Abstract
Cross-sectional structural magnetic resonance (MR) imaging studies of individuals with an alcohol use disorder (AUD) report that those who relapse after treatment, relative to individuals who maintain a period of extended abstinence, show greater morphological abnormalities in multiple brain regions near the inception of treatment, particularly in the frontal lobe. However, given the cross-sectional design of previous studies, it is unclear if the baseline morphological differences between future abstainers and relapsers were maintained over the course of early recovery. The primary goal of this study was to determine if frontal lobe tissue volume recovery during early abstinence is associated with long-term abstinence from alcohol. We compared frontal, parietal, temporal and occipital grey matter (GM) and white matter (WM) volumes, at 1 and 4 weeks of abstinence, among individuals who resumed alcohol consumption within 12 months of treatment (Relapsers) and those who showed sustained abstinence over 12 months following treatment (Abstainers). At 1 and 4 weeks of sobriety, both Abstainers and Relapsers demonstrated significantly smaller GM volumes than Controls in the majority of ROIs, but Relapsers exhibited significantly smaller bilateral frontal GM volumes than Abstainers. No significant group differences were observed for any WM region of interest. The persistent bilateral frontal GM volume deficits in Relapsers over 4 weeks from last alcohol use may represent an endophenotype that differentiates those who respond more favorably to the typical psychosocial and pharmacological interventions provided for AUD.
Keywords: alcohol use disorder, brain volume, relapse, magnetic resonance imaging, neurocognitive
INTRODUCTION
Approximately 60% of individuals with an alcohol use disorder (AUD) will relapse to hazardous alcohol consumption within 6 months of treatment (Witkiewitz, 2011). Magnetic resonance (MR) imaging studies of AUD have demonstrated that those who relapse after treatment, relative to individuals who maintain a period of extended abstinence, show greater morphological abnormalities in multiple brain regions early in sobriety (Beck et al., 2012; Cardenas et al., 2011; Durazzo et al., 2008; Durazzo et al., 2011; Rando et al., 2011; Wrase et al., 2008). Collectively, these studies suggest that individuals who relapsed after treatment for AUD exhibited greater regional morphological abnormalities (e.g., smaller volumes, thinner cortices) during the first month of abstinence, particularly in anterior frontal cortical regions that are implicated in the development and maintenance of AUD (Seo and Sinha, 2015). The corresponding neuroimaging research indicates differences between future relapsers and abstainers in regional brain morphology near the inception of treatment; however, all studies have been cross-sectional in nature. Therefore, it is unclear if the baseline morphological differences between future abstainers and relapsers were maintained over the course of early recovery.
In a smaller subset of participants from our AUD cohort, we previously observed that at both 1 and 5 weeks of abstinence, future relapsers demonstrated significantly lower frontal grey matter (GM) blood flow than future abstainers (Durazzo et al., 2010a); while perfusion increased between time points in both groups, the rate of change in perfusion did not differ significantly between future relapsers and abstainers. Therefore, relapsers continued to exhibit significantly lower frontal GM perfusion than abstainers at 5 weeks of abstinence. Evaluation of potential differences in regional morphological recovery between future abstainers and relapsers during short-term abstinence is clinically salient because MR studies indicate the rate of volume recovery – a macroscopic measure of neuroplasticity - in those with AUD is greatest during the first 1 to 3 months of abstinence, particularly in the frontal lobe (Buhler and Mann, 2011; Durazzo et al., 2015). Disparities in recovery of frontal lobe volume may influence individual response to treatment, such as cognitive remediation and neurostimulation techniques that can be used to target frontal-subcortical circuits during the early phase of treatment (Gorelick et al., 2014; Rupp et al., 2012; Wexler, 2011).
The primary goal of this study was to determine if frontal lobe tissue volume recovery during early abstinence was associated long-term abstinence from alcohol. We compared frontal, parietal, temporal and occipital GM and white matter (WM) volumes in alcohol dependent participants who resumed alcohol consumption within 12 months of treatment (Relapsers) to those who showed sustained abstinence over 12 months after treatment (Abstainers). We specifically predicted: 1) Compared to light-drinking controls and Abstainers, Relapsers demonstrate smaller frontal GM and WM volumes at approximately 1 week (assessment point 1; AP1) and 4 weeks (assessment point 2; AP2) of abstinence; 2) Relapsers show less recovery than Abstainers in frontal GM and WM volumes over approximately 4 weeks of sustained abstinence; 3) In Relapsers, less frontal GM and WM volume recovery over 4 weeks is related to a shorter duration of abstinence following treatment. In exploratory analyses, we examined group-specific associations between changes in regional brain volumes and neurocognition.
MATERIALS AND METHODS
Participants
Alcohol dependent individuals (n = 123) were recruited from the San Francisco VA Medical Center (SFVAMC) Substance Abuse Day Hospital and the San Francisco Kaiser Permanente Chemical Dependence Recovery outpatient treatment clinics. All alcohol dependent individuals were actively in treatment at the time of assessment, and treatment duration ranged from 14–35 days. Thirty-three never-smoking, light-drinking controls (Controls) were recruited from the local community and screened for biomedical or psychiatric conditions known or suspected to influence brain neurobiology. Participants were between 28 and 71 years of age and alcohol dependent participants met DSM-IV criteria for alcohol dependence. All participants provided their written informed consent prior to study. Study procedures were approved by the University of California San Francisco and the SFVAMC and were in accordance with the ethical standards of the Declaration of Helsinki.
Inclusion/exclusion criteria
Primary inclusion criteria for the alcohol dependent participants were fluency in English, DSM-IV diagnosis of alcohol dependence/abuse at baseline (all met criteria for alcohol dependence), consumption of greater than 150 standard alcohol-containing drinks (i.e., 13.6 grams of pure ethanol) per month for at least 8 years prior to enrollment for males, and greater than 80 drinks per month for at least 6 years prior to enrollment for females. See Table 1 for group demographic data. Exclusion criteria for alcohol dependent participants were any history of the following: dependence on any substance other than alcohol or nicotine in the 5 years immediately prior to enrollment, any intravenous drug use in the 5 years prior to baseline study, opioid agonist therapy, intrinsic cerebral masses, HIV/AIDS, cerebrovascular accident, cerebral aneurysm, arteriovenous malformations, myocardial infarction, medically uncontrolled chronic hypertension (systolic > 180 and/or diastolic > 120 mmHg), type-I diabetes, chronic obstructive pulmonary disease, non-alcohol related seizures, significant exposure to established neurotoxins, demyelinating and neurodegenerative diseases, Wernicke-Korsakoff syndrome, delirium, penetrating head injury, and closed head injury resulting in loss of consciousness > 10 minutes. Psychiatric exclusion criteria were history of schizophrenia-spectrum disorders, bipolar disorder, cyclothymia, PTSD, obsessive-compulsive disorder and panic disorder. Hepatitis C, type-2 diabetes, hypertension, unipolar mood disorders (i.e., major depression, substance-induced mood disorder) were allowed in the alcohol dependent group, given their high prevalence in those with an AUD (Grant et al., 2015; Mertens et al., 2005). Participants were breathalyzed and urine-tested for illicit substances before all assessments and no participant tested positive for substances at any assessment.
Table 1.
Group Demographics, Alcohol and Cigarette Use Histories, Self-Report Questionnaires and Comorbidity Frequency
| Measure | CON (n = 33) | ABS (n = 42) | REL (n = 72) | Group comparisons* |
|---|---|---|---|---|
| Age (years) | 46.1 (9.4) | 52.0 (12.2) | 50.1 (8.2) | CON < ABS, REL |
| Education (years) | 16.7 (2.5) | 14.6 (2.2) | 13.4 (1.8) | CON > ABS, REL; ABS > REL |
| Caucasian (%) | 73 | 80 | 71 | |
| Duration of Abstinence at AP1 | NA | 7 (4) | 7 (3) | |
| Duration of Abstinence at AP2 | NA | 33 (8) | 33 (10) | |
| Days until relapse | NA | NA | 141 (79) | |
| 1-yr average drinks/month | 16 (17) | 352 (178) | 431 (249) | CON < ABS, REL |
| Lifetime average drinks/month | 16 (14) | 181 (107) | 246 (192) | CON < ABS, REL; ABS < REL |
| Medical comorbidity (%) | NA | 45 | 53 | |
| Substance use disorder | NA | 24 | 22 | |
| Comorbidity (%) | NA | 21 | 18 | |
| Psychiatric comorbidity (%) | NA | 24 | 49 | ABS < REL |
| Smokers (%) | NA | 52 | 62 | |
| FTND | NA | 5 (2) | 5 (2) | |
| Pack years | NA | 27 (17) | 27 (21) | |
| Smoking duration (years) | NA | 27 (12) | 26 (12) | |
| BDI | 4 (3) | 9 (8) | 10 (8) | CON < ABS, REL |
| STAI | 33 (7) | 46 (10) | 50 (13) | CON < ABS, REL |
| Body mass index | 25 (5) | 26 (5) | 27 (5) | |
| Intracranial volume (cc) | 1509 (136) | 1516 (132) | 1497 (127) |
Note. ABS: Abstainers. AP1: assessment point 1. AP2: assessment point 2.BDI: Beck Depression Inventory. CON: Controls. FTND: Fagerstrom Tolerance Test for Nicotine Dependence. NA: not applicable. REL: Relapsers. STAI: State -trait Anxiety Inventory – Trait.
All listed group comparisons p < .05. Mean (SD).
Definition of Abstainers and Relapsers
Participants were followed for 12 months following AP2. Initial follow-ups (in person, telephone and/or collateral source interview, and/or medical record) took place approximately 5–7 months after AP2. Seventy-six of the 123 alcohol dependent participants originally studied at AP2 were revaluated approximately 7 months later with all MR scans, psychiatric and behavioral measures administered at AP1 as well as the Time Line Follow-Back (Sobell et al., 1985; Sobell et al., 1988) to specifically assess alcohol consumption patterns during relapse. For the remaining 47 participants, follow-up assessment involved brief in-person and/or telephone contact (n = 26), review of available medical records (n = 18), and/or telephone interview of collateral sources (i.e., family or friends; n = 3), where only relapse status (i.e., any alcohol consumption) or date of relapse onset was obtained. Abstainers: participants were designated as Abstainers if they met all the following criteria: a) self-reported no alcohol consumption between AP1/AP2 and long-term follow-u; b) there was no report of alcohol consumption between the baseline and follow-up in available medical records; and c) available laboratory indicators of alcohol consumption (e.g., gamma glutamyltransferase; GGT) were within normal limits at follow-up. All Abstainers were contacted (in-person or via telephone) again approximately 5–6 months after the initial follow-up determine if they maintained continuous sobriety over the 12 month observation period. Relapsers: participants were designated as Relapsers if they met any of the following criteria: a) self-reported of any alcohol consumption after AP1 or AP2 within 12 months via in-person or telephone interview; b) alcohol consumption or relapse was indicated in medical records; c) report of participant alcohol use by a relative or close friend via telephone contact. In our previous studies, any level of alcohol consumption following treatment was associated with poorer psychosocial functioning (Durazzo et al., 2008).
Eighty-two alcohol dependent participants (27 Abstainers; 55 Relapsers) completed a magnetic resonance imaging (MRI) study at 7 ± 4 days after consumption of their last alcohol drink (AP1). One hundred fourteen alcohol dependent participants (42 Abstainers; 72 Relapsers) completed an MRI after approximately 33 ± 9 days of abstinence (AP2). Seventy-three (73) of the 114 participants were studied at both AP1 and AP2, and 41 participants were enrolled and studied first at AP2, because they completed acute detoxification at another SFVAMC or Kaiser Permanente sponsored program, precluding their participation at AP1. Nine individuals relapsed or were lost to follow-up between AP1 and AP2 (n = 123 total participants). Control participants completed a single MR study and clinical assessment.
Clinical Measures
After study enrollment, participants completed the Clinical Interview for DSM-IV Axis I Disorders, Version 2.0 (SCID-I/P) and semi-structured interviews for lifetime alcohol consumption (Lifetime Drinking History) and substance use (in-house questionnaire assessing substance type, and quantity and frequency of use). From the Lifetime Drinking History, average number of alcoholic drinks/month over 1 year prior to enrollment, and average number of drinks/month over lifetime were calculated. At AP1 and AP2, participants also completed standardized questionnaires assessing depressive (Beck Depression Inventory, BDI) and anxiety symptomatology (State-Trait Anxiety Inventory, Trait form Y-2, STAI), and nicotine dependence via the Fagerstrom Tolerance Test for Nicotine Dependence (FTND). See (Pennington et al., 2013) for corresponding references for the above measures.
Neurocognitive Measures
At AP1 and AP2, participants completed a battery of measures that assessed working memory, processing speed, and auditory-verbal and visuospatial learning and memory, typically within 48 hours of their MR scan. Alternate forms were used, where available, at AP2. The following measures were administered: Wechsler Adult Intelligence Scale 3rded. (WAIS-III) - Digit Span (working memory), Symbol Search, and Digit Symbol (processing speed); California Verbal Learning Test-II (CVLT-II) - Immediate Recall trials 1–5 (auditory-verbal learning), Short-and-Long Delay Free Recall (auditory-verbal memory); Brief Visual Memory Test (BVMT) Revised-Total Recall (visuospatial learning) and Delayed Recall (visuospatial memory). See (Pennington et al., 2013) for corresponding references for the above measures.
MR Data Acquisition and Processing
Structural images were acquired on a 1.5 Tesla MR system (Vision, Siemens Medical Systems, Iselin NJ) with a T1-weighted Magnetization Prepared Rapid Acquisition Gradient Echo sequence (1 × 1 mm2 in-plane resolution, 1.5 mm contiguous slabs) oriented orthogonal to the long axis of the hippocampus [see (Mon et al., 2013) for specific acquisition parameters]. Tissue intensity-based segmentation of cortical and subcortical GM, WM, and cerebrospinal fluid (CSF) from T1-weighted images was conducted with the semi-automated Expectation-Maximization Segmentation method (Van Leemput et al., 1999). This technique employed a probabilistic segmentation of GM, WM, and CSF to each MRI voxel based on T1-weighted tissue intensity [see (Mon et al., 2013) for method details]. Volumes (cubic centimeters; cc) for bilateral GM and WM of the four major lobar (frontal, parietal, temporal, occipital) regions of interest (ROI) were obtained by non-linear co-registration of tissue maps to a reference atlas [see (Studholme et al., 2001; Studholme et al., 2003) for method details and reliability].
Data Analyses
Demographic and clinical data
Comparisons of demographic and clinical data between Controls, Abstainers and Relapsers were conducted with univariate analysis of variance and Fishers Exact Test, where indicated. There were no significant differences in demographic or clinical variables between the alcohol dependent cohorts at AP1 and AP2, with the exception of lower BDI and STAI scores at AP2; therefore, values for the larger AP2 sample are reported in all tables.
Cross-sectional regional brain volumetric comparisons between Controls, Abstainers and Relapsers
Group comparisons on regional GM and WM volumes at AP1 and AP2 were conducted with generalized linear modeling. Controls were younger and showed more years of education than Abstainers and Relapsers (see Table 1). Therefore, these variables as well as intracranial volume (ICV) were used as covariates in comparisons between Controls and the alcohol dependent groups. Average number of drinks/month over lifetime, years of education and frequency of psychiatric comorbidities (primarily unipolar mood disorders) were significantly higher in Relapsers than Abstainers (see Table 1); these variables and ICV were used as covariates in all cross-sectional comparisons of Abstainers and Relapsers. Significant main effects for group (i.e., Controls, Abstainers, Relapsers) (p < .05) for each ROI were followed-up with pairwise t-tests. Effect sizes (ES) for pairwise t-tests of group means were calculated with Cohen’s d (Cohen, 1977). Although we made a priori predictions for frontal GM and WM differences between Abstainers and Relapsers, statistical significance levels (p < .05) for all pairwise t-tests were corrected for multiple comparisons according to the number of ROIs (16; GM and WM for left and right frontal, parietal, temporal and occipital lobes) and the average intercorrelations among ROIs for all groups combined (r = 0.73). This modified Bonferroni method (Sankoh et al., 1997) yielded an adjusted significance level of p < .024 for pairwise t-tests. The same regional volume values for Controls were used for AP1 and AP2 comparisons with Abstainers and Relapsers; this is justified as we previously observed no significant regional volumes changes in this Control cohort over approximately 6 months (Durazzo et al., 2015).
Longitudinal regional brain volumetric comparisons between Abstainers and Relapsers
Longitudinal regional volume changes in Abstainers and Relapsers over approximately 1 month of abstinence was assessed with linear mixed modeling. In this analysis, the focus was on the group (Abstainers vs. Relapsers) x days abstinent interaction and the main effect for group. Average number of drinks/month over lifetime, years of education, frequency of psychiatric comorbidities, and ICV were employed as covariates in these analyses. Main and simple effects and interactions for all ROIs were considered statistically significant at p < .024, as applied to cross-sectional analyses.
Associations between longitudinal changes in regional brain volumes and neurocognitive measures in Abstainers and Relapsers
Associations between change in both regional brain volumes and neurocognitive measures over the AP1-AP2 interval were separately evaluated for Abstainers and Relapsers via linear mixed modeling. The neurocognitive measure (e.g., Digit Span score) served as the dependent measure, with age, education, days abstinent, smoking status, lifetime average drinks/month, and ICV-corrected regional brain volume (e.g., frontal GM) as predictors. False discovery rate (FDR) (Benjamini and Hochberg, 1995) was used to control for multiplicity of associations for each ROI and p < .05 was considered statistically significant after FDR adjustment.
Associations between regional brain volume changes and duration of post-treatment abstinence in Relapsers
Associations between change in regional brain volumes and duration of abstinence after treatment in Relapsers were assessed for Relapsers via linear mixed modeling. The duration of post-treatment abstinence served as the dependent measure, with age, education, smoking status, lifetime average drinks/month, and ICV-corrected regional brain volume (e.g., frontal GM) as predictors. These exploratory associations were considered statistically significant at p < .05.
RESULTS
Group demographics and clinical measures (Table 1)
Controls were younger, had more education, showed lower alcohol consumption levels, and demonstrated lower BDI and STAI scores than both Abstainers and Relapsers (all p < .05). Abstainers and Relapsers were equivalent on age, BDI and STAI scores, duration of abstinence at AP1 and AP2, and frequency of smokers, comorbid medical conditions and history of substance use disorders. Abstainers had a higher level of education, lower lifetime alcohol consumption and a lower frequency of psychiatric comorbidities than Relapsers (all p < .05). All groups were equivalent on ICV and the percent of Caucasian participants.
Volume comparisons of Controls, Abstainers and Relapsers at AP1 (1 week of abstinence)
Frontal lobe
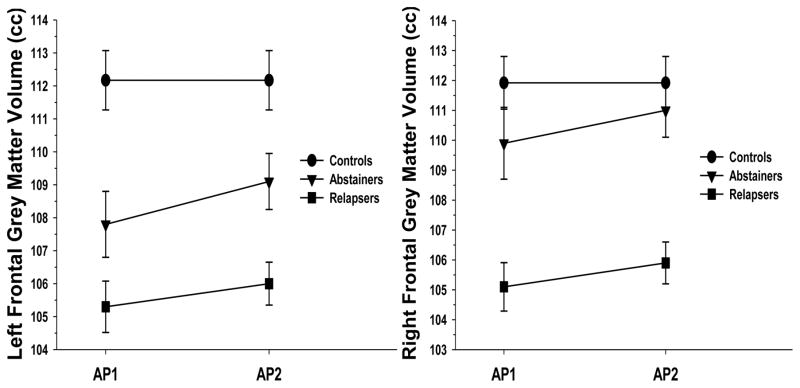
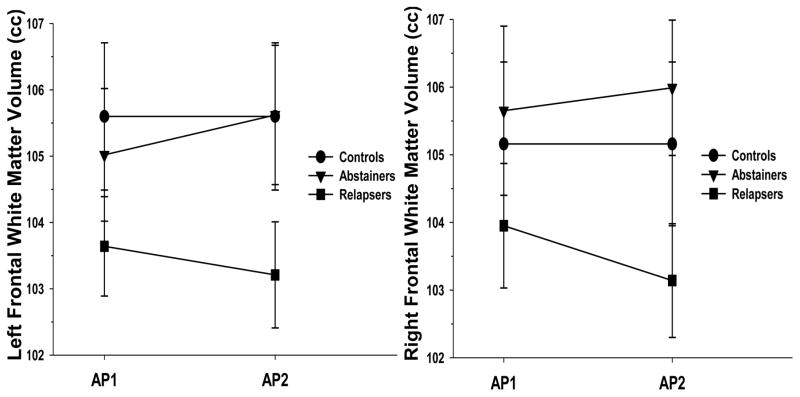
GM: Group differences were observed for left [(χ2(2) = 31.9, p < .001] and right [(χ2(2) = 36.5, p < .001] frontal GM volume. Follow-up t-tests indicated that for left frontal GM, both Abstainers and Relapsers demonstrated smaller volumes than Controls, and Relapsers had smaller volume than Abstainers (all p < .024). For the right frontal GM, Relapsers showed smaller volume than Abstainers and Controls (p < .024); Abstainers and Controls were not significantly different on right frontal GM volume (p = .23). WM: No group differences were observed for left [(χ2(2) = 1.8, p = .41] and right [(χ2(2) = 3.4, p = .18] frontal WM volume.
Parietal lobe
GM: Group differences were seen for left [(χ2(2) = 18.0, p < .001] and right [(χ2(2) = 21.5, p < .001] parietal GM volume. For left parietal GM, Abstainers and Relapsers showed smaller volumes than Controls (p < .024); no significant differences were found between Abstainers and Relapsers (p = .098). For right parietal GM, Abstainers and Relapsers demonstrated smaller volumes than Controls (p < .024), but there were no differences between Abstainers and Relapsers (p = .29). WM: Groups were not different on left [(χ2(2) = 4.5, p = .11] and right [(χ2(2) = 1.9, p = .38] parietal WM volume.
Temporal lobe
GM: Group differences were observed for left [(χ2(2) = 13.5, p = .001] and right [(χ2(2) = 21.2, p < .001] temporal GM volume. For left temporal GM, Relapsers showed smaller volumes than Controls (p < .024), but no significant differences were apparent between Abstainers and Controls (p = .078) or among Abstainers and Relapsers (p = .19). For right temporal GM, Abstainers and Relapsers demonstrated smaller volumes than Controls (p < .024), but there were no differences between Abstainers and Relapsers (p = .73). WM: No group differences were observed for left [(χ2(2) = 2.0, p = .36] and right [(χ2(2) = 5.4, p = .07] temporal WM volume.
Occipital lobe
GM: Significant group differences were found for left occipital GM [(χ2(2) = 9.4, p = .009], while no group differences were seen for right occipital GM [(χ2(2) = 0.44, p = .80]. For left occipital GM, Abstainers and Relapsers showed smaller volumes than Controls (p < .024), but no significant differences were apparent between Abstainers and Relapsers (p = .96). WM: No group differences were observed for left [(χ2(2) = 2.6, p = .27] and right [(χ2(2) = 3.0, p = .23] occipital WM volume (see Tables S1 and S2)
Comparisons of volume changes for Abstainers and Relapsers over AP1 and AP2
Frontal lobe
GM: A main effect for group was seen for left [F(1, 125) = 5.5, p = .021] and right [F(1, 124) = 14.1, p < .001] frontal GM, where Abstainers showed significantly larger left and right frontal GM volumes across approximately 4 weeks of sobriety (see Figure 1). Simple effect tests indicated that both Abstainers and Relapsers showed significant increases in left and right frontal GM volume over 4 weeks (both p < .024). No group x days abstinent interaction was observed (p = .26), indicating the rate of change was statistically equivalent for Abstainers and Relapsers. WM: No group main effect or group x days abstinent interaction were observed for left frontal WM (both p > .24). A trend for a group x days abstinent interaction was seen for right frontal WM [F(1, 68) = 3.1, p = .085]; simple effect tests indicated Abstainers exhibited no change, while Relapsers showed a slight, but statistically significant decrease in right frontal WM volume over 4 weeks (p = .024; see Figure 2).
Figure 1.
Changes in Left and Right Frontal Grey Matter Volumes for Controls, Abstainers and Relapsers across Assessment Points 1 (approximately 1 week of sobriety) and 2 (approximately 4 weeks of sobriety).
Figure 2.
Changes in Left and Right Frontal White Matter Volumes for Controls, Abstainers and Relapsers across Assessment Points 1 (approximately 1 week of sobriety) and 2 (approximately 4 weeks of sobriety).
Parietal, temporal, and occipital lobes
No main effects for group or group x days abstinent interactions were observed for parietal, temporal, and occipital GM and WM volumes after correction for multiple comparisons. A main effect for days abstinent was observed for left [F(1, 67) = 6.5, p = .013] and right [F(1, 66) = 12.2, p = .001] parietal GM, which indicated both Abstainer and Relapsers demonstrated and equivalent level of parietal GM volume increase over the AP1-AP2 interval.
Volume comparisons of Controls, Abstainers and Relapsers at AP2 (4 weeks of abstinence)
Frontal lobe
GM: Significant group differences were observed for left [(χ2(2) = 27.0, p < .001] and right [(χ2(2) = 34.6, p < .001] frontal GM volumes. For left frontal GM, both Abstainers and Relapsers demonstrated smaller volumes than Controls, and Relapsers had smaller volume than Abstainers (all p < .024). For the right frontal GM, relapsers showed smaller volume than Abstainers and Controls (p < .024); Abstainers and Controls were not significantly different on right frontal GM volume (p = .37). WM: No group differences were observed for left [(χ2(2) = 0.6, p = .73] and right [(χ2(2) = 2.8, p = .24] frontal WM volumes.
Parietal lobe
GM: Group differences were observed for left [(χ2(2) = 9.6, p = .003] and right [(χ2(2) = 14.3, p = .001] parietal GM volumes. For left parietal GM, Abstainers and Relapsers had smaller volumes than Controls (p < .024); no significant differences were found between Abstainers and Relapsers (p = .099). For right parietal GM, Abstainers and Relapsers exhibited smaller volumes than Controls (p < .024), but there were no differences between Abstainers and Relapsers (p = .99). WM: Groups were not different on left [(χ2(2) = 0.3, p = .87] and right [(χ2(2) = 0.2, p = .92] parietal WM volumes.
Temporal lobe
GM: Group differences were apparent for left [(χ2(2) = 10.1, p = .007] and right [(χ2(2) = 15.4, p < .001] temporal GM volumes. For left temporal GM, Relapsers showed significantly smaller volumes than Controls (p < .024), but no differences were observed between Abstainers and Controls (p = .078). Abstainers and Relapsers showed no differences (p = .19). For right temporal GM, Abstainers and Relapsers had smaller volumes than Controls (p < .024), but no differences were found between Abstainers and Relapsers (p = .48). WM: No group differences were found for left [(χ2(2) = 0.6, p = .75] and right [(χ2(2) = 4.3, p = .12] temporal WM volumes.
Occipital lobe
GM: Significant group differences were detected for left occipital GM [(χ2(2) = 8.1, p = .017], while no group differences were observed for the corresponding right volume [(χ2(2) = 0.44, p = .80]. For left occipital GM, Abstainers and Relapsers showed significantly smaller volumes than Controls (p < .024), but no differences were seen between Abstainers and Relapsers (p = .95). WM: No group differences were observed for left [(χ2(2) = 2.6, p = .27] and right [(χ2(2) = 1.3, p = .54] occipital WM volumes (see Tables S1 and S2).
Associations between changes regional brain volume and neurocognitive measures over AP1 and AP2 for Abstainers and Relapsers
In Abstainers, increasing in Digit Span scores (measure of aural working memory) were positively related to increasing volumes in left [β = 2.2; F(1, 45) = 5.3, p = .049] and right frontal GM [β = 2.5; F(1, 45) = 6.2, p = .041], left [β = 2.4; F(1, 44) = 6.0, p = .043] and right parietal GM [β = 2.7; F(1, 44) = 7.1, p = .036], left temporal GM [β = 3.1; F(1, 42) = 9.1, p = .029], and right frontal WM [β = 2.4; F(1, 44) = 5.7, p = .045]. In Relapsers, there were no significant associations between changes in neurocognitive measures and regional volumes before FDR correction (all p >.13). Abstainers and Relapsers showed equivalent rates of change and variance measures in both Digit Span (data not shown) and regional GM brain volumes, so the lack of associations between changes in Digit Span and brain volumes in Relapsers was not attributable to a restriction of range in this group.
Associations between regional brain volume changes and duration of post-treatment abstinence in Relapsers
Increasing left frontal GM volume was related to longer duration of abstinence after treatment in relapsers [β = 2.2; F(1, 109) = 4.7, p = .032]. Greater increases in right frontal GM and WM showed trends for associations with longer duration of abstinence after treatment in relapsers (both p = .086). No associations were observed for volume changes in other ROIs and duration of abstinence after treatment in relapsers.
DISCUSSION
The primary findings from this predominately Caucasian, male, Veteran cohort were: 1) At approximately 1 week (AP1) of sobriety, both Abstainers and Relapsers demonstrated significantly smaller GM volumes than Controls in the majority of ROIs, and Relapsers showed the largest magnitude volume deficits compared to Controls. Relapsers exhibited significantly smaller bilateral frontal GM volumes than Abstainers. No significant group differences were observed for any WM ROI. 2) Over approximately 4 weeks of sobriety, both Abstainers and Relapsers showed significant increases in bilateral frontal GM volumes, but Relapsers continued to show lower frontal GM volumes than Abstainers; Relapsers also demonstrated a small, but statistically significant decrease in right frontal WM. 3) At approximately 4 weeks of sobriety (AP2), both Abstainers and Relapsers continued to show significantly smaller GM volumes than Controls in the majority of ROIs, and Relapsers maintained the largest magnitude volume deficits relative to Controls. Consistent with AP1, Relapsers exhibited significantly smaller bilateral frontal GM volumes than Abstainers and no significant group differences were observed for regional WM. 4) Over approximately 4 weeks of alcohol abstinence, improving working memory was related to increasing frontal, parietal and temporal GM and frontal WM volumes in Abstainers; no significant associations of changes in neurocognition with regional volumes were apparent in Relapsers.
The smaller frontal GM volumes in Relapsers compared to Abstainers at both AP1 and AP2 reported here are highly consistent with the pattern previously shown by these groups for regional cerebral blood flow, where Relapsers showed significantly lower frontal GM perfusion at both AP1 and AP2 (Durazzo et al., 2010a). Furthermore, Abstainers and Controls were not significantly different on right frontal GM volume at AP1 or AP2, and, similarly, Abstainers and Controls were equivalent on frontal GM perfusion levels at AP1 and AP2 reported previously. Taken together, these volumetric and perfusion results suggest that Relapsers entered treatment with greater neurobiological abnormalities in their frontal GM than Abstainers, and their deficits were persistent across the 4 weeks while in treatment for AUD. Cross-sectional studies have observed morphological, biochemical and functional connectivity differences between Abstainers and Relapsers in multiple subregions of the frontal lobe (e.g., anterior cingulate cortex, dorsolateral and ventromedial prefrontal cortex, orbitofrontal cortex) implicated in the development and maintenance of AUD (Beck et al., 2012; Cardenas et al., 2011; Durazzo et al., 2010b; Durazzo et al., 2011; Rando et al., 2011). Therefore, assessment of longitudinal changes in these behaviorally relevant frontal subregions is necessary to determine if the Relapsers also continue to exhibit greater abnormalities in functionally important frontal subregions while engaged in the early phase of treatment. Both Abstainers and Relapsers showed significantly smaller GM volumes than Controls at AP1 and AP2 in most parietal and temporal lobes and occipital GM ROIs, and Abstainers and Relapsers were not different in these regions at AP1 and AP2; this is consistent with numerous research studies showing individuals with alcohol use disorders demonstrate widespread GM volume loss during early abstinence (Buhler and Mann, 2011; Durazzo et al., 2015). Contrary to our predictions, Abstainers and Relapsers demonstrated statistically equivalent rates of change in all GM ROIs over 4 weeks. While GM volumes in parietal and temporal lobes were equivalent between Abstainers and Relapsers at AP1 and AP2, and these groups did not show a differential recovery over 4 weeks, it is possible that Relapsers and Abstainers may have exhibited differences in subregions of these lobes (e.g., insula, posterior parietal lobe) that are components of neurocircuitry that manifest neurobiological abnormalities in AUD (Seo and Sinha, 2015). There were no significant regional differences among Controls, Abstainers and Relapsers in WM volume at AP1 or AP2, and Abstainers and Relapsers were statistically equivalent on the rate change of WM volumes in all ROIs except the right frontal WM, where Relapsers showed a significant volume decrease over 4 weeks. However, while statistically significant, the decrease in right frontal WM was 0.67%. Since this decrease in right frontal WM in Relapsers was not associated with neurocognitive changes, the functional significance of this WM volume decrease is unclear and may not be clinically salient. Previous diffusion tensor imaging studies indicated lower fractional isotropy in anterior frontal tracts in those who relapsed after treatment (Sorg et al., 2012); therefore, examination of longitudinal changes in frontal WM microstructure during early sobriety may yield differences between Abstainers and Relapsers, particularly in the right frontal WM.
For Abstainers, increasing GM volumes in the bilateral frontal GM and parietal GM, left temporal GM and right frontal WM were associated with increasing scores on Digit Span, a measure of aural working memory. These associations were not observed in Relapsers. Abstainers and Relapsers were not significantly different on Digit Span at both AP1 and AP2, and these groups showed an equivalent level of change over 4 weeks (results not shown). Beck and colleagues (Beck et al., 2012) observed disparate functional connectivity between alcohol-dependent individuals who relapsed within 3 months of treatment and those who remained abstinent over this interval. Compared with the relapsers, abstainers showed increased functional connectivity between the right midbrain and the left OFC, and between the right midbrain and the left amygdala, when exposed to alcohol-associated visual stimuli. Relapsers also showed significantly smaller OFC volumes relative to Abstainers and Relapsers. Taken together the above findings suggest the regional volume differences demonstrated by Abstainers and Relapsers early in treatment may be linked to differential integrity of functional connectivity among brain regions involved in emotional regulation and working memory.
In Relapsers, greater recovery of left frontal GM volume over 4 weeks of sobriety was related to a longer duration of abstinence post-treatment for AUD. Rando and colleagues reported that larger medial frontal GM volume at 4 weeks of abstinence was associated with a longer duration of abstinence post-treatment in alcohol-dependent individuals. Collectively, the foregoing results suggest that frontal cortex macrostructural integrity is related to the ability to sustain abstinence following treatment and is consistent with the involvement of anterior frontal subregions in working memory, planning, judgement, emotional regulation, impulse control, monitoring and adjustment of behavior in accordance with contextual demands and reward processing (Volkow et al., 2012; Volkow et al., 2013). Deficits in the foregoing abilities are associated with increased risk of relapse [see (Durazzo et al., 2011) and references therein].
This study has limitations that may affect the generalizability of the findings. There is no universally agreed upon definition of relapse in AUD (Witkiewitz and Marlatt, 2007); therefore, our relapse criteria - any alcohol consumption within 12 months of initial assessment - may not be congruent with criteria employed in other studies. Other limitations include the primary reliance on self-report and/or medical records for the determination of drinking status at post-treatment in most participants, and the inability to examine sex effects because of the small number of females. It is vital to include comparable number of females in future research to evaluate for the potential influence of sex.
The persistent bilateral frontal GM volume deficits in this group of predominantly Caucasian, male, Veteran Relapsers over 4 weeks from last alcohol use, combined with the volumetric findings from previous cross-sectional studies, may represent an endophenotype that differentiates those who respond more favorably to the typical psychosocial and pharmacological interventions provided for AUD. Our combined longitudinal findings of persistently smaller volume and lower perfusion in the frontal GM of Relapsers, over the 4 weeks they were in treatment, suggest differences in frontal GM neuroplasticity between Abstainers and Relapsers. Differences in demographic factors and comorbid medical, psychiatric, and substance abuse conditions did not account for the frontal GM volumetric differences between Abstainers and Relapsers. However, disparities between Abstainers and Relapsers in premorbid factors [e.g., polymorphisms in neurotrophic factors genetic and/or vulnerability to hazardous alcohol consumption (Mon et al., 2013; Tessner and Hill, 2010)], or other comorbid factors (e.g., diet/nutrition, exercise, subclinical liver, pulmonary, cardiac, or cerebrovascular dysfunction) that were not assessed in this study may have influenced the volumetric findings and warrant consideration in prospective studies on the neurobiological correlates of relapse in AUD. Given the critical role of the frontal lobe in higher level neurocognitive functions, the persistently smaller bilateral frontal GM volume exhibited by Relapsers compared to Abstainers may have implications for emerging AUD treatments such as neurostimulation (Gorelick et al., 2014) and cognitive retraining/remediation (Rupp et al., 2012; Wexler, 2011) interventions initiated soon after detoxification that seek to improve the functional integrity of frontal-subcortical circuits that subserve response inhibition, impulse control, working memory, and executive skills.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (DA24136 to TCD and AA10788 to DJM), and by the use of resources and facilities at the VA San Francisco and Palo Alto Health Care Systems.
Footnotes
AUTHOR CONTRIBUTION
TCD and DJM were responsible for study concept and design. TCD performed all clinical and neurocognitive assessments, completed all statistical analyses, and wrote the manuscript. AM and SG were responsible for the acquisition and processing of MRI data. DJM, AM and SG provided important intellectual content to the manuscript. All authors approved the final version.
The authors have no disclosures and no conflicts of interest to report.
References
- Beck A, Wustenberg T, Genauck A, Wrase J, Schlagenhauf F, Smolka MN, Mann K, Heinz A. Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Archives of general psychiatry. 2012;69:842–852. doi: 10.1001/archgenpsychiatry.2011.2026. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;57:289–300. [Google Scholar]
- Buhler M, Mann K. Alcohol and the human brain: a systematic review of different neuroimaging methods. Alcohol Clin Exp Res. 2011;35:1771–1793. doi: 10.1111/j.1530-0277.2011.01540.x. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Durazzo TC, Gazdzinski S, Mon A, Studholme C, Meyerhoff DJ. Brain Morphology at Entry into Treatment for Alcohol Dependence Is Related to Relapse Propensity. Biological psychiatry. 2011;70:561–567. doi: 10.1016/j.biopsych.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Academic Press; New York: 1977. (rev. ed.) [Google Scholar]
- Durazzo T, Gazdzinski S, Mon A, Meyerhoff D. Cortical perfusion in alcohol-dependent individuals during short-term abstinence: relationships to resumption of hazardous drinking after treatment. Alcohol. 2010a;44:201–210. doi: 10.1016/j.alcohol.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Yeh PH, Meyerhoff DJ. Combined neuroimaging, neurocognitive and psychiatric factors to predict alcohol consumption following treatment for alcohol dependence. Alcohol and Alcoholism. 2008;43:683–691. doi: 10.1093/alcalc/agn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mon A, Gazdzinski S, Yeh PH, Meyerhoff DJ. Serial longitudinal magnetic resonance imaging data indicate non-linear regional gray matter volume recovery in abstinent alcohol-dependent individuals. Addiction biology. 2015;20:956–967. doi: 10.1111/adb.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Pathak V, Gazdzinski S, Mon A, Meyerhoff DJ. Metabolite levels in the brain reward pathway discriminate those who remain abstinent from those who resume hazardous alcohol consumption after treatment for alcohol dependence. Journal of studies on alcohol and drugs. 2010b;71:278–289. doi: 10.15288/jsad.2010.71.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Tosun D, Buckley S, Gazdzinski S, Mon A, Fryer SL, Meyerhoff DJ. Cortical thickness, surface area, and volume of the brain reward system in alcohol dependence: relationships to relapse and extended abstinence. Alcohol Clin Exp Res. 2011;35:1187–1200. doi: 10.1111/j.1530-0277.2011.01452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick DA, Zangen A, George MS. Transcranial magnetic stimulation in the treatment of substance addiction. Annals of the New York Academy of Sciences. 2014;1327:79–93. doi: 10.1111/nyas.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA psychiatry. 2015;72:757–766. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens JR, Weisner C, Ray GT, Fireman B, Walsh K. Hazardous drinkers and drug users in HMO primary care: prevalence, medical conditions, and costs. Alcohol Clin Exp Res. 2005;29:989–998. doi: 10.1097/01.alc.0000167958.68586.3d. [DOI] [PubMed] [Google Scholar]
- Mon A, Durazzo TC, Gazdzinski S, Hutchison KE, Pennington D, Meyerhoff DJ. Brain-derived Neurotrophic Factor (BDNF) Genotype is Associated with Lobar Gray and White Matter Volume Recovery in Abstinent Alcohol Dependent Individuals. Genes Brain Behav. 2013;12:98–107. doi: 10.1111/j.1601-183X.2012.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington DL, Durazzo TC, Schmidt T, Mon A, Abe C, Meyerhoff DJ. The Effects of Chronic Cigarette Smoking on Cognitive Recovery during Early Abstinence from Alcohol. Alc Clin Exp Research. 2013;37:1220–1227. doi: 10.1111/acer.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando K, Hong KI, Bhagwagar Z, Li CS, Bergquist K, Guarnaccia J, Sinha R. Association of frontal and posterior cortical gray matter volume with time to alcohol relapse: a prospective study. The American journal of psychiatry. 2011;168:183–192. doi: 10.1176/appi.ajp.2010.10020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp CI, Kemmler G, Kurz M, Hinterhuber H, Fleischhacker WW. Cognitive remediation therapy during treatment for alcohol dependence. Journal of studies on alcohol and drugs. 2012;73:625–634. doi: 10.15288/jsad.2012.73.625. [DOI] [PubMed] [Google Scholar]
- Sankoh A, Huque M, Dubey S. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Statistics in Medicine. 1997;16:2529–42. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Seo D, Sinha R. Neuroplasticity and Predictors of Alcohol Recovery. Alcohol research: current reviews. 2015;37:143–152. [PMC free article] [PubMed] [Google Scholar]
- Sobell L, Sobell M, Maisto S, Cooper A. Time-line follow-back assessment method. NIAAA treatment handbook series. 1985;2:85–1380. [Google Scholar]
- Sobell LC, Sobell MB, Riley DM, Schuller R, Pavan DS, Cancilla A, Klajner F, Leo GI. The reliability of alcohol abusers’ self-reports of drinking and life events that occurred in the distant past. Journal of studies on alcohol. 1988;49:225–232. doi: 10.15288/jsa.1988.49.225. [DOI] [PubMed] [Google Scholar]
- Sorg SF, Taylor MJ, Alhassoon OM, Gongvatana A, Theilmann RJ, Frank LR, Grant I. Frontal white matter integrity predictors of adult alcohol treatment outcome. Biological psychiatry. 2012;71:262–268. doi: 10.1016/j.biopsych.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessner KD, Hill SY. Neural circuitry associated with risk for alcohol use disorders. Neuropsychology review. 2010;20:1–20. doi: 10.1007/s11065-009-9111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leemput K, Maes F, Vandermeulen D, Suetens P. Automated model-based tissue classification of MR images of the brain. IEEE Trans Med Imaging. 1999;18:897–908. doi: 10.1109/42.811270. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D. Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol. 2012;52:321–336. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD. Unbalanced neuronal circuits in addiction. Current opinion in neurobiology. 2013;23:639–648. doi: 10.1016/j.conb.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler BE. Computerized cognitive remediation treatment for substance abuse disorders. Biological psychiatry. 2011;69:197–198. doi: 10.1016/j.biopsych.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K. Predictors of heavy drinking during and following treatment. Psychol Addict Behav. 2011;25:426–438. doi: 10.1037/a0022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Marlatt GA. Modeling the complexity of post-treatment drinking: it’s a rocky road to relapse. Clin Psychol Rev. 2007;27:724–738. doi: 10.1016/j.cpr.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Makris N, Braus D, Mann K, Smolka M, Kennedy D, Caviness V, Hodge S, Tang L, Albaugh M. Amygdala volume associated with alcohol abuse relapse and craving. American Journal of Psychiatry. 2008;165:1179. doi: 10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.