Abstract
Background
Concerns regarding the safety of codeine have been raised. Cases of life-threatening respiratory depression and death in children have been attributed to codeine’s polymorphic metabolic pathway. International health agencies recommend restricted use of codeine in children. Despite these recommendations, the epidemiology of codeine use among children remains unknown.
Aims
Our objective was to examine patterns of codeine use in the US among children.
Methods
A cross-sectional analysis of children ages 0–17 years from 1996–2013 in the US was performed. Data was extracted from MEPS, a nationally representative set of health care surveys. Prevalence rates of codeine use between 1996 and 2013 were examined. Multivariable logistic regression examined relationships between codeine use and patient demographics.
Results
Codeine use remained largely unchanged from 1996 to 2013 (1.08 vs. 1.03 million children, respectively). Odds of codeine use was higher in ages 12–17 (OR, 1.40; [1.21–1.61]), outside of the Northeast US, and among those with poor physical heath status (OR, 3.29 [1.79–6.03]). Codeine use was lower in children whose ethnicity was not white and those uninsured (OR, 0.47 [0.34–0.63]). Codeine was most frequently prescribed by emergency physicians (18%) and dentists (14%). The most common condition associated with codeine use was trauma-related pain.
Conclusions
Pediatric codeine use has declined since 1996, however more than 1 million children still used codeine in 2013. Health care providers must be made aware of guidelines advising against the use of codeine in children. Codeine is potentially hazardous and safer alternatives to treat children’s pain are available.
Keywords: child, pediatrics, adolescents, opioids, analgesics, narcotics
Introduction
Codeine is a weak opioid that has been widely used as an analgesic agent for acute pain. In the last decade, however, serious concerns regarding the safety of codeine have been identified, particularly in the pediatric population. The potential dangers of codeine in pediatrics first gained attention following a report in 2009 of a 2 year-old child who died following codeine administration post-tonsillectomy.(1) Post-mortem evaluation demonstrated the child had a functional duplication of the CYP2D6 allele, resulting in ultra-rapid metabolization of codeine. In 2012, Kelly et al. published a case series of children ages 3 to 5 of which two died and one experienced severe respiratory depression after receiving the recommended dose of codeine post-adenotonsillectomy.(2)
Following these cases the World Health Organization (WHO) removed codeine entirely from its guidelines for pediatric pain management.(3) Shortly thereafter, the Food and Drug Administration (FDA) launched an evaluation of the safety of codeine use in adenotonsillectomy, which lead to a black box warning advising against the use of codeine for post-operative analgesia following tonsillectomy.(4) The European Medicine Agency expressed similar concerns and in 2013 issued a statement that all codeine-containing medications were contraindicated for patients under 12 years of age, extending this to up to 18 years in patients undergoing adenotonsillectomy.(5) The United Kingdom Medicines and Healthcare Products Regulatory Agency advised against codeine use in any child at risk of respiratory compromise and proposed a maximum dose and duration.(6) Furthermore, since 2013 Health Canada no longer recommends use of codeine in children less than 12 years of age.(7) In light of ongoing concerns regarding codeine use in children the FDA advisory committee met in December 2015 to assess whether codeine should be restricted in children beyond current advisories and whether it should be available as an over the counter antitussive.(8) The final determination of this meeting is pending.
The concerns surrounding codeine relate to its metabolic pathway, which is highly variable between individuals. Codeine is a pro-drug that requires conversion by the liver into its active metabolite, morphine, to exert its analgesic effect.(2) This pathway depends on the cytochrome P450 2D6 enzyme, which has significant variability in its expression and activity. Identified polymorphisms include extensive metabolizers (EM), poor metabolizers (PM), and ultra-rapid metabolizers (UM).(9) The EM phenotype expresses enzymes with normal activity. The PM phenotype expresses either a dysfunctional or inactive enzyme, resulting in inadequate or complete lack of analgesic effect. Of greatest concern is the UM phenotype which exists as a result of the duplication in the CYP2D6 pathway. These individuals are at risk of developing potentially life threatening systemic levels of morphine. The prevalence of the UM phenotype within the Caucasian, Mediterranean, Asian or Middle Eastern, and Ethiopian populations are <1%, 10%, 10–30%, and 30%, respectively. The PM phenotype has a prevalence ranging from <1% in those of Asian descent up to 10% in Caucasian populations.(10) Such widespread variability in the biotransformation of codeine results in a variable and unpredictable clinical response.
Despite these concerns and recommendations advising against the use of codeine, patterns and predictors of codeine use remains largely unknown. Codeine use remains prevalent in emergency departments (11), however no studies have yet examined codeine use in the general childhood population. We previously examined opioid use among children in the United States and found that codeine constituted 39.9% of opioids used by children in 2012(12). The current study analyses this data further by examining patterns and predictors of codeine use among children in the USA between 1996 and 2013. The goal of this study is to advance understanding of codeine epidemiology in order to inform efforts at preventing codeine use. We hypothesized that codeine use among children would significantly decrease over time from 1996 to 2013. We also sought to identify characteristics associated with codeine use among children in the U.S.
Methods
Study Design and Sample
The study population includes children 0–17 years of age who participated in the 1996–2013 Medical Expenditure Panel Surveys (MEPS). Approximately 9,000 children participated in MEPS each year from 1996 to 2013 and our final sample included 154,362 children. The study was deemed exempt from review by the Institutional Review Board at Seattle Children’s Hospital.
Data Source
Data was extracted from the 1996–2013 MEPS. MEPS have previously been used to examine trends in opioid use among adults and children in the U.S.(12, 13) MEPS are a large nationally representative set of surveys aimed at providing comprehensive estimates of health care use among the non-institutionalized population of the U.S. Surveys are conducted by two governmental agencies, the National Centre for Health Statistics and the Agency for Healthcare Research and Quality, on a bi-annual basis. MEPS participants are surveyed at the household level, allowing for analysis of both the individual and the family. Five in-person interviews are conducted over the course of two years during which sociodemographic information is gathered and all medical encounters, including prescribed medications, are documented. Further information regarding health care utilization is gathered from households’ health care providers, hospitals, pharmacies and health insurance agencies. Please see the MEPS website http://meps.ahrq.gov/mepsweb/ for further information on design, sampling, and methodology(14).
Study Variables
We extracted the following data for each child participant documented in the 1996–2013 MEPS to use in a cross sectional analysis: participant sociodemographics, parent-reported physical and mental health status, use of codeine and other opioids, medical practitioner visits associated with opioid use, and medical conditions associated with opioid use.
Sociodemographics
Variables included age (categories: <6 years, 6–11 years, and 12–17 years), gender, race/ethnicity (White, Black, Hispanic, other), US region (Northeast, Midwest, South, and West), and insurance coverage (private, public, or uninsured).
Health status
Parent-reported child physical and mental health status were classified as excellent, very good, good, fair, or poor.
Medical visit data
Medical practitioners prescribing opioids were classified as pediatricians, general practitioners, emergency department physicians, otolaryngologists, orthopedic surgeons, dentists, physician assistants, other medical specialties, and unknown providers. We were unable to distinguish between practitioners who had received pediatric specific training or otherwise. We also identified a group of providers who provided prescriptions at hospital discharge, these providers were classified as “hospital discharges”, MEPS does not collect data on inpatient medications received during hospitalization.
Medical diagnoses associated with codeine use: MEPS collected data on participants’ medical conditions and procedures as verbatim text. Verbatim text was then recoded to fully specified International Statistical Classification of Diseases and Related Health Problems (ICD-9) codes by professional coders. ICD-9 codes were then aggregated into clinically meaningful categories generated by Clinical Classification Code Software (CCC). CCC resulted in 263 mutually exclusive categories. To further ease interpretability, we categorized these 263 CCC categories into 13 general medical categories including: trauma, surgical procedures, cardiac and respiratory, dental, neurologic and headache, skin and dermatological, urogenital, orthopedic (non- traumatic), infectious, and hematological and oncological conditions.
Identification and classification of codeine and other opioids
MEPS participants were surveyed about medications purchased and used by them during the past year. Additional data about prescribed medications used, including Cerner’s Multum Lexicon therapeutic class and medicine name were then collected from participants’ dispensing pharmacies. Included in MEPS are data on all medications associated with outpatient visits (e.g. hospital outpatient visits, office-based visits, and dental office visits), however no information was collected on medications prescribed during hospital inpatient visits nor was data collected on over-the-counter medications. We identified opioid prescriptions using Cerner’s Multum Lexicon therapeutic drug class classification system as captured in MEPS. According to the Multum Lexicon system opioid prescriptions are any that fit the “narcotic analgesic” and “narcotic analgesic combination” classification. The former definition includes all pure opioid agonists (e.g. codeine without additives), while the latter includes all opioid combination drugs (e.g. codeine and acetaminophen combinations). From 1996 to 2013 we identified >250 unique opioid analgesics among children, each containing varying aspects of manufacturer, formulation, and route of administration (e.g. oral, transdermal). We assigned these >250 unique opioid products to one of 4 opioid classes: (1) codeine and codeine combinations, (2) hydrocodone and hydrocodone combinations (3) oxycodone and oxycodone combinations, and (4) other opioids (incl. meperidine, propoxyphene, methadone, fentanyl, and opium products). Codeine users were classified as those survey participants who received one or more prescriptions for codeine or codeine and acetaminophen combinations in a given calendar year.
Analysis
Analyses were conducted with Stata version 12.1 (StataCorp, College Station, TX); α level was set at 0.05. We adjusted for the complex probability survey design of MEPS using sampling weights, stratification, and clustering to provide nationally representative estimates. We calculated prevalence rates of codeine use and total codeine use for each year from 1996 to 2013. Furthermore, to gain a better understanding of recent patterns in codeine use, we determined which providers prescribed codeine to children and the medical diagnosis associated with codeine use between 2010 and 2013. However, because overall numbers of medical providers were unknown we were not able to conduct comparative testing on medical providers associated with codeine prescriptions. Finally, we performed multivariable logistic regression analysis to examine child characteristics (age, sex, race/ethnicity, insurance status, region, urbanization, physical health, and mental health) associated with codeine use from 1996 to 2013. In our logistic regression model, all years 1996–2013 were included for analysis as a continuous variable. Odds ratio for year presented in our tables represents the linear test of trend across 18 years (1996–2013). In order to gain a better understanding of recent patterns in codeine use we also performed a multivariate logistic regression analysis to determine characteristics associated with codeine use between 2010 and 2013. However, results (odds ratios’ sizes and directions) were similar for 2010–2013 analysis compared to the 1996–2013 analysis, and therefore we only report results from the full range of 1996–2013 in this manuscript. Codeine prescriptions were also analyzed according the type of medical provider and reported as percentage of total codeine prescriptions. We were unable to determine odds ratios for type of medical provider.
Results
Changes in Codeine Use Practices
Data from 154,362 children were available in the MEPS database from 1996 to 2013. We estimated that 1.08 million children received a codeine prescription in 1996 versus 1.03 million in 2013. (Figure 1) As a percentage of the total childhood population 1.60% of children received a codeine prescription in 1996 versus 1.46% in 2013 (p<0.001). We also compared codeine prescription rates between 1996–2006 and 2007–2013: the percentage of children with a codeine prescription from 1996–2006 vs. 2007–2013 decreased significantly from 1.8% to 1.3% (p<0.0001).
Figure 1.
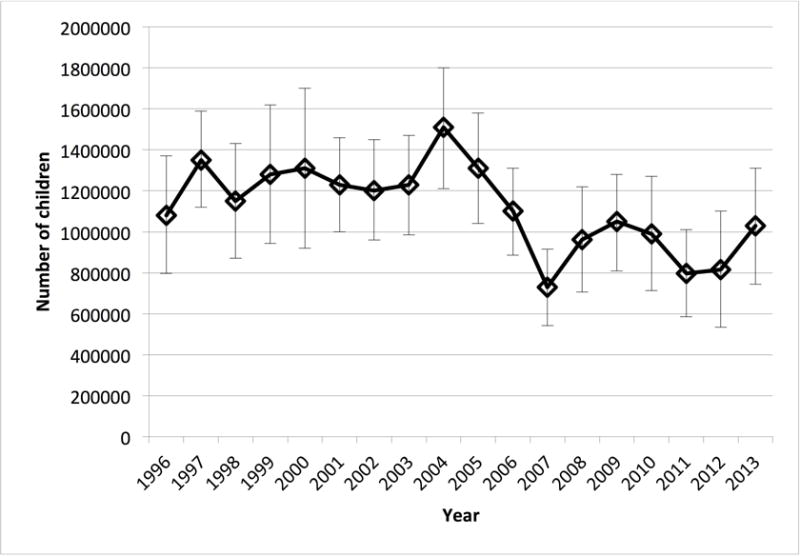
Total number of children who used codeine each year 1996 to 2013. Total number of children using codeine in 1996 (1.08 million, 95% CI 0.79–1.37 million) decreased to 1.03 million (95% CI 0.74–1.31) in 2013.
After controlling for multiple factors, the odds ratio of codeine use decreased per year of the study (odds ratio [OR] 0.98; 95% confidence interval [0.96–0.99]). Codeine as a percentage of total opioids used decreased from 52.7% in 1996 to 44.3% in 2013 with a corresponding increase in oxycodone and hydrocodone use (figure 2). However, codeine remained the most common opioid used by children in 2013.
Figure 2.
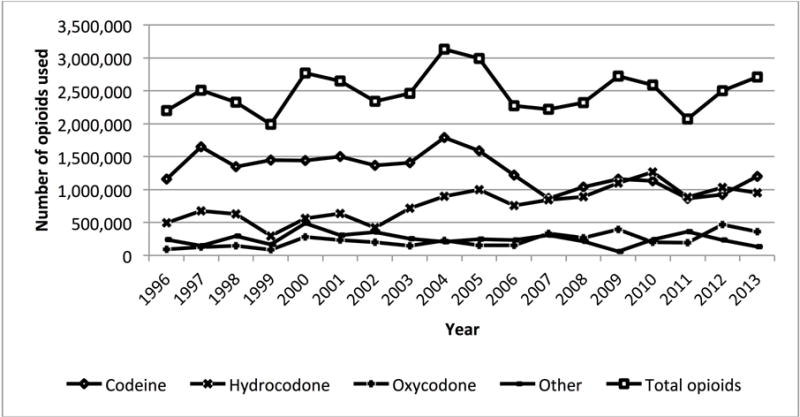
Number of total opioid, codeine, hydrocodone, oxycodone, and other opioids used among children 0–17 years of age in the United States between 1996 and 2013. Codeine as a percentage of total opioid use decreased from 52.7% in 1996 to 44.3% in 2013.
Characteristics Associated with Codeine Use
Patient characteristics associated with codeine use are outlined in Table 1. Codeine was more likely used by children ages 12–17 than younger patients ages under 6 years (OR 1.40 [1.21–1.61]). The proportion of children using codeine was lowest in those under the age of 6 (1.4%, p<0.001). Patients residing outside of the Northeastern US were more likely to receive codeine prescriptions. The odds of codeine use increased with poorer parent-reported child physical health status. Females had a decreased likelihood of receiving codeine compared to males (OR 0.86 [0.77–0.97]). Codeine use was also less likely in children and adolescents who identified as non-white race and in patients who were uninsured (OR 0.47 [0.34–0.63]). Poorer parent-reported child mental health status did not increase the likelihood of codeine use.
Table 1.
Multivariable logistic regression testing survey year and participant characteristics associated with codeine use among children 0–17 years of age in the United States, 1996–2013. Data source: Medical Expenditure Panel Surveys 1996–2013.
| Characteristic | No. of Codeine Use (N=154 362) |
Proportion of children using Codeine (%) |
P (X2) | Adjusted OR (95% CI) for Codeine Rx | P (Adjusted OR) |
|---|---|---|---|---|---|
| Year (1996–2013) | 2343 | 1.6 | <0.001 | 0.98 (0.96–0.99) | 0.001 |
| Age Group (years) | |||||
| <6 | 582 | 1.4 | Reference | ||
| 6 to 11 | 790 | 1.5 | <0.001 | 1.11 (0.97–1.27) | 0.12 |
| 12–17 | 971 | 1.9 | 1.40 (1.21–1.61) | <0.001 | |
| Gender | |||||
| Male | 1284 | 1.7 | 0.02 | Reference | |
| Female | 1059 | 1.5 | 0.86 (0.77–0.97) | 0.01 | |
| Race/Ethnicity | |||||
| White | 1173 | 1.8 | Reference | ||
| Black | 414 | 1.4 | <0.001 | 0.69 (0.59–0.82) | <0.001 |
| Hispanic | 625 | 1.2 | 0.59 (0.50–0.69) | <0.001 | |
| Other | 116 | 1.2 | 0.62 (0.48–0.79) | <0.001 | |
| US region | |||||
| Northeast | 245 | 1.2 | Reference | ||
| Midwest | 553 | 1.8 | 0.003 | 1.44 (1.20–1.74) | <0.001 |
| South | 880 | 1.6 | 1.41 (1.18–1.69) | <0.001 | |
| West | 665 | 1.7 | 1.51 (1.24–1.83) | <0.001 | |
| Payment source | |||||
| Private | 1234 | 1.7 | Reference | ||
| Public | 1003 | 1.7 | <0.001 | 1.17 (1.03–1.33) | 0.02 |
| Uninsured | 106 | 0.8 | 0.47 (0.34–0.63) | <0.001 | |
| Physical Health Status | |||||
| Excellent | 879 | 1.3 | Reference | ||
| Very good | 733 | 1.7 | 1.41 (1.19–1.67) | <0.001 | |
| Good | 583 | 2.1 | <0.001 | 1.83 (1.51–2.21) | <0.001 |
| Fair | 126 | 3.8 | 3.24 (2.41–4.37) | <0.001 | |
| Poor | 20 | 3.6 | 3.29 (1.79–6.03) | <0.001 | |
| Mental Health Status | |||||
| Excellent | 1093 | 1.5 | Reference | ||
| Very good | 656 | 1.6 | 0.87 (0.73–1.02) | 0.09 | |
| Good | 494 | 2.0 | <0.001 | 0.89 (0.73–1.09) | 0.26 |
| Fair | 75 | 2.6 | 0.89 (0.62–1.27) | 0.52 | |
| Poor | 22 | 2.0 | 0.58 (0.34–0.99) | 0.05 |
Examination of the type of health care providers from 2010 to 2013 demonstrated that emergency physicians (18%) and dentists (14%) were the most frequent prescribers of codeine. Physician assistants (1%), pediatricians (6%), and orthopedic surgeons (6%) least frequently prescribed codeine. Otolaryngologists accounted for 9% of codeine prescriptions (Figure 3). Figure 4 describes the primary medical diagnosis associated with codeine use. Pain associated with trauma was the most common reason for receiving codeine, followed by post-procedural pain.
Figure 3.
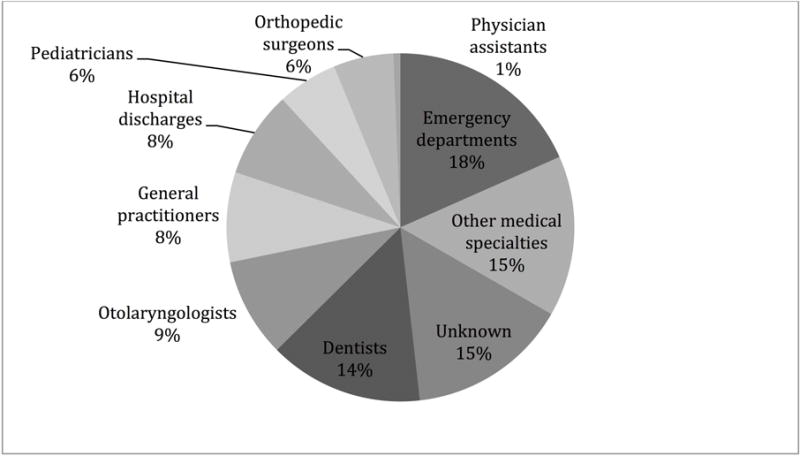
Percentage of total codeine prescriptions by health care provider type for 2010 to 2013.
Figure 4.
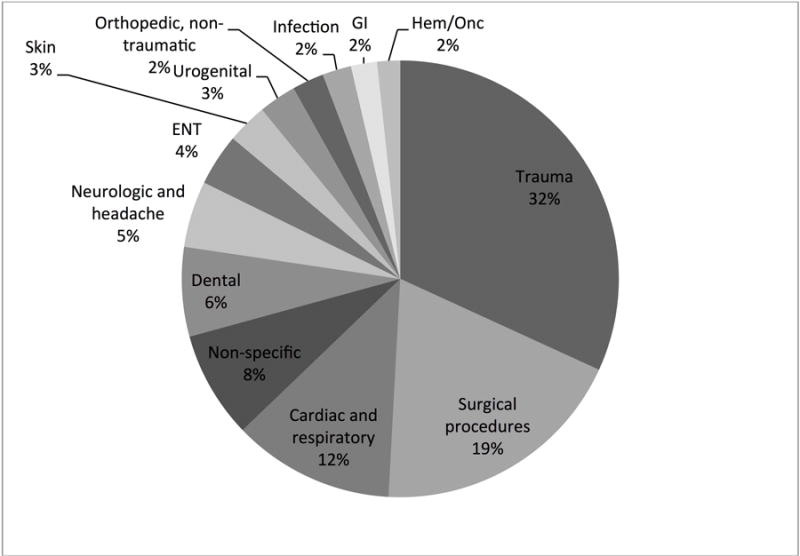
Primary diagnosis associated with codeine use from 2010 to 2013. ENT ear, nose, and throat; GI gastroenterology; Hem/Onc hematology and oncology.
Discussion
This nationally representative cross-sectional analysis found an overall decrease in the percentage of children who were prescribed codeine and a decrease in the odds ratio of codeine use per year from 1996–2013. However, while these decreases were statistically significant, they were perhaps not clinically significant as in 2013 there were still 1.03 million children who received a codeine prescription. Furthermore, in 2013 codeine remained the most frequently prescribed opioid to children.
These findings are consistent with literature examining national codeine use practices in emergency departments. Using data from the National Hospital Ambulatory Medical Care Survey, national rates of codeine prescription were reported to decrease slightly but remain common in emergency departments in the United States.(11) Findings are also similar to previous studies demonstrating that opioid prescriptions in the United States from 1996 to 2012 have remained largely unchanged in recent years. Of the opioids prescribed, codeine comprised 39.9% of prescriptions(12).
Our study identified several factors that are predictive of codeine use in children. As expected, older children had higher odds of codeine use compared to younger children. Children under 6 years had the lowest proportion of codeine use in our study. Because deaths associated with codeine use have been primarily in children under the age of 6, (1, 2) it is possible that healthcare provider prescription practices have been influenced by dissemination of concerns about codeine use in young children. Our results also demonstrated that children and adolescents of non-white ethnicity were less likely to receive codeine. The prevalence of the codeine UM phenotype is greatest in non-white populations.(10) However, prior literature has demonstrated that non-white children have been less likely to be prescribed any opioid.(15, 16) Therefore, decreased codeine use among non-white children may be a reflection of general opioid disparities rather than acknowledgement of UM phenotypes. Codeine use was greatest among those with health insurance, which is consistent with previous studies demonstrating that overall opioid use is greatest in insured children and adolescents.(12) Additional studies have demonstrated disparities in dispensation of medications to children and adolescents based on insurance status.(17) Geographical differences in codeine use were also noted in the present study. Children and adolescents located outside of the North-Eastern US were more likely to receive codeine, which is also consistent with previous studies.(11, 18) Regional differences in opioid use have also been noted in the adult population.(19) Further study of the reason for regional variation in codeine and opioid use more broadly is needed.
Our study is the first to assess the type of health care professional providing codeine prescriptions nationally. We found that the health care providers most frequently prescribing codeine were emergency physicians and dentists. Pediatricians, physician assistants, orthopedic surgeons and otolaryngologists accounted for a smaller percentage of codeine prescriptions. Although orthopedic surgeons and otolaryngologists were low prescribers of codeine, it is unknown how they compare to other surgical specialties. It is possible that they represent a high proportion of codeine prescriptions when compared to other surgical providers. Further studies are needed to determine reasons for the varying codeine prescription practices among providers. The differences observed in this study highlight potential areas in our health care system for education regarding the hazards of codeine use in children and could guide such efforts.
The WHO eliminated codeine from its guidelines for pediatric pain management in 2013. Since then, several other agencies, including a black box warning issued by the FDA against codeine use in children following adenotonsillectomy, have advised against codeine prescription in pediatric populations. In December 2015 FDA began to re-examine the safety of codeine use in children and determine need for further restrictions.(8) Previous studies have demonstrated that guidelines have had limited ability to impact physician behavior.(20) Barriers to implementation of clinical practice guidelines are complex and largely unclear. Provisions of resources to both physicians and pharmacists in the form of reference cards, computerized order entry with decision support, and modification of formularies have been more successful in changing prescription behavior.(21, 22) Alteration of insurance reimbursement models also has the potential to impact prescription practices.(23) Such strategies are all possible avenues through which to reduce codeine prescription to children and adolescents.
Safe alternatives to codeine for pain management in children have been identified and are gaining popularity due to the pharmacogenetic variance in codeine metabolism. Oxycodone has been safely used in management of acute musculoskeletal pain in children.(24) Oxycodone is metabolized by the CYP3A4 and CYP2D6 pathways to oxymorphone, noroxycodone and noroxymorphone. These metabolites are active in vitro, however, in vivo studies have demonstrated they have little analgesic effect due to their inability to cross the blood brain barrier.(25) Hydrocodone has been used in the management of pain in children and been shown to have fewer treatment failures when compared with codeine.(26) Hydrocodone is a semisynthetic opioid analgesic, metabolised by the CYP2D6 and CYP3A4 pathways. Of note, polymorphisms in its metabolic pathway have been identified.(27) One fatality in a child has been partially attributed to pharmacogenetic variance in the CYP2D6 metabolic pathway of hydrocodone.(28) Tramadol has also been examined as an alternative to codeine for post-operative pain. However, similar to codeine, genetic polymorphisms in tramadol’s metabolic pathway have been linked to a case of severe respiratory depression following adenotonsillectomy, prompting an FDA evaluation of its safety in children of 17 years and under.(29) These examples illustrate the need for genotyping and phenotyping in developing individualized analgesic therapy and preventing serious adverse events.
This study has several limitations. The MEPS database relies on self-reporting that may result in under or over-estimation of codeine use. This study included data available up to 2013 and therefore has limited ability to comment on changes in prescription practices since the introduction of advisories against pediatric codeine use in 2012. This study is unable to comment on changes in the number of medical encounters such as emergency department visits and surgeries from 1996 to 2013 that may have resulted in opioid prescription. Changes in use of non-opioid analgesics were not examined. We are also limited in understanding the reason for the codeine use. Although we hypothesize that it is for analgesic use, codeine is also prescribed and bought over-the-counter as an antitussive medication. It is therefore possible that prescriptions made to children were for multifactorial reasons and not solely for analgesic use. This study also has several strengths as compared to other epidemiological studies examining codeine use among children and adolescents: 1) we included a very large nationally representative sample spanning 18 years, from 1996 to 2013 rendering multiple years of data available for analysis, 2) we analyzed codeine obtained from a wide range of outpatient prescribers including hospital outpatient departments, other office based visits, dental offices, emergency departments, and other healthcare providers, and 3) we used data from the Medical Expenditure Panel Survey, the most complete source of data on health care use in the United States(14). Another recent epidemiological survey used data from the National Hospital Ambulatory Medical Care Survey, however only evaluated codeine use in the emergency department setting.
Conclusions
This nationally representative study demonstrates that despite a decrease in recent years, codeine use among children and adolescents remains high and is the most frequently prescribed opioid. The genetic variability in codeine metabolism places children at risk of an unpredictable clinical response and potential for morbidity and mortality. Further interventions are required to change provider-practicing behaviors to limit the use of this potentially hazardous medication in children.
What is already known
International recommendations have advised against pediatric codeine use, yet it continues to be frequently prescribed.
Studies of opioids in the United States, revealed codeine constituted 40% of opioids used by children.
What this article adds
This nationally representative study highlights that despite a decrease in recent years, codeine use among children remains high and is the most frequently prescribed opioid.
Associated patient and provider characteristics and potential reasons for ongoing pediatric codeine use are reviewed.
Acknowledgments
Funding Source: CBG was supported by National Institutes of Health Ruth L. Kirschstein National Research Service Award Institutional Research Training Grant T32GM086270 (PI: TMP). JAR was supported by National Institutes of Health K23HD078239. TMP was partially supported by National Institutes of Health 2K24HD060068.
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Potential Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
References
- 1.Ciszkowski C, Madadi P, Phillips MS, Lauwers AE, Koren G. Codeine, ultrarapid-metabolism genotype, and postoperative death. The New England journal of medicine. 2009;361(8):827–8. doi: 10.1056/NEJMc0904266. [DOI] [PubMed] [Google Scholar]
- 2.Kelly LE, Rieder M, van den Anker J, Malkin B, Ross C, Neely MN, et al. More codeine fatalities after tonsillectomy in North American children. Pediatrics. 2012;129(5):e1343–7. doi: 10.1542/peds.2011-2538. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Persisting pain in children package: WHO guidelines on the pharmacological treatment of persisting pain in children with medical illnesses. 2013 [PubMed] [Google Scholar]
- 4.Food and Drug Administration. Safety review update of codeine use in children; new Boxed Warning and Contraindication on use after tonsillectomy and/or adenoidectomy. FDA Drug Safety Communication. 2013 [Google Scholar]
- 5.Agency EM. Restrictions on use of codeine for pain relief in children – CMDh endorses PRAC recommendation. 2013 [Google Scholar]
- 6.United Kingdom Medicines and Healthcare Products Regulatory Agency. Codeine for analgesia: restricted use in children because of reports of morphine toxicity. 2013 [Google Scholar]
- 7.Health Canada’s review recommends codeine only be used in patients aged 12 and over. 2013 [cited 2016 January 11]. Available from: http://www.healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2013/33915a-eng.php.
- 8.Meeting JP-ADACaDSaRMAC, editor. Food and Drug Administration. FDA summary memorandum: The safety of codeine in children 18 years of age and younger. 2015. [Google Scholar]
- 9.Zahari Z, Ismail R. Influence of Cytochrome P450, Family 2, Subfamily D, Polypeptide 6 (CYP2D6) polymorphisms on pain sensitivity and clinical response to weak opioid analgesics. Drug metabolism and pharmacokinetics. 2014;29(1):29–43. doi: 10.2133/dmpk.dmpk-13-rv-032. [DOI] [PubMed] [Google Scholar]
- 10.A LL, Naranjo ME, Rodrigues-Soares F, Penas LEM, Farinas H, Tarazona-Santos E. Interethnic variability of CYP2D6 alleles and of predicted and measured metabolic phenotypes across world populations. Expert opinion on drug metabolism & toxicology. 2014;10(11):1569–83. doi: 10.1517/17425255.2014.964204. [DOI] [PubMed] [Google Scholar]
- 11.Kaiser SV, Asteria-Penaloza R, Vittinghoff E, Rosenbluth G, Cabana MD, Bardach NS. National patterns of codeine prescriptions for children in the emergency department. Pediatrics. 2014;133(5):e1139–47. doi: 10.1542/peds.2013-3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groenewald CB, Rabbitts JA, Gebert JT, Palermo TM. Trends in opioid prescriptions among children and adolescents in the United States: a nationally representative study from 1996 to 2012. Pain. 2016;157(5):1021–7. doi: 10.1097/j.pain.0000000000000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sites BD, Beach ML, Davis MA. Increases in the use of prescription opioid analgesics and the lack of improvement in disability metrics among users. Reg Anesth Pain Med. 2014;39(1):6–12. doi: 10.1097/AAP.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crous PW, Carris LM, Giraldo A, Groenewald JZ, Hawksworth DL, Hernandez-Restrepo M, et al. The Genera of Fungi - fixing the application of the type species of generic names - G 2: Allantophomopsis, Latorua, Macrodiplodiopsis, Macrohilum, Milospium, Protostegia, Pyricularia, Robillarda, Rotula, Septoriella, Torula, and Wojnowicia. IMA fungus. 2015;6(1):163–98. doi: 10.5598/imafungus.2015.06.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradford LD. CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics. 2002;3(2):229–43. doi: 10.1517/14622416.3.2.229. [DOI] [PubMed] [Google Scholar]
- 16.Pletcher MJ, Kertesz SG, Kohn MA, Gonzales R. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. Jama. 2008;299(1):70–8. doi: 10.1001/jama.2007.64. [DOI] [PubMed] [Google Scholar]
- 17.Vila D, Rand CS, Cabana MD, Quinones A, Otero M, Gamache C, et al. Disparities in asthma medication dispensing patterns: the case of pediatric asthma in Puerto Rico. The Journal of asthma: official journal of the Association for the Care of Asthma. 2010;47(10):1136–41. doi: 10.3109/02770903.2010.517338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fortuna RJ, Robbins BW, Caiola E, Joynt M, Halterman JS. Prescribing of controlled medications to adolescents and young adults in the United States. Pediatrics. 2010;126(6):1108–16. doi: 10.1542/peds.2010-0791. [DOI] [PubMed] [Google Scholar]
- 19.Zerzan JT, Morden NE, Soumerai S, Ross-Degnan D, Roughead E, Zhang F, et al. Trends and geographic variation of opiate medication use in state Medicaid fee-for-service programs, 1996 to 2002. Medical care. 2006;44(11):1005–10. doi: 10.1097/01.mlr.0000228025.04535.25. [DOI] [PubMed] [Google Scholar]
- 20.Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. Jama. 1999;282(15):1458–65. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 21.Rosenbluth G, Wilson SD, Maselli JH, Auerbach AD. Analgesic prescribing practices can be improved by low-cost point-of-care decision support. Journal of pain and symptom management. 2011;42(4):623–31. doi: 10.1016/j.jpainsymman.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Connor AB, Lang VJ, Quill TE. Eliminating analgesic meperidine use with a supported formulary restriction. The American journal of medicine. 2005;118(8):885–9. doi: 10.1016/j.amjmed.2005.01.061. [DOI] [PubMed] [Google Scholar]
- 23.Siracuse MV, Vuchetich PJ. Impact of Medicaid prior authorization requirement for COX-2 inhibitor drugs in Nebraska. Health services research. 2008;43(1 Pt 2):435–50. doi: 10.1111/j.1475-6773.2007.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali S, Drendel AL, Kircher J, Beno S. Pain management of musculoskeletal injuries in children: current state and future directions. Pediatric emergency care. 2010;26(7):518–24. doi: 10.1097/PEC.0b013e3181e5c02b. quiz 25–8. [DOI] [PubMed] [Google Scholar]
- 25.Lalovic B, Kharasch E, Hoffer C, Risler L, Liu-Chen LY, Shen DD. Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: role of circulating active metabolites. Clinical pharmacology and therapeutics. 2006;79(5):461–79. doi: 10.1016/j.clpt.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Turturro MA, Paris PM, Yealy DM, Menegazzi JJ. Hydrocodone versus codeine in acute musculoskeletal pain. Annals of emergency medicine. 1991;20(10):1100–3. doi: 10.1016/s0196-0644(05)81383-6. [DOI] [PubMed] [Google Scholar]
- 27.Linares OA, Fudin J, Daly AL, Boston RC. Individualized Hydrocodone Therapy Based on Phenotype, Pharmacogenetics, and Pharmacokinetic Dosing. The Clinical journal of pain. 2015;31(12):1026–35. doi: 10.1097/AJP.0000000000000214. [DOI] [PubMed] [Google Scholar]
- 28.Madadi P, Hildebrandt D, Gong IY, Schwarz UI, Ciszkowski C, Ross CJ, et al. Fatal hydrocodone overdose in a child: pharmacogenetics and drug interactions. Pediatrics. 2010;126(4):e986–9. doi: 10.1542/peds.2009-1907. [DOI] [PubMed] [Google Scholar]
- 29.Orliaguet G, Hamza J, Couloigner V, Denoyelle F, Loriot MA, Broly F, et al. A case of respiratory depression in a child with ultrarapid CYP2D6 metabolism after tramadol. Pediatrics. 2015;135(3):e753–5. doi: 10.1542/peds.2014-2673. [DOI] [PubMed] [Google Scholar]


