Abstract
Objectives
Fine Motor Ability (FMA) is essential in certain Activities of Daily Living (ADL), and is considered mostly as a component of physical function. We hypothesize that cognitive ability explains significant variance in ADL-related FMA, above and beyond what is explained by physical ability (grip strength).
Methods
Origins of Variance in the Oldest Old Study (OCTO)-Twin participants (n=218), aged 80+ (dementia, stroke, Parkinson’s disease excluded) were assessed on depressive symptoms (CES-D), a cognitive battery, grip strength, and FMA.
Results
In a series of ordinary least-squares regression models, FMA was not associated with gender or depressive symptoms, but was associated with age (marginally; β= − 0.164; p = .051), grip strength (β=−0.381; p<0.01) and one cognitive measure, perceptual speed (β= − 0.249; p<0.01).
Discussion
In non-demented older adults, cognitive speed predicts ADL-related FMA after controlling for age and physical ability. Physical rehabilitation of FMA in ADL tasks should consider the importance of cognitive ability, even in non-demented older adults.
Keywords: Fine Motor Ability, Functional Ability, Disablement, Cognitive Ability, Grip Strength
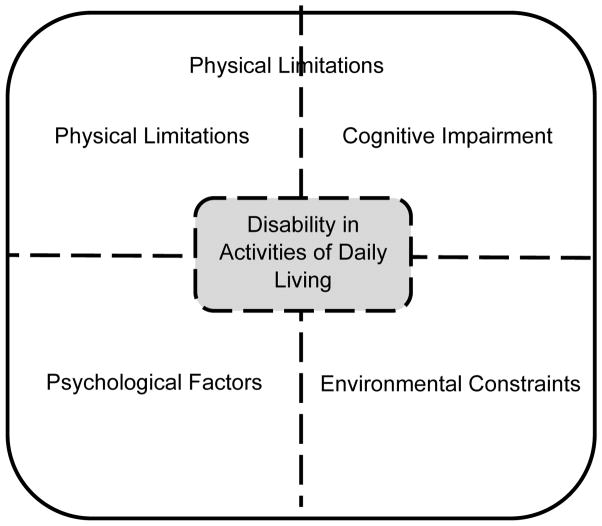
The Disablement Process model (Verbrugge & Jette, 1994) describes how pathology in older adults leads to impairments in body systems, which may eventually manifest as functional limitations (restrictions in basic physical and mental function). When an individual with functional limitations has difficulty with, or needs assistance in performing Activities of Daily Living (ADL), he or she has developed a disability. This entire process is impacted by demographic and other risk factors, as well as intraindividual factors (psychological characteristics) and extraindividual factors (environmental resources or barriers). Stated in a broader way, late-life ADL disability may be driven by health and physical abilities, but may also be impacted in differing degrees by cognitive ability, psychological factors, and environmental constraints (see Figure 1). To illustrate this process, we can use the ADL disability of being unable to bathe without assistance. Difficulty in bathing may be influenced by physical limitations (e.g. lack of strength/flexibility needed to transfer to a bath or shower), and/or cognitive ability (e.g. executive function impairments in completing bathing-related tasks in sequential order), and/or psychological factors (e.g. feeling depressed and unmotivated to bathe), and/or environmental constraints (e.g. having an unsafe bathing environment, without handle bars and with a high risk for falls). At the level of the individual, these four domains will contribute to ADL performance to differing degrees. A person with dementia will have a larger contribution of the cognitive domain than someone without dementia. In addition, there are interactions among these domains. To revisit our prior example, a complicated water faucet (environmental barrier) may contribute to difficulty in bathing, but only so when a person has cognitive impairment and/or arthritis in their hands; for others this faucet may not pose as a barrier. Given the potential influence of multiple domains on ADL performance, the purpose of the current study is to examine the contributions of physical, cognitive, and psychological factors on ADL-related performance, specifically, on ADL tasks related to fine motor ability. Below we will review the role of fine motor abilities in ADL tasks. Then we will justify the need to consider non-physical influences (see Cognitive, Psychological domains from Figure 1) on ADL-related FMA.
Figure 1.
Contributing Factors to Disability in Performing Activities of Daily Living
Note: This figure was adapted from an earlier figure published by Fauth, Schwartz, Tschanz, Østbye, Corcoran, & Norton (2013)
Fine Motor Ability: Essential for Daily Function
According to the National Institute of Health (2013), fine motor ability (FMA) is defined as the coordination of muscles, bones, and nerves to produce small, precise movements, such as picking up a small item with the index finger and thumb. As a comparison, gross motor ability involves the coordination of muscles, bones, and nerves to produce large, general movements, such as waving an arm. Both fine and gross motor abilities are inherent in daily tasks. Activities of Daily Living as proposed by Katz and colleagues (1970; sometimes specified as Personal Activities of Daily Living, PADL; or Basic Activities of Daily Living, BADL) include dressing, feeding, bathing, toileting, continence and transferring. Because gross motor movements are essential for some or all of these tasks (standing from a chair to transfer, entering a bathtub or shower area, etc.), researchers interested in Disablement Processes (Verbrugge & Jette, 1994) typically study functional limitations via gross motor ability tasks (timed chair stand tests, the ability to walk 3 meters and back, the ability to lift a 1-kilogram weight, and others; Femia, Zarit, & Johansson, 2001; Peek, Ottenbacher, Markides, &Ostir, 2003). Less common is the focus on fine motor abilities, yet FMA is also essential in PADL performance, particularly for tasks such as dressing and feeding. Dressing involves the hand manipulation of zippers, buttons, and other fasteners, and feeding involves the handling of utensils, manipulation of knives and forks in small, precise movements. Instrumental Activities of Daily Living (IADL; Lawton& Brody, 1969) include additional tasks related to independent, daily function: using the telephone, shopping, preparing food, housekeeping, laundry, transportation, taking medications, and managing finances. Several of these tasks also involve FMA: dialing or pressing numbers on a phone, opening pill containers, manipulating cooking utensils, and so on.
Practical Implications for the Prevention and Intervention of Disablement: A Focus on the Physical Rehabilitation
The goal of geriatric physical rehabilitation is to reduce the functional impairments that accrue during disablement processes, and ultimately to delay, prevent, or reverse disability. Performance of ADLs is often the goal of therapy; for example, chair stand movements and leg strengthening exercises will be repeated so that an older individual gains or maintains the strength needed to enter and exit a vehicle, or transfer from bed to a chair. The exercises and training in therapy often revolve around repeated gross motor movements, but where clinically appropriate, will also include fine motor tasks. Given the potential for physical, cognitive, psychological, and environmental domains to influence ADL disability, it is surprising that physical rehabilitation does not systematically consider cognitive impairments in either the design of treatments or the management of therapy goals. According to the American Physical Therapy Association’s (2015) guidelines, there is “no required screening, examination, evaluation, or diagnosis of cognition” in patients, and “no required review of cognitive system,” although notes may be made regarding the: “ability to make needs known, consciousness, expected emotional/behavioral responses, learning preferences (e.g., education needs, learning barriers), and orientation (person, place, time)”. In short, there is potential for the effects of physical rehabilitation in older adults to be enhanced with a more standardized, evidence-based consideration of cognitive, psychological, and environmental factors that are known to contribute to the disablement process as well.
Cognitive, Psychological, and Demographic Correlates with FMA and ADL
Evidence supports that fine motor performance is associated with factors outside of the “physical” domain, including cognitive, psychological, and other factors. Both cognitive performance, and depressive symptoms are associated with fine motor abilities in community-dwelling adults aged 60+ (Grigsby, Kaye, Baxter, Shetterly, & Hamman, 1998). Not all cognitive tests are related to functional performance the same, however. Cahn-Weiner, Boyle, and Malloy (2002) reported that performance-based IADL (including both gross and fine-motor tasks) was associated with executive functioning (Trail Making Task-B; Reitan & Wolfson, 1985), but not word fluency (Controlled Oral Word Fluency Test; Borkowski, Benton, & Spreen, 1967) or preservation and abstract thinking (Wisconsin Card Sorting Test; Heaton et al., 1993) in community-dwelling older adults.
Psychological factors are associated with ADL and FMA performance. Depressive symptoms are associated with general ADL disability in an interdependent way, both prior to and after the onset of ADL disability (Fauth, Gerstorf, Ram, & Malmberg, 2012). Major depression is also associated with psychomotor slowing, including slowing of fine motor movements (Sabbe, Hulstijn, Van Hoof, & Zitman, 1996).
Demographic characteristics of age and gender are particularly relevant to ADL and FMA. Advanced age is associated with poorer ADL function, as well as slower FMA (Seidler, et al., 2010). Gender may also influence FMA. Older men have stronger grip strength, but older women are faster on FMA measures, such as peg-board tasks (Buchman, Wilson, Bienias, &Mennett, 2005).
The Current Study
The purpose of this paper is to examine the influence of physical, cognitive, and psychological factors on ADL performance, specifically, on ADL-related FMA. The fourth domain, environmental constraints, while potentially important, was not available in the current data. With our population-based study of older adults in advanced age (aged 80+), we target a sample with sufficient variability in participants’ stages of disablement. We utilize a “normative sample”, selecting out individuals with dementia, stroke, or Parkinson’s disease, so as not to artificially over-inflate the contribution of the cognitive domain. We hypothesize that physical abilities will explain significant variance in ADL-related FMA performance, and that other domains (cognitive abilities, psychological factors) will also be relevant (particularly cognition), explaining significant variance in FMA in and above that which is explained by physical abilities.
Methods
Participants
The current analyses include baseline data from the Origins of Variance in the Old Old Study (OCTO-Twin; McClearn, et al.,1997), which utilized the population-based Swedish Twin Registry (a registry of all multiple births in Sweden) to identify 351 monozygotic and dizygotic twin pairs aged 80+ living throughout Sweden. From 1991–1993, baseline data were collected. While the original study continued to follow the sample via longitudinal re-assessments, those data are not utilized in the current study, as our aims were not to observe changes over time; rather we examine the overall contribution (variance explained) in a “snapshot” of time. Because we were not interested in genetic contributions of FMA, nor the “twin status” of participants, and because data from twin pairs may not be independent, we randomly selected one twin from each pair for inclusion in these analyses. Of note, comparisons of twin samples with non-twin samples suggest that there are no inherent selection biases in population-based twin samples; twins did not differ from non-twins in health or biobehavioral characteristics (Simmons, Johansson, Zarit, Ljungquist, Plomin, & McClearn, 1997). We also excluded anyone who had a diagnosis of stroke, Parkinson’s disease, and/or dementia (based on the Diagnostic and Statistical Manual of Mental Disorders: DSM-III-R criteria; American Psychiatric Association, 1987). Our final sample was N=270. The Regional Ethical Review Board in Stockholm, Sweden and the Institutional Review Board at Pennsylvania State University reviewed and approved the procedures involved in the OCTO-twin study, and all participants completed the informed consent process.
Measures
Dependent Variable: ADL-related Fine Motor Ability
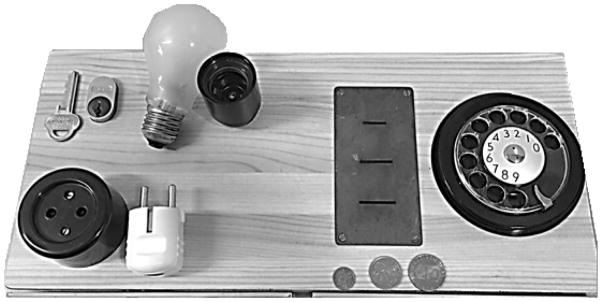
Participants were presented with an apparatus that required them to complete five tasks on equipment replicating real daily tasks involving fine motor abilities. They had to insert a key into a lock and turn it, insert a plug into an electrical outlet, screw a light bulb into a socket, insert coins into coin slots, and dial numbers on a rotary telephone dial (a rotary phone was nearly universally used by older adults in Sweden at the time of study initiation; See Figure 2). These tasks include some of the same tasks used in other performance-based measures, such as the Fine Motor Subscale of the Structured Assessment of Independent Living Skills (SAILS; Mahurin, DeBettignies, & Pirozzolo, 1991), which includes picking up coins and turning a key in a lock, cutting with scissors, removing wrappers, and folding a letter and inserting into an envelope. The difference with the current measure is that it assesses performance in time in seconds, with faster times indicating higher fine motor ability, thus the outcome is continuous. By contrast, SAILS uses sums of a subjective scoring system from the rater (e.g. for coin task the responses are 0 (drops 2) 1 (drops one) 2 (slow) and 3 (normal).
Figure 2.
ADL-related Fine Motor Ability Apparatus
Independent Variable: Depressive Symptoms
A measure of depressive symptoms was included as a global psychological factor. We used the Center for Epidemiologic Studies - Depression scale (CES-D; Radloff 1977), which is a 20-item self-report scale assessing the frequency of depressive symptoms over the past week (higher scores indicate greater depressive symptoms).
Independent Variable: Cognitive Abilities
A battery of seven cognitive performance tests were administered to participants, including the classic Kohs Block Design (a test of visuospatial ability, Kohs, 1920; Dureman & Sälde, 1959), digit span forward and backward (short-term memory; Wechsler, 1991), perceptual speed (a timed picture matching task assessing processing speed; Dureman & Sälde, 1959), the Thurstone Picture Memory Test (episodic memory; Thurstone & Thurstone 1949), and a 30- minute, 10-item object recall task (Memory-in-Reality Test; Johansson, 1998/89).
Independent Variable: Grip Strength
Our assessment of physical ability was grip strength, a performance-based test where participants squeezed a dynamometer with their dominant hand, three times. Their maximum force across trials was used as their grip strength score. Grip strength is a common functional performance test that is correlated with general muscle strength in the rest of the body (a good proxy for global physical function; Massy-Westropp, et al., 2011), and is strongly associated with PADL and IADL ability (Fauth, Zarit, &Malmberg, 2008).
Control Variables: Gender and Age
Because gender is related to the physical ability measure included here (males have higher average grip strength; Massy-Westropp, Gill, Taylor, Bohannon, & Hill, 2011), and because gender is related to general PADL and IADL performance (women have higher average ADL impairments; Merrill, Seeman, Kasl, & Berkman, 1997), we included gender (0=male, 1 = female) as a demographic covariate in the analyses. Age is also included as a covariate, as it shares variance with the independent variables: depressive symptoms (Blazer, Burchett, Service, & George, 1991); cognitive ability (Salthouse, 2004); grip strength (Proctor et al., 2006), and is also associated with the dependent variable of FMA (Seidler, et al., 2010).
Analyses
A series of Ordinary Least Squares regression models were run (SPSS version 22; IBM Corp., 2013) with timed FMA as the dependent variable. By including the variables in preliminary models by blocks (with the blocks driven by our model; see Figure 1) we identified the relationships by domain without covariates, yet we also reduced the likelihood of Type I errors by not testing all variables independently, as would have been the case with a correlation table. In block 1, gender and age were included; block 2 included psychological factors (depressive symptoms); block 3 included physical ability (grip strength), block 4 included all seven tests of cognitive performance, and block 5 carried forward statistically significant variables from prior blocks, into the final model.
Results
Participants had a mean age of 83.50 (SD=3.07), and 66.3% of participants were women. Just under one-third (29.8%) of participants were currently married. The majority were living alone (59.2%), and 10.2% of the sample lived in institutional housing (which included nursing homes and Swedish “service apartments”, similar to assisted living facilities). The mean years of formal education was typical for Swedes of this cohort (mean years of formal education = 7.19; SD = 2.25; See Table 1).
Table 1.
Mean and Standard Deviations across Independent and Dependent Variables
| Independent Variables | Mean (SD) (or Percent) |
|---|---|
| Age | 83.5 (3.07) |
| Gender | 66.3 % Female |
| Depressive Symptoms | 8.15 (8.60) |
| Grip Strength (Max score) | 0.63 (0.19) |
| Block Design | 12.08 (7.01) |
| Digit Span Forward | 5.45 (1.24) |
| Digit Span Backward | 3.35 (1.48) |
| Perceptual Speed | 10.41 (3.85) |
| Thurstone test | 18.15 (5.13) |
| Object recall | 6.40 (2.54) |
|
| |
| Dependent Variable
| |
| Timed ADL-related Fine Motor Ability | 55.37 (21.32) |
In block 1 of the hierarchical linear regression models, gender was not statistically significant, but age was significant, thus age was carried forward for the final model. In block 2, depressive symptoms were not statistically significant. In block 3, the physical ability variable of grip strength was significantly associated with fine motor abilities. In block 4, only one cognitive variable (perceptual speed) was associated with fine motor abilities, thus the final model included age, perceptual speed, and grip strength as the independent variables. Age became marginally significant (p=0.051), while both perceptual speed and grip strength remained statistically significant predictors of ADL-related fine motor abilities (see Table 2).
Table 2.
Hierarchical Linear Regression Models: Dependent Variable is Timed ADL-related Fine Motor Ability
| Block: Domain | Independent Variables | B | Standard Error | β | Model R2 |
|---|---|---|---|---|---|
|
| |||||
| 1: Demographics | Constant | −141.272 | 42.018 | ||
| Age | 2.368 | 0.507 | 0.311** | ||
| Gender | −0.937 | 3.002 | −.021 | 0.095 | |
|
| |||||
| 2: Psychological | Constant | 52.804 | 1.920 | ||
|
| |||||
| Depressive Symptoms | 0.358 | 0.178 | 0.094 | 0.004 | |
|
| |||||
| 3: Physical | Constant | 82.679 | 4.762 | ||
|
| |||||
| Grip Strength | −42.944 | 7.183 | −0.381** | 0.145 | |
|
| |||||
| 4: Cognitive | (Constant) | 98.116 | 8.584 | ||
|
| |||||
| Block Design | −0.436 | 0.299 | −0.147 | ||
|
| |||||
| Digit Span Forward | −1.709 | 1.462 | −0.109 | ||
|
| |||||
| Digit Span Backward | −1.563 | 1.333 | −0.113 | ||
|
| |||||
| Perceptual Speed | −1.286 | 0.553 | −0.249* | ||
|
| |||||
| Thurstone test | −0.247 | 0.428 | −0.062 | ||
|
| |||||
| Object recall | −1.138 | 0.816 | −0.133 | 0.315 | |
|
| |||||
| 5: Final Model | Constant | −26.986 | 61.850 | ||
|
| |||||
| Age | 1.399 | 0.710 | 0.164 | ||
|
| |||||
| Grip Strength | −25.657 | 8.732 | −0.238** | ||
|
| |||||
| Perceptual Speed | −1.910 | 0.439 | −0.355** | 0.301 | |
Note:
indicates p < .05;
indicates p < .01
Discussion
A broad summarization of the Disablement Process model (Verbrugge & Jette, 1994) is that late life disability in Activities of Daily Living is influenced in differing degrees by physical impairments, cognitive abilities, psychological factors, and environmental constraints. ADL tasks involve both gross and fine motor abilities, but the majority of functional tasks used in disablement process research assess gross motor function (standing, lifting, and walking, and so on). The current study is a unique opportunity to study the physical, cognitive and psychological influences on performance based, ADL-related Fine Motor Abilities (FMA), both in independent models, and then collectively in a final model.
In addition to directing research attention to the utility of assessing ADL-related FMA in disablement research, the main purpose of this study was to examine the multiple domains of influence (physical, cognitive, and psychological only, as the environmental domain was not able to be assessed) on ADL-related FMA. In this study, the psychological factor of depressive symptoms was not significantly associated with FMA. We did find, however, that physical ability (grip strength) was associated with FMA performance. Perhaps even more interesting is that after controlling for age and physical ability, the cognitive ability of perceptual speed shared statistically significant variance with FMA. In other words, in a non-demented sample of older adults, perceptual speed is associated with ADL-related fine motor tasks, above and beyond that which is explained by age and physical function. We acknowledge that because we assessed FMA as a timed task in this study, it is possible that its association with perceptual speed may be higher than with other cognitive tasks, where speed plays less of a role in cognitive performance. On one hand, we have shown recently in laboratory-based studies that non-demented older adults with lower cognitive status moved more slowly on a functional reaching and grasping task (adapted from a standardized clinical test of hand function) compared to those with higher cognitive status, regardless of how much task practice they had (Schaefer, Dibble, & Duff, 2015). Cognitive status in this study was measured using the Montreal Cognitive Assessment, which has minimal emphasis on perceptual or processing speed. The relationship between movement speed and overall cognitive status is further supported in patients with clinical diagnoses of Alzheimer’s disease and mild cognitive impairment, such that the more severe the impairment, the slower the individual moves during a complex motor skill like mirror tracing (Rouleau, Salmon, & Vrbancic, 2002) or targeted pointing (Yan & Dick, 2006). Thus, results in the current study and from others (e.g. Scherder, Dekker, & Eggermont, 2008) may suggest relationships between fine motor and global cognition, regardless of whether the tasks used to test them are timed or not. On the other hand, however, the current study does offer deeper insights into which cognitive domain(s), namely perceptual speed, may be particularly and selectively important for Fine-motor-related ADL ability and its change (particularly slowing of these tasks) over time. Rodrigue, Kennedy, and Raz (2005) reported interactions between age and perceptual speed on the extent to which movement speed on a fine motor task (i.e., mirror-tracing) declined over a five-year test-retest period, suggesting a unique involvement of this domain. Interestingly, perceptual speed did not predict the extent to which older adults reacquired motor skill on the tracing task with practice five years later, suggesting that this domain may be less important to consider when targeting improvements in fine motor abilities through rehabilitation. Taken together, more research is clearly needed to identify candidate predictors of ADL function (including those specifically FMA-related) and rehabilitation.
Current findings have implications for basic research, as well as therapeutic intervention. First, because PADL and IADL tasks involve FMA, we should consider fine motor assessment in functional performance batteries, much as we would tasks of gross motor abilities. Second, therapeutic approaches such as physical or occupational therapy that are aimed at improving ADL-related performance in older adults by training gross and/or fine motor ability should consider the role of cognitive ability (particularly perceptual speed) and variability in the overall approach to intervention. Such therapies should also potentially consider psychological and environmental factors as well, based on the Disablement Process model (Verbrugge & Jette, 1994), although these were not directly tested in the current study.
In other words, one implication from this study is that physical rehabilitative approaches should systematically consider how patients’ gross and fine motor skill may be influenced by their cognitive abilities, even in cases without dementia or clinically significant levels of cognitive impairment. To date, however, there are no standardized, evidence-based principles guiding physical therapists beyond clinical reasoning in individualizing their treatment plans based on patients’ cognitive abilities. A second implication from this study is the potential combined benefit of concurrent cognitive and physical therapies on minimizing disability in older adults, relative to physical therapy alone (e.g. Eggenberger, Schumacher, Angst, Theill, & de Bruin, 2015). For example, how might perceptual speed training administered prior to or during motor training enhance gains made in therapy or declines after therapy? In short, the current study provides evidence of the interactions between cognitive and physical domains, which warrants consideration in the development of future therapies in older adults.
Limitations, Strengths, and Future Directions
We presented environmental constraints as a domain of influence in the overall theoretical approach to the study, yet we did not have any adequate measures of this potentially influential construct. It would be of interest for future studies to examine the overall contribution of environmental constraints on FMA performance. For example, one could experimentally examine the extent to which larger/smaller keys influence the ability to manipulate the key and lock task. One could test whether push-button phones (with buttons of varying physical qualities) influence performance differently than the rotary dial task, and so on.
Secondly, we did not find that depressive symptoms were associated with FMA, although prior studies show well-established associations between general ADL performance (IADL/PADL abilities) and depressive symptoms (Fauth, et al., 2012). This null finding in the current study deserves further consideration. It could be that, in fact, psychological factors play a larger role in more global self-reported ADL disability, and a lesser role in performance-based FMA. It could also, be, however, that other psychological factors besides depressive symptoms should be considered as alternative psychological variables.
We note that a strength of the study lies in its non-clinical sample of older adults, including those residing in institutional settings. Using a Swedish population registry for sampling participants offers a unique opportunity to observe these constructs in a generalizable sample, whereas many studies of the oldest old include only the healthiest individuals who have volunteered for research through newspaper campaigns or other community-based recruitment. That being said, we acknowledge that some of the frailest individuals in the population may not be represented in this sample. Additionally, our findings should be replicated in samples outside of Sweden and from other birth cohorts.
Finally, we propose that additional attention should be given to the null finding of gender in our models; gender was not associated with timed FMA. In other disablement studies, gender routinely predicts ADL function (Merrill, et al., 1997), as well as many other gross-motor functional tasks (including but not limited to grip strength; Massy-Westropp, et al., 2011). The fact that gender was not associated with ADL-related timed FMA is noteworthy. It could be that FMA is less susceptible to gender-socialized behaviors such as those assessed in IADL performance (shopping, preparing food, and housekeeping), and less susceptible to physiological gender differences (greater muscle strength/mass in men). Identifying a “genderless” functional performance task may offer significant practical utility for researchers interested in functional performance and disability, and these lack of gender differences in ADL-related FMA need to be assessed, validated, or refuted in future research.
Acknowledgments
Special thanks to the late Stig Berg (Institute for Gerontology in the College of Health Sciences at Jönköping University), to Gerald McClearn (Pennsylvania State University), and the research teams at the Institute for Gerontology in the College of Health Sciences at Jönköping University in Sweden, the Center for Developmental and Health Genetics at the Pennsylvania State University, and the Division of Genetic Epidemiology at the Karolinska Institute in Stockholm, Sweden, for their design and collection of the Origins of Variance in the Old Old Study (OCTO)-Twin data.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: National Institute on Aging AG08861 for funding of the Origins of Variance in the Old Old Study (OCTO)-Twin study and to National Institutes of Health for the K01AG047926 award.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- American Physical Therapy Association. Positions, Standards, Guidelines, Policies & Procedures. APTA; Alexandria, VA: 2015. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R) 3. APA; Washington, DC: 1987. [Google Scholar]
- Blazer D, Burchett B, George LK. The association of age and depression among the elderly: An epidemiologic exploration. Journal of Gerontology. 1991;46(6):M210–M215. doi: 10.1093/geronj/46.6.m210. [DOI] [PubMed] [Google Scholar]
- Borkowski JG, Benton AL, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5:135–140. [Google Scholar]
- Buchman AS, Wilson RS, Bienias JL, Bennett DA. Gender differences in upper extremity motor performance of older persons. Geriatrics & Gerontology International. 2005;5(1):59–65. doi: 10.1111/j.1447-0594.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- Cahn-Weiner DA, Boyle PA, Malloy PF. Tests of executive function predict Instrumental Activities of Daily Living in community-dwelling older individuals. Applied Neuropsychology. 2002;9:187–191. doi: 10.1207/S15324826AN0903_8. [DOI] [PubMed] [Google Scholar]
- Dureman I, Sälde H. Psykometriska och experimentalpsykologiska metoder för klinisk tillämpning (Psychometric and Experimental- Psychological methods for Clinical Application) Almqvist &Wiksell; Uppsala, Sweden: 1959. [Google Scholar]
- Eggenberger P, Schumacher V, Angst M, Theill N, de Bruin ED. Does multicomponent physical exercise with simultaneous cognitive training boost cognitive performance in older adults? A 6-month randomized controlled trial with a 1-year follow-up. Clinical Interventions in Aging. 2015;10:1335–1349. doi: 10.2147/CIA.S87732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauth EB, Gerstorf D, Ram N, Malmberg B. Changes in depressive symptoms in the context of disablement processes: Role of demographic characteristics, cognitive function, health, and social support. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2012;67(2):167–177. doi: 10.1093/geronb/gbr078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauth EB, Schwartz S, Tschanz JT, Østbye T, Corcoran C, Norton MC. Baseline disability in activities of daily living predicts dementia risk even after controlling for baseline global cognitive ability and depressive symptoms. International Journal of Geriatric Psychiatry. 2013;28(6):597–606. doi: 10.1002/gps.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauth EB, Zarit SH, Malmberg B. Mediating relationships within the disablement process model: a cross-sectional study of the oldest-old. European Journal of Ageing. 2008;5(3):161–179. doi: 10.1007/s10433-008-0092-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Femia EE, Zarit SH, Johansson B. The Disablement process in very late life: A study of the oldest-old in Sweden. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2001;56(1):P12–P23. doi: 10.1093/geronb/56.1.p12. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Kaye K, Baxter J, Shetterly SM, Hamman RF. Executive cognitive abilities and functional status among community-dwelling older persons in the San Luis Valley Health and Aging Study. Journal of the American Geriatrics Society. 1998;46(5):590–596. doi: 10.1111/j.1532-5415.1998.tb01075.x. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtis G. Wisconsin card sorting test manual: Revised and expanded. Odessa, FL: Psychological Assessment Resources; 1993. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp; 2013. [Google Scholar]
- Johansson B. The MIR-Memory in Reality Test. Psykologiförlaget AB; Stockholm: 1998/89. [Google Scholar]
- Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. The Gerontologist. 1970;10(1 Part 1):20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- Kohs SC. The Block-Design Tests. Journal of Experimental Psychology. 1920;3(5):357. [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. The Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- Mahurin RK, DeBettignies BH, Pirozzolo FJ. Structured Assessment of Independent Living Skills: Preliminary report of a performance measure of functional abilities in dementia. The Journals of Gerontology Series B: Psychological Sciences. 1991;46:P58–P66. doi: 10.1093/geronj/46.2.P58. [DOI] [PubMed] [Google Scholar]
- Massy-Westropp NM, Gill TK, Taylor AW, Bohannon RW, Hill CL. Hand grip strength: Age and gender stratified normative data in a population-based study. BMC Research Notes. 2011;4(1):127. doi: 10.1186/1756-0500-4-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClearn GE, Johansson B, Berg S, Pedersen NL, Ahern F, Petrill SA, Plomin R. Substantial genetic influence on cognitive abilities in twins 80 or more years old. Science. 1997;276:1560–1563. doi: 10.1126/science.276.5318.1560. [DOI] [PubMed] [Google Scholar]
- Merrill SS, Seeman TE, Kasl SV, Berkman LF. Gender differences in the comparison of self-reported disability and performance measures. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 1997;52(1):M19–M26. doi: 10.1093/gerona/52a.1.m19. [DOI] [PubMed] [Google Scholar]
- National Institute of Health. Medline Plus: Fine motor control. 2013 Retrieved from http://www.nlm.nih.gov/medlineplus/ency/article/002364.htm.
- Peek MK, Ottenbacher KJ, Markides KS, Ostir GV. Examining the disablement process among older Mexican American adults. Social Science & Medicine. 2003;57(3):413–425. doi: 10.1016/s0277-9536(02)00367-2. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Fauth EB, Hoffman L, Hofer SM, McClearn GE, Berg S, Johansson B. Longitudinal changes in physical functional performance among the oldest old: insight from a study of Swedish twins. Aging Clinical and Experimental Research. 2006;18(6):517–530. doi: 10.1007/BF03324853. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery. Tucson, AZ: Neuropsychology Press; 1985. [Google Scholar]
- Rodrigue KM, Kennedy KM, Raz N. Aging and longitudinal change in perceptual-motor skill acquisition in healthy adults. The Journals of Gerontology Series B: Psychological Sciences. 2005;60B(4):P174–P181. doi: 10.1093/geronb/60.4.p174. [DOI] [PubMed] [Google Scholar]
- Rouleau I, Salmon DP, Vrbancic M. Learning, retention and generalization of a mirror tracing skill in Alzheimer’s disease. Journal of Clinical and Experimental Neuropsychology. 2002;24(2):239–250. doi: 10.1076/jcen.24.2.239.997. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. What and when of cognitive aging. Current Directions in Psychological Science. 2004;13(4):140–144. doi: 10.1177/0963721414535212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbe B, Hulstijn W, Van Hoof J, Zitman F. Fine motor retardation and depression. Journal of Psychiatric Research. 1996;30(4):295–306. doi: 10.1016/0022-3956(96)00014-3. [DOI] [PubMed] [Google Scholar]
- Schaefer SY, Dibble LE, Duff K. Efficacy and feasibility of functional upper extremity task-specific training for older adults with and without cognitive impairment. Neurorehabilitation and Neural Repair. 2015;29(7):636–644. doi: 10.1177/1545968314558604. [DOI] [PubMed] [Google Scholar]
- Scherder E, Dekker W, Eggermont L. Higher-level hand motor function in aging and (preclinical) dementia: Its relationship with (Instrumental) Activities of Daily Living – A mini-review. Gerontology. 2008;54:333–341. doi: 10.1159/000168203. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, … Lipps DB. Motor control and aging: Links to age-related brain structural, functional, and biochemical effects. Neuroscience and Biobehavioral Reviews. 2010;34(5):721–733. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons SF, Johansson B, Zarit SH, Ljungquist B, Plomin R, McClearn GE. Selection bias in samples of older twins? A comparison between octogenarian twins and singletons in Sweden. Journal of Aging and Health. 1997;9:553–567. doi: 10.1177/089826439700900407. [DOI] [PubMed] [Google Scholar]
- Thurstone LL, Thurstone TG. Manual to SRA primary mental abilities. Science Research Associates; Chicago: 1949. [Google Scholar]
- Verbrugge LM, Jette AM. The disablement process. Social Science & Medicine. 1994;38(1):1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale Revised. The Psychological Corporation; New York: 1991. [Google Scholar]
- Yan JH, Dick MB. Practice effects on motor control in healthy seniors and patients with Mild Cognitive Impairment and Alzheimer’s disease. Aging, Neuropsychology, and Cognition. 2006;13:385–410. doi: 10.1080/138255890969609. [DOI] [PubMed] [Google Scholar]