Abstract
Infections caused by bacteria in the airway preferentially induce a Th17 response. However the mechanisms involved in the regulation of CD4 T cells responses in the lungs are incompletely understood. Here we have investigated the mechanisms involved in the regulation of Th17 differentiation in the lungs in response to immunization with LPS as adjuvant. Our data shows that both Myd88 and TRIF are necessary for Th17 induction. This distinctive fate determination can be accounted for by the pattern of inflammatory cytokines induced by airway administration of LPS. We identified the production of IL-1β and IL-6 by small macrophages and IL-23 by alveolar dendritic cells, favoring Th17 responses, and IL-10 repressing IFN-γ production. Furthermore, we show that exogenous IL-1β can drastically alter Th1 responses driven by influenza and lymphocytic choriomeningitis virus infection models and induce IL-17 production. Thus the precision of the lung immune responses to potential threats is orchestrated by the cytokine microenvironment, can be repolarized and targeted therapeutically by altering the cytokine milieu. These results indicate how the development of Th17 responses in the lung are regulated by the cytokines produced by lung dendritic cells and macrophages in response to intranasal immunization with LPS adjuvant.
Introduction
The lung mucosa is a major site of interaction of the immune system with microbial pathogens and other environmental antigens capable of stimulating strong responses1, 2. In view of the necessity to maintain lung function during infection, immune responses tailored to maximize elimination of pathogens that avoid unnecessary immune-mediated inflammation and cell death would be highly desirable. Here we explore the role of key adjuvants in determining the quality of CD4 T cell responses and provide evidence that such responses are, indeed, precisely controlled to mount responses appropriate to particular microbial threats.
During airway immunization, antigen-presenting lung DCs migrate to the mediastinal lung-draining lymph node and stimulate antigen-specific naive T cells3–5. Primed CD4 T cells that have established a memory/effector state are rapidly mobilized to the airway where they engage the pathogens for which they are specific6, 7. Because the lung is frequently exposed to an extensive range of potentially infectious agents, it is not a surprise that it contains a complex network of DCs and macrophages that could contribute to the induction of a polarizing microenvironment appropriate to the pathogen as revealed by distinctive patterns of CD4 T cell phenotypes8, 9. However, it is not clear how the interaction of mucosal innate and CD4 T cells is regulated to precisely drive the differentiation of responding CD4 T cells to distinctive polarized states in the draining node and for the appropriate migration of these cells to the lung10.
It is known that subcutaneous immunizations using lipopolysaccharide (LPS) adjuvant or intravenous bacterial infection induce a broad Th1 and Th17 response, while bacterial intranasal infection can predominantly induce a Th17 response11–13.
Here, using a T cell transfer model, we have investigated the mechanisms driving the differentiation of Th1 and Th17 CD4 T cells in the airway following engagement of single TLRs. Although the stimulation of most toll-like receptors (TLR) triggers activation of Myd88 signaling pathway, TLR4 - which recognizes LPS - has the unique characteristic among TLRs to use both Myd88 and TIR-domain-containing adapter-inducing interferon-β (TRIF) while TLR3 –which recognizes poly(I:C) - only uses TRIF14. Subsequently we have dissected the contribution of both Myd88 and TRIF pathways in the regulation of CD4 helper T cell polarization in response to airway immunization. We have analyzed the control of Th17 effector differentiation by cellular and cytokine responses to intranasal immunization using LPS as adjuvant in parallel with poly(I:C)-dependent Th1 differentiation. Furthermore, the analysis of the pattern of inflammatory cytokines produced locally in response to airway administration of LPS or poly(I:C) and the identification of their cellular origin provide insights into the mechanisms controlling CD4 Th17 cell responses induced in the airway.
Results
CD4 T cell activation in response to intranasal immunization
To analyze the regulation of CD4 helper T cell differentiation in response to airway immunization, we have used a well-established adoptive transfer system: 105 to 106 TCR-transgenic CD4 T cells congenic for CD45.1 were transferred into CD45.2 recipients. The recipients were immunized the following day through the airway with antigen plus adjuvant.
Cells were isolated from the lung-draining mediastinal lymph node (Med LN), the popliteal lymph node (PLN) and the lungs at various times and stained with a mixture of antibodies specific for CD4, CD44, CD45.1 and CD45.2 to identify and characterize the transferred TCR transgenic CD4 T cells. Initial experiments utilized 5C.C7 TCR transgenic cells, specific for a pigeon cytochrome c (PCC) peptide. In a recent report using this model, we have shown that within 2 hours of intranasal immunization with LPS + PCC, the frequency of 5C.C7 cells in the blood diminished dramatically, followed at 18 hours by comparable diminution from the lung. Numbers of 5C.C7 cells remained constant in the Med LN over this period (data not shown)15. Furthermore, transferred CD4 cells quickly increased expression of CD69 and lost CD62L expression in the Med LN following intranasal immunization; by 6h, 31% of the cells were CD69+ and by 12h, 97% were CD69hi; almost ¾ of the CD69hi cells were also CD62Llo at 12h (Fig. S1a). By contrast, at 12h, CD4 T cells resident in lung or lung vasculature maintained a naive phenotype16–18. By 1.5 days after airway immunization, Med LN 5C.C7 cells had completely downregulated CD62L and Ly6C (data not shown); CD44 was fully upregulated. 71.8% of transferred cells were CD44hi CD45RBlo (Fig. S1b). 5C.C7 cells in the peripheral lymph nodes were only partially activated at this time. 24.3% were CD44hi CD45RBlo, only reaching full activation on day 5. Thus, the initial priming occurs mainly in the Med LN, but not in the lungs. As previously shown with comparable systems with bacterial infection, the expansion of PCC-specific T cells reached its maximum by day 7 and 8; their frequency diminished rapidly until day 10–12, remaining relatively stable or diminishing more slowly thereafter (Fig. S1c)6, 7. 5C.C7 cells in Med LN, PLN and lung retained an activated/memory phenotype out to 60 days, as shown in Fig S1b.
Use of LPS as an adjuvant in airway immunization leads to an exclusive IL-17-producing phenotype
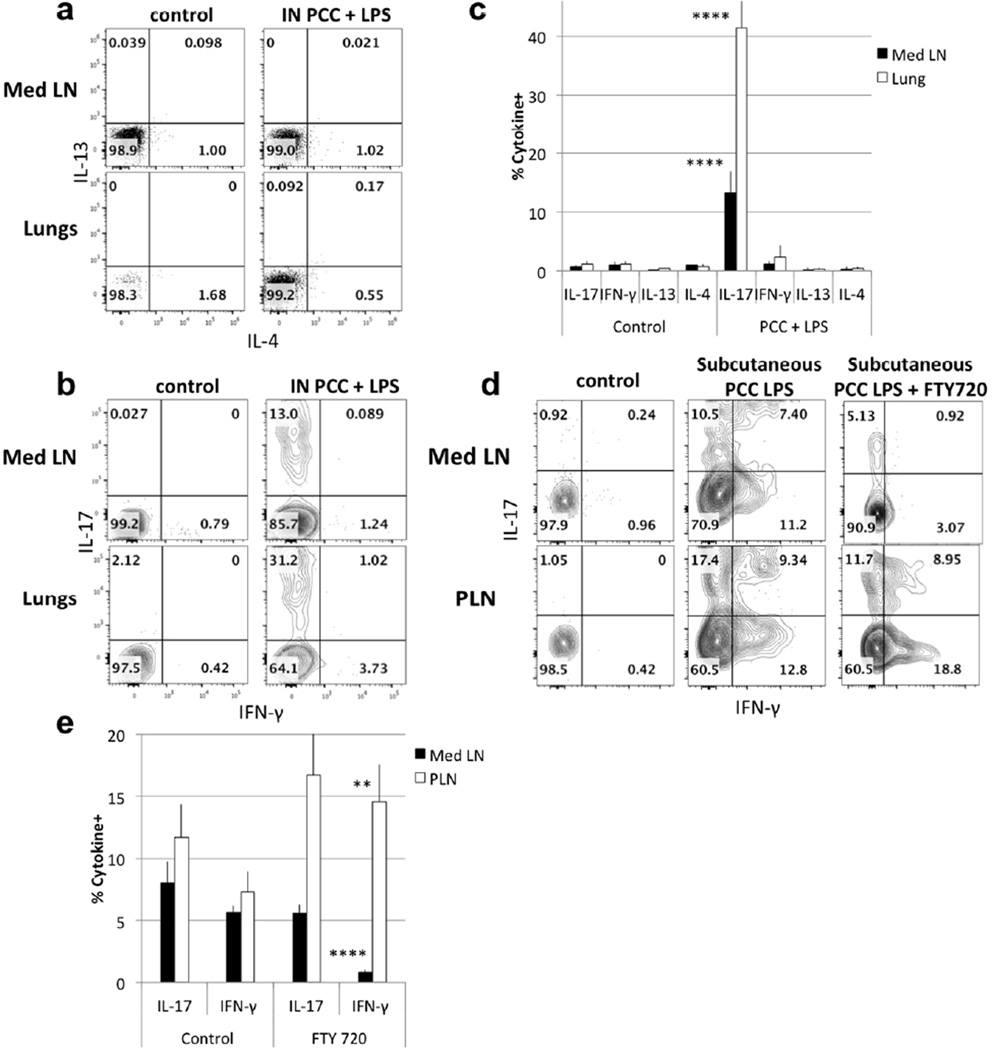
Airway immunization with LPS plus PCC resulted in Med LN and lung 5C.C7 cells that failed to produce either IFN-γ or IL-4 6h after ex vivo stimulation. 31% of lung and 13% of Med LN 5C.C7 cells from these mice produced IL-17 (Fig. 1a–c)13. Labelling 5C.C7 T cells with CFSE prior transfer revealed that IL-17-producing cells had fully diluted their CFSE (data not shown). The cytokine-producing patterns of memory phenotype cells were similar to those of the “acutely”-primed cells. When these cells were analyzed 45 days later, the Med LN 5C.C7 CD4 T cells still retained 11.8% IL-17 producing capacity (data not shown).
Figure 1. Intranasal immunization with LPS induces an IL-17 response.
(a–b) Cell suspensions from Med LN and lungs of recipients of 5C.C7 cells were restimulated for 6h with PMA and ionomycin 6 days after intranasal immunization with LPS + PCC and stained for intracellular cytokines with anti-IL-4, anti-IL-13 (a) or anti-IFN-γ, and anti-IL-17 (b). Cells were gated on CD4+ CD45.1+/ CD45.2−. (c) Mean (+/− s.e.m.) percent cytokine producing cells in Med LN and lungs of control animals and of animals immunized with PCC and LPS. **** p<0.001, **p<0.02 comparing PCC + LPS immunization with control for IL-17 production by Med LN and lung cells (unpaired Student t-test) [Data are representative of two (IL-4/ IL-13 and FTY720) or >4 (IFN-γ/ IL-17) separate experiments.] (d) Med LN and PLN cells were restimulated with PMA/ ionomycin 6 days after immunization in the hock. FTY720 was administered 12h after immunization. 5C.C7 CD45.1 cells were analyzed by intracellular staining for IFN-γ and IL-17. (e) Mean (+/− s.e.m.) percent IFN-γ-producing cells in Med LN and PLN of control and FTY-720 treated mice immunized with PCC and LPS in the hock. [Data are representative of eight (control) and four (FTY720) mice analyzed in two separate experiments] **** p<0.001, **p<0.02 (unpaired Student t-test).
In contrast to the unique IL-17-producing phenotype of airway/LPS primed 5C.C7 cells, single-dose subcutaneous immunization utilizing LPS as an adjuvant resulted in both IL-17 and IFN-γ-producing PLN cells at 5 days. 22.1% of PLN 5C.C7 cells produced IFN-γ while 26.7% produced IL-17 (Fig. 1d–e). Such “mixed” LPS-induced subcutaneous priming was also observed in cells in the Med LN of these mice, where 18.6% produced IFN-γ and 17.9% produced IL-17. Similar results were seen in other studies: intravenous or intranasal infection with Listeria monocytogenes induced specific CD4+ naive T cells to differentiate into Th1 or Th17 respectively6. To determine where the priming occurred, we have pre-treated the mice with FTY720 to inhibit DC and T cell migration from lymph nodes. The cells from subcutaneously immunized mice in the PLN still produced both IFN-γ and IL-17 while cells in the Med LN exclusively produced IL-17, implying that when priming occurred in the Med LN with LPS as an adjuvant, the response was limited to the production of IL-17. This suggests that it is the locus of priming, not the site of introduction of antigen, that determines the outcome of LPS adjuvanted priming6, 19, 20. This argument was further strengthened by transferring in vitro-primed Th1 or Th17 CD4 T cells into normal mice that were then challenged either intranasally or subcutaneously with antigen plus LPS, the transferred cells retained their initial cytokine-producing phenotype (data not shown). Thus, the local microenvironment is most important during the priming phase of the immune response.
Poly(I:C) adjuvant favors Th1 differentiation in Mediastinal LN
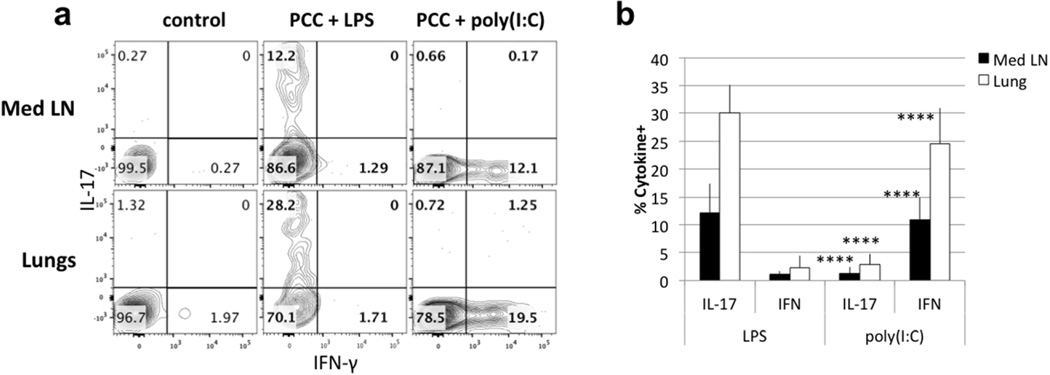
To further investigate the contribution of LPS to the response restricted to Th17, we have compared LPS with another adjuvant: poly(I:C). While responsiveness to LPS, the major structural component of the outer wall of Gram-negative bacteria, is mediated by Toll-like receptor 4 (TLR4), responsiveness to poly(I:C), an analog of double-stranded RNA, is mediated by TLR3. TLR 4 uses both a Myd88- and a TRIF/TRAM-signaling pathway while TLR3 signaling is confined to the TRIF pathway, although TRAM is not involved21. In contrast to LPS adjuvant action in the airway, intranasal immunization with poly(I:C) induced PCC-specific CD4 T cells to secrete only IFN-γ (Fig. 2a–b). 6 days after immunization, 5C.C7 CD4 T cells found in the lungs and the draining lymph nodes from mice immunized with LPS plus PCC secreted IL-17 upon stimulation (28.2% in the lungs; 12.2% IL-17 in Med LN) while those immunized with PCC plus poly(I:C) exclusively produced IFN-γ (19.5% in the lungs and 12.1% in Med LN).
Figure 2. Intranasal immunization with LPS leads to Th17 differentiation while immunization with poly(I:C) leads to Th1 differentiation.
(a) 5C.C7 cells were transferred to wildtype mice and immunized with LPS + PCC or poly(I:C) + PCC. (b) Statistical analysis compares frequency of IL-17- and IFN-γ-producing cells in Med LN and Lung of mice immunized with LPS or poly(I:C) as adjuvant. Data are representative of >13 mice from 4 different experiments. (mean ± s.e.m. **** p<0.001, unpaired Student t-test)
MyD88 and TRIF control the polarized secretion of cytokines
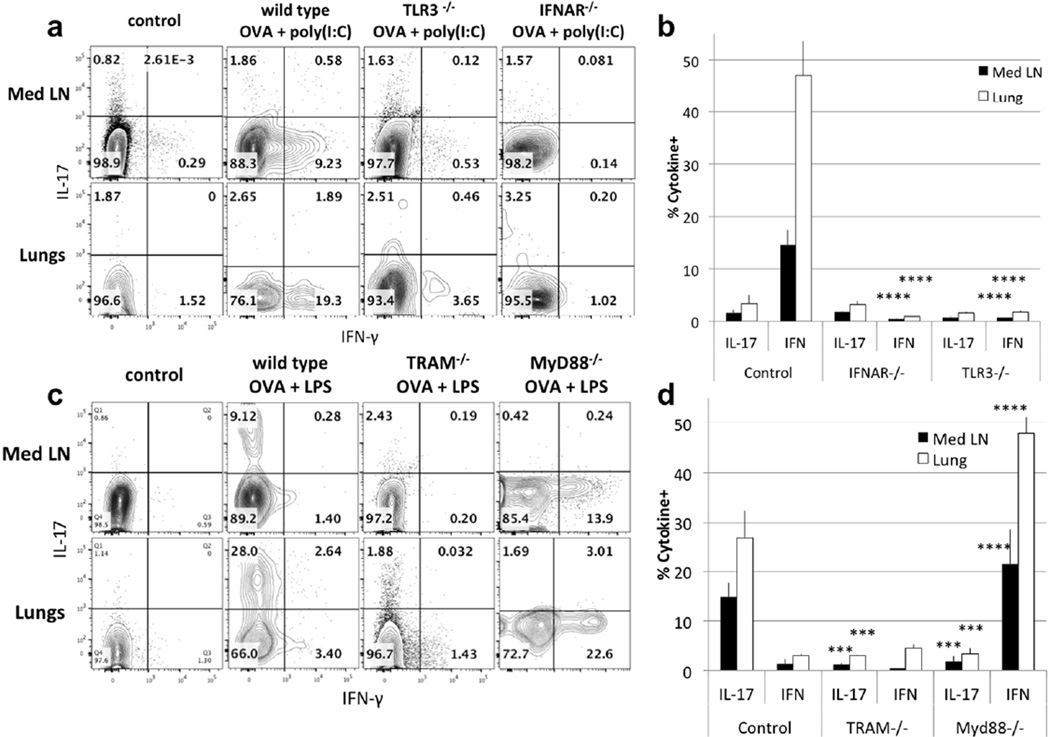
We asked whether the distinctive pattern of CD4 T cell polarization was dependent upon differences in the signalling pathways activated by these adjuvants14. Because most TLR-associated molecule knockouts are available on a C57BL/6 background, we used OT-II TCR transgenic CD4 T cells transferred into wild-type and various gene-deleted C57BL/6 recipients and assessed the types of responses induced by airway immunization with ovalbumin (OVA). Our initial hypothesis was that Myd88 would be driving the Th17 differentiation in response to LPS and TRIF would not be used. Not surprisingly, recipients that lacked TLR3 or the type I interferon receptor (TLR3−/− or IFNAR−/− recipients) failed to support differentiation of OT-II cells to the production of IFN-γ nor did these cells acquire IL-17-producing capacity (Fig. 3a, b). As previously reported, this indicates that poly(I:C) mediates its effects through type I interferon production, as a result of TLR3-mediated signaling22, 23.
Figure 3. Roles of recipient cells in the cytokine differentiation.
(a) OT-II CD4+ T cells were transferred into wild type, TLR3−/− and IFNAR−/− mice that were immunized with poly(I:C) + OVA. Transferred cells were gated and analyzed as in figure 1 for production of IFN-γ and IL-17. (b) Statistical analysis of IL-17- and IFN-γ-producing Med LN and Lung cells from IFNAR−/− compared to control mice and from TLR3−/− compared to control mice was performed on 4 to 10 mice from >2 separate experiments. (mean ± s.e.m. **** p<0.001, unpaired Student t-test)
(c) OT-II CD4+ T cells were transferred into wild type, TRAM−/− and MyD88−/− mice that were immunized with LPS + OVA. Transferred cells were analyzed for IFN-γ and IL-17. (d) Statistical analysis of IL-17- and IFN-γ-producing Med LN and Lung cells from TRAM−/− mice compared to control mice and from Myd88−/− mice compared to control mice was performed on 4 to 10 mice from >2 separate experiments. (mean ± s.e.m. **** p<0.001, *** p<0.01, unpaired Student t-test)
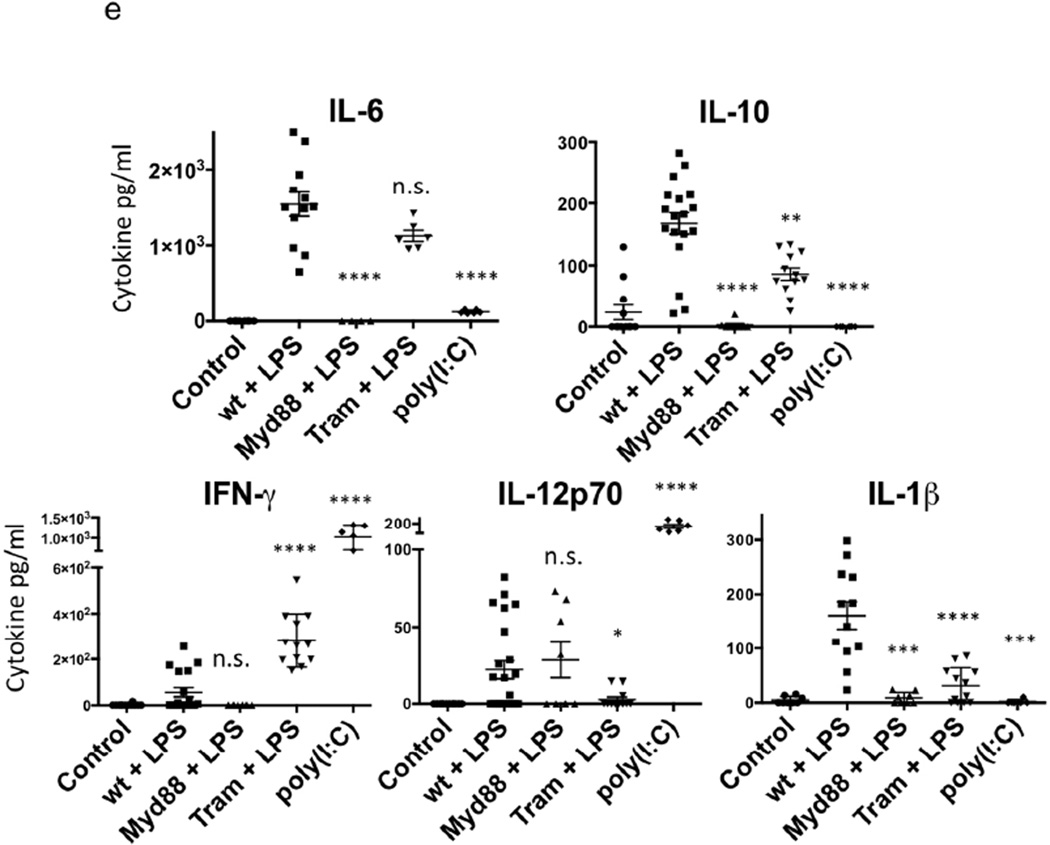
(e) Wild type, TRAM−/− and MyD88−/− B6 mice were treated with LPS or poly(I:C). Broncho alveolar lavage was performed 18h later, and fluid analyzed with Cytokine Bead Array for IL-6, IL-10, IFN-γ, IL-12p70 and IL-1β. Data are derived from 6 to 12 mice from 4 separate experiments. (Statistical analysis for each cytokine compares Myd88 + LPS, TRAM + LPS and poly(I:C) to wt + LPS. **** p<0.001, *** p<0.01, * p<0.05)
Since LPS can use both the MyD88 and TRIF pathways, we tested the capacity of recipients deficient in one or the other for their response to airway immunization with OVA plus LPS. To test the TRIF pathway, we used TRAM−/− recipients; TRAM is the adaptor molecule that links TLR4 to TRIF24. As shown in Fig. 3c, d, in TRAM−/− recipients, intranasal immunization with LPS plus OVA led to neither IL-17 nor IFN-γ production by the OT-II cells, suggesting that TRIF is critical to Th17 differentiation. Similarly, low levels of LPS present in inhaled allergens have been reported to induce IL-17 production using the TRIF pathway13.
MyD88−/− recipients immunized with OVA and LPS lost the capacity to make an IL-17 response in both Med LN and lungs. In its place, OT-II cells in these mice developed a robust IFN-γ response. This strongly suggests that the MyD88-signalling pathway is necessary for the production of IL-17 and that it directly or indirectly represses the induction of an IFN-γ response.
Deletion of TRAM or MyD88 had no effect on the capacity of poly(I:C) to induce an IFN-γ response (Fig. S2). This is not surprising since TLR3 links directly to TRIF without the need from TRAM as an intermediate.
Cytokine-Production in Response to Airway Instilled Adjuvants
To understand how Myd88 and TRIF signalling pathways in the host regulate acquisition of cytokine-producing phenotype by wild-type CD4 T cells, we analyzed production of key cytokines in the lungs in response to airway introduction of either LPS or poly(I:C). 12 to 18h after intranasal introduction of LPS or poly(I:C), we analyzed cytokine content in the broncho-alveolar (BAL) fluid. We found that LPS induced IL-6, IL-10 and IL-1β but little if any IFN-γ and modest amounts of IL-12p70 (Fig. 3e). By contrast, poly(I:C) strikingly induced IFN-γ and IL-12 p70 but little or no IL-6, IL-10 or IL-1β.
IL-1β, IL-6 and IL-23 are known to play important roles in Th17 differentiation so that the capacity of LPS to induce IL-17-producing CD4 T cells and the failure of poly(I:C) to do so is reasonable. TGF-β production by dendritic cells in response to bacterial intranasal infection has been shown to participate in Th17 differentiation in the airway12. Further, production of type I interferon and IL-12 in response to poly(I:C) but not LPS is consistent with the latter but not the former inducing a Th1 response (Fig. 3e, S3).
The TRAM−/− mouse produces ~10-fold less IL-1β in response to LPS than does the wild-type animal, consistent with its limited capacity to support a Th17 response, although its production of IL-6 remains normal (Fig. 3e). The MyD88−/− mouse failed to produce either IL-6 or IL-1β in response to LPS, consistent with its inability to mount a Th17 response in response to LPS. It also failed to produce IL-10 but did produce ample IL-12, consistent with the production of IFN-γ rather than IL-17 by OT-II cells transferred to this animal.
To further characterize the cellular origin of these helper T cell-inducing cytokines, we analyzed lung cytokine production by intracellular staining of mice treated intranasally with LPS or poly(I:C) and the lungs were harvested 48h later. IL-1β pro-form and IL-6 induced by LPS were mainly produced by CD11c− CD11b+ F4/80+ small macrophages, while IL-23p19 was produced by CD11c+ CD11b+ alveolar dendritic cells (Fig. S3a, b). Similarly, IL-6 induced by poly(I:C) was also found in small macrophages. By contrast, Th1-polarizing cytokines IFNα and IL-12p35 induced by poly(I:C) were produced by CD11c+ CD11b− alveolar macrophages, also expressing Siglec-F under inflammatory conditions.
Th1 differentiation in the airway is controlled by relative amounts of IL-10 and IL-12
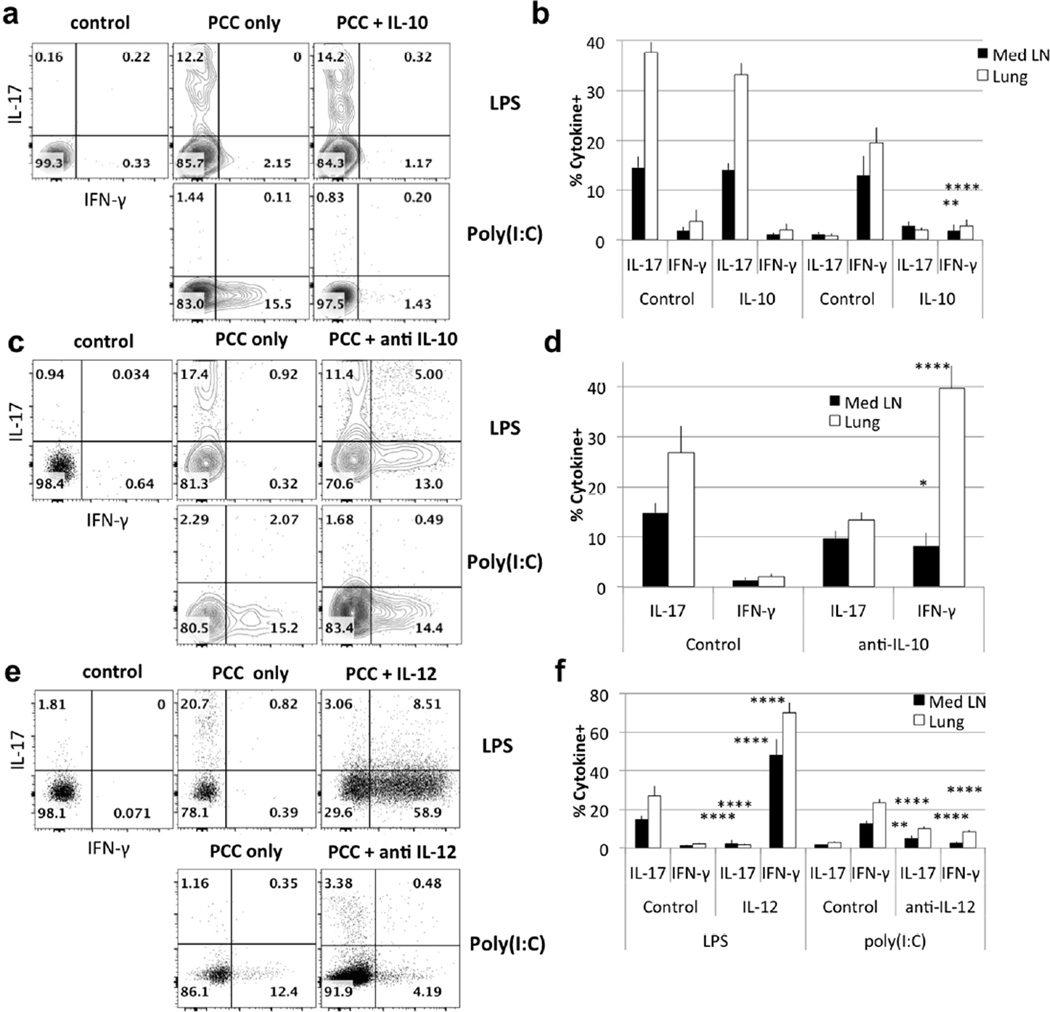
To assess the significance of the altered cytokine environments in normal and gene knockout mice treated with either LPS or poly(I:C), we treated recipients of 5C.C7 cells with either exogenous cytokines or neutralizing anti-cytokine antibodies at the time of immunization. Intranasal treatment with exogenous IL-10 completely inhibited induction of IFN-γ-producing cells in response to airway immunization with PCC and poly(I:C) while induction of IL-17-producing cells in response to LPS immunization was unaffected (Fig. 4a, b). Anti-IL-10 treatment of mice immunized with PCC plus LPS resulted in the appearance of IFN-γ- and IL-17- single producing CD4 T cells (Fig 4c, d). That is, with only modest inhibition of Th17 differentiation, neutralization of IL-10 allowed a Th1 response to occur, mimicking what is seen in subcutaneous immunization with antigen plus LPS (Fig. 1d). Similarly, a previous report showed that the rapid release of IL-10 following LPS intraperitoneal injection suppresses LPS-induced IFN-γ release, while neutralization results in increased IFN-γ levels25.
Figure 4. IL-10 and IL-12 control IFN-γ production in response to intranasal immunization.
Wild type mice received 106 5C.C7 T cells and were immunized with LPS + PCC or poly(I:C) + PCC. Transferred 5C.C7 T cells were analyzed for IFN-γ and IL-17 production 6 days after immunization.
(a) Mice were immunized with adjuvant + PCC and treated with exogenous IL-10 12h later. (c) As in (a), mice were treated with anti-IL-10 12h before immunization. (e) As in (a), mice were treated with IL-12, or anti-IL-12. (b, d, f) Data are representative of 4 mice in 2 independent experiments; p values represent comparisons of IL-17- and IFN-γ-producing cells from control mice and mice treated with IL-10, anti-IL-10, IL-12 or anti-IL-12 (mean ± s.e.m. **** p<0.001, ** p<0.02, * p<0.05 unpaired Student t-test).
IL-12 treatment of mice immunized with PCC plus LPS led the responding T cells to develop an almost exclusive Th1 response (Fig. 4e, f), implying that the presence of large amounts of IL-12 could both induce a Th1 response and suppress a Th17 response. Consistent with this idea, anti-IL-12 treatment of mice immunized with PCC plus poly(I:C) resulted in diminished IFN-γ production by the responding T cells as well as the appearance of T cells capable of producing IL-17 (Fig. 4e, f).
Control of Th17 Differentiation by IL-1β
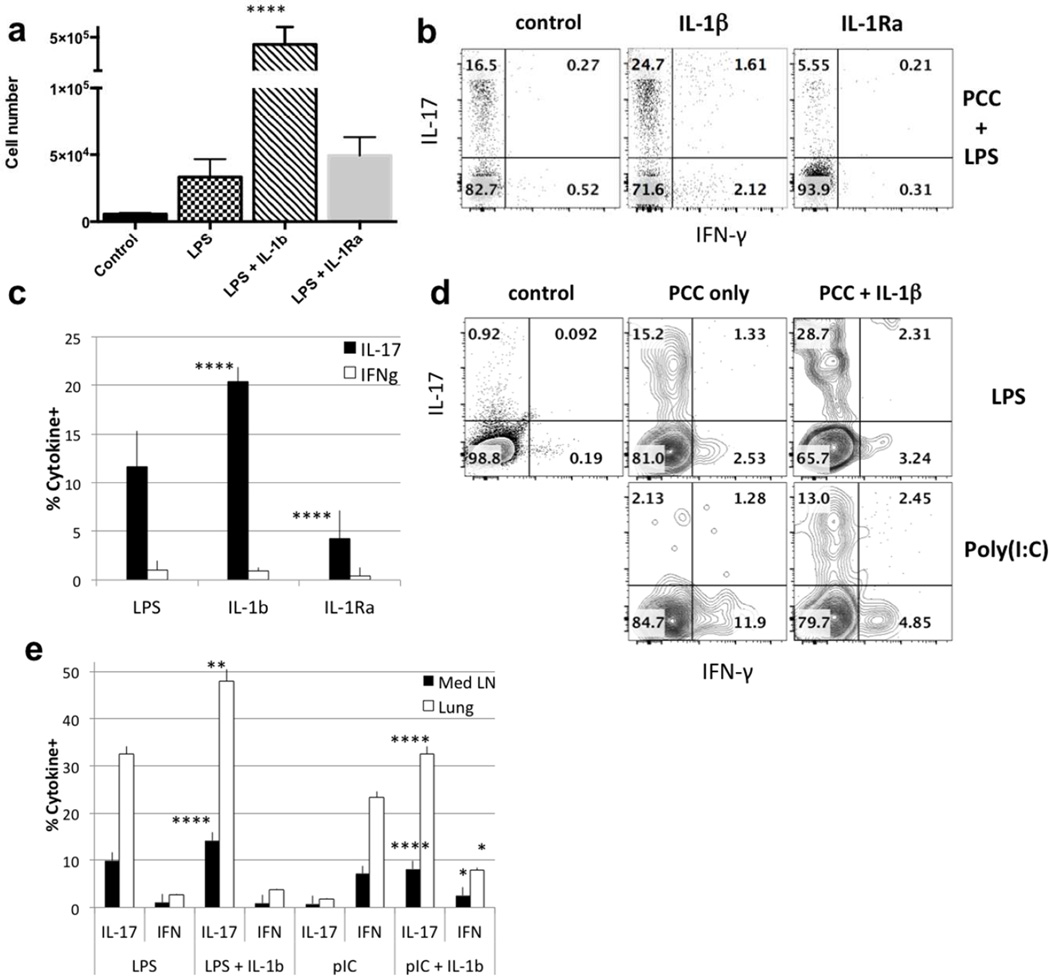
We previously described a direct effect of IL-1β on in vivo CD4 and CD8 T cells during primary and secondary responses to subcutaneous immunization26, 27. IL-1β dramatically enhanced both T cell expansion and the proportion of CD4 T cells producing IL-17. Considering the established role of IL-1β in increasing the frequency of Th17 cells during in vitro priming28, we have investigated the importance of IL-1β in our system of airway immunization. The addition of IL-1β to airway priming with PCC plus LPS increased the frequency of 5C.C7 cells by almost ten-fold (Fig. 5a). The addition of IL-1β also caused ~5-fold increase in the frequency of IL-17-producing 5C.C7 cells in mice when compared to mice that received the IL-1 receptor antagonist (IL-1Ra; Anakinra) (Fig. 5b, c). Taking the increase in the absolute number of 5C.C7 cells together with the increase in the frequency of IL-17-producing 5C.C7 cells, IL-1β treatment results in an ~25-fold increase in the absolute number of IL-17-producing cells in response to airway immunization. This IL-1β-mediated effect enhances both IL-17A and F production (data not shown) and is on the T cells themselves since it is observed when wild-type OT-II cells are transferred to IL-1R1−/− recipients (Fig. S4).
Figure 5. IL-1β is critical for IL-17 production.
(a) Total 5C.C7 cell numbers in Med LN 7 days after intranasal immunization with PCC +LPS, PCC + LPS + IL-1β or PCC + LPS + IL-1Ra. Statistical analysis was performed on data from 8 mice from 3 separate experiments. p values compare LPS +IL-1β treated mice with LPS only treated mice (mean ± s.e.m. **** p<0.001, unpaired Student t-test). (b) 5C.C7 transferred cells were analyzed for IFN-γ and IL-17 6 days after immunization with PCC + LPS, PCC + LPS + IL-1β or PCC + LPS + IL-1Ra. (c) Data are representative of >9 mice analyzed in 3 separate experiments; p values represent comparison of IL-17-producing cells from mice treated with LPS+IL-1β or LPS + IL-1Ra both compared to LPS only (**** p<0.001). (d) 5C.C7 transferred cells were analyzed for IFN-γ and IL-17 6 days after immunization with PCC + LPS or PCC + poly(I:C) ± IL-1β. (e) Statistical analysis was performed on data from 4 to 10 mice in 3 separate experiments. p values compare IL-17- or IFN-γ-producing T cells from LPS + IL-1β and LPS only treated mice or of poly(I:C) + IL- 1β and poly (I:C) only treated mice (**** p<0.001, ** p<0.02, * p<0.05)
The addition of exogenous IL-1β to airway immunization with PCC plus poly(I:C) was sufficient to induce the secretion of IL-17 (Fig. 5d, e) while modestly diminishing the production of IFN-γ in response to such immunization, suggesting that the absence of IL-1β prevents the generation of IL-17 or that IL-1β addition overcomes the need for another pathway.
Cytokine environment determines the type of response
As shown in Fig. 3e, LPS administration to wild-type mice results in production of IL-1β and IL-10 in the BAL. By contrast, airway administration of LPS to Myd88−/− mice fails to induce either cytokine. The altered pattern of IL-17 and IFN-γ production by OT-II cells primed in Myd88−/− recipients might be explained by the differences in IL-10, IL-12 and IL-1β production in these mice29.
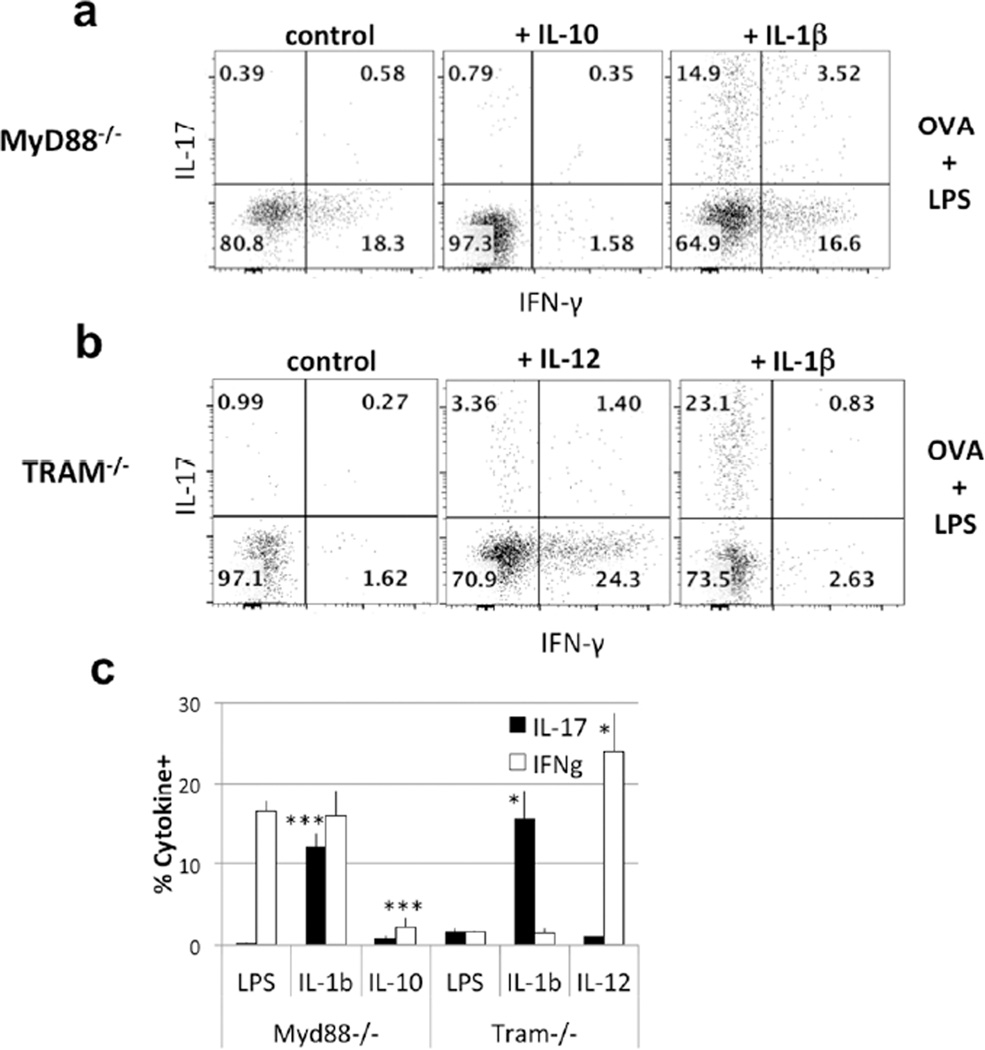
To test this hypothesis, we have transferred OT-II cells to Myd88−/− recipients that were immunized with OVA plus LPS made an IFN-γ response in the Lung. Administration of IL-10 inhibited this response but did not “rescue” the IL-17 response, arguing that IL-10 inhibited the IFN-γ response but was not important for the induction of IL-17 (Fig. 6a, c). By contrast, administration of IL-1β to Myd88−/− mice recipients of OT-II cells primed with OVA plus LPS, while not diminishing the IFN-γ-response, resulted in a robust IL-17 response.
Figure 6. IL-1β, IL-10 and IL-12 restore cytokine production in MyD88−/− and TRAM−/− recipients.
(a–b) MyD88−/− and TRAM−/− recipients of OT-II CD4 T cells were immunized with LPS + OVA and treated with exogenous IL-10, IL-1β or IL-12. Transferred CD4 T cells were analyzed 6 days later for IFN-γ and IL-17 production. (c) Statistical analysis was performed on data from 4 mice in 2 separate experiments. p values represent comparisons of IL-17- and IFN-γ-producing cells from Myd88−/− or TRAM−/−mice treated with LPS + IL-1β or with LPS + IL-10 to similar mice treated with LPS only (mean ± s.e.m. *** p<0.01, * p<0.05, unpaired Student t-test)
Administration of IL-1β to a TRAM−/− recipient also allowed the transferred OT-II cells to produce IL-17 in response to OVA plus LPS, implying that it is the deficiency of IL-1β in both Myd88−/− and TRAM−/− mice that prevents a Th17 response in such recipients of OT-II cells and thus implying that both the Myd88 and TRIF pathways are needed for an LPS-induced IL-1β production (Fig. 6b, c). Finally, the failure of TRAM−/− recipients of OT-II cells to make an IFN-γ response can be overcome by administration of IL-12. This is consistent with failure of IL-12 production in both the wild-type and TRAM−/− mice, presumably accounted for by the robust production of IL-10 in both animals.
Thus, inhibition of IFN-γ-production in wild-type mice immunized through the airway with antigen plus LPS can be attributed to Myd88-dependent IL-10 production and the failure of IL-12 production, presumably as a consequence, while the production of IL-17 by T cells in such animals can be attributed to Myd88- and TRAM-dependent IL-1β production.
IL-1β induces a Th17 response in the course of an influenza infection
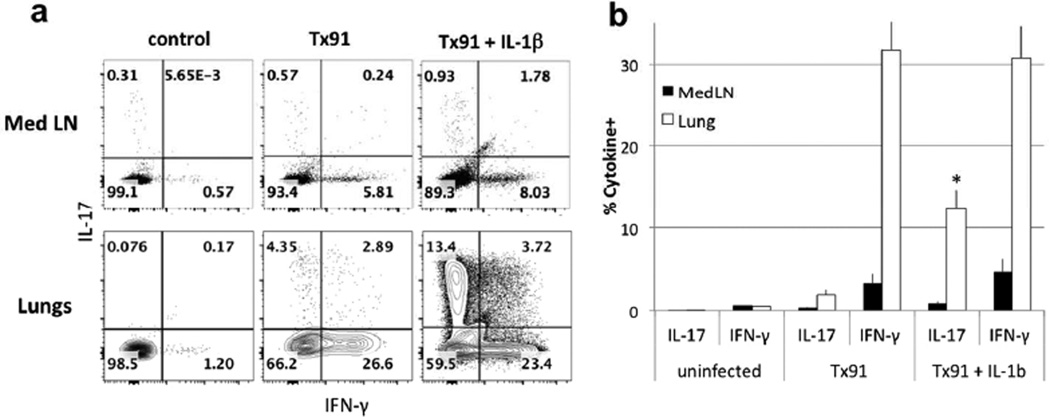
Based on all the results, we theorize that fine-tuning the cytokine microenvironment in the lungs can dramatically alter the choice of helper T cell differentiation. Using a live infection that induces a Th1 response in the airway, we have assessed whether providing IL-1β during an infection that strongly induces a CD4 IFN-γ response would also polarize to a Th17 response. We studied priming of CD4 T cells in mice that were infected intranasally with the non-lethal influenza strain H1N1 virus Tx9130, 31. As shown in Fig. 7a,b, endogenous lung CD4 T cells made a response to influenza dominated by the capacity of CD4 T cells to produce IFN-γ. The addition of IL-1β increased the proportion of IL-17 producers in the lung but did not affect IFN-γ-producing cells; only a modest proportion of the IFN-γ-producers also produced IL-17. No increase in IL-17 producers was observed in the Med LN. During rheumatoid arthritis, IL-23p19 and IL-6 expression can be induced by IL-1β via NF-κB, suggesting that the addition of IL-1β during influenza can induce IL-23p19 and IL-6 in a similar fashion32, 33.
Figure 7. IL-1β induces IL-17 production by endogenous cells during influenza infection.
(a–b) B10.A mice were infected with Tx91 influenza virus and treated 3 days later with exogenous IL-1β. Med LN and Lung CD4 T cells were analyzed 6 days later for IFN-γ and IL-17 production. Statistical analysis was performed on data from 8 mice in 3 separate experiments. p values represent a comparison of IL-17-producing cells from lungs of mice infected with Tx91 and treated with IL-1β to that of mice infected with Tx91 only (mean ± s.e.m. * p<0.05, unpaired Student t-test).
In addition, we analyzed the lung immunopathological consequences of providing IL-1β during influenza infection: immunohistochemistry analysis of lung sections shows excessive peri alveolar and transmural inflammatory cell infiltrates, and goblet cells metaplasia when IL-1β is added during Tx91 infection (Fig. 7, S5). Similar excessive immunopathology is observed in mice infected with influenza and treated with E. coli LT-IIb34. An even more striking IL-1β-mediated induction of endogenous CD4 T cells to adopt a Th17 phenotype occurred in mice infected with lymphocytic choriomeningitis virus (Fig. S6), with the frequency of IL-17-producers in the lung increasing from 10% to 37%.
Discussion
Our results suggest that in response to intranasal immunization using LPS as adjuvant: Myd88-dependent IL-1β production by lung macrophages and the expression of TRIF are required for Th17 differentiation, and that Myd88-dependent IL-10 production is required for Th1 inhibition. Although our data support recent reports that intranasal infection by bacteria induces a Th17 response6, 12, its molecular regulation at the cellular and cytokine levels remain unclear. Here, we have explored the regulation of Th17 airway responses and have shown that the distinctive pattern of inflammatory cytokines produced in response to the two adjuvants LPS and poly(I:C), can account for the highly polarized CD4 T cell responses elicited. We have systematically analyzed the regulation of Th17 differentiation, as a consequence of the Myd88/ TRIF signalling cascade in response to TLR ligands and subsequent regulation and cellular origin of the polarizing cytokines in the airway. New findings in this study provide a better understanding of the interconnection between adjuvants and polarizing cytokines during Th17 differentiation in the airway.
Subcutaneous immunization utilizing LPS as an adjuvant generally results in a CD4 T cell mixed response in the sense that cells capable of secreting IFN-γ, IL-4 or IL-17 are induced to varying degrees26, 35, 36, 37. We and others have observed that the response to LPS or bacterial airway immunization is largely limited to cells that produced IL-17 upon in vitro challenge6. Nonetheless the distinctive Th17-only cytokine profile with intranasal immunization compared with the mixed response induced by a systemic immunization has not been explained. One can argue that great precision should be required of a response aimed at lung infections since in the lung, among all mucosal tissues, an inappropriate effector response is apt to be of the greatest consequence. For example, inappropriate mobilization of neutrophils in the case of a virus infection might lead to a marked reduction in airway function, at great cost to the individual, with little or no apparent immune benefit38, 39.
In the present study, the analysis of cytokines in the broncho-alveolar lavage fluid 18 hours after airway introduction of LPS showed substantial amounts of IL-6, IL-1β and IL-10 and little to no IL-12. IL-1β pro-form and IL-6 were both detected in lung small macrophages while IL-23 was made by lung alveolar DC. A recent report showed that IL-6 and TGF-β production by dendritic cells is required for Th17 differentiation in the lungs. Our data show that neutralization of IL-1β markedly reduced the frequency of LPS-induced IL-17-producing cells without enhancing an IFN-γ response, in keeping with the known effects of IL-1β in the development of Th17 cells28. Furthermore, we found that exogenous intranasal IL-1β can induce Th17 effector cells independently of the Th1 response induced by poly(I:C). We also show that neutralizing IL-10, while only moderately diminishing the Th17 response to LPS, resulted in the appearance of IFN-γ-producing cells. This was associated with the appearance of IL-12 in the BAL, implying that IL-10 produced in response to LPS blocked the production of IL-12 and thus blocked the induction of a Th1 response. Indeed, administration of excess IL-12 in the airway of mice immunized with LPS adjuvant fully polarized the response to the Th1 phenotype.
The distinctive pattern of cytokines induced upon LPS treatment was shown to depend upon the signalling pathways activated by TLR440. A recent study showed that TRIF expression by lung dendritic cells induces a Th17 response associated with neutrophilic asthma13. Our LPS intranasal immunization model supports this and shows that when TRAM−/− mice - in which the TLR4 TRIF pathway is disabled but the Myd88 pathway remains intact - were immunized, IL-1β production was blocked but IL-10 continued to be made, accounting for the lack of a Th17 and a Th1 response respectively41. Additionally, deletion of Myd88 had an even more dramatic effect since in its absence LPS failed to induce IL-1β, IL-10 and IL-12. Thus, the Th17 response to intranasal immunization with LPS required the expression of either TRIF or Myd88 and failed to occur presumably because of the absence of IL-1β. In its place, while IL-12 levels remained very low, in the absence of IL-10 a robust Th1 response occurred. This implies that in the airway, IL-1β production in response to LPS requires contributions from signalling through both the TRIF and Myd88 pathways and only if both pathways are intact does LPS act to promote a Th17 response by transferred OT-II cells. Indeed, it has been reported that in response to TLR4-dependent LPS signalling in DCs and macrophages, Myd88 is important in pro-IL-1β production while TRIF is needed for IL-1β maturation42, 43. Furthermore, we show that in the absence of IL-10, LPS intranasal immunization can induce IFN-γ, suggesting that just like in a subcutaneous immunization, LPS has the potency to induce IFN-γ, but that this pathway is inhibited by the production of IL-10 in the airway.
The capacity of poly(I:C) to enhance a Th1 response can also be traced to the pattern of inflammatory cytokines induced in BAL. Thus, our data show that poly(I:C) induces IFN-γ and IL-12 in BAL, and IFNα and IL-12 production by alveolar macrophages22, 44. Also while blocking IL-12 in our model diminishes the Th1 response induced by poly(I:C), treating mice with IL-10 both blocks IL-12 production and prevents the Th1 differentiation45, 46. Interestingly two different cell types are mainly involved: whilst we show that alveolar macrophages are the major cell type producing Th1-polarizing cytokines, small macrophages are restricted to make Th17-inducing cytokines.
Although our analysis is based on an adoptive transfer model, it is consistent with our observations in two infectious systems. We used influenza or LCMV infections that induce highly polarized Th1 responses in the lung, consistent with the importance of IFN-γ in controlling these infections30, 47. Our data shows that instilling IL-1β in the airway in both settings strikingly induces a lung Th17 response arguing that such responses in the lung fine-tune CD4 T cell differentiation and that IL-1β remains the key to Th17 production in infectious settings just as it does in model systems. A previous report has shown significant increased mortality with IL-1R−/− mice during lethal H1N1 influenza infection, and suggested that although IL-1α/β may increase pulmonary inflammatory pathology, it enhances survival48. Although the strain we used was not lethal, our data suggest that the IL-1β-induced Th17 response could be in part responsible for the increased survival and the acute pneumonia. Keeping in mind that neutrophilia is elevated in the majority of cases of COPD in patients; the excessive recruitment of neutrophils could largely outweight the benefit of the Th17 response. Furthermore, our results with mice infected with influenza and treated with IL-1β support another study where induction of IL-17 and IL-22, and increased bacterial clearance were seen in mice co-infected with influenza A H1N1 and Staphylococcus aureus when IL-23 is overexpressed49. As combined influenza and bacterial pneumonia are among the most deadly infectious diseases, it is critical to understand the mechanisms of increased susceptibility to superinfection in the lung and subsequent coordinated cellular and cytokine responses to effectively control the infection.
Our results further suggest that airway vaccinations for resistance to lung pathogens need to use or select adjuvants to tailor the immune response and generate efficient protective immunity. We argue that CD4 T cell responses in the lung, perhaps more than in any other tissue, need to be regulated with exquisite precision if they are not to avoid to severe immunopathology perhaps coupled with poor protection. Ultimately, therapeutic targeting of key polarizing cytokines during the early steps of the immune response can reprogram the cytokine microenvironment in the airway and allow control of the inflammatory response.
Materials and Methods
Mice
B10.A and B10.A.SJL-Rag2−/− -Ptprca-5C.C7(TcRa, TcRb) were obtained from Taconic Farms (Germantown, NY). C57BL/6, C57BL/6 OT-II (B6.Cg- Tg (TcraTcrb) 425Cbn/J, Tram−/− C57BL/6J- Ticam1Lps2/J, TLR3−/− B6;129S1- Tlr3tm1Flv/J, MyD88−/− CBy.129P2(B6) - MyD88tm1Defr/J, IFNAR−/− B6.129S2- Ifnar1tm1Agt/Mmjax, IL-1R−/− B6.129S7-Il1r1tm1Imx/J were purchased from Jackson Laboratories (Bar Harbor, ME). All mice were housed under specific pathogen-free animal conditions at the National institute of Allergy and Infectious Diseases (NIAID), and used between 6 and 12 weeks of age in accordance with guidelines provided by the Institutional Animal Care and Use Committee of the NIAID.
Viruses
Mice were infected with 106 pfu Influenza H1N1 virus A/Texas/36/91 (provided by Marlene Brandes, LSB, NIAID). Mice infected with LCMV were inoculated intraperitoneally with 2 × 105 pfu Clone 13 (provided by George A. Punkosdy, LI, NIAID).
Isolation of Lung cells
Mice were bled by intracardiac puncture and lungs were extensively perfused with HBSS before being harvested. Lungs were minced with gentleMACS dissociator (Miltenyi Biotec) and digested in Liberase TM with DNase I (Invitrogen) for 30 minutes at 37°C. The reaction was stopped by adding EDTA. The digested tissue was processed on a 40µm cell strainer (BD Biosciences), and a single cell suspension was enriched on a 40/60% Percoll gradient centrifugation (GE Healthcare).
Adoptive Cell Transfer
CD4 T cells from 5C.C7 or OT-II-Tg mice were isolated from lymph nodes. 105–106 cells were adoptively transferred by intravenous injection into the retro-orbital sinus of each recipient. When specified, cells were labeled with 1µM carboxyfluorescein succinimidyl ester (Sigma) for 5min at 37°C prior transfer. After transfer, cells were allowed to home for 24h.
Immunization and in vivo treatments
Mice were immunized intranasally or subcutaneously in the hock with antigen plus adjuvant. 100µg ovalbumin or pigeon cytochrome c (Sigma) were used with either 2.5µg lipopolysaccharide (Sigma) or 20µg polyinosinic:polycytidylic acid (Invivogen). For cytokine instillation, mice were given 1µg of recombinant IL-1β, IL-6, IL-10, IL-12 (Peprotech) or 100µg IL-1Ra (Kineret) 12 to 18h after OVA or PCC challenge. Where indicated, 250µg of anti-mouse IL-6 (MP5-20F3), anti-mouse IL-10 (JES5-2A5) (BioXCell) or anti-mouse IL-12 were injected intraperitoneally 12h before antigen challenge. In some experiments, mice were injected intraperitoneally with 40µg FTY720 (Calbiochem) 12–18h after antigen challenge.
Analysis of airway cytokines
Whole lung lavage was performed in mice treated with LPS or poly(I:C) overnight. The trachea was intubated with an oral needle, 800µl medium was infused and the volume was retrieved. Concentrations of IL-1β, IL-6, IL10, IL-12p70 and IFN-γ were assessed by multiplex Cytometric Bead Array and analyzed with FCAP Array software according to the manufacturer’s instructions (BD Biosciences). IL-6 analysis is shown with bronchoalveolar lavages diluted at 1:10.
For intracellular staining, lungs were harvested at 48h, kept in vitro for 6h with monensin before staining.
Flow Cytometry
Lymph nodes and lungs were harvested and single-cell suspensions were prepared in HBSS plus FCS, sodium azide and EDTA. For intracellular cytokine staining, cells were stimulated with 10ng/ml phorbol 12-myristate 13-acetate and 1µM ionomycin in presence of 2µM Monensin for 6h, before being fixed, permeabilized and then stained. Antibodies to Ly6C (AL21), CD62L (MEL14), CD45RB (16A), CD69 (H1.2F3), CD4 (RM4), CD45.1 (A20), CD45.2 (104), IL-2 (JES6-5H4), IL-4 (11B11), IL-13, IL-17A (TC11-18H10), IL-17F (O79-289) were purchased from BD Biosciences. IL-1β pro-form (NJTEN3), IL-23 p19 (fc23cpg) were from eBioscience; CD8 (53-6.7), CD11c (N418), IL-6 (MP5-20F3) were from BioLegend and IL-12 p35 (27537) from R&D systems. Siglec F (E50-2440), CD11b (M1/70) and F4/80 (BM8) were a gift from Awen Gallimore (I&I, Cardiff, UK). After staining, cells were visualized through LSRII or LSR Fortessa flow cytometer (BD Biosciences) and analyzed with FlowJo software (TreeStar).
In vitro T cell differentiation
Naive 5C.C7 CD4 T cells were cultured with irradiated T depleted splenocytes in presence of 1µM pigeon cytochrome c peptide for 3-4 days with various combinations of antibodies and cytokines: IL-2, IL-12 and anti-IL-4 for Th1 differentiation; anti-IL-4, anti-IFN-γ, anti-IL12, IL-6, TGFβ, IL-21 and IL-23 for Th17 differentiation. Cells were then rested in IL-2 (Th1) or cytokine-free medium (Th17) before being transferred in vivo.
Immunohistochemistry
Lung lobes were perfused and immersed in 5% Formalin before staining with Hematoxylin and Eosin (American Histolab, Gaithersburg, MD). Images were acquired using a Nikon Optiphot microscope equipped with Axiocam HRc Zeiss camera.
Statistical analysis
Sample sizes were determined empirically. No animals or samples were excluded from the analysis, and no randomization or blinding was used. Differences between data sets of similar variance were analyzed by unpaired two-tailed Student's t-test. A p value >0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank G. A. Punkosdy for LCMV Clone 13; M. Brandes-Kuchen for influenza Tx91; A. Gallimore (I&I, Cardiff) for antibodies and for critically reading the manuscript; members of the Laboratory of Immunology at NIAID for discussions. This research was supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases.
Abbreviations
- Med LN
Mediastinal Lymph Node
- PCC
Pigeon Cytochrome c
- PLN
Popliteal Lymph Node
References
- 1.Li W, Deng G, Li M, Liu X, Wang Y. Roles of Mucosal Immunity against Mycobacterium tuberculosis Infection. Tuberculosis research and treatment. 2012;2012:791728. doi: 10.1155/2012/791728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cauley LS, Lefrancois L. Guarding the perimeter: protection of the mucosa by tissue-resident memory T cells. Mucosal immunology. 2013;6(1):14–23. doi: 10.1038/mi.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook DN, Bottomly K. Innate immune control of pulmonary dendritic cell trafficking. Proceedings of the American Thoracic Society. 2007;4(3):234–239. doi: 10.1513/pats.200701-026AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakano H, Burgents JE, Nakano K, Whitehead GS, Cheong C, Bortner CD, et al. Migratory properties of pulmonary dendritic cells are determined by their developmental lineage. Mucosal immunology. 2013;6(4):678–691. doi: 10.1038/mi.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khanna KM, Aguila CC, Redman JM, Suarez-Ramirez JE, Lefrancois L, Cauley LS. In situ imaging reveals different responses by naive and memory CD8 T cells to late antigen presentation by lymph node DC after influenza virus infection. European journal of immunology. 2008;38(12):3304–3315. doi: 10.1002/eji.200838602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pepper M, Linehan JL, Pagan AJ, Zell T, Dileepan T, Cleary PP, et al. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nature immunology. 2010;11(1):83–89. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambrecht BN, Pauwels RA, Fazekas De St Groth B. Induction of rapid T cell activation, division, and recirculation by intratracheal injection of dendritic cells in a TCR transgenic model. Journal of immunology. 2000;164(6):2937–2946. doi: 10.4049/jimmunol.164.6.2937. [DOI] [PubMed] [Google Scholar]
- 8.GeurtsvanKessel CH, Lambrecht BN. Division of labor between dendritic cell subsets of the lung. Mucosal immunology. 2008;1(6):442–450. doi: 10.1038/mi.2008.39. [DOI] [PubMed] [Google Scholar]
- 9.Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157(4):832–844. doi: 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Werner JL, Steele C. Innate receptors and cellular defense against pulmonary infections. Journal of immunology. 2014;193(8):3842–3850. doi: 10.4049/jimmunol.1400978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skokos D, Nussenzweig MC. CD8- DCs induce IL-12-independent Th1 differentiation through Delta 4 Notch-like ligand in response to bacterial LPS. The Journal of experimental medicine. 2007;204(7):1525–1531. doi: 10.1084/jem.20062305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linehan JL, Dileepan T, Kashem SW, Kaplan DH, Cleary P, Jenkins MK. Generation of Th17 cells in response to intranasal infection requires TGF-beta1 from dendritic cells and IL-6 from CD301b+ dendritic cells. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(41):12782–12787. doi: 10.1073/pnas.1513532112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsia BJ, Whitehead GS, Thomas SY, Nakano K, Gowdy KM, Aloor JJ, et al. Trif-dependent induction of Th17 immunity by lung dendritic cells. Mucosal immunology. 2015;8(1):186–197. doi: 10.1038/mi.2014.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301(5633):640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 15.Caucheteux SM, Torabi-Parizi P, Paul WE. Analysis of naive lung CD4 T cells provides evidence of functional lung to lymph node migration. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(5):1821–1826. doi: 10.1073/pnas.1221306110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ, et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nature protocols. 2014;9(1):209–222. doi: 10.1038/nprot.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner DL, Bickham KL, Thome JJ, Kim CY, D'Ovidio F, Wherry EJ, et al. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal immunology. 2014;7(3):501–510. doi: 10.1038/mi.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zachariah MA, Cyster JG. Neural crest-derived pericytes promote egress of mature thymocytes at the corticomedullary junction. Science. 2010;328(5982):1129–1135. doi: 10.1126/science.1188222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24(6):677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annual review of immunology. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 21.Shi G, Vistica BP, Nugent LF, Tan C, Wawrousek EF, Klinman DM, et al. Differential involvement of Th1 and Th17 in pathogenic autoimmune processes triggered by different TLR ligands. Journal of immunology. 2013;191(1):415–423. doi: 10.4049/jimmunol.1201732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longhi MP, Trumpfheller C, Idoyaga J, Caskey M, Matos I, Kluger C, et al. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. The Journal of experimental medicine. 2009;206(7):1589–1602. doi: 10.1084/jem.20090247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeon SG, Oh SY, Park HK, Kim YS, Shim EJ, Lee HS, et al. TH2 and TH1 lung inflammation induced by airway allergen sensitization with low and high doses of double-stranded RNA. The Journal of allergy and clinical immunology. 2007;120(4):803–812. doi: 10.1016/j.jaci.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 24.Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim SO, et al. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424(6950):743–748. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- 25.Marchant A, Bruyns C, Vandenabeele P, Ducarme M, Gerard C, Delvaux A, et al. Interleukin-10 controls interferon-gamma and tumor necrosis factor production during experimental endotoxemia. European journal of immunology. 1994;24(5):1167–1171. doi: 10.1002/eji.1830240524. [DOI] [PubMed] [Google Scholar]
- 26.Ben-Sasson SZ, Hu-Li J, Quiel J, Cauchetaux S, Ratner M, Shapira I, et al. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(17):7119–7124. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ben-Sasson SZ, Hogg A, Hu-Li J, Wingfield P, Chen X, Crank M, et al. IL-1 enhances expansion, effector function, tissue localization, and memory response of antigen-specific CD8 T cells. The Journal of experimental medicine. 2013;210(3):491–502. doi: 10.1084/jem.20122006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30(4):576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boonstra A, Rajsbaum R, Holman M, Marques R, Asselin-Paturel C, Pereira JP, et al. Macrophages and myeloid dendritic cells, but not plasmacytoid dendritic cells, produce IL-10 in response to MyD88- and TRIF-dependent TLR signals, and TLR-independent signals. Journal of immunology. 2006;177(11):7551–7558. doi: 10.4049/jimmunol.177.11.7551. [DOI] [PubMed] [Google Scholar]
- 30.Brandes M, Klauschen F, Kuchen S, Germain RN. A systems analysis identifies a feed forward inflammatory circuit leading to lethal influenza infection. Cell. 2013;154(1):197–212. doi: 10.1016/j.cell.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Lu X, Li J, Berube N, Giest KL, Liu Q, et al. Genetically engineered, biarsenically labeled influenza virus allows visualization of viral NS1 protein in living cells. Journal of virology. 2010;84(14):7204–7213. doi: 10.1128/JVI.00203-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu FL, Chen CH, Chu SJ, Chen JH, Lai JH, Sytwu HK, et al. Interleukin (IL)-23 p19 expression induced by IL-1beta in human fibroblast-like synoviocytes with rheumatoid arthritis via active nuclear factor-kappaB and AP-1 dependent pathway. Rheumatology (Oxford) 2007;46(8):1266–1273. doi: 10.1093/rheumatology/kem055. [DOI] [PubMed] [Google Scholar]
- 33.Rose-John S, Schooltink H. Cytokines are a therapeutic target for the prevention of inflammation-induced cancers. Recent Results Cancer Res. 2007;174:57–66. doi: 10.1007/978-3-540-37696-5_5. [DOI] [PubMed] [Google Scholar]
- 34.Gopal R, Rangel-Moreno J, Fallert Junecko BA, Mallon DJ, Chen K, Pociask DA, et al. Mucosal pre-exposure to Th17-inducing adjuvants exacerbates pathology after influenza infection. Am J Pathol. 2014;184(1):55–63. doi: 10.1016/j.ajpath.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ben-Sasson SZ, Caucheteux S, Crank M, Hu-Li J, Paul WE. IL-1 acts on T cells to enhance the magnitude of in vivo immune responses. Cytokine. 2011;56(1):122–125. doi: 10.1016/j.cyto.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim YS, Hong SW, Choi JP, Shin TS, Moon HG, Choi EJ, et al. Vascular endothelial growth factor is a key mediator in the development of T cell priming and its polarization to type 1 and type 17 T helper cells in the airways. Journal of immunology. 2009;183(8):5113–5120. doi: 10.4049/jimmunol.0901566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Donnell H, Pham OH, Li LX, Atif SM, Lee SJ, Ravesloot MM, et al. Toll-like receptor and inflammasome signals converge to amplify the innate bactericidal capacity of T helper 1 cells. Immunity. 2014;40(2):213–224. doi: 10.1016/j.immuni.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. Journal of immunology. 2002;169(1):443–453. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 39.Nagarkar DR, Wang Q, Shim J, Zhao Y, Tsai WC, Lukacs NW, et al. CXCR2 is required for neutrophilic airway inflammation and hyperresponsiveness in a mouse model of human rhinovirus infection. Journal of immunology. 2009;183(10):6698–6707. doi: 10.4049/jimmunol.0900298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thorley AJ, Ford PA, Giembycz MA, Goldstraw P, Young A, Tetley TD. Differential regulation of cytokine release and leukocyte migration by lipopolysaccharide-stimulated primary human lung alveolar type II epithelial cells and macrophages. Journal of immunology. 2007;178(1):463–473. doi: 10.4049/jimmunol.178.1.463. [DOI] [PubMed] [Google Scholar]
- 41.Sato N, Takahashi N, Suda K, Nakamura M, Yamaki M, Ninomiya T, et al. MyD88 but not TRIF is essential for osteoclastogenesis induced by lipopolysaccharide, diacyl lipopeptide, and IL-1alpha. The Journal of experimental medicine. 2004;200(5):601–611. doi: 10.1084/jem.20040689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shenderov K, Riteau N, Yip R, Mayer-Barber KD, Oland S, Hieny S, et al. Cutting edge: Endoplasmic reticulum stress licenses macrophages to produce mature IL-1beta in response to TLR4 stimulation through a caspase-8- and TRIF-dependent pathway. Journal of immunology. 2014;192(5):2029–2033. doi: 10.4049/jimmunol.1302549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eigenbrod T, Franchi L, Munoz-Planillo R, Kirschning CJ, Freudenberg MA, Nunez G, et al. Bacterial RNA mediates activation of caspase-1 and IL-1beta release independently of TLRs 3, 7, 9 and TRIF but is dependent on UNC93B. Journal of immunology. 2012;189(1):328–336. doi: 10.4049/jimmunol.1103258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Desch AN, Henson PM, Jakubzick CV. Pulmonary dendritic cell development and antigen acquisition. Immunol Res. 2013;55(1–3):178–186. doi: 10.1007/s12026-012-8359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao S, Liu J, Chesi M, Bergsagel PL, Ho IC, Donnelly RP, et al. Differential regulation of IL-12 and IL-10 gene expression in macrophages by the basic leucine zipper transcription factor c-Maf fibrosarcoma. Journal of immunology. 2002;169(10):5715–5725. doi: 10.4049/jimmunol.169.10.5715. [DOI] [PubMed] [Google Scholar]
- 46.Bradley MN, Zhou L, Smale ST. C/EBPbeta regulation in lipopolysaccharide-stimulated macrophages. Molecular and cellular biology. 2003;23(14):4841–4858. doi: 10.1128/MCB.23.14.4841-4858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Su HC, Cousens LP, Fast LD, Slifka MK, Bungiro RD, Ahmed R, et al. CD4+ and CD8+ T cell interactions in IFN-gamma and IL-4 responses to viral infections: requirements for IL-2. Journal of immunology. 1998;160(10):5007–5017. [PubMed] [Google Scholar]
- 48.Schmitz N, Kurrer M, Bachmann MF, Kopf M. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. Journal of virology. 2005;79(10):6441–6448. doi: 10.1128/JVI.79.10.6441-6448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kudva A, Scheller EV, Robinson KM, Crowe CR, Choi SM, Slight SR, et al. Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. Journal of immunology. 2011;186(3):1666–1674. doi: 10.4049/jimmunol.1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shirey KA, Lai W, Patel MC, Pletneva LM, Pang C, Kurt-Jones E, et al. Novel strategies for targeting innate immune responses to influenza. Mucosal immunology. 2016 doi: 10.1038/mi.2015.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.