Abstract
Many individuals with aphasia describe anomia with comments like “I know it but I can’t say it.” The exact meaning of such phrases is unclear. We hypothesize that at least two discrete experiences exist: the sense of (1) knowing a concept, but failing to find the right word, and (2) saying the correct word internally but not aloud (successful inner speech, sIS). We propose that sIS reflects successful lexical access; subsequent overt anomia indicates post-lexical output deficits. In this pilot study, we probed the subjective experience of anomia in 37 persons with aphasia. Self-reported sIS related to aphasia severity and phonological output deficits. In multivariate lesion-symptom mapping, sIS was associated with dorsal stream lesions, particularly in ventral sensorimotor cortex. These preliminary results suggest that people with aphasia can often provide meaningful insights about their experience of anomia and that reports of sIS relate to specific lesion locations and language deficits.
Keywords: Language, Stroke, Aphasia, Anomia, Self-report, Inner speech, Lesion-symptom mapping
1. Introduction
People with aphasia universally struggle with anomia, an acquired deficit of naming and word finding. These individuals often report that their internal knowledge of words exceeds what they demonstrate through aloud speech, saying, for example, “I know it but I can’t say it.” At times, these reports include the specific feeling that one can hear or say the correct word in one’s head, an experience that we label here as “successful inner speech,” or sIS. No prior studies have examined whether these subjective feelings of sIS provide useful information about the cognitive processes underlying anomia in a large group of individuals with aphasia. In this exploratory study, we gathered information from individuals with aphasia about sIS and related experiences, to test how these experiences map onto specific language deficits, preserved language abilities, and lesion locations.
1.1. Anomia and our model of naming
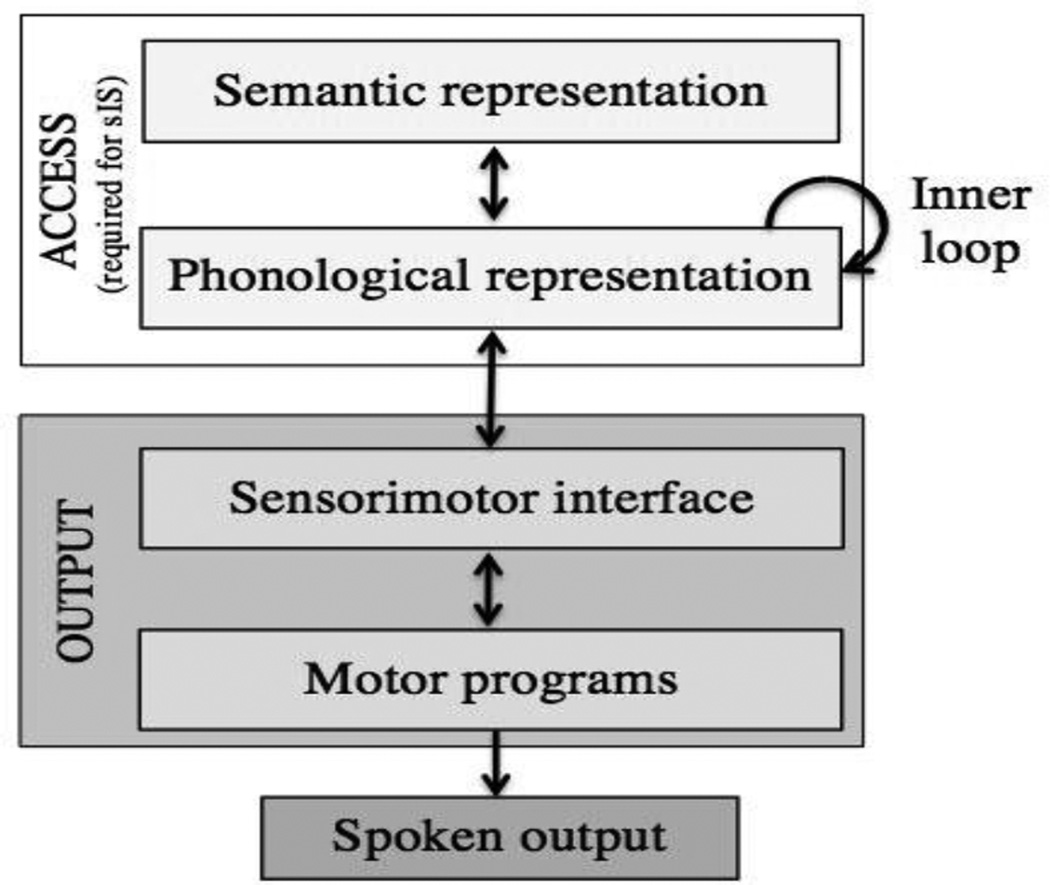
One-third of all stroke survivors are diagnosed with aphasia, a language disorder that often results in chronic communication deficits (Berthier, 2005; Engelter et al., 2006). The specific language impairments associated with aphasia can vary widely from person to person, but a hallmark symptom of aphasia is anomia (Goodglass & Wingfield, 1997; Laine & Martin, 2006; Maher & Raymer, 2004). Anomia is easily observable: a person with aphasia is sometimes unable to produce certain words, either during spontaneous speech or during an attempt to name an object or picture (both labeled “overt anomia” here). The overt deficit is conspicuous, but the cognitive mechanisms underlying anomia are best understood in the context of a theoretical model showing the stages of successful naming. Figure 1 presents a simplified model of the processing stages that have been suggested by existing naming models: access to a word’s semantic representation (encompassing both semantic knowledge for the concept and the corresponding abstract word-form), access to the phonological representation, and the postlexical output processes that are necessary to turn that phonological form into a spoken word (Dell & O’Seaghdha, 1992; Dell, Schwartz, Martin, Saffran, & Gagnon, 1997; Goldrick & Rapp, 2007; Levelt, 2001; Levelt, Roelofs, & Meyer, 1999; Walker & Hickok, 2015).
Figure 1.
Simplified model of naming, demonstrating a distinction between access, which is necessary and sufficient for sIS, and output, which is then required for successful aloud naming.
Correct naming requires success at each step of the naming process; consequently, overt anomia may result from a breakdown at any point, either within a stage or in the processing phase between stages (Dell et al., 1997; Laine & Martin, 2006). The locus of word-finding impairment within the access stages may be at the level of the semantic representation, at the level of the phonological representation, or in the mapping stage between the two. The process of word retrieval is typically understood to be complete once access to the phonological representation has been achieved (i.e., the end of the white access stages, Fig. 1). Next, there are additional output processes that are required to achieve successful spoken output (shown in gray, Fig. 1), i.e., the “word-production” components of naming (Kohn & Goodglass, 1985). A deficit at the level of the sensorimotor interface or motor programs, which may include phonological, phonetic and articulatory processes, can also result in spoken errors that can be difficult to distinguish from a word retrieval deficit (Feinberg, Rothi, & Heilman, 1986; Geva, Bennett, Warburton, & Patterson, 2011; Miceli, Amitrano, Capasso, & Caramazza, 1996).
1.2. The subjective experience of anomia
Anecdotally, many persons with aphasia endorse the idea that their out loud naming abilities do not match their inner speech (IS), stating, “I know it but I can’t say it” (Blanken, Dittmann, Haas, & Wallesch., 1987; Martin & Dell, 2007) or more specifically, “I can say it in my head” or “I can hear it in my head.” These statements are often accompanied by a sense of frustration, but their exact meaning is unclear and, to date, they have not been systematically explored. We hypothesize that many individuals with anomia are aware of the level at which their inability to find a word arises, and that these statements relate to underlying word retrieval and production processes.
More precisely, we hypothesize that there are at least two discrete internal experiences of anomia, both of which result in a person being unable to produce a word correctly out loud:
Wanting to communicate a concept or idea but failing to find the right word in one’s head (idea without word, IwW)
Finding the right word in one’s head but failing to turn that lexical form into a spoken word (sIS)
Note that throughout this manuscript we use the terms IwW and sIS to refer to the subjective experience of anomia. Therefore, the term sIS does not imply that the IS is necessarily correct, only that the individual reporting the experience of sIS feels that it is; the accuracy of these reports may vary across individuals.
The difference between these two subjective experiences can be understood in terms of the possible loci of impairment in the naming model above (section 1.1.): we suggest that IwW relates to a deficit at an early stage of processing involving access to either the semantic or phonological representation, whereas sIS reflects successful access to the word but a deficit in the post-lexical output processes (Fig. 1). We hypothesize that the sense of successful sIS arises after successful retrieval of both the semantic representation and the phonological form. In the model, self monitoring occurs via an inner loop that utilizes speech perception areas in the superior temporal gyrus (Indefrey, 2011), but the precise mechanism of monitoring is not critical for the current study (for further discussion of self-monitoring in IS, see Discussion section 4.4). IwW as described above is heterogeneous: an individual may feel that he/she cannot retrieve any word at all, or has retrieved a related word, or has retrieved a word that is close but not exactly right. Stated plainly, IwW encompasses all experiences of anomia that do not meet the criteria for sIS. In contrast, sIS is discrete: an individual reporting he/she feels able to say or hear the right word internally, despite being unable to say it out loud. We can make targeted predictions about who should experience sIS, both with respect to language processing abilities and to lesion location (see section 1.5.).
1.3. Relationship between sIS, IwW and other failures of word retrieval
To further clarify our operational definitions of sIS and IwW, it is useful to distinguish them from two related concepts, the tip-of-the-tongue (ToT) phenomenon and “feeling-ofknowing” (FoK). The ToT experience is well known to all language users and has been well characterized in the psychology literature since the early work of Brown & McNeill (1966). The ToT state is “a failure to recall a word of which one has knowledge” (Brown & McNeill, 1966), where an individual is unable to access a word, but has a feeling of being very close to recalling it. Here, we endorse the view that ToT arises from successful lexical-semantic access and incomplete (but partial) phonological access (Burke, MacKay, Worthley, & Wade, 1991; Dell et al., 1997; Harley & MacAndrew, 2014; James & Burke, 2000; Levelt et al., 1999; Meyer & Bock, 1992).
By definition, individuals experiencing ToT do not have full access to the phonological form of the target word at the time of attempted production, so ToT is distinct from our definition of sIS. Furthermore, most language users have experienced the ToT sensation, but individuals without neurological speech impairments should rarely experience a feeling of sIS without successful spoken output. ToT is also distinct from IwW, although healthy language users may experience both types of word-finding failure. It is possible for IwW to reflect a feeling of partial phonological access, in which case it would be very closely related to ToT; however, as described in section 1.2., IwW also encompasses retrieval of a related word or an inability to retrieve any word at all. These word-finding failures do not necessarily include the ToT state’s sense of closeness to retrieving the target word, which likely results from the achievement of partial phonological access (A. S. Brown, 1991; Jersakova, Souchay, & Allen, 2015). Thus, we hypothesize that the ToT experience does not precisely map onto the anomic experiences we describe here, but lies in between IwW and sIS at the level of partial phonological access.
Similarly to ToT, a FoK experience is a metacognitive state in which a person can identify that a word is stored in memory, believes that he/she may be able to recall it at a later time, and would be able to recognize the target word when it is presented to him/her (Hart, 1965). Importantly, FoK does not necessarily include the sense of closeness that accompanies a ToT state (Hanley, 2014). FoK is easily distinguishable from our definition of sIS, as it does not include access to the phonological form. In contrast, FoK and IwW share many characteristics and may be indistinguishable in some cases. Specifically, in the context of naming, both FoK and IwW involve a sense of recognition of a certain object or person presented, without the ability to retrieve the name. FoK likely surpasses the level of knowledge included in IwW, however, as a person reporting a FoK state is typically able to recognize the name when they subsequently see or hear it (Hart, 1965; Maril, Simons, Weaver, & Schacter, 2005). IwW, as we have defined it here, does not require an individual with anomia to demonstrate any recognition ability of the target concept or word. We do not include FoK in our interviews with individuals with aphasia, due to potential for confusion with IwW.
1.4. Inner speech in aphasia
There is some objective evidence, albeit in limited previous literature, that IS abilities can exceed overt speech output in some persons with aphasia (Feinberg et al., 1986; Geva, Bennett, et al., 2011; Geva, Jones, et al., 2011; Goodglass, Kaplan, Weintraub, & Ackerman, 1976). Very few studies have systematically examined self-reports of this experience: first, Goodglass et al. found that individuals with conduction aphasia and Broca’s aphasia often reported having “the idea of a word,” labeled tip-of-the-tongue (ToT) in their study, and correspondingly demonstrated relatively intact phonological awareness of target words during anomic events (Goodglass et al., 1976). They used objective measures of lexical access (e.g. rhyme judgments, syllable counting) as a proxy for IS, finding that individuals with Broca’s and conduction aphasia performed better on these tasks than their aloud naming abilities would suggest. This study showed that some individuals with aphasia demonstrate some lexical knowledge of words that they cannot say out loud and, importantly, that certain aphasic subgroups show intact self-awareness with respect to this ability. Their approach, however, focused on ToT and did not require participants to endorse the specific experience of sIS as we define it. A recent study used silent homophone judgments as a proxy for IS and showed that individuals with either conduction aphasia or motor planning impairments performed better on these tests than their out loud naming would predict (Geva, Bennett, et al., 2011). There is only one study since Goodglass et al. to directly explore the subjective experience of anomia. Hayward et al. (2016) asked two people with aphasia to report the accuracy of their IS for individual items on a covert naming task. Reports of sIS were associated with lexical frequency, accuracy of naming aloud on other days, and the likelihood of phonological errors when naming was incorrect; report of sIS also related to the rate of relearning of individual naming items during subsequent anomia therapy (Hayward, Snider, Luta, Friedman, & Turkeltaub, 2016). This recent study provides strong evidence that reports of sIS by people with aphasia can provide useful information regarding the mental processes of word-finding and production. Specifically, the results suggest that reports of sIS on individual naming attempts relate to lexical phonological access, and that subsequent errors relate to post-lexical output processes. Here, we assessed more general relationships between how often people with aphasia report experiencing sIS in daily life and their stroke locations and language deficits.
1.5. Expected relationships between self-reported sIS, language functions, and lesion locations
In the current study we aim to validate individuals’ subjective experiences of sIS by comparing their self-report to lesion location and performance on behavioral testing, to determine whether there are meaningful relationships. First, there should not necessarily be a relationship between reports of sIS and overall severity of aphasia or anomia. The integrity of sIS should depend on which specific language processes are affected as a result of the brain injury, not on the overall severity of impairment.
What specific language processes must be intact in order for an individual to achieve sIS? What language processes must be impaired for someone to experience overt anomia, despite sIS? And finally, what brain regions underlie those abilities? Returning to our simplified model of naming (Fig. 1), we suggested that sIS requires successful lexical access. As such, individuals who report frequent sIS should perform relatively well on tests of semantic processing as well as input-level phonological processing. We suggest further that individuals who experience overt anomia despite frequently achieving sIS have deficits in post-lexical output processes. The postlexical phase of word production encompasses several proposed processes and a disruption of any of them could lead to overt anomia after sIS, e.g., loss of the phonological trace or a failure of sensorimotor mapping, phonological assembly, phonetic encoding, or articulatory planning (Goldrick & Rapp, 2007; Indefrey & Levelt, 2004; Indefrey, 2011; Walker & Hickok, 2015). The last of these, a failure of articulatory motor planning, often falls under a diagnosis of verbal apraxia or acquired apraxia of speech (Duffy et al., 2015; Strand, Duffy, Clark, & Josephs, 2014; Whiteside, Dyson, Cowell, & Varley, 2015). Individuals with anomia who report frequent sIS should perform poorly on tasks that rely heavily on these output processing skills, such as repetition or oral reading. Furthermore, there may be a lexicality effect, defined by poorer performance on production tasks using pseudowords (pronounceable non-words) as opposed to real words. Real words benefit from stable patterns of activation and a boost from semantic content, so can be more resilient to phonological processing impairments than pseudowords, which do not share these benefits (Coltheart, 1996; Crisp & Lambon Ralph, 2006; Patterson, Suzuki, & Wydell, 1996). The lexicality effect thus serves as a specific measure of post-lexical phonological output processing impairments, which we predict relate to reports of sIS.
Parallel predictions can be made regarding the integrity of relevant brain regions. Individuals who report frequent sIS are predicted to have relatively intact lexical semantic and phonological access, so are unlikely to have lesions in areas that underlie these processes, such as the middle temporal gyrus and the anterior/middle regions of superior temporal gyrus (STG) (Hickok, 2009; Indefrey & Levelt, 2004; Price, 2012). These individuals, who report frequent sIS but still experience anomia, are likely to have lesions in areas that affect post-lexical output processes (Feinberg et al., 1986; Geva, Bennett, et al., 2011). These dorsal stream areas may include the posterior STG and supramarginal gyrus (sensorimotor interface for phonological assembly/encoding) and the ventral motor and premotor cortices (for articulatory coding and sequencing) (Hickok, 2009; Indefrey & Levelt, 2004; Price, 2012; Walker & Hickok, 2015).
1.6. Overview of study aims
Studies on IS in aphasia have been limited and only two prior studies have focused on the individual’s own subjective experience of anomia. In this study, we aimed to fill this gap by providing individuals with aphasia a structured opportunity to describe and report their experience of IS. Specifically, we aimed to establish preliminary evidence that (1) individuals with aphasia themselves can inform us about their IS abilities and (2) their self-reports have meaningful relationships with overt language abilities and with lesion locations. Overall, these findings may validate the common reports made by individuals with aphasia regarding their IS. Additionally, the study of sIS may contribute to existing models and, in turn, inform future approaches to anomia treatment.
2. Materials and Methods
2.1. Participants
Participants for this study were thirty-seven individuals drawn from the participant group of a larger project investigating the brain mechanisms of aphasia recovery. All participants gave informed consent under a protocol approved by the Georgetown University Institutional Review Board. For inclusion in this study, participants were required to be a native English speaker (spoken since age five or younger), to have adequate hearing and vision (with correction, if appropriate) and to have a diagnosis of aphasia from a left hemisphere ischemic or hemorrhagic stroke that occurred at least six months prior to enrollment, without history of other significant neurological or psychiatric illness.
Participants included 26 men and 11 women, with an average age of 60.5 years old (SD = 10.1, range = 39–83 years) and an average of 16.8 years of formal education (SD = 2.8, range = 12–24 years). Using the Edinburgh Handedness inventory, 30 participants were identified as right-handed, 5 as left-handed, and 2 as ambidextrous (Oldfield, 1971). Post-stroke chronicity ranged from 11 months to 21 years (median = 50.6 months, mean = 64.4, standard deviation = 52.2). Five participants had aphasia resulting from a hemorrhagic stroke and thirty-two participants had aphasia resulting from an ischemic stroke, including one individual whose aphasia developed from a series of strokes that followed left hemisphere brain tumor resection.
The group presented with a wide range of aphasia severity and subtype, as established by the Western Aphasia Battery – Revised (WAB-R) (Kertesz, 2006). WAB-R Aphasia Quotient (AQ) scores ranged from 20 to 96.2, with an average of 68.6 (SD = 23.9). Three of the included participants scored above a 93.8 on the WAB-R AQ, so are technically considered to be within the normal range according to the assessment scoring system. They each reported ongoing communication difficulties and exhibited clinical evidence of mild anomia, so were included in this study. Participants were diagnosed using the WAB-R aphasia subtype classification criteria: 19 anomic (including the three “recovered” patients), 13 Broca’s, two Wernicke’s, two conduction, and one transcortical sensory. We excluded participants with significant impairments in sentence- and/or word-level comprehension. Cut-off scores were a minimum of 4/10 on the WAB-R Auditory Verbal Comprehension composite and a minimum score of 24/48 on an in-house auditory word-picture matching task (in a field of 6, 50% performance on this task is better than chance at P<1×10−7). For diagnosis of verbal apraxia, we utilized the Apraxia of Speech Rating Scale (ASRS) 2.0, a rating-based checklist of distinguishing features of apraxia of speech (Strand et al., 2014 and Duffy, 2016). Authors S.F.S. and M.E.F. individually observed videos of verbal subtests from the WAB-R, rated each participant, and then discussed discrepancies as needed to arrive at a consensus score for each participant.
2.2. Methods for probing the subjective experience of anomia
As stated in the Introduction, many individuals with aphasia make vague statements such as, “I know it but I can’t say it” – our goal was to generate more specific descriptions of the experience of anomia that correspond to our hypothesized experiences (described in section 1.2.). We generated easily understandable phrasing to describe sIS vs. IwW by combining anecdotal experiences with individuals with aphasia with knowledge of theoretical models of anomia. To obtain self-report data from our participants, we used open-ended, yes/no, and rating scale-based questions, beginning with:
Do you ever know what you want to say but you can’t say it out loud?
How would you describe that feeling?
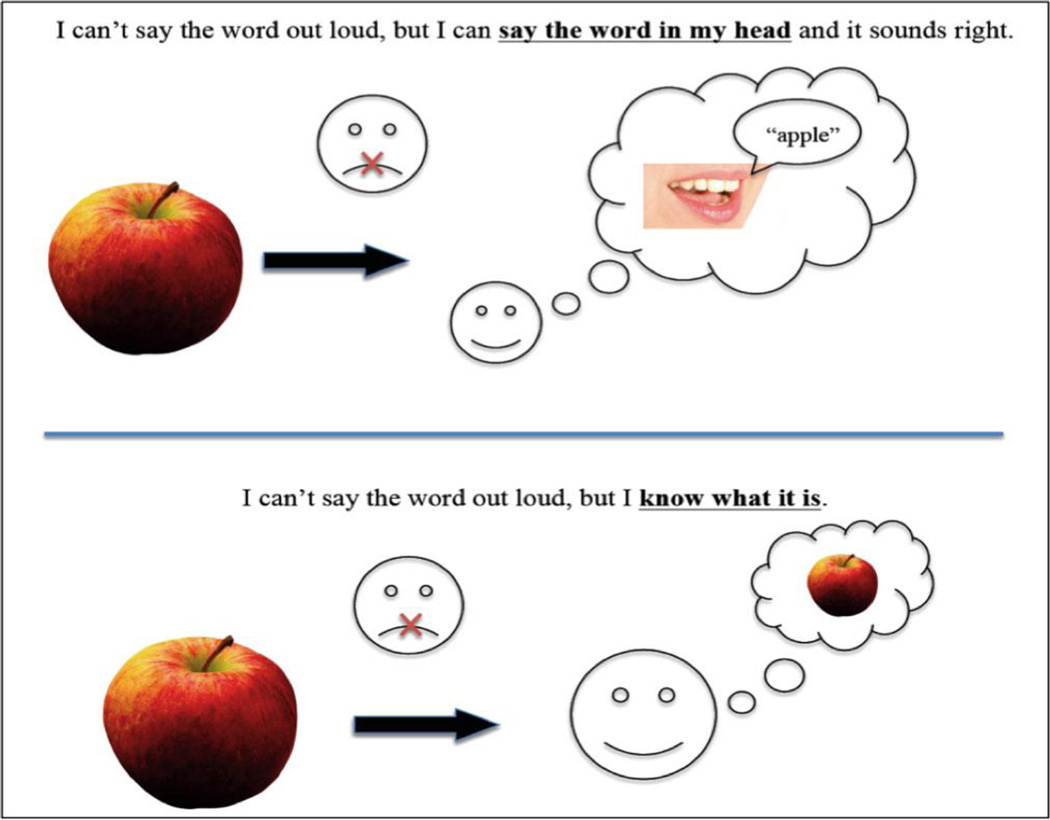
After these initial questions, we presented a set of written phrases and pictures to help explain the various concepts that follow (Figure 2). Note that the pictorial support accompanying IwW clarifies that the idea or concept is available, but the word is not. With the pictures still in view, participants were asked to reply yes/no to the following questions about each specific experience:
Figure 2.
Picture support for the concepts of sIS and IwW. No pictorial support was given for ToT.
Do you ever feel…
“I can’t say it out loud, but I can say the right word in my head and it sounds right” (sIS)
“I can’t say it out loud, but I know what it is in my head” (IwW)
“I can’t say it out loud, but it’s on the tip of my tongue” (ToT)
Participants were then asked to identify how frequently they experience each of the above scenarios, on the following scale: never, rarely, sometimes, often, and almost always. [For subjects who answered “no” on the prior yes/no question, the expected response for the rating scale was “never”]. All possible ratings were available in written form for participants to choose from and were presented in vertical orientation, to avoid possible negative impact of hemispatial neglect (Kleinman et al., 2007). When giving answers, participants could either point to the written word or provide a verbal response. In the Results and Discussion, participants’ subjective responses regarding the frequency of each experience will be referred to as “anomia ratings,” with specific reports of the frequency of sIS referred to as “sIS ratings.”
Interview sessions for obtaining anomia ratings took place at either Georgetown University Medical Center or MedStar National Rehabilitation Hospital, often in conjunction with sessions involving other language testing (described below in section 2.3.) or structural imaging (2.4.). All sessions took place in a quiet, private room and were videotaped. As described above, the spoken questions were accompanied by written/picture support in order to help participants understand the questions as well as remember and utilize the possible answers; some participants required additional time and explanation with these materials, so this portion of the session lasted from 10–30 minutes, depending on the participant. The first author of this paper (M.E.F.) obtained anomia ratings from 33 of the participants, with two other trained lab members completing the sessions with the remaining four participants. All transcribed live responses were confirmed for accuracy via later video review. Additionally, M.E.F. reviewed the videos for the four subjects who were seen by a different researcher, to confirm reliability of administration procedures. No amendments to the recorded responses were made during this process.
2.3. Language testing
This study’s participants were drawn from a larger cohort of study participants in the lab and were each administered an extensive language/cognitive assessment battery as a part of that larger study. In the current study, we analyzed only those behavioral assessments that either (1) provided a measure of overall severity or (2) fit our hypotheses about the relationship between self-reported IS and language processing, namely that frequency of self-reported sIS in individuals with anomia should correlate with poor performance on tasks of output processing and relatively intact performance on tests of lexical/semantic access. The language measures are given below; all unpublished tasks were developed in-house and normed in a healthy cohort of older adults.
Overall language ability: WAB-R AQ (Kertesz, 2006)
Overall naming ability: Philadelphia Naming Test (PNT) – 60 item short-form version (Roach, Schwartz, Martin, Grewal, & Brecher, 1996)
Motor speech: ASRS 2.0
- Lexical/semantic composite score (serving as a surrogate measure of lexical/semantic access). This score is an average of:
-
◦The Pyramids & Palm Trees Test (Howard & Patterson, 1992)
-
◦Auditory word-picture matching task (48 items, field of 6)
-
◦
- Lexicality effect (serving as a measure of post-lexical phonological output processing). This score is the difference between word and pseudoword performance, averaged across repetition and reading tasks:
-
◦Repetition
-
▪Pseudoword repetition (30 single words ranging from 1 to 5 syllables)
-
▪Word repetition (composite of 30 single words from the Apraxia Battery for Adults-2 subtests 2A: Increasing Word Length and 5: Repeated Trials, selected for presentation format and to match average word length of the pseudoword repetition task)
-
▪
-
◦Single word oral reading
-
▪Pseudowords (20 single syllable pseudowords)
-
▪Words matched to pseudowords (20 real single syllable words)
-
▪
-
◦
In distinguishing between tasks of access and output, we acknowledge that we do not have tasks that directly assess lexical access in production; however, a similar word-to-picture matching task and the Pyramids & Palm Trees test have both been shown to be good predictors of output abilities, as measured by error patterns on a naming task (Martin, Schwartz, & Kohen, 2006). All language assessments were given to the participants by a certified speech-language pathologist or a postdoctoral researcher, each of whom was thoroughly trained in administration and scoring procedures relevant to the current study. All sessions were videotaped.
2.4. Structural imaging: acquisition, lesion segmentation, and warping
Structural magnetic resonance imaging (MRI) was performed at the Center for Functional and Molecular Imaging at Georgetown University, which uses a 3T Siemens Magnetom Trio scanner. Participants underwent a high-resolution T1-weighted structural scan (MPRAGE) for lesion localization (TR of 1900 ms, TA of 2.56 ms, FOV 250×250, 9° flip angle and 160 contiguous sagittal slices for voxel size of 1 × 1 × 1 mm. Trained lab members (blinded to the behavioral data) manually traced the lesions onto these T1-weighted structural images by identifying differences in signal between healthy and lesioned brain areas in MRIcron (http://www.mccauslandcenter.sc.edu/mricro/mricron). All lesion segmentations were verified and finalized by a board certified neurologist (P.E.T.). Lesion masks were warped into the Montreal Neurological Institute (MNI) space using the VBM8 toolbox in SPM8 (http://www.fil.ion.ucl.ac.uk/spm) running under Matlab R2014a.
2.5. Statistical analysis
Statistical analyses were performed in SPSS 24. Ordinal logistic regression was used to determine relationships between language scores and subjective anomia ratings. Prior to the regression, a series of bivariate correlations (Spearman and Pearson as appropriate) were performed to examine relationships between the subjective anomia ratings and to explore the collinearity among the language scores. These exploratory tests were not corrected for multiple comparisons.
Imaging analyses were performed in N=36, excluding one participant who had a right ACA stroke at the same time as his left hemisphere stroke. We used a multivariate lesion-symptom mapping technique in which patterns of lesion status across voxels in the brain are considered simultaneously to predict a single behavioral score (Zhang, Kimberg, Coslett, Schwartz, & Wang, 2014). This was performed using the support vector regression-based lesion-symptom mapping (SVR-LSM) toolbox in MATLAB (https://cfn.upenn.edu/~zewang/), which is more resistant than voxel-based lesion-symptom mapping to theoretical bias in localization due to lesion covariance, especially when behaviors rely on multiple distinct brain regions (Herbet, Lafargue, & Duffau, 2015; Mah, Husain, Rees, & Nachev, 2014). Here, we used SVR-LSM to identify lesion locations that predict the three anomic experiences: sIS, IwW, and ToT. We did so by identifying voxels where the presence of a lesion was predictive of a higher self-reported frequency for each experience (as opposed to most LSM analyses, which look for voxels that predict lower scores). We excluded voxels that were lesioned in fewer than seven participants (minimum 20%). Significance testing was performed using 10,000 permutations of anomia ratings, and the significance threshold was set at a voxelwise P<.01 with a cluster size threshold of 200 mm3. Direct total lesion volume control was applied to control for relationships between lesion size, stroke distribution, and our measures of interest. Because SVR-LSM considers all voxels simultaneously in a single regression model, correction for multiple comparisons is not required (Zhang et al., 2014).
3. Results
3.1. Subjective anomia ratings
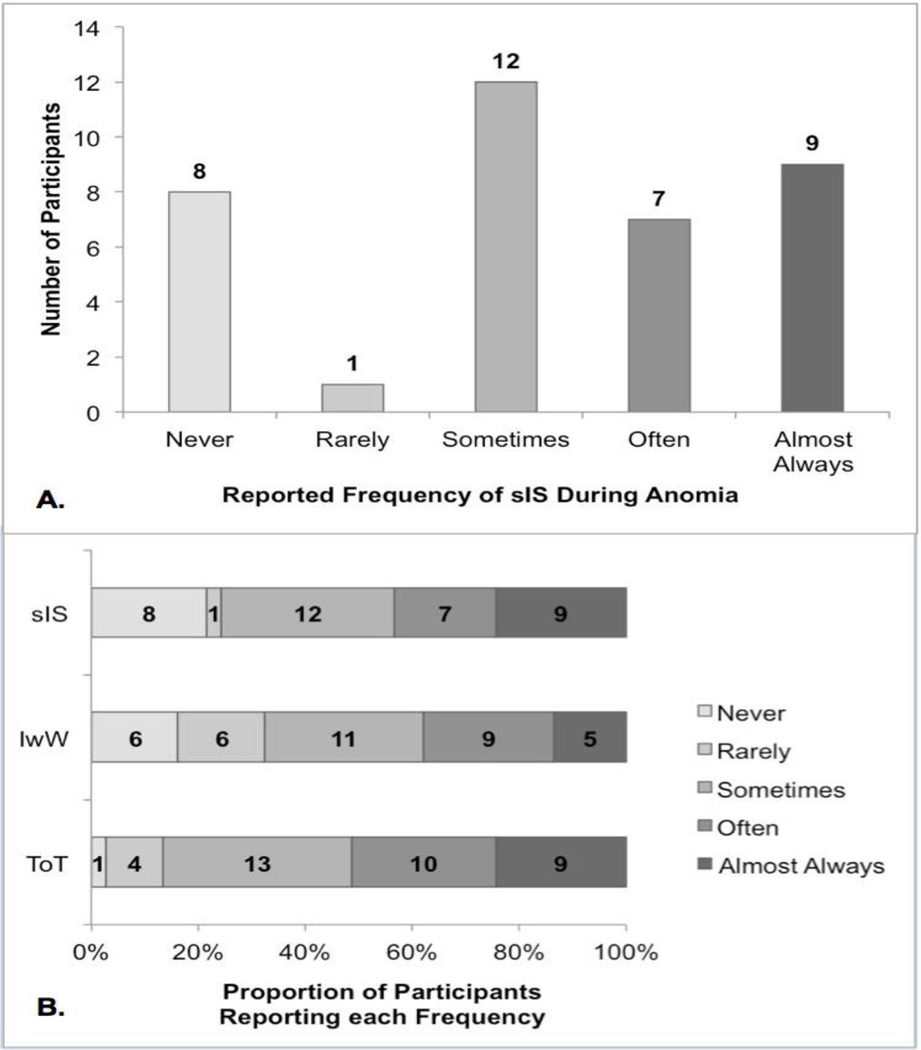
All participants endorsed the general experience of anomia by answering yes to the question: “Do you ever know what you want to say but you can’t say it out loud?” Of those, 29 participants (78.4%) endorsed the specific anomic experience of sIS - “I can’t say it out loud, but I can say it in my head and it sounds right.” Figure 3A shows participants’ reported frequencies of sIS during anomia, from never to almost always. In Figure 3B, this distribution is compared to participant-reported frequencies of “I know what it is in my head” (IwW) and “It’s on the tip of my tongue” (ToT).
Figure 3.
sIS and other anomia ratings.
3.2. Behavioral comparisons
We first examined relationships between the participant-reported frequency of the three experiences of anomia (sIS, IwW, and ToT; on a scale of 0/“never” to 4/“almost always”) using nonparametric correlations. These a priori analyses tested the hypothesis that sIS and IwW are discrete perceptions but that ToT, which represents partial phonological access, bears similarities to both. In line with our hypothesis, no relationship was found between frequencies of sIS and IwW (P=.73), whereas ToT correlated with both sIS (P=.006) and IwW (P=.03; Table 1).
Table 1.
Relationship between anomia ratings and behavioral language measures.
| Correlation coefficients |
sIS | IwW | ToT | WAB-R AQ |
PNT | ASRS | Lexicality effect |
|---|---|---|---|---|---|---|---|
| IwW | −0.059 | - | |||||
| ToT | 0.447** | 0.359* | - | ||||
| WAB-R AQ | −0.393* | 0.175 | −0.130 | - | |||
| PNT | −0.344* | 0.014 | −0.164 | 0.863*** | - | ||
| ASRS | 0.384* | −0.106 | 0.265 | −0.655*** | −0.547*** | - | |
|
Lexicality effect (output phonology) |
0.343* | −0.199 | 0.116 | 0.153 | 0.192 | −0.221 | - |
|
Lexical/semantic composite |
−0.252 | −0.023 | −0.199 | 0.715*** | 0.668*** | −0.438** | 0.151 |
Correlation coefficients are shown. Correlations with subjective anomia ratings (first three columns) are Spearman’s rho; correlations among language scores (last four columns) are Pearson’s r.
Statistical significance denoted as follows:
significant at P<.05;
significant at P<.01;
significant at P<.001.
The three correlations between the subjective anomia ratings are a priori tests and do not require correction for multiple comparisons. All other tests are considered exploratory, so uncorrected p-values are shown.
In preparation for the main regression analysis examining relationships between the participant-reported frequency of the anomia experiences and language scores, we first performed exploratory bivariate correlations to examine relationships among the variables (Table 1). These correlations revealed that sIS correlated with each of our language measures (all P<.05 uncorrected), except the lexical/semantic composite score (P=.13 uncorrected). In contrast, no relationships with language measures were observed for ToT or IwW (all P>.10 uncorrected). The exploratory correlations also revealed that WAB-R AQ, PNT, ASRS, and the lexical/semantic composite were highly inter-correlated (all P<.001, except ASRS with lexical/semantic composite at P=.007). Thus, relationships between sIS and these individual scores may not be distinguishable from its relationship with overall aphasia severity. It is also notable in this context that although the lexical/semantic composite score related to overall aphasia severity, it was not correlated with sIS, even at an uncorrected threshold. The lexicality effect did not correlate with any of the other language scores (all P>.10).
Next, relationships between sIS and behavioral scores were formally examined using ordinal logistic regression with reported frequency of sIS (scale of 0/never to 4/almost always) as the dependent measure. Because of the observed collinearities discussed above, WAB-R AQ, PNT, ASRS, and the lexical/semantic composite were scaled from 0–1 and averaged to create a measure of overall aphasia severity. This score and the lexicality effect score were used as the independent variables in the regression. The overall regression model fit was significant (X2(2)=12.19, P=.002). Both aphasia severity and lexicality effect had independent relationships with sIS (aphasia severity Wald z(1)=7.53, P=.006; lexicality effect Wald z(1)=6.23, P=.013). The direction of effects show that more frequent sIS was associated with poorer reading and repetition of pseudowords compared to words (lexicality effect), and more severe aphasia. Regressions on IwW and ToT using the same independent variables yielded no significant results (P=.421 and P=.324, respectively).
3.3. Multivariate lesion-symptom mapping
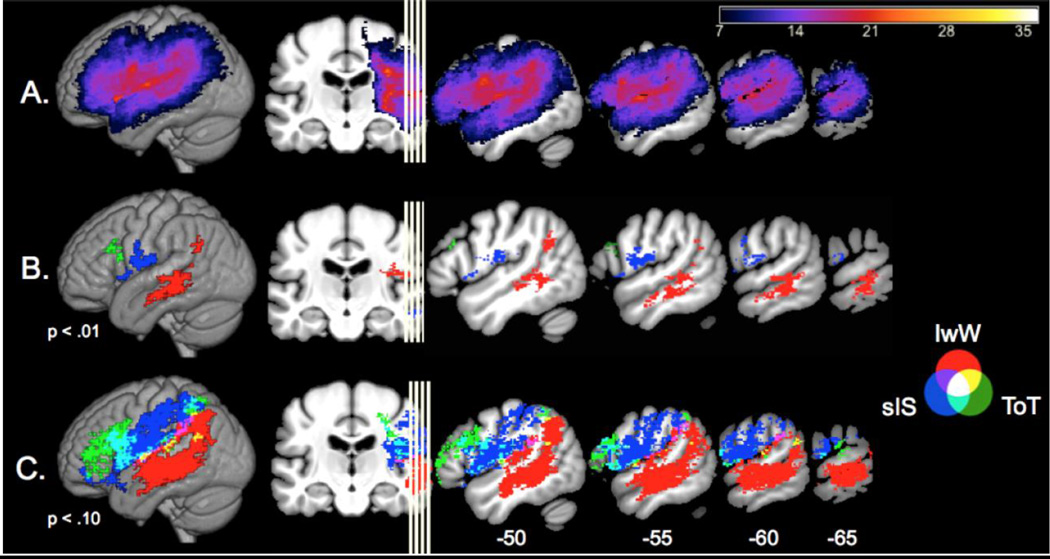
Figure 4A shows a lesion overlap map demonstrating our participant group provided coverage across the entire perisylvian language network, including frontal, parietal, and temporal lobe regions. In nonparametric correlations, none of the three experiences of anomia were correlated with overall lesion size (all P>.10). SVR-LSM analyses were conducted to identify specific lesion locations associated with higher reports of each experience of anomia. sIS was associated with lesions in the inferior frontal gyrus pars opercularis (IFG pOp) and the ventral pre- and postcentral gyri, whereas IwW was associated with lesions in the mid-posterior middle temporal gyrus and the angular gyrus (Table 2, Fig. 4). ToT was associated with lesions in the dorsal portion of IFG pars triangularis and pOp, near the inferior frontal junction. The main analysis showed little to no spatial overlap between the lesion sites associated with the three subjective experiences of anomia. To ensure this dissociation was not related to the statistical threshold used, we examined the lesion-symptom maps at a reduced threshold (P<.10). At this lower threshold, there was some overlap between sIS and ToT in the ventral premotor cortex and POp, but still no spatial overlap between sIS and IwW. In this map, as predicted, lesions in broad dorsal stream language areas (frontal/parietal lobes) involved in post-lexical output processes relate to sIS and ToT, whereas lesions in superior and middle temporal areas involved in lexical access relate to IwW.
Figure 4.
A. Lesion overlay map (N=36). B. SVR-LSM results showing lesion locations associated with higher reports of sIS, IwW, and ToT (p < .01, see figure legend for color representations). C. SVR-LSM results at p < .10 to illustrate the minimal overlap between lesions associated with sIS and IwW. Total lesion volume was controlled in both maps, using the direct lesion volume control method.
Table 2.
SVR-LSM results showing significant clusters (threshold = 200 mm3) at a threshold of P<.01.
| Anatomical description of cluster location | Peak voxel | Number of voxels |
|---|---|---|
| sIS | ||
| Inferior frontal gyrus (IFG), pars opercularis | (−58, 6, 4) | 93 |
| Ventral precentral gyrus | (−57, −4, 9) | 1097 |
| IwW | ||
| Angular gyrus | (−46, −54, 24) | 266 |
| Posterior middle temporal gyrus (MTG) | (−54, −34, −4) | 1414 |
| ToT | ||
| IFG, dorsal portion of pars triangularis | (−56, 18, 21) | 89 |
| IFG, medial portion of pars opercularis (near inferior frontal junction) |
(−38, 9, 26) | 86 |
4. Discussion
By probing subjective experiences, we found that people with aphasia differentiated between distinct experiences of anomia: the sense of being able to say a word correctly in one’s head (sIS) and the sense of wanting to communicate an idea without finding the right word in one’s head (IwW). Both of these experiences related to the ToT state in terms of reported frequency. We found that reports of sIS, but not IwW or ToT, related to behavioral measures of language impairment, including overall severity of anomia and post-lexical output processes. Further, multivariate lesion-symptom mapping results demonstrated that damage in discrete brain regions was associated with each of the three subjective experiences of anomia.
4.1. Successful inner speech is subjectively dissociable from a failure to retrieve the word (IwW)
During our testing sessions, all subjects endorsed the general experience of anomia, in which they want to communicate a word or idea but are unable to do so aloud, consistent with our knowledge of the universality of anomia in aphasia (Goodglass & Wingfield, 1997). As predicted, our precise probing enabled them to go beyond this general endorsement to provide a more detailed characterization of how frequently they experience sIS, IwW, and ToT. Although there was a bias towards a “sometimes” response, participants gave a wide range of responses, which verifies that the wording of our questions did not consistently lead respondents to a particular response pattern. We demonstrated a lack of within-subject correlation between reports of sIS and IwW, supporting the conclusion that participants conceived of these as two different experiences. Reports of both of these, however, were positively correlated with reported frequency of ToT. This is consistent with our hypothesis that ToT, as a state of partial phonological access, falls somewhere in between the two experiences of anomia that we have defined here. Like IwW, ToT is a failure of word retrieval, in which a person cannot access the target word. Like sIS, ToT includes a strong sense of closeness to being able to produce the target word, generated by some amount of access to the phonological representation of the word (Burke et al., 1991; Dell et al., 1997; James & Burke, 2000). ToT was reported to be nearly universal in our participants (see Fig. 3B), which is expected due to its prevalence in healthy populations (Brown, 1991).
4.2. sIS is common in aphasia and relates to aphasia severity and phonological output processes
The widespread reports of sIS suggest that it is common in aphasia – nearly 80% of participants endorsed having some experience of sIS during overt anomia, with varied frequency ranging from rarely to almost always. We predicted that participants who reported frequent sIS during anomia would demonstrate deficits primarily in phonological output processes, such as sensorimotor mapping and articulatory motor programs (see Fig. 1 for the model) (Walker & Hickok, 2015). We found support for this hypothesis in the strong positive correlation between sIS and the lexicality effect. There is no consensus as to the exact nature of the phonological impairment that gives rise to the lexicality effect, but Friedman (1995) gives several possibilities: a deficit in generating the phonological code, in maintaining the phonological code, or at the level of motor output (Friedman, 1995). The relationship observed between sIS and the lexicality effect is thus consistent with a deficit in phonological output processing. However, rather than reflecting a post-retrieval output failure, the relationship with the lexicality effect could also be consistent with the alternate interpretation that sIS arises from activation of a nearly-correct phonological form that is strong enough to result in an internal sense of retrieval, but not strong enough to be accurately translated to a correct motor speech act. By this account, however, the only distinction between sIS and ToT would be a subtle difference in the strength of the retrieved phonological form. The distinct relationships with behavior and lesion location observed here for sIS compared to ToT suggests otherwise.
The positive relationship with severity of apraxia of speech indicates that an articulatory impairment may relate to the experience of sIS, but additional research using more precise behavioral tasks would be necessary to delineate the exact relationship between sIS and the phonological, phonetic, and articulatory subprocesses of the post-lexical processing stage. Furthermore, apraxia severity was strongly correlated with overall naming/language impairment, so we cannot disentangle any specific relationship between sIS and apraxia from a more general relationship with overall severity. Indeed, we found a relationship between sIS ratings and overall naming or language impairment that was not predicted a priori. It is possible that this relationship relates to a sampling bias. Our cohort was comprised mostly of individuals with Broca’s or anomic aphasia, in whom severity of apraxia and aphasia were closely correlated. The relationship between sIS and aphasia severity might not exist in a population in which these deficits were uncorrelated. Using our methods in a sample that includes more subjects with fluent aphasia and severe anomia related to large temporal lobe lesions would help to clarify the relationship between sIS ratings and overall naming/language ability. The lack of a significant negative correlation between sIS and our lexical/semantic composite score, even at an uncorrected significance threshold, supports our hypothesis that sIS reflects intact lexical/semantic access.
In considering the significant relationships that exist between sIS and behavior, it is equally important to note the lack of relationship between IwW and these same measures, in either a positive or negative direction. Given our focus on sIS, we aimed primarily to differentiate between the specific experience in which lexical retrieval feels successful (sIS) and all other experiences of anomia (IwW). The incorporation of diverse anomic experiences – including partial retrieval, retrieval of a related word, or failure to retrieve any word at all – into a single category may have led to the lack of strong relationships between IwW and any individual behavioral measure.
4.3. Lesion locations conform to predicted anatomical patterns
The multivariate lesion-symptom mapping results provide a second line of evidence for the validity of the subjective anomia ratings. Taken as a whole, the SVR-LSM results illustrate a striking dissociation between the two main experiences of anomia, with minimal overlap in lesion localization between sIS and IwW even at a very low statistical threshold. Strong endorsement of sIS is associated with lesions in inferior frontal regions and sensorimotor cortices, which are involved in articulatory coding/sequencing and motor speech output (Indefrey & Levelt, 2004; Price, 2012; Schwartz, Faseyitan, Kim, & Coslett, 2012). The experience of IwW is associated with lesions in the middle temporal gyrus and angular gyrus, areas that underlie lexical semantic retrieval (Binder, Desai, Graves, & Conant, 2009; Mechelli, Josephs, Lambon Ralph, McClelland, & Price, 2007; Price, 2012; Troiani et al., 2008). The ToT experience is associated with lesions to the dorsal pars opercularis of the inferior frontal gyrus, near the inferior frontal junction, which contributes to phonological assembly prior to articulatory planning (Ghosh, Tourville, & Guenther, 2008; Indefrey, 2011). This discrete localization supports that a ToT experience is distinct from either anomic experience. Also, this ToT finding is consistent with prior neuroimaging research on the neural correlates of ToT in healthy adults, which shows that a loss of structural integrity in a nearby region, the left insula, relates to increased experience of ToT during normal aging (Shafto, Burke, Stamatakis, Tam, & Tyler, 2007) and that young adults show stronger insula activation than older adults during ToT states (Shafto, Stamatakis, Tam, & Tyler, 2009).
Overall, these lesion findings support our hypothesis: an early failure of word retrieval results in a feeling of IwW, whereas successful retrieval followed by a failure of post-lexical output, primarily related to damage in dorsal stream language areas, results in a feeling of sIS. A feeling of ToT arises in between, consistent with the view of ToT as a state in which the lexical item is retrieved but there is only partial access to the phonological representation (Burke et al., 1991; Dell et al., 1997; James & Burke, 2000; Levelt et al., 1999; Meyer & Bock, 1992).
4.4. The role of self-monitoring in sIS
Despite the support for our hypotheses regarding language abilities and lesion locations related to sIS, we cannot indisputably establish the validity of our participants’ self-reports within the context of the current study. There is potential concern regarding overall comprehension ability for understanding our questions, as well as self-monitoring ability for making such fine-grained metacognitive judgments regarding internal experiences. With regard to sentence-level comprehension, we took care to use clear, simple language that would maximize participants’ ability to understand the concepts and questions being presented, while also using a slow pace and written/pictorial support. In addition, individuals with severe sentence-level comprehension deficits were not included in the study.
A second, more specific concern about using self-report as a measure of language function involves the integrity of self-monitoring in individuals with aphasia, an impairment of which would negatively impact the reliability of self-reports regarding the anomic experience, i.e., their perception of having retrieved the correct word may simple be wrong. Prior studies have shown that many individuals with aphasia do show preserved error detection for their aloud speech (Marshall, Neuburger, & Phillips, 1994; Nickels & Howard, 1995; Oomen, Postma, & Kolk, 2001). Models of self-monitoring in speech production have largely relied on a comprehension-based monitor, where a speaker monitors his/her own output through the same speech perception pathways used to comprehend others’ speech (Indefrey & Levelt, 2004; W. J. Levelt et al., 1999). Both the 2004 and a more recent model include a mechanism by which the accuracy of IS can be monitored prior to spoken output via an “internal loop” between phonological representations and speech perception areas in superior temporal gyrus (see Fig. 1, inner loop) (Indefrey, 2011). Given this model, an individual with relatively intact single word auditory comprehension should have similarly spared self-monitoring ability for both aloud and IS. To maximize the likelihood of adequate self-monitoring ability in our participants, we therefore excluded individuals with severely impaired single word auditory comprehension.
There are notable alternative proposals to this comprehension-based model of self-monitoring, however, as criticisms have been raised regarding its ability to account for all available evidence regarding self-monitoring (Nickels & Howard, 1995; Nozari, Dell, & Schwartz, 2011). In place of a comprehension-based monitor, Nickels & Howard (1995) suggested a pre-articulatory production-based monitor, but this type of model was not fully characterized for many years. Recently, Nozari et al. (2011) expanded upon these prior suggestions by proposing a domain-general, conflict-based monitor that is accomplished within the production system itself, through relaying of information to an executive center (such as the anterior cingulate cortex, ACC) about the level of response conflict at the time of word retrieval. This conflict-based model is also consistent with our model of sIS, since the conflict signal in Nozari et al.’s proposed model is generated within the access stages of word-finding based on the relative activation strength of retrieved forms. As such, these signals would arise prior to the post-lexical output processing that we predicted (and found) to be impaired in our individuals who reported frequent sIS during anomia.
Our results however, are less consistent with ideas that IS is generated and monitored based on efference copies generated by motor speech regions (Tian & Poeppel, 2013). In this case, severe verbal apraxia and lesions in the ventral motor and premotor areas would be expected to result in less sIS, not more, as we found in our analyses. Another recent functional imaging study on unimpaired participants found monitoring of both inner and aloud speech was associated with increased activation in a domain-general error detection area, the ACC, as well as the posterior IFG (Gauvin, De Baene, Brass, & Hartsuiker, 2015). These results suggest that lesions in the posterior IFG might cause unreliable self-monitoring, not a relationship with frequency of sIS as we found. These fMRI studies do not specifically establish a necessary role of IFG in monitoring of IS, so this seeming conflict may simply reflect a difference in the type of evidence provided by different methods. Clearly, additional research on mechanisms of IS self-monitoring will be needed to fully understand the psychological and neural bases of subjective experiences of anomia.
4.5. Clinical implications of self-reported IS
The findings of this study demonstrate that many individuals with aphasia can provide reliable, nuanced reports of their experience of anomia, when given a structured opportunity to do so. These findings complement our recent report that self-reported IS on individual naming attempts corresponds to success of naming aloud, error types, and rate of relearning during subsequent anomia treatment (Hayward et al., 2016). Although not expected to be universally reliable, evidence for meaningful self-monitoring of IS raises interesting questions about the potential for self-cueing in aphasia, an approach that has received mixed support from the treatment literature (Tompkins, Scharp, & Marshall, 2006). It is possible that a deeper understanding of sIS could inform candidacy for such treatment paradigms.
4.6. Limitations and future directions
Our method for probing the subjective experience of anomia was newly designed for the current study and certain limitations were revealed during data collection. As mentioned in sections 1.2. and 4.2., we defined sIS to refer to one very specific experience of anomia, whereas IwW includes various different types of experiences. Additionally, participants were asked to use a categorical scale when identifying how frequently they experienced the three scenarios – never, rarely, sometimes, often, and almost always – it is possible that individual respondents interpreted the scale differently. We did ask participants to use these ratings with regard only to experiences of anomia rather than all communication attempts in general, but we cannot be certain that all participants did so. Although such inconsistencies did not impact our ability to identify meaningful relationships between subjective anomia ratings, behavioral performance, and lesion locations, future research should address these concerns by including a more specific set of experiences under the heterogeneous IwW scenario and using a continuous frequency scale rather than a categorical scale.
We were also limited in this initial study by the use of language and cognitive measures collected for other purposes, and thus not prospectively selected to test our hypotheses on self-perceived IS. For example, this pilot study used a relatively short naming task that did not provide enough error responses for meaningful analysis. Future studies will address these limitations by using behavioral tasks designed specifically to address these issues.
5. Conclusions
Although preliminary in nature, the relationships discovered between subjective and objective measures suggest that participants with aphasia may have the metacognitive ability to distinguish between different subjective experiences of anomia. The findings suggest that self-reported sIS in individuals with anomia is associated with poor post-lexical phonological output processing as well as with lesions in the dorsal stream brain regions that support those language processes. More generally, our results suggest that many people with aphasia can often serve as reliable sources of information about their experience of anomia, which may enable researchers and clinicians to better diagnose and treat anomia on an individual basis.
Highlights.
People with aphasia often report that they can say words internally, but not aloud.
We find “successful inner speech” (sIS) appears distinct from other anomic experiences.
Self-reported sIS correlates with anomia severity and phonological output deficits.
Lesion-symptom mapping relates reports of sIS to ventral sensorimotor lesions.
Results suggest that sIS in anomia may relate to post-lexical output deficits.
Acknowledgments
The authors would like to extend their sincere gratitude to the study participants for their time, commitment, and willingness to speak openly about their experience with aphasia. We would also like to thank Dr. Laura Skipper-Kallal for data collection and for providing hands-on training in SVR-LSM to the first author. Finally, we are grateful to the funding that allows us to do this work. M.E.F. is supported by National Institutes of Health (NIH) Grant F31DC014875. W.H. is supported by NIH Grant F30DC014198. P.E.T. is supported by NIH/NIDCD R03DC014310, NIH/NCATS via the Georgetown-Howard Universities Center for Clinical and Translational Science (KL2TR000102), the Doris Duke Charitable Foundation Grant 2012062, and the Vernon Family Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berthier ML. Poststroke aphasia : epidemiology, pathophysiology and treatment. Drugs & Aging. 2005;22(2):163–182. doi: 10.2165/00002512-200522020-00006. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15733022. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where Is the Semantic System? A Critical Review and Meta-Analysis of 120 Functional Neuroimaging Studies. Cerebral Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanken G, Dittmann J, Haas J-C, Wallesch C-W. Spontaneous speech in senile dementia and aphasia : Implications for a neurolinguistic model of language production. Cognition. 1987;27:247–274. doi: 10.1016/s0010-0277(87)80011-2. [DOI] [PubMed] [Google Scholar]
- Brown AS. A review of the tip-of-the-tongue experience. Psychological Bulletin. 1991;109:204–223. doi: 10.1037/0033-2909.109.2.204. [DOI] [PubMed] [Google Scholar]
- Brown R, McNeill D. The “ Tip of the Tongue” Phenomenon. Journal of Verbal Learning and Verbal Behavior. 1966;5(1934) [Google Scholar]
- Burke DM, MacKay DG, Worthley JS, Wade E. On the Tip of the Tongue: What Causes Word Finding Failures in Young and Older Adults? Journal of Memory and Language30. 1991;30:542–579. [Google Scholar]
- Coltheart M. Phonological Dyslexia: Past and Future Issues. Cognitive Neuropsychology. 1996;13(6):749–762. [Google Scholar]
- Crisp J, Lambon Ralph Ma. Unlocking the nature of the phonological-deep dyslexia continuum: the keys to reading aloud are in phonology and semantics. Journal of Cognitive Neuroscience. 2006;18(3):348–362. doi: 10.1162/089892906775990543. [DOI] [PubMed] [Google Scholar]
- Dell GS, O’Seaghdha PG. Stages of lexical access in language production. Cognition. 1992;42:287–314. doi: 10.1016/0010-0277(92)90046-k. [DOI] [PubMed] [Google Scholar]
- Dell GS, Schwartz MF, Martin N, Saffran EM, Gagnon DA. Lexical access in aphasic and nonaphasic speakers. Psychological Review. 1997;104:801–838. doi: 10.1037/0033-295x.104.4.801. [DOI] [PubMed] [Google Scholar]
- Duffy JR, Strand EA, Clark H, Machulda M, Whitwell JL, Josephs KA. Primary Progressive Apraxia of Speech: Clinical Features and Acoustic and Neurologic Correlates. American Journal of Speech-Language Pathology. 2015:1–13. doi: 10.1044/2015_AJSLP-14-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelter ST, Gostynski M, Papa S, Frei M, Born C, Ajdacic-Gross V, Lyrer PA. Epidemiology of Aphasia Attributable to First Ischemic Stroke: Incidence, Severity, Fluency, Etiology, and Thrombolysis. Stroke. 2006;37(6):1379–1384. doi: 10.1161/01.STR.0000221815.64093.8c. [DOI] [PubMed] [Google Scholar]
- Feinberg T, Rothi L, Heilman K. “Inner Speech” in Conduction Aphasia. Archives of Neurology. 1986;43:591–593. doi: 10.1001/archneur.1986.00520060053017. Retrieved from http://archneur.ama-assn.org/cgi/reprint/43/6/591.pdf. [DOI] [PubMed] [Google Scholar]
- Friedman RB. Two Types of Phonological Alexia. Cortex. 1995;31(2):397–403. doi: 10.1016/s0010-9452(13)80372-3. [DOI] [PubMed] [Google Scholar]
- Gauvin HS, De Baene W, Brass M, Hartsuiker RJ. Conflict monitoring in speech processing: An fMRI study of error detection in speech production and perception. NeuroImage. 2015 doi: 10.1016/j.neuroimage.2015.11.037. [DOI] [PubMed] [Google Scholar]
- Geva S, Bennett S, Warburton Ea, Patterson K. Discrepancy between inner and overt speech: Implications for post-stroke aphasia and normal language processing. Aphasiology. 2011;25(3):323–343. [Google Scholar]
- Geva S, Jones PS, Crinion JT, Price CJ, Baron J-C, Warburton Ea. The neural correlates of inner speech defined by voxel-based lesion-symptom mapping. Brain : A Journal of Neurology. 2011;134(Pt 10):3071–3082. doi: 10.1093/brain/awr232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh SS, Tourville JA, Guenther FH. A Neuroimaging Study of Premotor Lateralization and Syllables. Journal of Speech Language Hearing Research. 2008;51(5):1183–1202. doi: 10.1044/1092-4388(2008/07-0119). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldrick M, Rapp B. Lexical and post-lexical phonological representations in spoken production. Cognition. 2007;102(2):219–260. doi: 10.1016/j.cognition.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E, Weintraub S, Ackerman N. The “tip-of-the-tongue” phenomenon in aphasia. Cortex. 1976;12:145–153. doi: 10.1016/s0010-9452(76)80018-4. Retrieved from http://www.sciencedirect.com/science/article/pii/S0010945276800184. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Wingfield A. In: Anomia: Neuroanatomical and Cognitive Correlates. Goodglass H, Wingfield A, editors. San Diego: Academic Press; 1997. [Google Scholar]
- Hanley JR. Retrieval Failures for the Names of Familiar People. In: Schwartz BL, Brown AS, editors. Tip-of-the-Tongue States and Related Phenomena. New York: Cambridge University Press; 2014. pp. 50–74. [Google Scholar]
- Harley TA, MacAndrew SB. Why the Journey to a Word Takes You No Closer. In: Schwartz BL, Brown AS, editors. Tip-of-the-Tongue States and Related Phenomena. New York: Cambridge University Press; 2014. pp. 95–115. [Google Scholar]
- Hart JT. Memory and the feeling-of-knowing experience. Journal of Education Psychology. 1965;56(4):208–216. doi: 10.1037/h0022263. [DOI] [PubMed] [Google Scholar]
- Hayward W, Snider SF, Luta G, Friedman RB, Turkeltaub PE. Objective support for subjective reports of successful inner speech in two people with aphasia. Cognitive Neuropsychology. 2016 Jul;3294:1–16. doi: 10.1080/02643294.2016.1192998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbet G, Lafargue G, Duffau H. Rethinking voxel-wise lesion-deficit analysis: a new challenge for computational neuropsychology. Cortex. 2015;64:417–419. doi: 10.1016/j.cortex.2014.10.021. [DOI] [PubMed] [Google Scholar]
- Hickok G. The functional neuroanatomy of language. Handbook of Clinical Neurophysiology. 2009;10(3):61–70. [Google Scholar]
- Howard D, Patterson K. The Pyramids and Palm Trees Test. Pearson. 1992 [Google Scholar]
- Indefrey P. The spatial and temporal signatures of word production components: a critical update. Frontiers in Psychology. 2011 Oct;2:255. doi: 10.3389/fpsyg.2011.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJM. The spatial and temporal signatures of word production components. Cognition. 2004;92(1–2):101–44. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- James L, Burke D. Phonological Priming Effects on Word Retrieval and Tip-of-the- Tongue Experiences in Young and Older Adults. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26(6):1378–1391. doi: 10.1037//0278-7393.26.6.1378. [DOI] [PubMed] [Google Scholar]
- Jersakova R, Souchay C, Allen RJ. Negative affect does not impact semantic retrieval failure monitoring. Canadian Journal of Experimental Psychology. 2015 doi: 10.1037/cep0000065. In Press. [DOI] [PubMed] [Google Scholar]
- Kertesz A. Western Aphasia Battery - Revised. San Antonio, TX: Pearson; 2006. [Google Scholar]
- Kleinman JT, Newhart M, Davis C, Heidler-Gary J, Gottesman RF, Hillis AE. Right Hemispatial Neglect: Frequency and Characterization Following Acute Left Hemisphere Stroke. Brain and Cognition. 2007;64(1):50–59. doi: 10.1016/j.bandc.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn SE, Goodglass H. Picture Naming in Aphasia. Brain and Language. 1985;24:266–283. doi: 10.1016/0093-934x(85)90135-x. Retrieved from http://www.sciencedirect.com/science/article/pii/0093934X8590135X. [DOI] [PubMed] [Google Scholar]
- Laine M, Martin N. Anomia: Theoretical and Clinical Aspects. New York: Psychology Press; 2006. [Google Scholar]
- Levelt WJM. Spoken word production: A theory of lexical access. PNAS. 2001;98:13464–13471. doi: 10.1073/pnas.231459498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt WJ, Roelofs A, Meyer AS. A theory of lexical access in speech production. The Behavioral and Brain Sciences. 1999;22:1–38. doi: 10.1017/s0140525x99001776. discussion 38–75. [DOI] [PubMed] [Google Scholar]
- Mah Y-H, Husain M, Rees G, Nachev P. Human brain lesion-deficit inference remapped. Brain : A Journal of Neurology, Advanced A. 2014:1–10. doi: 10.1093/brain/awu164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher L, Raymer A. Management of Anomia. Topics in Stroke Rehabilitation. 2004;11(1):10–21. doi: 10.1310/318R-RMD5-055J-PQ40. [DOI] [PubMed] [Google Scholar]
- Maril A, Simons JS, Weaver JJ, Schacter DL. Graded recall success: an event-related fMRI comparison of tip of the tongue and feeling of knowing. NeuroImage. 2005;24(4):1130–1138. doi: 10.1016/j.neuroimage.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Marshall RC, Neuburger SI, Phillips DS. Verbal self-correction and improvement in treated aphasic clients. Aphasiology. 1994;8(6):535–547. [Google Scholar]
- Martin N, Dell GS. Common mechanisms underlying perseverative and non-perseverative sound and word substitutions. Aphasiology. 2007;21(10–11):1002–1017. [Google Scholar]
- Martin N, Schwartz M, Kohen FP. Assessment of the ability to process semantic and phonological aspects of words in aphasia: A multi-measurement approach. Aphasiology. 2006;20(2–4):154–166. [Google Scholar]
- MATLAB and Statistics Toolbox. Natick, Massachusetts, United States: The MathWorks, Inc.; 2012. [Google Scholar]
- Mechelli A, Josephs O, Lambon Ralph MA, McClelland JL, Price CJ. Dissociating stimulus-driven semantic and phonological effect during reading and naming. Human Brain Mapping. 2007;28(3):205–217. doi: 10.1002/hbm.20272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AS, Bock K. The tip-of-the-tongue phenomenon: blocking or partial activation? Memory & Cognition. 1992;20(6):715–726. doi: 10.3758/bf03202721. [DOI] [PubMed] [Google Scholar]
- Miceli G, Amitrano A, Capasso R, Caramazza A. The Treatment of Anomia Resulting from Output Lexical Damage : Analysis of Two Cases. Brain and Language. 1996;52:150–174. doi: 10.1006/brln.1996.0008. [DOI] [PubMed] [Google Scholar]
- Nickels L, Howard D. Phonological errors in aphasic naming: comprehension, monitoring and lexicality. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 1995;31(2):209–237. doi: 10.1016/s0010-9452(13)80360-7. [DOI] [PubMed] [Google Scholar]
- Nozari N, Dell GS, Schwartz MF. Is comprehension necessary for error detection? A conflict-based account of monitoring in speech production. Cognitive Psychology. 2011;63(1):1–33. doi: 10.1016/j.cogpsych.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oomen CC, Postma A, Kolk HH. Prearticulatory and Postarticulatory Self- Monitoring in Broca’s Aphasia. Cortex. 2001;37(5):627–641. doi: 10.1016/s0010-9452(08)70610-5. [DOI] [PubMed] [Google Scholar]
- Patterson K, Suzuki T, Wydell TN. Interpreting a case of Japanese phonological alexia: The key is in phonology. Cognitive Neuropsychology. 1996;13(6):803–822. [Google Scholar]
- Price CJ. A review and synthesis of the first 20years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage. 2012;62(2):816–847. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach A, Schwartz MF, Martin N, Grewal RS, Brecher A. The Philadelphia Naming Test: Scoring and Rationale. Clinical Aphasiology. 1996;24:121–133. [Google Scholar]
- Schwartz MF, Faseyitan O, Kim J, Coslett HB. The dorsal stream contribution to phonological retrieval in object naming. Brain : A Journal of Neurology. 2012;135(Pt 12):3799–814. doi: 10.1093/brain/aws300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafto MA, Burke DM, Stamatakis EA, Tam PP, Tyler LK. On the tip-of-the- tongue: neural correlates of increased word-finding failures in normal aging. Journal of Cognitive Neuroscience. 2007;19(12):2060–2070. doi: 10.1162/jocn.2007.19.12.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafto MA, Stamatakis EA, Tam PP, Tyler LK. Word Retrieval Failures in Old Age : The Relationship between Structure and Function. 2009:1530–1540. doi: 10.1162/jocn.2009.21321. [DOI] [PubMed] [Google Scholar]
- Strand Ea, Duffy JR, Clark HM, Josephs K. The apraxia of speech rating scale: A tool for diagnosis and description of apraxia of speech. Journal of Communication Disorders. 2014;51:43–50. doi: 10.1016/j.jcomdis.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Poeppel D. The Effect of Imagination on Stimulation: The Functional Specificity of Efference Copies in Speech Processing. Journal of Cognitive Neuroscience. 2013;25(7):1020–1036. doi: 10.1162/jocn_a_00381. [DOI] [PubMed] [Google Scholar]
- Tompkins CA, Scharp VL, Marshall RC. Communicative value of self cues in aphasia: A re-evaluation. Aphasiology. 2006;20(7):684–704. doi: 10.1080/02687030500334076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiani V, Fernández-Seara Ma, Wang Z, Detre Ja, Ash S, Grossman M. Narrative speech production: An fMRI study using continuous arterial spin labeling. NeuroImage. 2008;40:932–939. doi: 10.1016/j.neuroimage.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker GM, Hickok G. Bridging computational approaches to speech production: The semantic–lexical–auditory–motor model (SLAM) Psychonomic Bulletin & Review. 2015 doi: 10.3758/s13423-015-0903-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside SP, Dyson L, Cowell PE, Varley Ra. The Relationship Between Apraxia of Speech and Oral Apraxia: Association or Dissociation? Archives of Clinical Neuropsychology. 2015:acv051. doi: 10.1093/arclin/acv051. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kimberg DY, Coslett HB, Schwartz MF, Wang Z. Multivariate lesion-symptom mapping using support vector regression. Human Brain Mapping. 2014;35(12):5861–5876. doi: 10.1002/hbm.22590. [DOI] [PMC free article] [PubMed] [Google Scholar]