Abstract
Objective
Cognitive-behavioral therapy (CBT) is effective in reducing disability among youth with juvenile fibromyalgia (JFM); however, engagement in moderate-vigorous physical activity remains poor even after CBT. The purpose of this study was to evaluate the feasibility and preliminary outcomes of an innovative program combining CBT with specialized neuromuscular exercise; the Fibromyalgia Integrative Training for Teens (FIT Teens) program.
Methods
Adolescents with JFM (n = 22, all female, ages 12–18) from two urban children’s hospitals participated in the eight-week FIT Teens intervention. Participants completed measures of pain intensity, functional disability, depressive symptoms, pain catastrophizing, fear of movement, and readiness to change at baseline and after the intervention.
Results
Feasibility of the intervention across two sites was documented, including high retention rates (80%). Participants showed significant decreases in functional disability (p < .05), depression (p < .001), fear of movement (p < .01), and pain catastrophizing (p < .001) from pre- to post-intervention. Results of the readiness to change measure indicated a significant decrease in precontemplation (p < .01) and increase in action/maintenance scores (p < .001). All results demonstrated medium to large effect sizes.
Conclusion
Results of this pilot study indicated that adolescents with JFM reported significant improvements in physical function and reduced fear of movement following the intervention. Improvement in physical function was achieved in a shorter time frame than a prior trial of CBT without an exercise component. Further work is needed to compare FIT Teens with existing approaches and determine whether objective changes in exercise participation are achieved.
Juvenile fibromyalgia (JFM) is a chronic pain condition characterized by widespread musculoskeletal pain, sleep difficulties, chronic fatigue, and associated symptoms such as gastrointestinal problems, and anxiety and depressive symptoms. The prevalence of JFM in children and adolescents is between 2–6%, and occurs primarily in females (1–6). Recommended treatment of JFM includes self-management strategies such as healthy lifestyle habits (sleep, physical exercise), and pain coping strategies through cognitive-behavioral therapy (CBT; 7–9). Routine physical exercise is a particularly important component of managing widespread musculoskeletal pain (10) and has been found to reduce pain intensity (11–15); however, long-term maintenance of exercise regimens is often problematic for patients (11, 16, 17). Most individuals with fibromyalgia are sedentary (18–20), and few tailored physical activity interventions for youth with fibromyalgia have been attempted. There is a pressing need to develop, test, and disseminate effective interventions that promote safe engagement in physical activity and address psychological barriers (e.g., low confidence in movement, poor pain coping skills) to engaging in activity among adolescents with JFM.
CBT for JFM is effective in reducing symptoms of depression, functional disability, and catastrophizing, and increasing pain coping efficacy (21); however it has not demonstrated consistent effectiveness in increasing engagement in physical activity (22) and reductions in pain intensity have been relatively small (21). This may be because traditional pain-focused CBT does not directly target the physical exercise component of pain self-management. While CBT can provide skills that encourage engagement in healthy lifestyle habits, patients clearly need more direct guidance and training to properly increase physical activity. There is evidence that adolescents with JFM show deficits in strength and altered biomechanics (23)—this may contribute to their fear of movement and movement-related pain (24). Therefore, establishing fundamental movement skills and confidence should be the first step to facilitate engagement in activities of daily living and physical activity. Integrative neuromuscular training is an established physical exercise intervention developed by exercise science specialists aimed at improving fundamental biomechanics and core strength (25). This type of training has been used with adolescent athletes to prevent injury (26), and has been specifically tailored by our group for adolescents with JFM (27).
In order to address the need for an integrated intervention which offers both training in fundamental movement and pain coping skills, we developed a program combining established pain-focused CBT with neuromuscular training (28). The new Fibromyalgia Integrative Training for Teens program (FIT Teens) is an 8-week (16-session) group-based intervention. The FIT Teens program was found to be safe and well-tolerated in a small pilot qualitative study of 11 adolescents with JFM where we iteratively developed and modified the intervention with patient input and feedback (29).
The goals of this study were to 1) further evaluate feasibility of the FIT Teens program by extending our pilot work across two sites and 2) examine preliminary efficacy in improving outcomes of patients with JFM treated at two sites using a pre-post treatment study design. Specifically, we sought to assess changes in functional disability, pain, fear of movement, coping, and motivation to engage in self-management for pain after the FIT Teens intervention. We hypothesized that participants would show significant reductions in functional disability, fear of movement, depressive symptoms, and pain intensity after the FIT Teens intervention. In a subset of the patients, we explored whether pain catastrophizing decreased and whether readiness to change significantly changed (i.e., decreased levels of precontemplation and contemplation, and increased levels of action/maintenance) after the FIT Teens intervention.
Patients and Methods
Recruitment
Adolescents (ages 12 to 18 years old) were eligible to participate in the study if they had been diagnosed with JFM by a pediatric rheumatologist or pain physician. Physicians used Yunus and Masi criteria to diagnose JFM, which includes widespread pain, associated symptoms (fatigue, sleep disturbance, etc.), and at least 5 tender points (6). Additionally, only participants who had average pain intensity of 4 or greater on a 0–10 cm Visual Analog Scale (VAS) and reported a Functional Disability Score of 7 or greater, indicating at least mild disability were included. Patients were not eligible to participate if they reported a Children’s Depression Inventory (CDI) T-score over 80 (indicative of severe depressive symptoms), were diagnosed with a comorbid rheumatic disease, untreated major psychiatric diagnoses or developmental delay, or any medical condition determined by their physician to be a contraindication for exercise. Potential participants meeting screening criteria were identified by trained research assistants from pediatric rheumatology and pain clinics at two large children’s hospitals (Site 1 located in the US Midwest and Site 2 in the US North East). Physicians confirmed medical eligibility and introduced the study to patients and their caregivers. If families indicated interest in participating, the research assistants provided a thorough overview of the study, answered any questions, and obtained written informed consent and assent. Institutional Review Board approval for the study was obtained at both children’s hospitals.
Intervention
Adolescent participants attended 90-minute small group treatment sessions twice weekly for 8 weeks for a total of 16 total sessions. Parent(s) of the adolescent were directly included in 6 of the 16 sessions. Treatment sessions were led jointly by a psychology post-doctoral fellow/pediatric pain psychologist and an exercise physiologist/physical therapist. Session content was manualized and structured to devote approximately 45 minutes to learning behavioral pain management techniques through CBT and 45 minutes to neuromuscular exercises to improve strength, fitness, and body mechanics. The interventionists from Site 2 attended a two-day training at Site 1 to learn to implement the FIT Teens intervention according to the protocol. The intervention took place at two children’s hospitals in their Sports Medicine Biodynamics Center and Center for Motion Analysis and Department of Physical Therapy (similar to a physical therapy/gym setting) for the neuromuscular training; the CBT component of the sessions was held in an adjacent conference room.
The CBT content of the intervention was based on a published clinical trial for JFM and included psychoeducation about the gate-control theory of pain and pain coping skills such as relaxation techniques, distraction, activity pacing, problem solving, and modifying negative and catastrophic thoughts about pain (21). Coping skills were practiced in vivo supported by the psychology fellow/psychologist while participants engaged in neuromuscular exercises for a more integrated approach. Education normalizing temporary muscle soreness when beginning a new exercise regimen and differentiating muscle soreness from a JFM pain flare was also discussed, as well as the applicability of each of the exercises for improved performance of activities of daily living—e.g., walking, lifting, and climbing stairs.
The neuromuscular training protocol was modified, specifically for the needs of JFM patients and the ease of translating exercises into the home setting. The resistive training protocol did not use any additional weights other than body weight (e.g., participants worked with their own body weight as resistance) and progressed through four levels of exercise: basic isometric “hold” exercises; concentric “muscle shortening” exercises; eccentric “muscle lengthening” exercises; and full range of motion exercises for “functional movement” (complete protocol published previously; 27). All of the movements are progressive until level four, where the functional movements are relevant to activities of daily living. The neuromuscular training protocol was specifically designed to reduce the potential for delayed-onset muscle soreness by starting with basic exercises and gradually increasing the complexity of muscle actions. Participants progressed to new levels of exercises every two weeks with ongoing supervision and constructive feedback regarding technique to ensure mastery of proper form before advancing to the next level of exercise difficulty.
At the end of each session, participants received daily diaries to monitor progress and home practice instructions for coping skills and physical exercises. Daily diaries included ratings of daily pain intensity, sleep, and fatigue, and practice of coping skills and physical exercise. Diaries were reviewed by the trainers at the beginning of each session to discuss progress and problem-solve barriers to independent home practice. Attendance was recorded at every session and brief make-up sessions were provided to ensure that all participants received the full course of the intervention.
Study Measures
Participants at both sites completed self-report measures of pain, functional disability, depressive symptoms, and fear of movement. In addition, Site 2 administered additional measures of pain catastrophizing and pain stages of change to explore the utility of these assessments.
Pain Intensity
The Visual Analogue Scale (VAS; 30) is a commonly used and validated measure of pain intensity in children and adolescents. Participants were asked to rate their pain intensity over the past two weeks on a 0–10cm scale ranging from 0 (no pain) to 10 (worst possible pain). All participants at both sites completed this measure (n = 22).
Functional Disability
The Functional Disability Inventory (FDI; 31) is a validated 15-item measure of adolescents’ perception of how difficult it is for them to complete normal daily tasks at home, school, recreational, and social environments. Participants rate each task on a scale of 0 (no trouble) to 4 (impossible) and total scores range from 0 to 60 with higher scores indicating more disability. Clinical reference points for children with chronic pain have been established (32), and the FDI has been found to have good psychometric properties in this population. All participants at both sites completed this measure (n = 22).
Depression
The Children’s Depression Inventory (CDI; 33) is a 27-item measure of symptoms of depression in children and adolescents which has been widely validated in pediatric pain research (34). Participants select 1 of 3 statements for each item which are scored from 0 to 2, with higher scores indicating greater frequency and/or severity of symptoms. Total scores range from 0 to 54, and raw scores are converted to T-scores based on a normative sample. This measure was administered at Site 1 only, and added after the first group (n = 7 at Site 1). The CDI 2 (a revised version of the CDI; 35, 36) was administered at Site 2 (n = 13). CDI and CDI 2 raw scores were converted to T-scores using their respective norms to ensure scores were comparable.
Fear of Movement
The Tampa Scale for Kinesiophobia (TSK-11; 37) is a reliable measure of the fear of movement due to pain consisting of 11 statements rated on a scale of 1 (strongly disagree) to 4 (strongly agree). Total scores range from 11 to 44 with higher scores indicating increased fear of movement, activity avoidance, and somatic focus. The scale has been validated across various pain conditions in adults (38); and the TSK-11 has been used previously with youth to validate other measures (39). To make the measure applicable for chronic pain (rather than acute injury) and to make it easily understandable for adolescents, we replaced “injure” with “hurt” for 2 items, replaced “my accident” with “my medical condition” for 1 item, and replaced “try to overcome” with “try to push myself”. This modified version of the scale was successfully used in a prior publication (23). In the current sample, the modified version demonstrated good internal consistency, α = .84. This measure was added after the first two groups at Site 1 and administered to all groups at Site 2 (n = 18).
Pain Catastrophizing
The Pain Catastrophizing Scale for Children (PCS-C; 40) is a validated and reliable measure consisting of 13 statements describing negative and catastrophic thoughts and feelings about pain. Participants rate how intensely they experience each thought or feeling when they have pain on a scale of 0 (not at all) to 4 (extremely). Total scores range from 0 to 52 with higher scores indicating greater levels of pain catastrophizing. This measure was administered at Site 2 only (n = 13).
Pain Stages of Change
The Pain Stages of Change Questionnaire (PSOCQ-A; 41) is a 30-item measure that assesses adolescents’ perceived readiness to self-manage their own pain. Individuals rate statements based on how they currently feel about their pain problem on a scale of 1 (strongly disagree) to 5 (strongly agree). Scores are examined across three subscales: Precontemplation, Contemplation, and Action/Maintenance. This measure was administered at Site 2 only (n = 13).
Data Analysis
Descriptive statistics were computed for demographic and outcome variables. The effects of the intervention were analyzed using dependent samples t-tests comparing pre-intervention scores on the VAS, FDI, CDI, TSK, PCS-C, and PSOCQ-A to post-intervention scores. This study was primarily designed as a feasibility pilot study and therefore corrections for multiple tests were not made. Instead, for interpretation purposes, Cohen’s d values were calculated to examine effect sizes for all comparisons.
Results
At Site 1, 34 adolescents were approached and assessed for eligibility, 17 declined to participate, and 17 were consented to receive the FIT Teens intervention (See Figure 1). At Site 2, 45 were approached and assessed for eligibility, 22 declined to participate, 2 did not meet initial screening criteria, and 21 were consented to receive the intervention. Combined, 48% (17 of 34 and 21 of 45) of those approached agreed to participate. At Site 1, 6 of the 17 individuals dropped out of the intervention (65% retention rate); at Site 2, 5 individuals did not meet inclusion criteria during additional screening prior to beginning the intervention, 3 withdrew prior to beginning the intervention, and all 13 who began the intervention completed the intervention (100% retention rate). Combined, a total of 24 participants completed the FIT Teens intervention across the two study sites resulting in an average 80% retention rate once the intervention began. Two participants from Site 1 were excluded from final analyses due to not meeting inclusion criteria that were refined and finalized after the first few groups; 22 participants were included in the final analyses.
Figure 1.
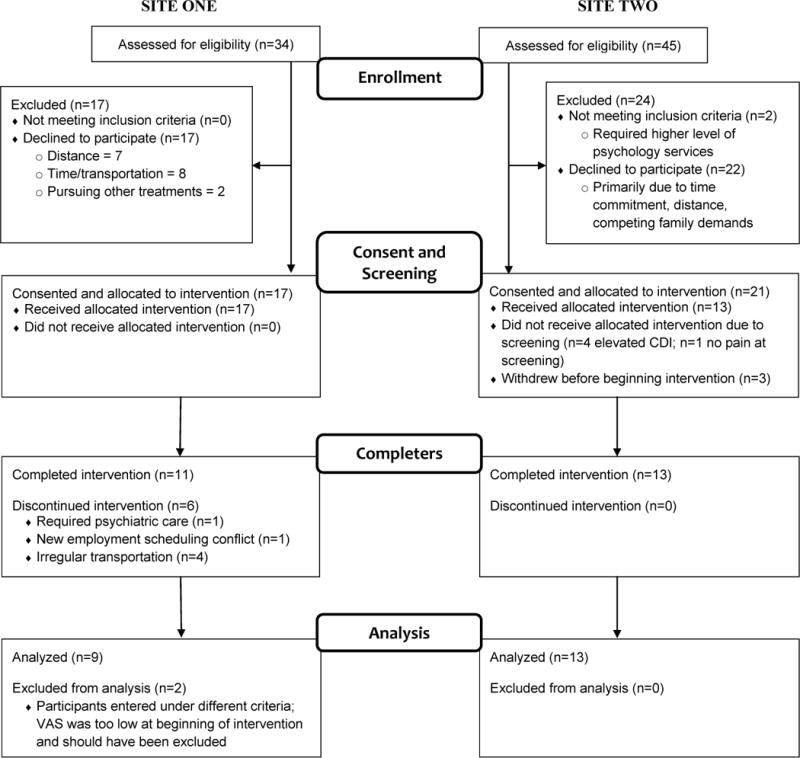
Study flow diagram.
Participants were all female, ranged in age from 13–18 (M = 16.19, SD = 1.59), and the majority (82%) were Caucasian. Three groups (n = 9) were completed at Site 1 and four groups (n = 13) were completed at Site 2; group size varied from 2 to 4 participants. Demographic information by site is provided in Table 1. Demographic characteristics were not different across sites.
Table 1.
Baseline demographic characteristics of the sample across sites
| Site 1 | Site 2 | |||
|---|---|---|---|---|
| M (SD) | N (%) | M (SD) | N (%) | |
| Age | 16.56 (1.81) | 15.94 (1.43) | ||
| Gender – female | 9 (100%) | 13 (100%) | ||
| Race – Caucasian | 6 (67%) | 12 (92%) | ||
| African American | 3 (33%) | 1 (8%) | ||
| Ethnicity – Non-Hispanic | 9 (100%) | 9 (82%)* | ||
Two participants did not complete this question
Individuals at both sites displayed comparable scores on the VAS, FDI, and TSK-11 at baseline (all ps > .05). Participants at Site 2 had higher CDI 2 T-scores compared to CDI T-scores for participants at Site 1 (M = 65.31, SD = 7.79; M = 55.43, SD = 10.10 respectively; t (18) = 2.44, p = .03). PCS-C and PSOCQ-A were collected at Site 2 only.
Across both sites, t-tests comparing pre-post scores indicated that participants’ scores on the FDI significantly decreased from pre- to post-intervention, demonstrating a medium effect size (d = 0.61; see Table 2). Scores also decreased on the CDI/CDI 2 and TSK-11, exhibiting large effect sizes (d = 0.99 and d = 1.02 respectively). Although not significant, participants’ pain intensity scores decreased, demonstrating a medium effect size (d = 0.48).
Table 2.
Pre- and post-intervention scores on primary outcome measures
| Measure | Outcomes | N | Pre-Intervention M (SD) |
Post-Intervention M (SD) |
t-score | p | d |
|---|---|---|---|---|---|---|---|
| VAS | Pain | 22 | 6.20 (1.22) | 5.48 (1.71) | 2.03 | .056 | 0.48 |
| FDI | Functional Disability | 22 | 26.50 (10.37) | 20.36 (9.82) | 2.78* | .011 | 0.61 |
| CDI | Depression | 20 | 61.85 (9.69) | 53.05 (8.09) | 5.11*** | <.001 | 0.99 |
| TSK-11 | Fear of Movement | 18 | 28.33 (6.35) | 23.00 (3.79) | 3.89** | .001 | 1.02 |
| PCS-C | Pain Catastrophizing | 13 | 31.46 (7.99) | 17.54 (6.96) | 6.50*** | <.001 | 1.86 |
| PSOCQ | Precontemplation | 13 | 3.12 (0.42) | 2.47 (0.70) | 3.28** | .007 | 1.13 |
| PSOCQ | Contemplation | 13 | 4.10 (0.39) | 3.84 (0.47) | 2.02 | .066 | 0.60 |
| PSOCQ | Action/Maintenance | 13 | 2.89 (0.76) | 4.42 (0.42) | −6.00*** | <.001 | −2.49 |
p < .05;
p < .01;
p < .001
d = Effect size (Cohen’s d)
At Site 2, participants’ scores on the PCS-C significantly decreased with a large effect size (d = 1.86; see Table 2). Regarding the PSOCQ-A, scores on the Precontemplation scale significantly decreased (see Figure 2), with a large effect size (d = 1.13), and scores on the Action/Maintenance scale significantly increased with a large effect size (d = −2.49). Scores on the Contemplation scale of the PSOCQ-A decreased, and although this change was not significant, it still demonstrated a medium effect size (d = 0.66).
Figure 2.
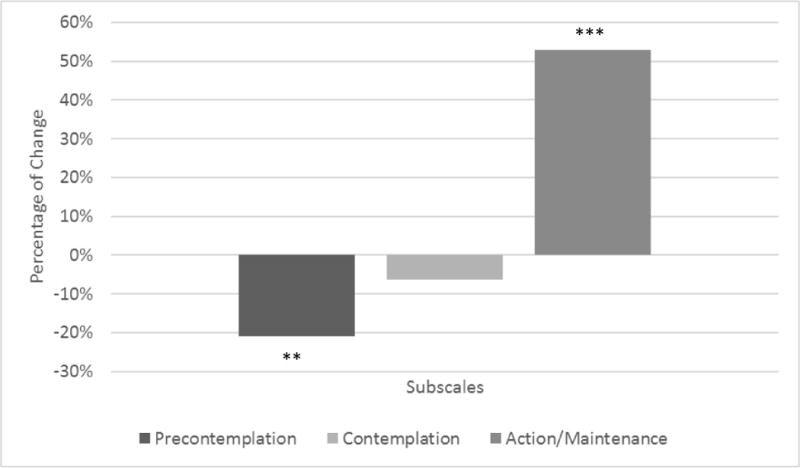
Percentage of change on adolescent report of PSOCQ from pre- to post-intervention, **p < .01, ***p < .001.
Discussion
This multi-site investigation provides promising initial evidence for the feasibility and efficacy of a novel intervention that combines CBT with neuromuscular training to reduce disability and fear of movement, while improving psychological coping and readiness to engage in self-management for pain. As we have reported in a previously published qualitative study, once enrolled, patients find FIT Teens highly engaging, report no adverse effects other than temporary muscle soreness, and greatly enjoy the group-format of this program (28). Recruitment for a treatment study such as this is quite complex; both sites showed similar rates of recruitment. During the initial phase of testing the FIT Teens intervention at Site 1, retention was lower and two participants were excluded from analyses as screening criteria were initially very inclusive. As we refined inclusion criteria and screening processes (e.g., using the CDI as a screening tool to exclude those with severe depressive symptoms and refer for psychiatric stabilization), retention rates improved and were replicated at Site 2. The primary reasons for declining to participate included time commitment, distance, and competing family demands. Although not all participants referred agreed to participate in the study, those that did commit were highly engaged and likely to complete the program (80% retention).
The results of this study build on our initial qualitative work and demonstrate that after participation in FIT Teens, patients showed significant improvements in important outcomes such as their daily functioning and depressive symptoms. Indeed, improvement in physical function was achieved in a shorter time frame (8 weeks) when compared to a prior trial of traditionally delivered pain focused CBT for adolescents with JFM without an exercise component (where similar outcomes were achieved only by the 6-month follow-up); and effect sizes for reductions in functional disability, depression, and pain were stronger in the present study (21). Furthermore, improvement occurred in fewer and less frequent sessions compared to an aerobic exercise intervention, which was 12 weeks, 3 sessions per week (12). While there was a greater effect on pain reduction after the aerobic intervention, there was no follow-up to assess long-term adherence to the program or pain levels. Studies in adults with FM have shown difficulty with adherence to aerobic programs with pain levels returning to baseline a few weeks after the intervention (17). FIT Teens was designed to increase movement confidence through neuromuscular training and gradual increases in physical activity—which may not be sufficient for immediate pain reduction. However, training in foundational movement skills and coping skills should facilitate sustained activity participation and engagement in pain management skills—ulitmately resulting in better maintained pain reduction. In addition to providing evidence for improvements in functional disability and depression—domains targeted in traditionally delivered CBT—this novel intervention integrated specialized neuromuscular training to reduce potential physical and psychological barriers to engagement in physical activity. The in vivo exposure to physical activity and simultaneous implementation of pain coping skills may be the mechanisms by which pain catastrophizing and fear of movement decreased over the course of the study. Reducing psychological barriers and instilling confidence regarding physical exercise may facilitate engagement in this critical area of self-management necessary for adequate pain control. Furthermore, the adolescents’ greater confidence and engagement in activity during the course of the FIT Teens intervention was observed by their parent(s), who participated in 6 sessions during the intervention. While parent castastrophizing about their adolescents’ pain was not directly assessed in the current study, observing the adolescents’ positive gains may help to decrease parental worries regarding the adolescents’ pain and functioning (42). Recent research suggests that this may be an important mechanism to examine in future studies, as the effect of parent catastrophizing about their child’s pain on child outcomes was found to be explained by its impact on the child’s catastrophizing levels (43, 44).
A patient’s level of motivation to take on self-management of pain, or readiness to change, has also emerged as an important construct for understanding engagement in pain self-management strategies and associated factors have shown promise in capturing changes following treatment in intensive pain rehabilitation (45, 46) and outpatient pain clinic settings (47). Our findings (from the Site 2 subsample) regarding readiness to change indicated that the majority of adolescents moved from stages of precontemplation and contemplation to action and maintenance—suggesting that this intervention was as effective as previous studies of intensive day hospital (46) and outpatient multidisciplinary treatments (45) in progressing readiness to change among pediatric pain patients. Coupled with the findings from our qualitative study that adolescents plan to continue engagement in these exercises after the FIT Teens intervention (29), it is feasible that this intervention addresses some of the physical and psychological barriers that prohibit initiating and maintaining participation in physical exercise.
This multi-site investigation demonstrates the potential for dissemination of this innovative intervention. Specialized interventions tailored for youth with JFM are desperately needed, thus it is crucial that promising treatments are able to be disseminated to other sites via manualized programs. These results demonstrate that interventionists within different hospital systems were able to implement the intervention effectively. Trainers at the two sites had different levels of training prior to the intervention (clinical psychologist and psychology fellow, sports physiologist and physical therapist), adding further support for the ease of training interventionists to implement the treatment protocol. Finally, the exercises used minimal equipment for ease of dissemination and generalization to the participants’ home environments.
It should be noted that CDI scores at Site 2 were higher than scores at Site 1. Although different versions of the CDI were used (CDI 2 and CDI respectively), this is unlikely the sole cause of the difference as standardized T-scores were used to compare scores across sites. It is possible that the sample at Site 2 was more complex, including a higher number of participants with significant depressive symptoms, although anyone with a T-score higher than 80 was excluded. Referral patterns may have differed across clinics, including variability in the availability of other treatment options and physician preferences for treatment.
Limitations include a relatively homogenous sample limiting generalizations to more diverse samples of patients with JFM. As this was a pilot study to determine whether conducting groups at multiple sites was feasible, a control group was not included. The next steps include future trials with CBT only or exercise only groups to determine if the integrated CBT and neuromuscular training group is superior to group-based CBT or exercise only interventions. Outcome measures were self-reported and focused on functioning and coping, and are thus subject to potential social desirability bias. Including assessments of objective outcomes (i.e., physical activity and biomechanical outcomes) would help determine whether self-reported improvements in fear of movement, pain catastrophizing, and readiness to change translate into increased engagement in physical activity and ensure that these improvements are not solely due to social desirability in responding. Participation in the group requires significant commitment from families in order to attend sessions and practice skills at home. While traditionally-delivered CBT also includes home practice, attending sessions twice weekly may be a barrier for some families.
Future work should focus on long-term follow up to see if treatment gains are maintained over time. Furthermore, additional research examining the role of parental factors that may further facilitate or impede adolescent engagement and outcomes will be important. Finally, research to examine the generalizability of the FIT Teens intervention will be important to consider in the future, particularly considering the promise of this combined approach in achieving powerful treatment gains across both physical and psychological outcomes.
Significance and Innovations.
In order to address the needs of adolescents with JFM for training in pain coping skills and engagement in physical activity, an intervention integrating an established pain-focused CBT protocol with a neuromuscular exercise program specifically tailored for adolescents with JFM was created—Fibromyalgia Integrative Training for Teens (FIT Teens).
The FIT Teens intervention shows promise in reducing functional disability and fear of movement, and improving coping and engagement with self-management strategies in adolescents with JFM.
This project provides initial support for the feasibility of implementing the FIT Teens intervention in two distinct treatment centers with different staffing resources.
Acknowledgments
We would like to thank Hermine Brunner, Kenneth Goldschneider, Kimberly Harris-Eaton, Michael Henrickson, Jennifer Huggins, Kimberly Kempner, Anne Lynch-Jordan, Ann Mendicino-Wrynn, Laura Miele, Esi Morgan-DeWitt, Ellen Price, John Rose, Barbara Rzepski, Matthew Solomito, Alex Szabova, Heather Tory, Ana Verissimo, and Tegan Willard for assistance with identification of eligible patients for the study. We would also like to thank the participants and families across the two medical centers who participated in the study for their time and effort.
Funding: This study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)/NIH Grants K24AR056687 and R21AR063412 to the last author, support from the Division of Behavioral Medicine and Clinical Psychology, Cincinnati Children’s Hospital Medical Center, and support from Connecticut Children’s Medical Center.
References
- 1.Buskila D, Press J, Gedalia A, Klein M, Neumann L, Boehm R, et al. Assessment of nonarticular tenderness and prevalence of fibromyalgia in children. The Journal of Rheumatology. 1993;20(2):368–70. [PubMed] [Google Scholar]
- 2.Clark P, Burgos-Vargas R, Medina-Palma C, Lavielle P, Marina F. Prevalence of fibromyalgia in children: A clinical study of Mexican children. The Journal of Rheumatology. 1998;25(10):2009–14. [PubMed] [Google Scholar]
- 3.Mikkelsson M, Salminen JJ, Kautiainen H. Non-specific musculoskeletal pain in preadolescents: Prevalence and 1-year persistence. Pain. 1997;73(1):29–35. doi: 10.1016/s0304-3959(97)00073-0. [DOI] [PubMed] [Google Scholar]
- 4.Sardini S, Ghirardini M, Betelemme L, Arpino C, Fatti F, Zanini F. Epidemiological study of a primary fibromyalgia in pediatric age. Minerva Pediatr. 1996;48(12):543–50. [PubMed] [Google Scholar]
- 5.Gerloni V, Ghirardini M, Fantini F. Arthritis Rheum. 1998 Lipppincot Williams & Wilkins 227 East Washington Sq; Philadelphia, PA 19106 USA: 1998. Assessment of nonarticular tenderness and prevalence of primary fibromyalgia syndrome in healthy Italian schoolchildren; pp. S267–S. [Google Scholar]
- 6.Yunus MB, Masi AT. Juvenile primary fibromyalgia syndrome: A clinical study of thirty-three patients and matched normal controls. Arthritis Rheum. 1985;28(2):138–45. doi: 10.1002/art.1780280205. [DOI] [PubMed] [Google Scholar]
- 7.Buskila D, Ablin JN. Treating juvenile fibromyalgia: Cognitive-behavioral therapy, exercise and pharmacotherapy. Pain Management. 2013;3(5):323–4. doi: 10.2217/pmt.13.37. [DOI] [PubMed] [Google Scholar]
- 8.Ting TV, Kashikar-Zuck S. Juvenile fibromyalgia: Diagnostic challenges and treatment options. Practical Pain Management. 2011;11(7):81–6. [Google Scholar]
- 9.Ablin J, Fitzcharles M, Buskila D, Shir Y, Sommer C, Häuser W. Treatment of fibromyalgia syndrome: Recommendations of recent evidence-based interdisciplinary guidelines with special emphasis on complementary and alternative therapies. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/485272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Pain Society. Guideline for the management of fibromyalgia syndrome pain in adults and children. Glenview, IL: American Pain Society; 2005. [Google Scholar]
- 11.Häuser W, Klose P, Langhorst J, Moradi B, Steinbach M, Schiltenwolf M, et al. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: A systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther. 2010;12:R79. doi: 10.1186/ar3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stephens S, Feldman BM, Bradley N, Schneiderman J, Wright V, Singh-Grewal D, et al. Feasibility and effectiveness of an aerobic exercise program in children with fibromyalgia: Results of a randomized controlled pilot trial. Arthritis Care Res (Hoboken) 2008;59(10):1399–406. doi: 10.1002/art.24115. [DOI] [PubMed] [Google Scholar]
- 13.Hooten W, Qu W, Townsend CO, Judd JW. Effects of strength vs aerobic exercise on pain severity in adults with fibromyalgia: A randomized equivalence trial. Pain. 2012;153(4):915–23. doi: 10.1016/j.pain.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 14.Bircan Ç, Karasel SA, Akgün B, El Ö, Alper S. Effects of muscle strengthening versus aerobic exercise program in fibromyalgia. Rheumatol Int. 2008;28(6):527–32. doi: 10.1007/s00296-007-0484-5. [DOI] [PubMed] [Google Scholar]
- 15.Busch AJ, Barber KA, Overend TJ, Peloso PM, Schachter CL. Exercise for treating fibromyalgia syndrome. Cochrane Database of Systematic Reviews. 2007;4(4) doi: 10.1002/14651858.CD003786.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Gowans SE. Effectiveness of exercise in management of fibromyalgia. Curr Opin Rheumatol. 2004;16(2):138–42. doi: 10.1097/00002281-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Fontaine KR, Conn L, Clauw DJ. Effects of lifestyle physical activity in adults with fibromyalgia: Results at follow-up. Journal of Clinical Rheumatology: Practical Reports on Rheumatic & Musculoskeletal Diseases. 2011;17(2):64. doi: 10.1097/RHU.0b013e31820e7ea7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kashikar-Zuck S, Flowers SR, Verkamp E, Ting TV, Lynch-Jordan AM, Graham TB, et al. Actigraphy-based physical activity monitoring in adolescents with juvenile primary fibromyalgia syndrome. The Journal of Pain. 2010;11(9):885–93. doi: 10.1016/j.jpain.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kop WJ, Lyden A, Berlin AA, Ambrose K, Olsen C, Gracely RH, et al. Ambulatory monitoring of physical activity and symptoms in fibromyalgia and chronic fatigue syndrome. Arthritis Rheum. 2005;52(1):296–303. doi: 10.1002/art.20779. [DOI] [PubMed] [Google Scholar]
- 20.Korszun A, Young EA, Engleberg NC, Brucksch CB, Greden JF, Crofford LA. Use of actigraphy for monitoring sleep and activity levels in patients with fibromyalgia and depression. J Psychosom Res. 2002;52(6):439–43. doi: 10.1016/s0022-3999(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 21.Kashikar-Zuck S, Ting TV, Arnold LM, Bean J, Powers SW, Graham TB, et al. Cognitive behavioral therapy for the treatment of juvenile fibromyalgia: A multisite, single-blind, randomized, controlled clinical trial. Arthritis Rheum. 2012;64(1):297–305. doi: 10.1002/art.30644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kashikar-Zuck S, Flowers SR, Strotman D, Sil S, Ting TV, Schikler KN. Physical activity monitoring in adolescents with juvenile fibromyalgia: Findings from a clinical trial of cognitive–behavioral therapy. Arthritis Care Res (Hoboken) 2013;65(3):398–405. doi: 10.1002/acr.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sil S, Thomas S, DiCesare C, Strotman D, Ting TV, Myer G, et al. Preliminary evidence of altered biomechanics in adolescents with Juvenile Fibromyalgia. Arthritis Care Res (Hoboken) 2015;67(1):102–11. doi: 10.1002/acr.22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simons LE, Kaczynski KJ. The Fear Avoidance model of chronic pain: Examination for pediatric application. The Journal of Pain. 2012;13(9):827–35. doi: 10.1016/j.jpain.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myer GD, Faigenbaum AD, Chu DA, Falkel J, Ford KR, Best TM, et al. Integrative training for children and adolescents: Techniques and practices for reducing sports-related injuries and enhancing athletic performance. The Physician and Sportsmedicine. 2011;39(1):74–84. doi: 10.3810/psm.2011.02.1854. [DOI] [PubMed] [Google Scholar]
- 26.Myer GD, Faigenbaum AD, Ford KR, Best TM, Bergeron MF, Hewett TE. When to initiate integrative neuromuscular training to reduce sports-related injuries in youth? Curr Sports Med Rep. 2011;10(3):155. doi: 10.1249/JSR.0b013e31821b1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas SM, Sil S, Kashikar-Zuck S, Myer GD. Can modified neuromuscular training support the treatment of chronic pain in adolescents? Strength & Conditioning Journal. 2013;35(3):12–26. [Google Scholar]
- 28.Kashikar-Zuck S, Tran ST, Barnett K, Bromberg MH, Strotman D, Sil S, et al. A Qualitative Examination of a New Combined Cognitive-behavioral and Neuromuscular Training Intervention for Juvenile Fibromyalgia. Clinical Journal of Pain. doi: 10.1097/AJP.0000000000000221. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kashikar-Zuck S, Tran ST, Barnett K, Bromberg MH, Strotman D, Sil S, et al. A qualitative examination of a new combined cognitive-behavioral and neuromuscular training intervention for juvenile fibromyalgia. The Clinical Journal of Pain. 2016;32(1):70–81. doi: 10.1097/AJP.0000000000000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGrath PJ, Walco GA, Turk DC, Dworkin RH, Brown MT, Davidson K, et al. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. The Journal of Pain. 2008;9(9):771–83. doi: 10.1016/j.jpain.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Walker LS, Greene JW. The Functional Disability Inventory: Measuring a neglected dimension of child health status. J Pediatr Psychol. 1991;(16):39–58. doi: 10.1093/jpepsy/16.1.39. [DOI] [PubMed] [Google Scholar]
- 32.Kashikar-Zuck S, Flowers SR, Claar RL, Guite JW, Logan DE, Lynch-Jordan AM, et al. Clinical utility and validity of the Functional Disability Inventory (FDI) among a multicenter sample of youth with chronic pain. Pain. 2011;152(7):1600–7. doi: 10.1016/j.pain.2011.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovacs M. Children’s Depression Inventory Manual. North Tonawanda, New York: Multi-Health Systems Inc; 1992. [Google Scholar]
- 34.Logan DE, Claar RL, Guite JW, Kashikar-Zuck S, Lynch-Jordan A, Palermo TM, et al. Factor structure of the children’s depression inventory in a multisite sample of children and adolescents with chronic pain. The Journal of Pain. 2013;14(7):689–98. doi: 10.1016/j.jpain.2013.01.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kovacs M. Children’s Depression Inventory 2 (CDI) Manual. 2nd. North Tonawanda, New York: Multi-Health Systems Inc; 2011. [Google Scholar]
- 36.Bae Y. Test Review: Kovacs, M. “Children’s Depression Inventory 2 (CDI 2)”. North Tonawanda, NY: Multi-Health Systems Inc, 2011. J Psychoeduc Assess. 2012;30(3):304–8. [Google Scholar]
- 37.Miller RP, Kori SH, Todd DD. The Tampa Scale: A measure of kinisophobia. The Clinical Journal of Pain. 1991;7(1):51. [Google Scholar]
- 38.Roelofs J, Sluiter JK, Frings-Dresen MH, Goossens M, Thibault P, Boersma K, et al. Fear of movement and (re) injury in chronic musculoskeletal pain: Evidence for an invariant two-factor model of the Tampa Scale for Kinesiophobia across pain diagnoses and Dutch, Swedish, and Canadian samples. Pain. 2007;131(1):181–90. doi: 10.1016/j.pain.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Simons LE, Sieberg CB, Carpino E, Logan D, Berde C. The Fear of Pain Questionnaire (FOPQ): Assessment of pain-related fear among children and adolescents with chronic pain. The Journal of Pain. 2011;12(6):677–86. doi: 10.1016/j.jpain.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Crombez G, Bijttebier P, Eccleston C, Mascagni T, Mertens G, Goubert L, et al. The child version of the pain catastrophizing scale (PCS-C): A preliminary validation. Pain. 2003;104(3):639–46. doi: 10.1016/S0304-3959(03)00121-0. [DOI] [PubMed] [Google Scholar]
- 41.Guite JW, Logan DE, Simons LE, Blood EA, Kerns RD. Readiness to change in pediatric chronic pain: Initial validation of adolescent and parent versions of the Pain Stages of Change Questionnaire. Pain. 2011;152(10):2301–11. doi: 10.1016/j.pain.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guite JW, Logan DE, McCue R, Sherry DD, Rose JB. Parental beliefs and worries regarding adolescent chronic pain. The Clinical journal of pain. 2009;25(3):223–32. doi: 10.1097/AJP.0b013e31818a7467. [DOI] [PubMed] [Google Scholar]
- 43.Pielech M, Ryan M, Logan D, Kaczynski K, White MT, Simons LE. Pain catastrophizing in children with chronic pain and their parents: Proposed clinical reference points and reexamination of the Pain Catastrophizing Scale measure. PAIN®. 2014;155(11):2360–7. doi: 10.1016/j.pain.2014.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welkom JS, Hwang W-T, Guite JW. Adolescent pain catastrophizing mediates the relationship between protective parental responses to pain and disability over time. J Pediatr Psychol. 2013:jst011. doi: 10.1093/jpepsy/jst011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simons LE, Sieberg CB, Pielech M, Conroy C, Logan DE. What does it take? Comparing intensive rehabilitation to outpatient treatment for children with significant pain-related disability. J Pediatr Psychol. 2012:jss109. doi: 10.1093/jpepsy/jss109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Logan DE, Conroy C, Sieberg CB, Simons LE. Changes in willingness to self-manage pain among children and adolescents and their parents enrolled in an intensive interdisciplinary pediatric pain treatment program. Pain. 2012;153(9):1863–70. doi: 10.1016/j.pain.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evans JR, Mano KJ, Guite JW, Weisman SJ, Hainsworth KR. Psychometric properties of the pain stages of change questionnaire: New insights on the measurement of readiness to change in adolescents, mothers, and fathers. The Journal of Pain. 2015 doi: 10.1016/j.jpain.2015.03.012. [DOI] [PubMed] [Google Scholar]


