Abstract
Objective
The clinical use of frozen, human allogeneic skin grafts is considered a suitable alternative to freshly harvested allogeneic skin grafts when the latter are not available. However, limited functional and histologic information exists regarding the effects of cryopreservation on allogeneic skin grafts, especially those across mismatched histocompatibility barriers. Thus, we performed a side-by-side comparative study of fresh vs. frozen skin grafts, across both minor and major histocompatibility barriers, in a miniature swine model. Since porcine skin shares many physical and immunologic properties with human skin, our findings have relevance to current clinical practices involving allogeneic grafting, and may support future, temporary wound therapies involving frozen xenografts, comprised of genetically modified porcine skin.
Methods
Four miniature swine underwent harvest and grafting of split thickness skin, with and without cryopreservation, in order to observe autologous grafts and grafts across minor and major histocompatibility barriers. Grafts were biopsied at regular intervals for study of architecture, vascularization, and outcomes.
Results
All grafts vascularized without technical complications. Differences were noted in the early appearance of some fresh vs. frozen grafts but no significant difference was observed in overall survival times in any of the experimental groups.
Conclusion
These results demonstrate that despite early observable differences in the healing process, cryopreservation and thawing does not significantly affect long-term graft survival or time to rejection, thus supporting the clinical and experimental use of fresh and frozen split thickness skin grafts as comparable and interchangeable.
Keywords: Cryopreservation, Freeze, Porcine, Split Thickness Skin Grafts, Thaw Media
INTRODUCTION
Many pioneers in the field of clinical transplantation were attracted to this field by the observation of skin graft rejection. For example, treating severely burned soldiers during World War II stimulated Nobel laureate Dr. Joseph E. Murray to find a way to control the rejection of “foreign” skin.1 In addition to their clinical use, skin grafts are also used extensively in experimental transplant immunology to test immunocompetence and/or confirm in vivo donor-specific unresponsiveness following tolerance induction protocols. In our laboratory and others, it is common for fresh and cryopreserved porcine skin grafts to be used interchangeably in such studies, based on the assumption of their equivalence.
However, while functional studies exist which validate the overall equivalence of fresh and frozen skin grafts in human, rabbit, and rodent models, little information exists regarding the effect of cryopreservation on split thickness skin grafts at the histologic level across defined histocompatibility barriers.2–5 Indeed, the utilization of cryopreserved and fresh skin in our own porcine transplantation models has previously been assumed to be equivalent,6 but never directly tested.
The experiments described herein investigate the effects of freezing and thawing on subsequent skin graft architecture and vascularization of porcine skin grafts at a histologic level, across minor histocompatibility (miHA) barriers as well as major histocompatibility (MHC) barriers.
In addition to the clinical utility of using frozen allogeneic skin grafts, placement of split skin grafts is a regular test of immunologic tolerance and immune competence following immune-tolerance protocols. Therefore, confirming that grafts stored for this purpose can be effectively thawed and transplanted without adverse implications is of critical importance in interpreting other experimental results. Given the well-established similarity between human and porcine skin,6,8–15 we expect that the findings in these studies should have relevance to the clinical practice of skin grafting.
MATERIALS AND METHODS
Animals
This study was approved by the Institution’s Animal Care and Use Committee (IACUC) and performed in accordance with the NIH Principles of Laboratory Animal Care.16 Four miniature swine were used, with ages ranging from 1 to 2 years, and weights of 16, 36, 42 and 42 kg. The animals underwent routine pathogen screening and quarantine prior to commencement of studies.
The two swine used in the first experimental series (Group 1), were MHC matched (Swine Leukocyte Antigen SLAaa to SLAaa, but mismatched for multiple minor histocompatibility antigens.17–19 In Group 2, two animals were fully mismatched for MHC antigens (SLAgg to/from SLAkk) swine. In both groups, reciprocal skin graft transplants were performed.
Experimental Design
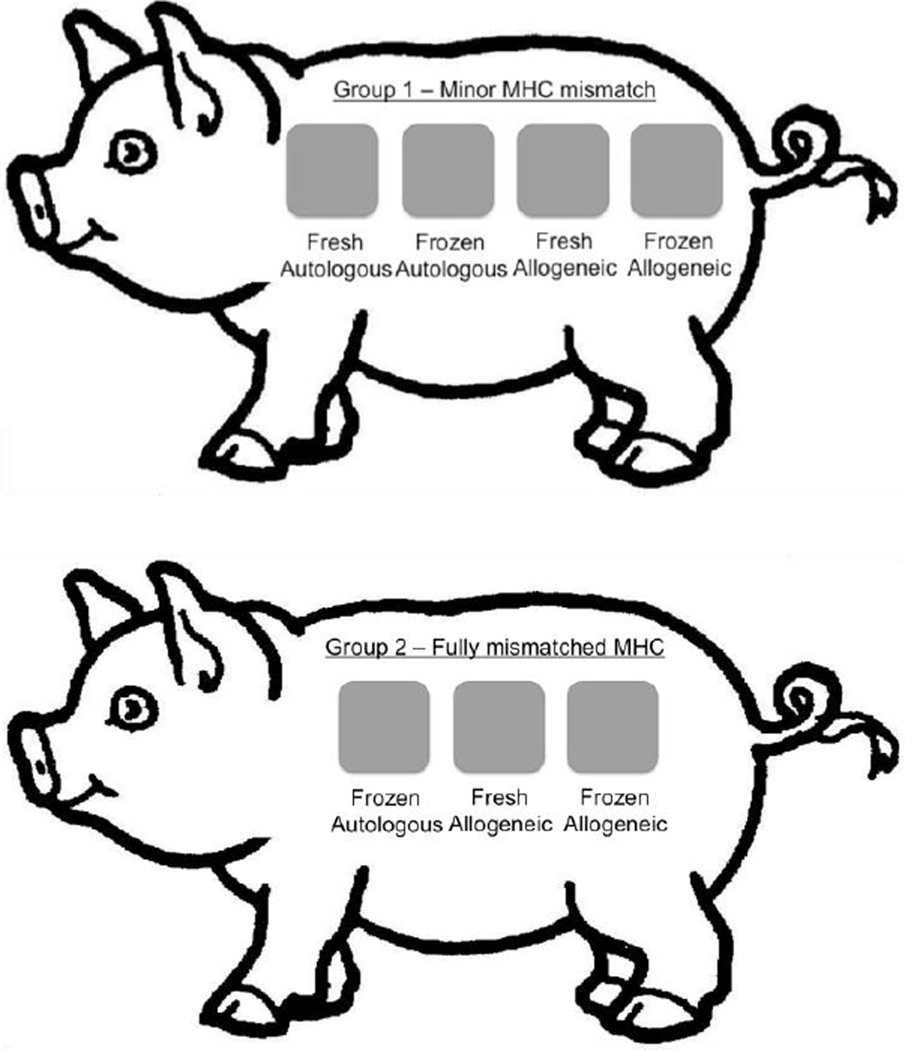
In Group 1 (Figure 1, top image), both animals received the following four reciprocal split thickness skin grafts: 1) fresh autograft (serving as a control to exclude inadequate wound bed preparation or other local technical issues), 2) frozen autograft, 3) fresh minor mismatched allograft, and 4) frozen minor mismatched allograft. This paradigm thus resulted in a total of eight grafts.
Figure 1.
Experimental surgical schematic depicting the placement of autologous and allogeneic, fresh and frozen, split-thickness skin grafts for both experimental groups. Minor mismatched MHC experimental series, Group 1, is shown top. Grafts (from left to right): Fresh Autologous (control), Frozen Autologous (control), Fresh Allogeneic, and Frozen Allogeneic split thickness skin graft. Fully mismatched MHC experimental series, Group 2, shown bottom. Grafts (from left to right): Frozen Autologous (control), Fresh Allogeneic, and Frozen Allogeneic split thickness skin graft.
In Group 2 (Figure 1, bottom image), both animals received the following three reciprocal split thickness skin grafts: 1) frozen autograft (serving as a control for the freeze technique), 2) fresh, fully MHC-mismatched allograft, and 3) frozen, fully MHC-mismatched allograft. This paradigm thus resulted in a total of six grafts.
Animals were monitored daily; grafts were photographed and biopsied every two days post-operatively. Combined, the two experimental groups yielded a total of 14 grafts and 110 total biopsies for this study.
Skin Graft Harvest Procedure
The paired swine donors were anesthetized with 2 mg/kg Telazol (tiletamine HCl and zolazepam HCl, Zoetis Inc., Kalamazoo, MI) intramuscular (IM) injection, and intubated. Anesthesia was maintained using 2% isoflurane and oxygen. The skin surfaces were disinfected before surgery with chlorhexidine, alcohol, betadine scrubs, and saline rinse. The animals were then draped, leaving the dorsum exposed. Split-thickness skin grafts, measuring approximately 5cm × 5 cm, were harvested from each animal using an air-driven Zimmer dermatome (Medfix Solution, Inc., Tucson, AZ) with the depth set to 0.056 cm. Following graft harvest, an additional pass with the dermatome was used to deepen the wound, ensuring full exposure of dermis with visible punctate bleeding in preparation for skin graft placement.
Following harvest from both swine, those grafts intended for placement as “fresh” grafts (non-cryopreserved) were stored in normal saline at 25°C, while those intended for placement as “frozen” grafts underwent a standardized cryopreservation process (described below). Hemostasis was achieved with gauze soaked in saline and epinephrine (1ml, 1:1000) and animals monitored continuously while skin was prepared for grafting.
Cryopreservation and Thaw Process
For all experimental groups, cryoprotective media RPMI (Lonza, BioWhittaker) containing 8% fetal porcine serum (FPS) was cooled to 4°C, employing sterile technique. The grafts were placed in a sterile mesh and rolled to conform to the shape of the freezing vial. 5ml of cryoprotective media (RPMI plus 8% NPS) was added, and the vials were subsequently cooled to −80°C in a phase freezer, and then maintained at this temperature for a period of at least 15 minutes. The vials were then placed in 37°C water baths, thawed, and skin specimens were removed from the vial and mesh material.
Grafts were subsequently thawed with 3 successive, rapid washes in RPMI plus 8% donor porcine serum, our standard protocol media which is supplemented with glutamine, non-essential amino acids, fetal porcine serum, and penicillin streptomycin gentamicin. This occurred for approximately 1 to 3 minutes, and then the tissue specimens were brought to the surgical field in normal saline.
Skin Graft Placement and Wound Dressings
All grafts were fenestrated on partial thickness wounds and sutured in place using 3/0 nylon sutures. Topical Bacitracin ointment was applied and the grafts were dressed with Telfa™ non-adhesive dressing (Covidien, Minneapolis, MN) and Tegaderm™ (3M, St. Paul, MN). Sterile dry gauze was then applied over the grafts and secured in place with Vetrap™ (3M, St. Paul, MN) bandaging tape to create pressure dressings. Recipients were dressed with cotton jackets to reduce interference with the grafts and received transdermal fentanyl patches for pain management. No immunosuppression regimen was administered at anytime during this experiment.
Post Operative Care and Biopsy Procedure
In both groups, dressings were changed beginning on post-operative day 2 and alternate days thereafter to permit assessment and, if indicated, biopsy of skin grafts. Specimens were obtained via 3mm punch biopsy, and included dermal layer tissue, ensuring samples from both the donor graft and recipient wound bed. All biopsy sites were remote from the graft border. Duplicate specimens were fixed in formalin and snap frozen in optimum cutting temperature (OCT) gel. Sutures were removed on POD 7, and dressings were removed and not replaced after POD 10. Skin grafts were assessed for viability and integrity, and were determined to have rejected when less than 10% of viable graft tissue covered the wound.6,20
Histological Assessment
Hematoxylin and eosin (H&E) stained slides of the biopsy specimens were evaluated for rejection by a pathologist blinded to specimen identity. In assessing the time course and criteria for rejection, the Banff 2007 Working Classification of Skin-Containing Composite Tissue Allograft Pathology was employed.21
This classification scheme characterizes a tissue specimen along a spectrum that includes five discrete values: Grade 0, Grade I, Grade II, Grade III, and Grade IV. Grade 0 possesses little to no inflammatory infiltrates, while Grade I is characterized as having mild perivascular infiltration with no involvement of the overlying epidermis. Progressing towards rejection, specimens considered Grade II exhibit moderate-to-severe perivascular inflammation with or without mild epidermal and/or adnexal involvement (limited to spongiosis and exocytosis). There is no epidermal dyskeratosis or apoptosis noted in Grade II rejection. Banff Grade III rejection is characterized as possessing severe, dense inflammation and epidermal involvement with epithelial apoptosis, dyskeratosis, and/or keratinolysis. Finally, Grade IV exhibits necrotizing acute rejection. Since this classification is designed for rejection of skin in the context of vascularized composite grafts, grading was appended with comments to reflect qualitative aspects of split thickness skin graft healing and rejection.
RESULTS
Outcomes by Gross Appearance
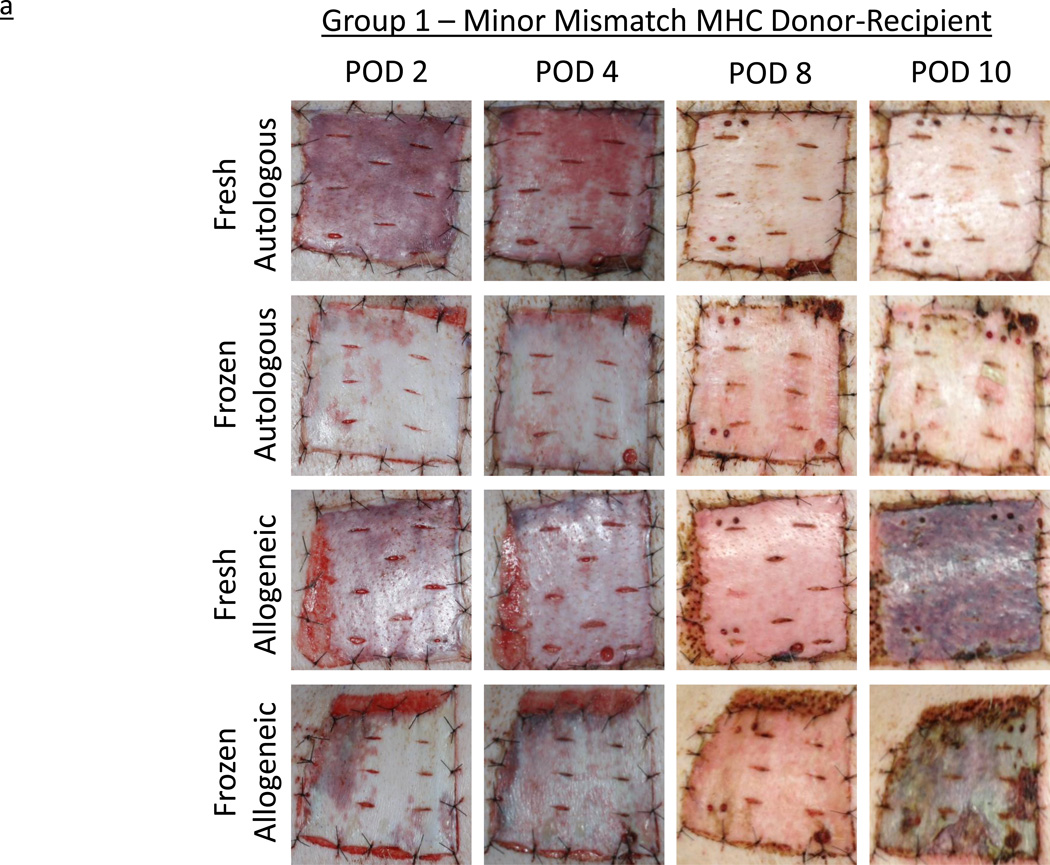
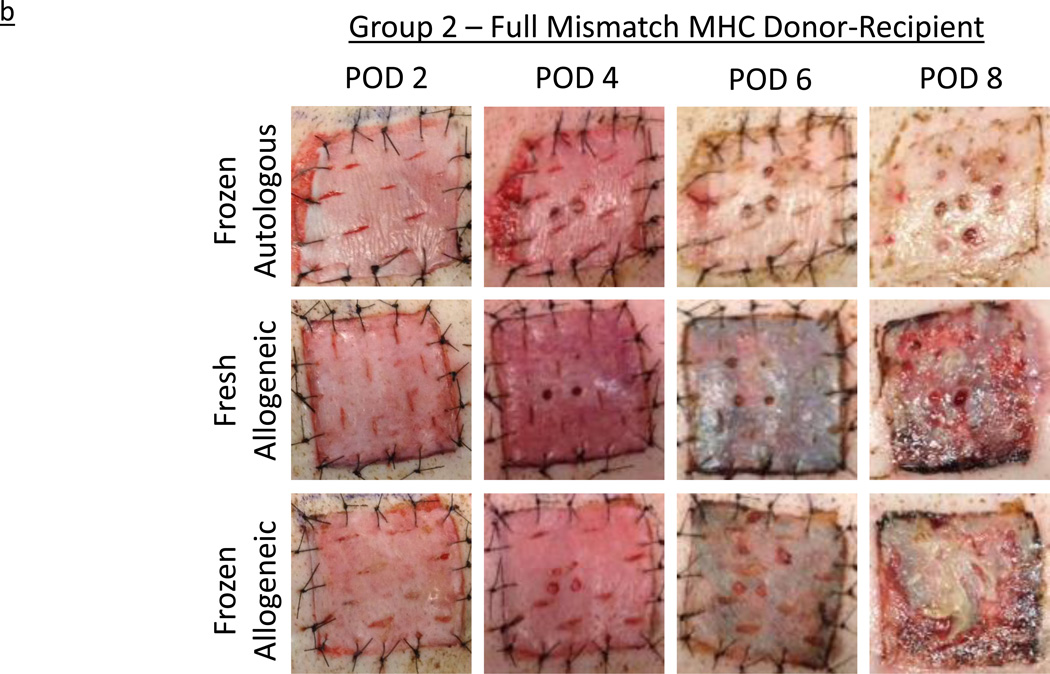
All grafts re-vascularized without evidence of technical complication. In Group 1, by day 2, both the fresh autografts and allografts appeared adherent and bruised, consistent with vascularization and perfusion. In contrast, grafts subjected to the freeze-thaw process, while similarly adherent, appeared paler, with only patchy bruising. Over the next six days, fresh and frozen autografts became virtually indistinguishable on gross examination. At this point (POD 8), all autografts appeared perfused, warm, and healthy and were clearly healing. Minor mismatched allogeneic grafts on both subjects appeared comparable to autografts at POD 2 and POD 4, and displayed a similar response to cryopreservation. However, by POD 8, all allogeneic grafts, fresh and frozen, demonstrated mild erythema, consistent with early rejection. Two days later, all four allogeneic grafts had significantly darkened and exhibited signs of rejection and necrosis. Both fresh and frozen allogeneic grafts were considered fully rejected by POD 10, in contrast to the fresh and frozen autografts, which remained warm, viable, and appeared healthy indefinitely (Figure 2a). In Group 2, recipients of fully MHC-mismatched grafts demonstrated comparable progression to the minor-mismatched grafts, but at an accelerated pace. The four frozen grafts again appeared paler at POD 2, but then showed increased evidence of bruising and vascularization by POD 4, when all six grafts appeared equivalent. By POD 6, all four allogeneic grafts, both fresh and frozen, appeared to have rejected completely, while the two frozen autografts remained healthy and viable (Figure 2b).
Figure 2.
a – Experimentally obtained images illustrating the rejection time course of autologous and allogeneic, fresh and frozen, split-thickness skin grafts in minor mismatched MHC experimental series, Group 1. Horizontal legend denotes post-operative day (POD); vertical legend denotes skin graft type.
b – Experimentally obtained images illustrating the rejection time course of autologous and allogeneic, fresh and frozen, split-thickness skin grafts in fully mismatched MHC experimental series, Group 2. Horizontal legend denotes post-operative day (POD); vertical legend denotes skin graft type.
Outcomes by Histology
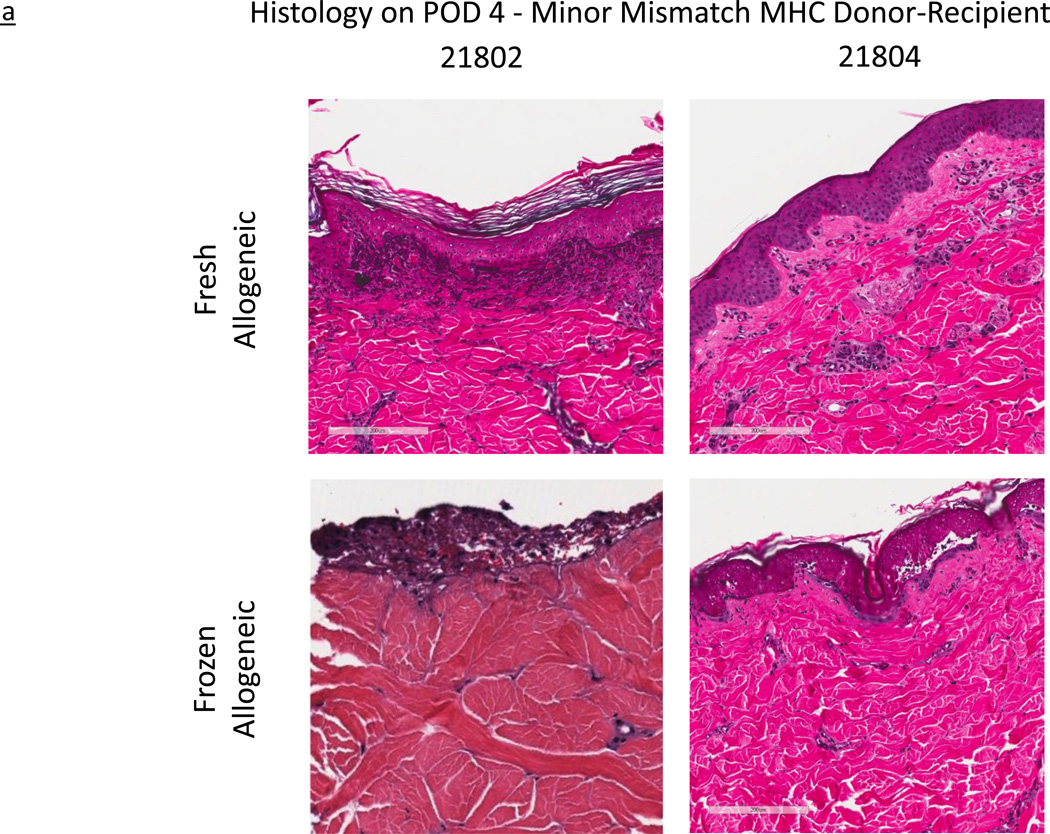
In Group 1, at the time of first biopsy on POD 2, some samples were un-gradable by Banff criteria due to localized epidermolysis. However, no gradable sample showed any signs of rejection, and grafts were therefore graded Banff Grade 0. Figure 3a shows the H&E histological staining images of both fresh and frozen minor antigen-mismatched grafts on POD 4. Consistent with gross outcomes, minor-mismatched grafts (both fresh and frozen) demonstrated Grade III rejection (as described in Materials and Methods) by POD 10, while autologous grafts remained rejection free. Histological rejection progressed at a comparable rate in both fresh and frozen allografts.
Figure 3.
a – Histological images of fresh and frozen skin grafts, in minor mismatched MHC experimental series, Group 1. All images representative of post-operative day (POD) 4. Horizontal legend represents unique subject identifier; horizontal legend denotes skin graft type.
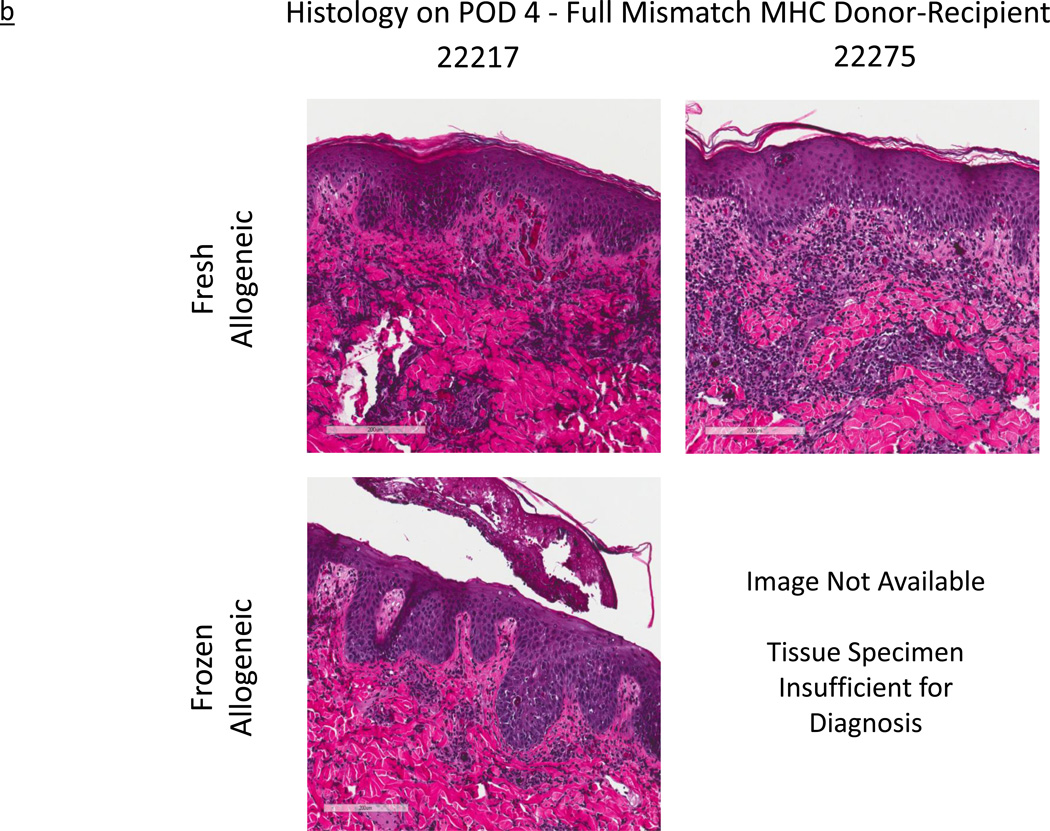
b - Histological images of fresh and frozen skin grafts across a fully mismatched MHC experimental series, Group 2. All images representative of post-operative day (POD) 4. Horizontal legend represents unique subject identifier; horizontal legend denotes skin graft type.
In Group 2, a similar but more rapidly progressive rejection was observed. As early as POD 2, biopsies obtained from both fresh and frozen allogeneic grafts displayed signs of Grade I rejection (as described in Materials and Methods) while their autologous counterparts did not. By POD 4, while the frozen autologous grafts were reported by the pathology as “within normal limits”, one of the frozen allogeneic grafts (22217, lower left hand image in Figure 3b) had uniformly progressed to Grade III (Figure 3b). Similarly, given the already discussed gross appearance indicating rejection by POD 4, we expected to see a similar progression in the histology of its counterpart frozen allogeneic graft (22275, lower right hand image in Figure 3b). Unfortunately, the tissue obtained from the biopsy specimen was reported as “insufficient for diagnosis”. However, by POD 6, both frozen allogeneic specimens had progressed to complete rejection, exhibiting Grade IV characteristics (as described in Materials and Methods). Lastly, in contrast to the observed rejection course of the allogeneic grafts, histological images show that all frozen autologous grafts, in both experimental groups, remained healthy and without evidence of inflammation.
DISCUSSION
Presently, in the clinical treatment of acute, full-thickness burn wounds or other cutaneous conditions affecting a high percentage of total body surface area (TBSA), the use of cadaver allogeneic skin grafts as a temporary cover of the wound to prevent infection and fluid loss is common. Such therapy is used in the absence of adequate autologous donor material, in order to allow healing and re-harvest of available autologous donor sites as well as to achieve permanent wound closure. In fact, “skin banking,” the organized practice of procuring suitable skin grafts and the subsequent necessary packaging, storage, and delivery for later use, has formalized this approach. Skin banking has been a common practice since the early 1900’s with over 50 skin banks currently operating in North America.4
Despite the approval and widespread clinical use of allogeneic skin of deceased human donors, this remedy is inherently temporary in nature, predictably rejecting from the wound bed within 7 to 12 days after placement.22–23 This rejection time course is principally due to immunologic incompatibility between the burn victim and the deceased donor.22–23 Thus, if other graft sources provided a combination of greater availability, economy, and similar efficacy, these sources would be highly desirable. Our previous research suggests the promise of a genetically modified porcine skin graft as a possible clinical alternative in the temporary coverage of burn wounds.6
In addition to their use clinically, skin grafts are used frequently in transplantation biology research, particularly in tolerance research, as they represent the most stringent test of tolerance.24 Skin grafts are often used either fresh or frozen interchangeably, on the assumption that there are no differences in the immunogenicity or functional behavior of these two kinds of grafts. However, it has been suggested that damage to the skin graft architecture from the freezing and thawing process may impact immunogenicity, either by causing greater inflammation in the wound bed, leading to a more rapid rejection course, or conversely, by removing antigens during the cryopreservation and thawing processes, potentially reducing immunogenicity.2–4 Since direct evidence supporting or refuting these suggestions was lacking, and since the results could be of importance both to basic research and to clinical applications, we undertook the current set of experiments.
In this study, we examined fresh and frozen split thickness skin grafts across defined genetic barriers both by gross observation and by histologic means, to determine whether any differences could be detected. Our laboratory has had extensive experience with such grafts and we chose genetic combinations in which we knew what to expect for graft survival times.6,25 Thus, it was our expectation that skin transplants across minor histocompatibility (miHA) barriers would reject completely by POD 10–12 and that those transplanted across major histocompatibility (MHC) mismatches would reject faster, at POD 6 to 8.6,25
Consistent with these expectations, we observed rejection, both by gross inspection and by histologic assessment, by POD 10 for the minor-mismatched allogeneic skin grafts and by POD 6 for the fully mismatched grafts, regardless of exposure to the effects of cryopreservation. All fresh and frozen grafts, regardless of the histocompatibility barrier, rejected with similar time courses, and all fresh and frozen autologous grafts appeared to vascularize and heal with overall comparable outcomes.
With the exception of the POD 4 biopsy from subject 22275, for which the sample submitted was found “insufficient for diagnosis,” all of the histologic data confirmed the gross appearance. Histological examinations of this animal's biopsies on POD 2 and POD 6, those directly preceding and following this time point, closely mirrored those of subject 22217. We presume, therefore, that the histological images of the biopsy of subject 22275 on POD4 would have continued this continuum of rejection and would also have been consistent with sample’s rejection course observed by gross outcome. In the context of the overall rapid progression towards rejection witnessed in both animals, these observations corroborate our overall hypothesis.
These findings help to validate previous work and provide a guide for future work in our large animal model – one of the principal aims of this study. We also anticipate that these findings, in combination with earlier work on xenogeneic skin grafts from this laboratory6,25 could have important clinical implications. Indeed, given the similarities of porcine skin to human skin, the outcomes described here may have direct applicability to the use of cryopreserved allogeneic and xenogeneic skin in clinical settings. Additionally, in the future, porcine skin may serve as an attractive option for use as temporary wound coverage6,25 and our findings of the equivalent outcomes for frozen and cryopreserved porcine skin may make it the skin graft of choice in emergency situations.
Acknowledgments
The authors wish to acknowledge funding and research support from Steward Health Care, the Department of Defense Grant W81XWH-09-1-0419 DOD (DR080729), and the National Institute of Health Grant No.1C 06 RR 20135-01.
Footnotes
PH - Contributed to design, execution of study, analysis of data, and preparation of the manuscript
DL - Contributed to design of the experiment, data analysis, and preparation of the manuscript
KS - Contributed to performance of study
KM – Contributed to the preparation of the manuscript
ZY – Contributed to the preparation of the manuscript
CLC - Contributed to design of experiments and preparation of manuscript
DHS - Contributed to design of experiments, data analysis, and preparation of manuscript
Disclosures: No conflict of interest exists for any authors
REFERENCES
- 1.Delmonico FL, Murray J. Interview with Dr. Joseph Murray. American Journal of Transplantation. 2002;2:803–806. doi: 10.1034/j.1600-6143.2002.20901.x. [DOI] [PubMed] [Google Scholar]
- 2.Baxter H, Entin MA. Experimental and clinical studies of reduced temperatures in injury and repair in man. III. Direct effect of cooling and freezing on various elements of the human skin. Plast Reconstr Surg. 1948;3:303–334. doi: 10.1097/00006534-194805000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Billingham RE, Medawar PB. The freezing, drying, and storage of mammalian skin. J Exp Biol. 1952;19:454–468. [Google Scholar]
- 4.Kagan RJ, Robb EC, Plessinger RT. Human skin banking. Clin Lab Med. 2005;25:587–605. doi: 10.1016/j.cll.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Taylor AC. Survival of rat skin and changes in hair pigmentation following freezing. J Exp Zool. 1949;110:77–112. doi: 10.1002/jez.1401100106. [DOI] [PubMed] [Google Scholar]
- 6.Barone AAL, Mastroianni M, Farkash EA, Mallard C, Albritton A, Torabi R, et al. Genetically modified porcine split-thickness skin grafts as an alternative to allograft for provision of temporary wound coverage: preliminary characterization. Burns. 2015;41(3):565–574. doi: 10.1016/j.burns.2014.09.003. http://dx.doi.org/10.1016/j.burns.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Chiarini A, Dal Pra I, Armato U. In vitro and in vivo characteristics of frozen/thawed neonatal pig split-skin strips: a novel biologically active dressing for areas of severe, acute, or chronic skin loss. Int J Mol Med. 2007;19:245–255. [PubMed] [Google Scholar]
- 8.Barnett JR, McCauley RL, Schutzler S, Sheridan K, Heggers JP. Cadaver donor discards secondary to serology. J Burn Care Rehabil. 2001;22:124–127. doi: 10.1097/00004630-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Elliott RA, Jr, Hoehn JG. Use of commercial porcine skin for wound dressings. Plast Reconstruct Surg. 1973;52:401–405. [PubMed] [Google Scholar]
- 10.Fishman JA, Scobie L, Takeuchi Y. Xenotransplantation associated infectious risk: a WHO consultation. Xenotransplantation. 2012;19:72–81. doi: 10.1111/j.1399-3089.2012.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lineen E, Namias N. Biologic dressing in burns. J Craniofac Surg. 2008 Jul;19(4):923–928. doi: 10.1097/SCS.0b013e318175b5ab. [DOI] [PubMed] [Google Scholar]
- 12.Mather M, De A, Gore M. Microbiological assessment of cadaveric skin grafts received in a skin bank. Burns. 2008;35:104–106. doi: 10.1016/j.burns.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Song IC, Bromberg BE, Mohn MP, Koehnlein E. Heterografts as biological dressings for large skin wounds. Surgery. 1966;59:576–583. [PubMed] [Google Scholar]
- 14.Vodicka P, Smetana K, Jr, Dvorankova V, Emerick T, Xu YZ, Ourednik J, et al. The miniature pig as an animal model in biomedical research. Ann NY Acad Sci. 2005;1049:161–171. doi: 10.1196/annals.1334.015. [DOI] [PubMed] [Google Scholar]
- 15.Weiner J, Yamada K, Ishikawa Y, Moran S, Etter J, Shimizu A, et al. Prolonged survival of GalT-KO swine skin on baboons. Xenotransplantation. 2010;17:147–152. doi: 10.1111/j.1399-3089.2010.00576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guide for the care and use of laboratory animals. Washington (DC): National Academies Press (US); 2011. Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research; Division on Earth and Life Studies, National Research Council. [Google Scholar]
- 17.Sachs D. Swine as Models in Biomedical Research. Ames, IA: Iowa State University Press; 1992. MHC homozygous miniature swine; pp. 3–36. [Google Scholar]
- 18.Sachs DH, Leight G, Cone J, Schwarz S, Stuart L, Rosenberg S. Transplantation in Miniature Swine. Transplantation. 1976;22(6):559–567. doi: 10.1097/00007890-197612000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Rosengard BR, Ojikutu CA, Fishbein J, Kortz EO, Sachs DH. Selective breeding of miniature swine leads to an increased rate of acceptance of MHC-identical, but not of class 1-disparate, rental allografts. J Immunol. 1992;149(3):1099–1103. [PubMed] [Google Scholar]
- 20.Leight GS, Kirman R, Rasmusen BA, Rosenberg SA, Sachs DH, Terrill R, et al. Transplantation in miniature swine III: effects of MSLA and A–O blood group matching on skin allograft survival. Tissue Antigens. 1978;12:65–74. doi: 10.1111/j.1399-0039.1978.tb01301.x. [DOI] [PubMed] [Google Scholar]
- 21.Cendales LC, Kanitakis J, Schneeberger S, Burns C, Ruiz P, Landin L, et al. The Banff 2007 working classification of skin-containing composite tissue allograft pathology. American Journal of Transplantation. 2008;8:1396–1400. doi: 10.1111/j.1600-6143.2008.02243.x. [DOI] [PubMed] [Google Scholar]
- 22.Wentscher J. A further contribution about the survivability of human epidermal cells. Dtsch Z Chir. 1903;70:21–44. [Google Scholar]
- 23.Bettman AG. Homogeneous Thiersch grafting as a life saving measure. Am J Surg. 1938;39:156–162. [Google Scholar]
- 24.Wekerle T, Sykes M. Mixed chimerism and transplantation tolerance. Annu Rev Med. 2001;52:353–370. doi: 10.1146/annurev.med.52.1.353. [DOI] [PubMed] [Google Scholar]
- 25.Albritton A, Leonard D, Barone AAL, Keegan J, Mallard C, Sachs D, et al. Lack of cross-sensitization between α-1, 3-galactosyltransferase knockout porcine and allogeneic skin grafts permits serial grafting. Transplantation. 2014;97:1209–1215. doi: 10.1097/TP.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]