review paper discussed the possibility of targeting miRNA to treat insulin resistance in burn patients.32
In summary, miRNAs and transcript gene profiles in rat plantaris were affected in burn and hindlimb unloading. These changes seen in signal pathways are associated with muscle pathophysiological changes, including muscle mass loss and function impairment. The muscle improvement observed with exercise training was also observed at the gene level with miRNA and genomic pathway alterations. The current exploration of regulation networks involving epigenetics- and gene-pathophysiological changes might aide the development of future biomarkers and potential therapeutic development in patients with muscle atrophy.
Supplementary Material
SUPPLEMENTAL TABLES (separate files attached): Table 1. miRNA and gene profiles in response to burn (B/A/N vs. S/A/N)
Table 2. miRNA and gene profiles in response to hindlimb unloading (S/H/N vs. S/A/N)
Table 3. miRNA and gene profiles in response to the combination of burn and hindlimb unloading (B/H/N vs. S/A/N)
Table 4. miRNA and gene comparison between burn and hindlimb unloading
Table 5. miRNA and gene profiles in response to exercise in burn and hindlimb unloading rats (B/H/E vs. B/H/N)
Acknowledgments
This work was supported by funds from the Golden Charity Guild Charles R. Baxter, MD, Chair; the Department of Defense #W81XWH-13-1-0462; and the National Institute for General Sciences of the National Institutes of Health #T32GM008593. We thank staff medical editor Dave Primm for his assistance. Authors have no financial or other interests construed as a conflict of interest.
Source of funding: This work was supported by funds from the Golden Charity Guild Charles R. Baxter, MD, Chair; the Department of Defense #W81XWH-13-1-0462; and the National Institute for General Sciences of the National Institutes of Health #T32GM008593.
Footnotes
Author contributions: JS, MRS, and ARC contributed to data collection and analysis and manuscript preparation. LAB was for animal experiment, sample collection, and manuscript revision. CEW and SEW were integral to the concept and design of the experiment as well as the critical revision of the manuscript.
Conflicts of interest: The authors have no financial or other interests construed as a conflict of interest.
References
- 1.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363(9424):1895–1902. doi: 10.1016/S0140-6736(04)16360-5. [DOI] [PubMed] [Google Scholar]
- 2.Biolo G, Fleming RY, Maggi SP, et al. Inverse regulation of protein turnover and amino acid transport in skeletal muscle of hypercatabolic patients. J Clin Endocrinol Metab. 2002;87(7):3378–3384. doi: 10.1210/jcem.87.7.8699. [DOI] [PubMed] [Google Scholar]
- 3.Xiao W, Mindrinos MN, Seok J, et al. A genomic storm in critically injured humans. J Exp Med. 2011;208(13):2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu NK, Xu XM. MicroRNA in central nervous system trauma and degenerative disorders. Physiol Genomics. 2011;43(10):571–580. doi: 10.1152/physiolgenomics.00168.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zovkic IB, Sweatt JD. Epigenetic mechanisms in learned fear: implications for PTSD. Neuropsychopharmacology. 2013;38(1):77–93. doi: 10.1038/npp.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang XH. MicroRNA in myogenesis and muscle atrophy. Curr Opin Clin Nutr Metab Care. 2013;16(3):258–266. doi: 10.1097/MCO.0b013e32835f81b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safdar A, Abadi A, Akhtar M, et al. miRNA in the regulation of skeletal muscle adaptation to acute endurance exercise in C57Bl/6J male mice. PLoS One. 2009;4(5):e5610. doi: 10.1371/journal.pone.0005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang P, Lv C, Jiang B, et al. MicroRNA profiling in denatured dermis of deep burn patients. Burns. 2012;38(4):534–540. doi: 10.1016/j.burns.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Wolfe RR. Control of muscle protein breakdown: effects of activity and nutritional states. Int J Sport Nutr Exerc Metab. 2001;11(Suppl):S164–169. doi: 10.1123/ijsnem.11.s1.s164. [DOI] [PubMed] [Google Scholar]
- 10.Wu X, Baer LA, Wolf SE, et al. The impact of muscle disuse on muscle atrophy in severely burned rats. J Surg Res. 2010;164(2):e243–251. doi: 10.1016/j.jss.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrando AA, Tipton KD, Bamman MM, et al. Resistance exercise maintains skeletal muscle protein synthesis during bed rest. J Appl Physiol (1985) 1997;82(3):807–810. doi: 10.1152/jappl.1997.82.3.807. [DOI] [PubMed] [Google Scholar]
- 12.Diego AM, Serghiou M, Padmanabha A, et al. Exercise training after burn injury: a survey of practice. J Burn Care Res. 2013;34(6):e311–317. doi: 10.1097/BCR.0b013e3182839ae9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suman OE, Spies RJ, Celis MM, et al. Effects of a 12-wk resistance exercise program on skeletal muscle strength in children with burn injuries. J Appl Physiol (1985) 2001;91(3):1168–1175. doi: 10.1152/jappl.2001.91.3.1168. [DOI] [PubMed] [Google Scholar]
- 14.Saeman MR, DeSpain K, Liu MM, et al. Effects of exercise on soleus in severe burn and muscle disuse atrophy. J Surg Res. 2015;198(1):19–26. doi: 10.1016/j.jss.2015.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morey-Holton ER, Globus RK. Hindlimb unloading rodent model: technical aspects. J Appl Physiol (1985) 2002;92(4):1367–1377. doi: 10.1152/japplphysiol.00969.2001. [DOI] [PubMed] [Google Scholar]
- 16.Padfield KE, Astrakas LG, Zhang Q, et al. Burn injury causes mitochondrial dysfunction in skeletal muscle. Proc Natl Acad Sci U S A. 2005;102(15):5368–5373. doi: 10.1073/pnas.0501211102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vemula M, Berthiaume F, Jayaraman A, et al. Expression profiling analysis of the metabolic and inflammatory changes following burn injury in rats. Physiol Genomics. 2004;18(1):87–98. doi: 10.1152/physiolgenomics.00189.2003. [DOI] [PubMed] [Google Scholar]
- 18.Merritt EK, Cross JM, Bamman MM. Inflammatory and protein metabolism signaling responses in human skeletal muscle after burn injury. J Burn Care Res. 2012;33(2):291–297. doi: 10.1097/BCR.0b013e3182331e4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song J, Saeman MR, De Libero J, et al. Skeletal Muscle Loss Is Associated with Tnf Mediated Insufficient Skeletal Myogenic Activation after Burn. Shock. 2015 doi: 10.1097/SHK.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeschke MG, Gauglitz GG, Kulp GA, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One. 2011;6(7):e21245. doi: 10.1371/journal.pone.0021245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen YW, Gregory CM, Scarborough MT, et al. Transcriptional pathways associated with skeletal muscle disuse atrophy in humans. Physiol Genomics. 2007;31(3):510–520. doi: 10.1152/physiolgenomics.00115.2006. [DOI] [PubMed] [Google Scholar]
- 22.Bonaldo P, Sandri M. Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech. 2013;6(1):25–39. doi: 10.1242/dmm.010389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wade CE, Baer LA, Wu X, et al. Severe burn and disuse in the rat independently adversely impact body composition and adipokines. Crit Care. 2013;17(5):R225. doi: 10.1186/cc13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guller I, Russell AP. MicroRNAs in skeletal muscle: their role and regulation in development, disease and function. J Physiol. 2010;588(Pt 21):4075–4087. doi: 10.1113/jphysiol.2010.194175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen JF, Mandel EM, Thomson JM, et al. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38(2):228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Rooij E, Sutherland LB, Liu N, et al. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A. 2006;103(48):18255–18260. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nader GA, von Walden F, Liu C, et al. Resistance exercise training modulates acute gene expression during human skeletal muscle hypertrophy. J Appl Physiol (1985) 2014;116(6):693–702. doi: 10.1152/japplphysiol.01366.2013. [DOI] [PubMed] [Google Scholar]
- 28.Davidsen PK, Gallagher IJ, Hartman JW, et al. High responders to resistance exercise training demonstrate differential regulation of skeletal muscle microRNA expression. J Appl Physiol (1985) 2011;110(2):309–317. doi: 10.1152/japplphysiol.00901.2010. [DOI] [PubMed] [Google Scholar]
- 29.Kouri FM, Hurley LA, Daniel WL, et al. miR-182 integrates apoptosis, growth, and differentiation programs in glioblastoma. Genes Dev. 2015;29(7):732–745. doi: 10.1101/gad.257394.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hudson MB, Rahnert JA, Zheng B, et al. miR-182 attenuates atrophy-related gene expression by targeting FoxO3 in skeletal muscle. Am J Physiol Cell Physiol. 2014;307(4):C314–319. doi: 10.1152/ajpcell.00395.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeschke MG, Finnerty CC, Herndon DN, et al. Severe Injury Is Associated With Insulin Resistance, Endoplasmic Reticulum Stress Response, and Unfolded Protein Response. Ann Surg. 2012;255(2):370–378. doi: 10.1097/SLA.0b013e31823e76e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu Y, Chai J. The function of miRNAs and their potential as therapeutic targets in burn-induced insulin resistance (review) Int J Mol Med. 2015;35(2):305–310. doi: 10.3892/ijmm.2014.2023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL TABLES (separate files attached): Table 1. miRNA and gene profiles in response to burn (B/A/N vs. S/A/N)
Table 2. miRNA and gene profiles in response to hindlimb unloading (S/H/N vs. S/A/N)
Table 3. miRNA and gene profiles in response to the combination of burn and hindlimb unloading (B/H/N vs. S/A/N)
Table 4. miRNA and gene comparison between burn and hindlimb unloading
Table 5. miRNA and gene profiles in response to exercise in burn and hindlimb unloading rats (B/H/E vs. B/H/N)