SUMMARY
In obesity, macrophages and other immune cells accumulate in insulin target tissues, promoting a chronic inflammatory state and insulin resistance. Galectin-3 (Gal3), a lectin mainly secreted by macrophages, is elevated in both obese subjects and mice. Administration of Gal3 to mice causes insulin resistance and glucose intolerance, whereas inhibition of Gal3, through either genetic or pharmacologic loss of function, improved insulin sensitivity in obese mice. In vitro treatment with Gal3 directly enhanced macrophage chemotaxis, reduced insulin stimulated glucose uptake in myocytes and 3T3-L1 adipocytes and impaired insulin-mediated suppression of glucose output in primary mouse hepatocytes. Importantly, we found that Gal3 can bind directly to the insulin receptor (IR) and inhibit downstream IR signaling. These observations elucidate a novel role for Gal3 in hepatocytes, adipocytes and myocyte insulin resistance, suggesting that Gal3 can link inflammation to decreased insulin sensitivity. Inhibition of Gal3 could be a new approach to treat insulin resistance.
INTRODUCTION
Obesity-associated insulin resistance is a hallmark of type 2 diabetes mellitus, and plays a central role in metabolic syndrome (Haffner and Taegtmeyer, 2003; Olefsky and Glass, 2010; Reaven, 2005). Many studies have shown that in obesity, there is an increased accumulation of macrophages in adipose tissue (Weisberg et al., 2003; Xu et al., 2003), liver (Lanthier et al., 2009), and muscle (Pillon et al., 2013), especially pro-inflammatory M-1 like Cd11c+ macrophages which account for the majority of this increase (Lackey and Olefsky, 2016; Li et al., 2010). Our previous studies clearly show that in vivo deletion of Cd11c+ cells improved obesity-induced insulin resistance, indicating that the chronic tissue inflammatory state plays a key causal role in obesity-induced insulin resistance (Patsouris et al., 2008).
Chronic tissue inflammation leads to increased levels of pro-inflammatory cytokines, such as TNFα and IL1β, which impair insulin signaling and induce insulin resistance (Hotamisligil et al., 1996; Jager et al., 2007). However, therapeutic efforts focusing on inhibition of TNFα or IL1β to ameliorate inflammation-induced insulin resistance have had limited success in clinical studies (Donath et al., 2013; Larsen et al., 2007; Stanley et al., 2011). Thus, we hypothesize that there are other secreted factors which play a role in inflammation-induced decreased insulin sensitivity. Galectin-3 (also known as Mac-2), a 30-kDa lectin, contains a carbohydrate-recognition binding domain that binds to β-galactoside (Barondes et al., 1994a; Yang et al., 2008), and is localized in the nucleus, cytoplasm and extracellular membrane. Galectin-3 (Gal3) plays an important disease-exacerbating role in autoimmune/inflammatory and malignant diseases (Gao et al., 2013; Li et al., 2014; Liu and Rabinovich, 2010). Our previous reports found that after switching obese mice from a high fat diet (HFD) to normal chow (NC), adipose tissue Cd11c+ macrophages express much lower levels of Gal3 compared to HFD fed mice. At the same time, there was amelioration of inflammation and insulin resistance in these diet-switched mice, despite retaining the same number of adipose tissue Cd11c+ macrophages (Li et al., 2010). Therefore, we hypothesize that Gal3 can promote insulin resistance, providing a link between inflammation and decreased insulin sensitivity.
Here, we report that Gal3 KO mice were protected from inflammation and insulin resistance, and a selective small molecule Gal3 inhibitor reduces insulin resistance. Moreover, we found that in vivo Gal3 administration causes glucose intolerance and insulin resistance whereas in vitro treatment can directly induce decreased insulin sensitivity in myocytes, hepatocytes, and adipocytes. Together these findings suggest that hematopoietic cell-derived Gal3 might be a valuable drug target for the treatment of insulin resistance.
RESULTS
Metabolic Studies in WT, Gal3 −/− and Gal3 +/− Mice
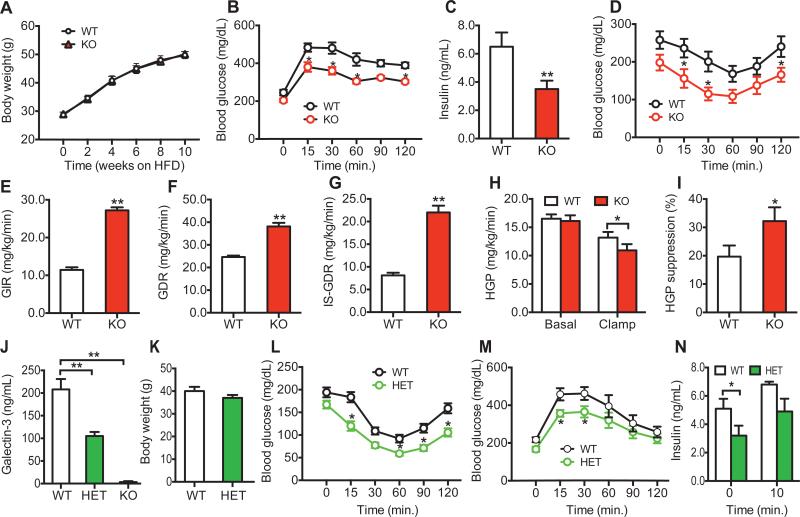
In obesity, proinflammatory macrophages accumulate in adipose tissue, liver and muscle, promoting a chronic inflammatory state, which contributes to insulin resistance. Since Gal3 is an abundant macrophage secretory factor (Liu et al., 1995; Sato and Hughes, 1994), we assessed glucose homeostasis and insulin sensitivity in chow and HFD-fed WT, Gal3 +/− (Gal3 HET), and Gal3 −/− (Gal3 KO) mice. Body weight and oral glucose tolerance tests (OGTTs) were comparable between the WT and Gal3 KO mice on chow diet (Figures S1A & B). Upon HFD feeding, WT and Gal3 KO mice gained the same amount of weight (Figure 1A); however, the Gal3 KO mice were much more glucose tolerant with lower blood insulin levels compared with WT mice (Figures 1B & C). The glucose-lowering effect of injected insulin was also greater in KO mice (Figure 1D), indicating improved systemic insulin sensitivity as a result of the Gal3 deletion. To measure tissue-specific insulin sensitivity hyperinsulinemic/euglycemic clamp studies were performed. As shown in Figures 1E-I, the amount of exogenous glucose required to maintain euglycemia (glucose infusion rate, GIR) and the glucose disposal rate (GDR) was substantially higher in the HFD Gal3 KO compared to HFD WT mice (Figures 1E & F). The insulin stimulated glucose disposal rate (IS-GDR), which primarily reflects skeletal muscle insulin sensitivity, and the ability of insulin to suppress hepatic glucose production (HGP), which reflects hepatic insulin sensitivity, was also improved in the Gal3 KO mice (Figures 1G-I). Thus, Gal3 deletion led to greater in vivo insulin sensitivity in muscle and liver.
Figure 1. Metabolic Studies in WT and Gal3 KO or Gal3 HET Mice.
(A) Body weight, (B) Glucose tolerance tests, (C) basal insulin levels, and (D) Insulin tolerance tests in 6-hr fasted WT and Gal3 KO mice on HFD. (E) Glucose infusion rate (GIR) during hyperinsulinemic, euglycemic glucose clamp studies, (F) Glucose disposal rate (GDR), (G) Insulin-stimulated glucose disposal rate (IS-GDR), (H) Hepatic glucose production (HGP), and (I) HGP suppression during hyperinsulinemic euglycemic clamp studies in WT and Gal3 KO mice on HFD. (J) Blood Gal3 levels in WT, Gal3 HET and Gal3 KO mice on HFD. (K) Body weight of WT and Gal3 HET mice on 10 weeks HFD, (L) Insulin tolerance tests (0.45 U/kg), (M) Glucose tolerance tests, and (N) Insulin levels during glucose tolerance tests in WT and Gal3 HET mice on HFD. Values are expressed as mean ± SEM. n=8-10 in each group, * P<0.05, ** P<0.01 for KO versus WT (B-J), or HET versus WT (J-N). See also Figures S1 & S2.
Since endogenous Gal3 circulates at relatively high levels in normal mice and humans, we asked whether heterozygous depletion of Gal3 would improve insulin resistance. To test this idea, HFD Gal3+/− (Gal3 HET) mice were studied. As expected, circulating Gal3 levels are 50% lower compared to HFD WT mice (Figure 1J), with values down to the normal concentrations seen in lean, chow-fed mice (Figure 2A). Consistent with the results in the Gal3 KO mice, Gal3 het mice gained the same amount of weight as WT on HFD (Figure 1K), but were insulin sensitive and glucose tolerant with lower blood insulin levels (Figures 1L-N). This indicates that ~50% deletion of Gal3 is adequate to induce a robust improvement in glucose tolerance and systemic insulin sensitivity. We also assessed tissue insulin signaling in these mice and, as seen in Figure S1C-E, we observed an increase in insulin-stimulated AKT phosphorylation in adipose tissue, liver, and muscle in the Gal3 HETs compared to WT.
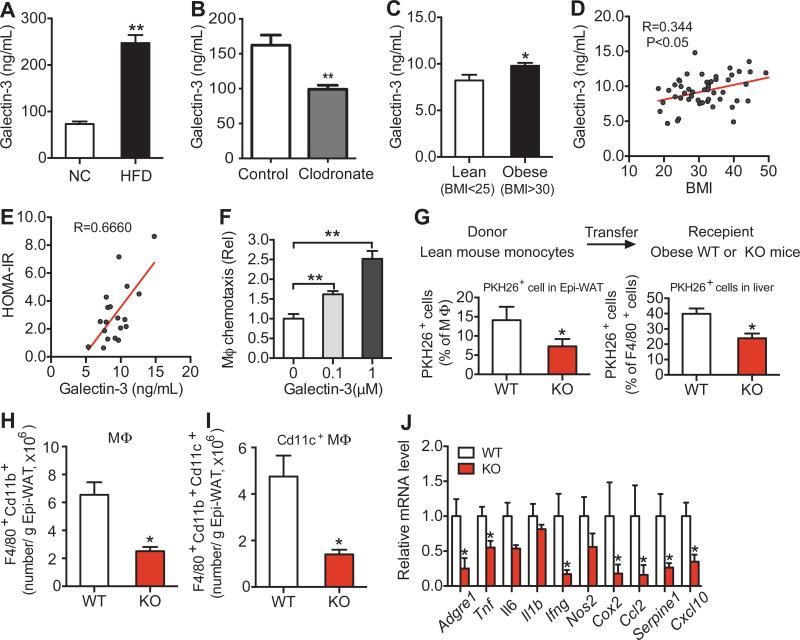
Figure 2. Obesity Increases Circulating Gal3 Levels and Gal3 Induces Chemotaxis.
(A) Blood Gal3 levels in chow and HFD fed mice. (B) Gal3 levels in clodronate or vehicle treated HFD mice. (C) Blood Gal3 levels in lean and obese human subjects. (D) Correlation analysis between Gal3 and BMI in human subjects. (E) Correlation analysis between Gal3 and HOMA-IR in human subjects. (F) In vitro macrophage chemotaxis stimulated by Gal3. (G) In vivo macrophage tracking experiments using normal WT donor monocytes and WT or Gal3 KO recipient mice. FACS analysis of labeled PKH26+, F4/80+, CD11b+ macrophages accumulating in recipient Epi-WAT (left panel) and liver (right panel). (H) FACS analysis of total F4/80+, Cd11b+ and (I) total F4/80+, CD11b+, CD11c+ adipose tissue macrophages in Epi-WAT of WT and KO mice on HFD. (J) Inflammatory gene expression in Epi-WAT. Values are expressed as mean ± SEM. n=8-10 in A, B and J, n=4-5 in F-I. * P<0.05, ** P<0.01 for KO versus WT. See also Figures S3 & S4 and Table S1.
We found that as chow-fed mice age, blood levels of Gal3 increase (Figure S1F). Interestingly, in these older chow-fed mice, Gal3 KO led to an improvement in glucose tolerance and insulin sensitivity (Figures S1G-I), unlike the results in young insulin sensitive mice (Figure S1B), but similar to the results in the HFD mice (Figures 1 B and D). In general, the degree of improvement was quite robust (40-50%) and was similar in magnitude across the HFD Gal3 KOs, HFD Gal3 HETs, and older Gal3 KO mice.
Interestingly, whereas HFD-induced obesity can lead to hepatosteatosis (Birkenfeld and Shulman, 2013; Samuel et al., 2004), we found that deletion of Gal3 did not alter the hepatic lipid profile in HFD mice (Figures S2A-G).
Circulating Levels of Gal3 are Higher in Obese Mice and Humans
Since obesity is associated with macrophage accumulation in metabolic tissues and macrophages produce and secrete Gal3 (Liu et al., 1995; Sato and Hughes, 1994), it seemed probable that blood Gal3 levels are elevated in obesity. To test this hypothesis, we measured circulating Gal3 by ELISA and found that Gal3 levels were nearly three times as high in HFD mice compared to lean age-matched chow-fed mice (Figure 2A). In vivo administration of clodronate to mice is an established method to deplete macrophages, and previous studies have shown that clodronate treatment leads to a marked decreased in systemic inflammation along with an increase in glucose tolerance and insulin sensitivity (Feng et al., 2011; Li et al., 2015). Interestingly, in clodronate-treated mice there was a marked decrease in circulating Gal3 levels, reducing these values from the elevated concentrations seen in HFD mice down to the normal levels seen in chow-fed mice (Figure 2B). These results suggest that macrophages may account for the majority of the increase in Gal3 levels seen in obesity.
With respect to human studies, obese subjects display significantly higher Gal3 levels than lean subjects (Figure 2C). Furthermore, Gal3 levels are positively correlated to both BMI and HOMA-IR (an index of insulin resistance, Figures 2D & E), suggestive for a role in insulin resistance in man.
Gal3 and Macrophage Migration
It has been reported that Gal3 produces chemotactic effects on immune cells (Sano et al., 2000), and consistent with this, we found that Gal3 stimulated in vitro chemotaxis of mouse intraperitoneal macrophages (IP–Macs) in a dose-dependent manner (Figure 2F). MCP-1 (100 ng/ml)-containing media can also induce migration of WT IP-Macs (Figure S3A). Interestingly, loss of Gal3 does not significantly alter the ability of MCP1 to induce chemotaxis (Figure S3A). With respect to another macrophage function, in vitro phagocytosis was reduced in the Gal3 HET macrophages and further reduced in the Gal3 KO cells (Figure S3B).
To determine if these in vitro chemotaxis results translated to the in vivo situation, we directly measured macrophage migration into adipose tissue using an in vivo macrophage tracking technique. With this approach, circulating monocytes were obtained from WT donor mice and labeled with fluorescent PKH26 dye ex vivo. The labeled monocytes were then injected into recipient HFD-fed WT or Gal3 KO mice. We found a substantially lower number of labeled macrophages in adipose tissue and liver in Gal3 KO mice (Figure 2G). We also conducted FACS analysis of epididymal adipose tissue (Epi-WAT) stromal vascular cells (SVCs) prepared from HFD WT and Gal3 KO mice. The results showed a lower total number of adipose tissue macrophages (ATMs) as well as ~70% fewer proinflammatory CD11c-positive macrophages in Gal3 KO Epi-WAT (Figures 2H-I & Figure S4). This indicates a reduced inflammatory state, and fully consistent with this idea, we found decreased expression of a variety of proinflammatory genes in adipose tissue from Gal3 KOs compared to WT (Figure 2J & Table S1).
Gal3 Directly Induces Cellular Insulin Resistance
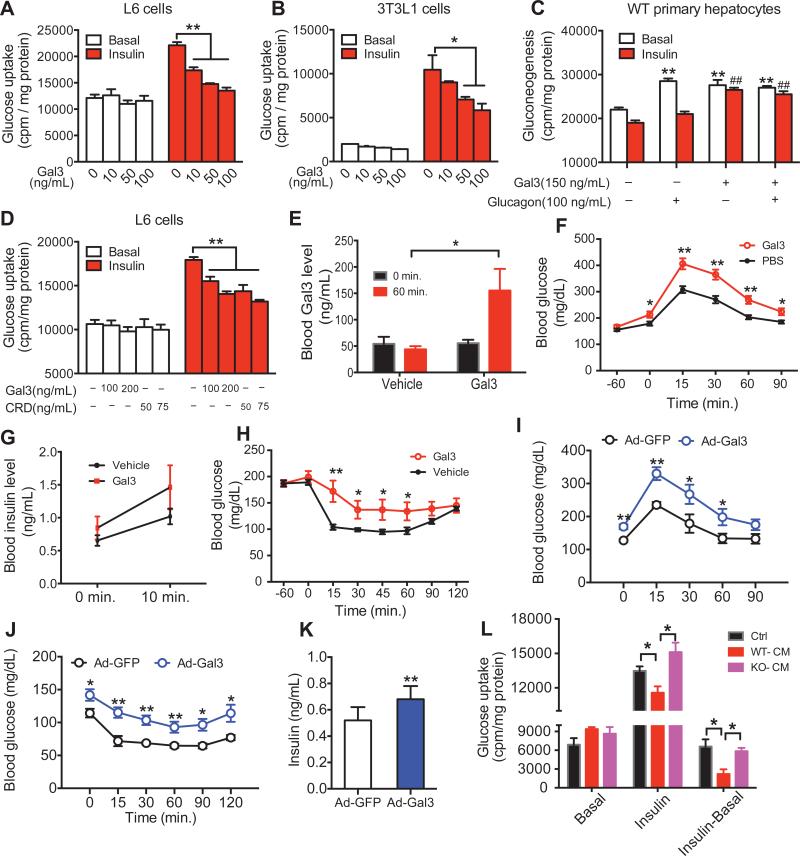
Given that circulating Gal3 levels are elevated in obesity (Figure 2A), we asked whether Gal3 could directly act on insulin target tissues in an endocrine manner to impair insulin signaling. To assess this, we studied the effects of Gal3 treatment on L6 myocytes, 3T3-L1 adipocytes, and primary hepatocytes. Gal3 treatment inhibited insulin-stimulated glucose transport in a dose-responsive manner in L6 myocytes and 3T3-L1 adipocytes (Figures 3A & B). The same effect was observed after longer (48 hours) Gal3 treatment (Figure S5A). To indicate the relevancy of these findings to human biology, we conducted in vitro Gal3 treatment studies in human inducible pluripotent stem cell (iPSC)-derived skeletal muscle cells. Insulin induced a ~2-fold increase in glucose transport in iPSC-derived myocytes, and this effect was blocked by Gal3 in a dose-dependent manner (Figure S5B). In WT primary hepatocytes, glucagon stimulates hepatic glucose output (HGO) and this increase was completely inhibited by insulin (Figure 3C). Gal3 alone also stimulated HGO and, as expected, this effect was not inhibited by insulin (Figure 3C 3rd set of bars). Combination treatment with Gal3 and glucagon had the same stimulatory effect on HGO as glucagon alone, and importantly, Gal3 blocked the effect of insulin to inhibit glucagon-stimulated HGO (Figure 3C, 4th the set of bars). Taken together, these results indicate that Gal3 can directly induce cellular insulin resistance in the 3 major insulin target tissues.
Figure 3. Gal3 Directly Induces Cellular Insulin Resistance.
(A) Dose response effect of Gal3 on insulin-stimulated glucose uptake in L6 myocytes. (B) Dose response effect of Gal3 on insulin stimulated glucose uptake in 3T3-L1 differentiated adipocytes. (C) Effect of Gal3 on gluconeogenesis in primary mouse hepatocytes. (D) Effect of CRD-Gal3 on insulin stimulated glucose uptake in L6 myocytes. (E) Circulating Gal3 levels after injection of 0.2 mg/kg recombinant Gal3. (F) Effect of exogenous Gal3 treatment (0.2 mg/kg) on glucose tolerance, (G) glucose-induced insulin secretion, (H) and insulin tolerance in chow fed insulin sensitive mice. Effect of Ad-Gal3 on glucose tolerance, (I) insulin tolerance, (J) and insulin levels (K) in chow fed mice. (L) Effect of CM from IPMacs on insulin stimulated glucose uptake in L6 myocytes. Values are expressed as mean ± SEM. n=8-10 in each group, * P<0.05, ** P<0.01. See also Figure S5.
Gal3 can interact with glycosylated proteins through its C-terminal domain, termed the carbohydrate recognition domain (CRD), whereas full length Gal3 forms oligomers through the N-terminal domain (Figure S5C) (Ahmad et al., 2004; Barondes et al., 1994b). To determine if the CRD-Gal3 has the same effect as full length Gal3, L6 myocytes were treated with CRD-Gal3, followed by measurement of insulin-stimulated glucose uptake. As shown in Figure 3D, the CRD-Gal3 inhibits glucose uptake similar to full length Gal3.
To determine if these in vitro results translated to the in vivo situation, we directly injected lean insulin-sensitive chow-fed mice with recombinant Gal3. In these studies, we injected a dose of Gal3 (0.2 mg/kg) designed to raise the circulating levels from the normal values observed in lean chow mice up to the elevated values seen in HFD WT mice as shown in Figure 3E. Consistent with the in vitro data, GTTs performed 1 hour after injection showed that these mice became glucose intolerant, with a 45% reduction in AUC (area under the curve, Figured 3F). In addition, these treated mice exhibit hyperinsulinemia (Figure 3G) and a marked decrease in insulin sensitivity as shown by a 55% increase in AUC during ITTs (Figure 3H). We also overexpressed Gal3 in chow fed mice by IV administration of an adenovirus encoding the Gal3 gene (Ad-Gal3). Ad-Gal3 leads to increased Gal3 levels, producing glucose intolerance (60% increase in AUC, Figure 3I), insulin intolerance (62% increase in AUC, Figure 3J) and hyperinsulinemia (Figure 3K), showing that Gal3 can directly induce insulin resistance in vivo. It is important to note that LPS could not be detected in our recombinant Gal3 preparations (Figure S5D). Despite this, we took the extra step of detoxifying the Gal3 (Figure S5D) and, as expected, LPS was not detected in this material compared to the Gal3 preparation which had been spiked with LPS at a concentration of 0.5 pg/ml.
Given that IP-MACs and other monocytes or macrophages can secrete Gal3 (Figures S5E & F) and that Gal3 inhibited insulin action, it seems possible that macrophages can directly affect insulin action through Gal3. To access this, we isolated conditioned medium (CM) either from WT or KO IP-MACs, followed by glucose uptake assays in L6 myocytes. As shown in Figure 3L, CM from WT IP-MACs significantly blocked insulin-stimulated glucose uptake compared to control medium. Importantly, the CM from KO IP-MACs had no effect to inhibit insulin action in L6 myocytes.
Mechanisms of Gal3-induced Cellular Insulin Resistance
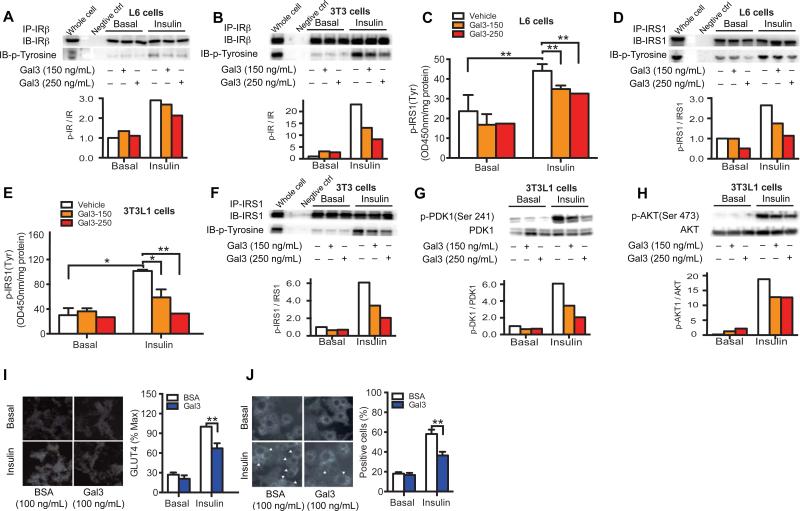
Insulin binds to the insulin receptor (IR), stimulating IR tyrosine auto phosphorylation and kinase activity, leading to phosphorylation and recruitment of substrate adaptors such as insulin receptor substrate (IRS1). IRS1 then stimulates P13 kinase and PDK1 which signal downstream to AKT and glucose transport. We used L6 myocytes and 3T3-L1 cells to assess the effects of Gal3 on these events. As seen in Figures 4A and B, Gal3 treatment inhibits insulin-stimulated IR tyrosine phosphorylation in both cell types. There was a corresponding decrease in IRS1 tyrosine phosphorylation, as measured both by ELISA assay and western blotting in L6 myocytes and 3T3-L1 adipocytes (Figures 4C-F). Consistent with these results, Gal3 treatment inhibited the effects of insulin to stimulate PDK1 (Figure 4G and Figure S6A) and AKT (Figure 4H and Figure S6B) phosphorylation in a dose-responsive manner in both cell types. In myocytes and adipocytes, insulin stimulation leads to GLUT4 translocation with subsequent increased glucose uptake. GLUT4 translocation was measured using the plasma membrane sheet assay (Figure 4I), and by immunofluorescence detection of GLUT4 recruitment to the cell surface using the GLUT4 ring assay (Figure 4J), as previously described (Imamura et al., 1999; Vollenweider et al., 1997). Both of these measurements show that Gal3 treatment reduces insulin-stimulated GLUT4 translocation in 3T3-L1 adipocytes (Figures 4I & J). In aggregate, these data indicate that Gal3 impairs all the major steps in the insulin signaling pathway.
Figure 4. In Vitro Gal3 Treatment Impairs Insulin Signaling.
(A) Gal3 impairs insulin-stimulated IR tyrosine phosphorylation as shown by IP and IB for IRβ in L6 myocytes and (B) 3T3-L1 adipocytes. (C) Gal3 impairs insulin-stimulated IRS1 tyrosine phosphorylation as shown by ELISA and (D) WB in L6 myocytes. (E) Gal3 impairs insulin-stimulated IRS1 tyrosine phosphorylation as shown by ELISA and (F) Western blot (WB) in 3T3-L1 adipocytes. (G) Gal3 impairs insulin-stimulated p-PDK1 and (H) p-Akt in 3T3-L1 adipocytes. (I) Gal3 decreases insulin-stimulated Glut4 translocation as measured by the by sheet assay and (J) immunofluorescence Glut4 staining ring assay in 3T3-L1 adipocytes. Values are expressed as mean ± SEM. * P<0.05, ** P<0.01. See also Figure S6.
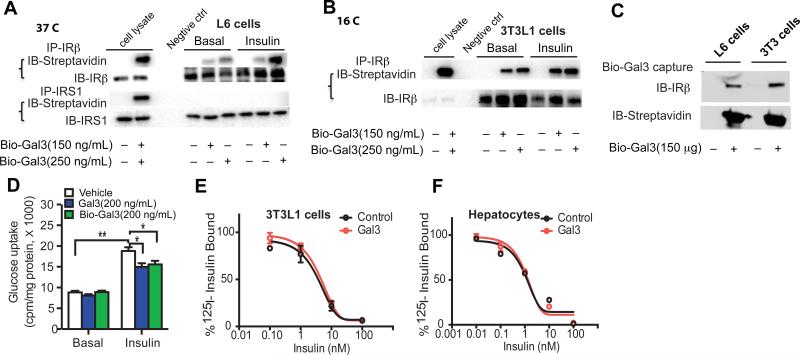
Most likely this is due to a direct inhibitory effect of Gal3 on the IR. Consistent with this, Figure 5 shows that when L6 cells and 3T3-L1 adipocytes are treated with Gal3, IR/Gal3 complexes can be co-precipitated from these cells. To evaluate Gal3 binding to the IR and IR phosphorylation, L6 myocytes were treated with biotinylated Gal3 (Bio-Gal3). As seen in Figure 5A, Bio-Gal3/IR complexes can be co-precipitated from these cells. In contrast, Gal3/IRS1 co-immunoprecipitated complexes were not detected (Figure 5A). Interestingly, the amount of IR co-precipitated with Gal3 was increased in the presence of insulin, suggesting that the binding of insulin to its receptor renders the IR/Gal3 interaction more efficient (Figure 5A). Furthermore, when similar experiments were performed at 16°C (to limit any IR internalization), the same results were seen with respect to Gal3/IR binding (Figure 5B). In the reverse experiment in which Bio-Gal3 was captured followed by immunoblotting of IR, comparable Gal3/IR binding was also detected (Figure 5C). In control studies, we also show that, Bio-Gal3 can impair insulin-stimulated glucose uptake in L6 cells (Figure 5D), similar to WT Gal3. To evaluate whether Gal3 treatment affects binding of insulin to its receptor, we performed insulin binding assays in both 3T3-L1 adipocytes and primary hepatocytes. As seen in Figures 5E & F, Gal3 treatment did not affect insulin binding, suggesting that Gal3 interacts with the insulin receptor at a different site than insulin.
Figure 5. Gal3 Interacts With the Insulin Receptor and does not Interfere with Insulin Binding.
(A) Co-immunoprecipitation of Bio-Gal3 with the IR, but not IRS1, at 37°C in L6 myocytes. (B) Co-immunoprecipitation of Bio-Gal3 with the IR at 16°C using 3T3-L1 adipocytes. (C) Immunoblotting of IR after bead capture of Bio-Gal3 from L6 myocytes or 3T3-L1 adipocytes treated with Bio-Gal3. (D) Comparable effect of Bio-Gal3 and recombinant Gal3 to inhibit insulin-stimulated glucose uptake in L6 myocytes. (E) Insulin binding to 3T3-L1 adipocytes in the presence of 200 ng/ml Gal3. (F) Insulin binding to primary hepatocytes in presence of 200mg/ml Gal3. Values are expressed as mean ± SEM.
Studies in Chimeric Gal3−/− Mice
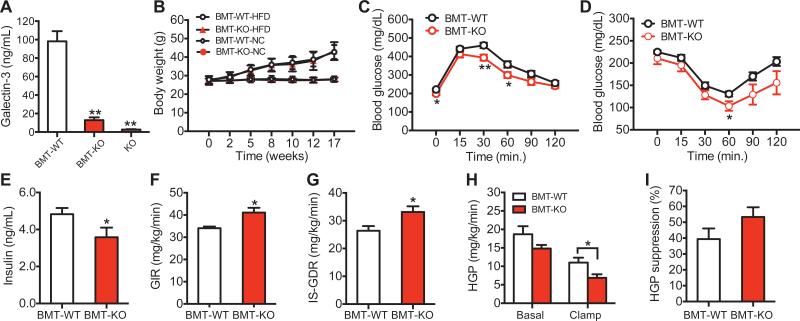
Since Gal3 is secreted by macrophages in vitro (Liu et al., 1995; Sato and Hughes, 1994), we determined the contribution of immune cells to the circulating Gal3 pool by performing bone marrow transplantation (BMT) experiments. With this adoptive transfer approach, we generated chimeric mice in which WT mice received bone marrow from either WT mice (BMT Gal3) or Gal3 KO mice (BMT Gal3 KO). As shown in Figure 6A, blood Gal3 levels were decreased by ~90% in BMT Gal3 KO mice, indicating that most of the circulating Gal3 is derived from immune cells. The BMT Gal3 and BMT Gal3 KO mice gained the same about of weight on HFD (Figure 6B). However, the BMT Gal3 KO mice were more glucose tolerant and insulin sensitive with lower insulin levels compared to BMT Gal3 mice (Figures 6C-E). Moreover, hyperinsulinemic euglycemic glucose clamp studies showed higher GIR, IS-GDR, lower HGP after insulin infusion, and elevated HGP suppression (Figures 6F-I) in BMT Gal3 KO versus BMT Gal3 animals. Taken together, these results indicate that immune cells are the major contributor to systemic Gal3 concentrations in mice and that circulating Gal3 induces systemic insulin resistance.
Figure 6. Metabolic Studies in Chimeric BMT Gal3−/− mice.
(A) Blood Gal3 levels. (B) Body weight curves. (C) Glucose tolerance tests. (D) Insulin tolerance tests. (E) Basal insulin levels in WT and Gal3 KO BMT mice on HFD. Hyperinsulinemic, euglycemic glucose clamp studies performed in WT and KO BMT mice on HFD for measurements of (F) GIR, (G) IS-GDR, (H) HGP, and (I) HGP suppression during the clamp study. Values are expressed as mean ± SEM. and n=8-10 in each group, *P < 0.05, **P < 0.01.
Gal3 Inhibition Improves Insulin Sensitivity
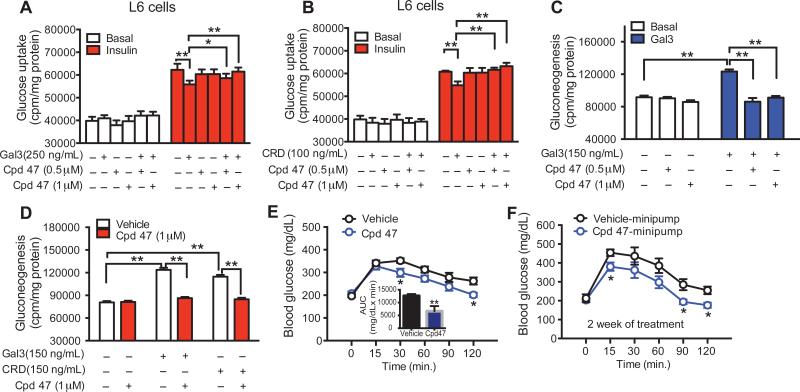
The above studies clearly show that Gal3 can directly induce cellular insulin resistance in vitro and systemic in vivo insulin resistance and that genetic Gal3 loss of function improves insulin sensitivity. To further define the role of Gal3 and assess its potential translational implications, we turned to a pharmacologic loss of function approach. Thus, we treated L6 myocytes with the Gal3 inhibitor compound 47 (Cpd47) (Salameh et al., 2010) and found that it blocked the inhibitory effect of both full length Gal3 and the CRD-Gal3 on insulin-stimulated glucose transport (Figures 7A & B). The Gal3 inhibitor also blocked the effect of Gal3 to inhibit the repressive effects of insulin on HGO in primary hepatocytes (Figures 7C & D). To extend these observations to the in vivo situation, obese HFD mice were treated with a single dose (50 mg/kg) of Cpd47 and OGTTs were performed 1 hour later. As Figure 7E shows, the Gal3 inhibitor improved glucose tolerance in these mice. To evaluate the effects of this inhibitor in the more chronic setting and at lower doses, we administered Cpd47 to HFD mice via an osmotic minipump at a dose of 6.4 mg/kg per day for two weeks. In agreement with the acute treatment data, chronic treatment also improved glucose tolerance in HFD/obese mice (Figure 7F).
Figure 7. Gal3 Inhibitor Improves Insulin Sensitivity.
(A) The Gal3 inhibitor (compound 47) mitigates the Gal3 and (B) CRD Gal3-induced decrease in insulin stimulated glucose transport in L6 myocytes. (C) The Gal3 inhibitor blocks Gal3 and (D) CRD Gal3-stimulated HGO in primary hepatocytes. (E) Single dose treatment with the Gal3 inhibitor (50 mg/kg, intraperitoneal injection) improves glucose tolerance in HFD mice. (F) Two week treatment with the Gal3 inhibitor (6.4 mg/kg) via an osmotic mini pump improves glucose tolerance in HFD mice. Values are expressed as mean ± SEM, n=8-10 in each group, *P<0.05, **P<0.01.
DISCUSSION
Here we show that circulating Gal3 levels are elevated in obese mice and humans and that Gal3 is a macrophage-derived factor that can cause in vivo systemic insulin resistance both acutely and chronically. In addition, Gal3 treatment in vitro causes decreased insulin action in adipocytes, muscle cells and hepatocytes by inhibiting insulin receptor activity, leading to decreased downstream signaling to the insulin action cascade. Gal3 also promotes adipose tissue inflammation by inducing macrophage chemotaxis and the accumulation of proinflammatory ATMs in obesity. Thus, these studies show that bone marrow-derived cells, such as macrophages, can secrete Gal3, which enters the circulation and can cause cellular and systemic insulin resistance. In this way, Gal3 is identified as an etiologic factor which contributes to obesity-induced insulin resistance and chronic tissue inflammation.
A major piece of evidence for this new role of Gal3 in insulin resistance comes from our KO mouse studies. Here we find that deletion of Gal3 leads to improved glucose tolerance with enhanced in vivo insulin sensitivity (Figures 1B-D) in HFD Gal3 KO mice. Using hyperinsulinemic euglycemic clamp studies, this increased insulin sensitivity is expressed in both muscle and liver (Figures 1E-I). This insulin sensitive phenotype was not limited to homozygous KOs, since heterozygous Gal3 deletion, which reduces Gal3 levels in Gal3 HETs down to values observed in lean mice, also causes greater glucose tolerance and insulin sensitivity (Figures 1L-N), indicating that a full reduction in circulating Gal3 levels is not necessary to observe this phenotype. Interestingly, the insulin sensitization induced by Gal3 deficiency is not restricted to the context of obesity and HFD, since older chow-fed mice with higher blood Gal3 levels also display improved glucose tolerance upon Gal3 KO (Figures S1H & I).
Deletion of Gal3 from the bone marrow compartment by transplanting Gal3 KO bone marrow into irradiated WT mice also produced a state of improved insulin sensitivity and glucose tolerance (Figures 6C-I). Importantly, these studies demonstrated a >90% reduction in circulating Gal3 levels in BMT Gal3 KO mice (Figure 6A), indicating that BM-derived cells are the dominant source of this circulating factor. As reported previously, Gal3 is poorly expressed in mouse lymphocytes and neutrophils (Joo et al., 2001; Sato et al., 2002) and this strengthens our conclusion that macrophages are a major source of the secreted circulating Gal3. Consistent with this, ATM Gal3 content increases on HFD and decreases on chow diet, parallel to the changes in blood Gal3 levels. With respect to the source of circulating Gal3, when we administered clodronate to WT HFD mice, we were able to achieve ~80% depletion of ATMs and this corresponded to a decrease in circulating Gal3 levels from the elevated concentration seen in HFD mice down to the normal concentrations seen in lean mice (Figure S3A). This suggests that a sizable component of the obesity-induced elevation of circulating Gal3 can be derived from clodronate-sensitive macrophages.
In addition to deletion of Gal3, we carried out Gal3 treatment studies. These experiments were conducted either by injecting exogenous Gal3 into WT animals, or by using an adeno-Gal3 to generate Gal3 transgenic mice. Both methods of Gal3 administration led to glucose intolerance and insulin resistance (Figures 3F-K), fully consistent with the phenotype observed with Gal3 deletion (Figure 1). In our Gal3 treatment studies, we used a dose of Gal3 designed to raise the circulating values from the normal concentration seen in lean chow fed mice to the elevated levels observed in obese HFD mice (Figure 3E). Elevating the Gal3 levels to the pathophysiologic obese concentrations in these lean mice led to a marked deterioration of glucose tolerance and insulin sensitivity (Figures 3F-H). Lastly, we supplemented our KO studies by conducting pharmacologic loss of function experiments with a Gal3 inhibitor. Treatment of obese animals with this inhibitor, either acutely or chronically, led to improved glucose tolerance and insulin sensitization (Figures 7E & F), consistent with the results in the Gal3 KO mice. The Gal3 inhibitor also reversed the effects of Gal3 to cause cellular insulin resistance in vitro (Figures 7A-D).
The concomitant improvement in insulin sensitivity and inflammatory status in the Gal3 KO and Gal3 inhibitor treated mice fits quite well with the known interaction between obesity-induced chronic inflammation and insulin resistance. In the current case, our data show that Gal3 can directly cause decreased insulin sensitivity in the three major insulin target tissues, adipocytes, muscle and liver. As such, these findings amplify the underlying mechanisms connecting inflammation and insulin resistance. Although the contribution of chronic inflammation to insulin resistance is reasonably well established, therapeutic attempts to correct this have not had adequate success in clinical studies. Based on our findings with Gal3, one can raise the possibility that previous therapeutic attempts to ameliorate inflammation-induced insulin resistance and hyperglycemia have not been properly directed at the underlying mechanism. These studies indicate that inhibition of Gal3 could be a future approach to the treatment of insulin resistance.
Given the magnitude of the effects of Gal3, we conducted detailed in vitro studies to elucidate the mechanisms of Gal3-induced insulin resistance. Our studies showed that Gal3 treatment led to decreased insulin signaling in adipocytes and myocytes (Figures 4 and 5). In 3T3-L1 adipocytes and L6 myocytes, Gal3 treatment inhibited insulin stimulated glucose transport and GLUT4 translocation in a dose-responsive manner (Figures 3A-B and 4I-J). In hepatocytes, Gal3 blocked the effects of insulin to suppress glucagon-stimulated hepatic glucose production (Figure 3C). With respect to more in-depth mechanisms, we found that Gal3 treatment led to decreased insulin-stimulated IR tyrosine phosphorylation in myocytes and adipocytes (Figures 4A & B). All of the subsequent steps in insulin signaling, such as IRS1 tyrosine phosphorylation, PDK1 activation, and AKT phosphorylation were also inhibited by Gal3 (Figures 4C-H and S6A-B). From these studies, we conclude that the major mechanism of action of Gal3 is to interact with the cell surface IR, inhibiting its function. This conclusion is strongly supported by our co-immunoprecipitation experiments which show a strong direct interaction between Gal3 and IR (Figures 5A-C). Interestingly, the interaction of Gal3 with the IR did not inhibit insulin binding. This suggests that Gal3 and insulin bind to different sites on the IR and that Gal3 inhibits IR autophosphorylation by interfering with signal transmission from the IRα to β subunits. Based on these studies, we suggest that extracellular Gal3 can interact with the glycosylated cell surface IR, through the Gal3 CRD, leading to inhibition of IR tyrosine phosphorylation and downstream signaling which causes insulin resistance. What other surface proteins Gal3 might bind to is not currently known. Interestingly, the interaction between Gal3 and IR can be shown in the absence of insulin, but is increased in insulin-stimulated cells. This raises the possibility that insulin binding to the IR introduces a conformational change favoring Gal3 association, and that the interaction of Gal3 with the IR interferes with ligand-induced IR autophosphorylation.
In addition to directly causing decreased insulin signaling, Gal3 also promotes adipose tissue inflammation. This is exemplified by the in vitro effects of Gal3 to promote macrophage chemotaxis (Figure 2E). This concept is reinforced by the in vivo monocyte tracking studies, showing that migration of fluorescently labeled WT donor macrophages into adipose tissue and liver in Gal3 KO recipient mice is markedly reduced compared to WT recipients (Figure 2G). This result is fully consistent with the decreased ATM content in HFD/obese Gal3 KO mice compared to HFD WT mice and to the decreased expression of inflammatory markers in adipose tissue in the KOs (Figures 2I-K).
Previous studies have examined the effects of Gal3 KO on various aspects of atherosclerosis, glomerulopathy, hepatic steatosis, and obesity, but the current report is the first to provide an in-depth study of Gal3 on insulin action, insulin resistance and diabetes. With respect to metabolic aspects, these earlier studies provide conflicting results without clear conclusions in common among them. For example, Pejonovic et. al. reported that Gal3 KO leads to an obese phenotype on HFD, although they did not measure metabolic effects (Pejnovic et al., 2013). However, in these studies, their WT mice did not gain weight on HFD diet, and this is rather inexplicable, since long-term 60% HFD causes obesity in male WT mice. In contrast, their Gal3 KO mice gained ~ 5g body weight over 18 weeks of 60% HFD. In our experience, male C57BL/6 mice always gain substantial weight after 18 weeks on 60% HFD, usually reaching body weights between 45-50g. Thus, even the 35g Gal3 KO mice reported by Pejonovic et.al., would be considered remarkably lean compared to most reports. Therefore, the “obese” phenotype of their Gal3 KOs is only in comparison to the lean WT mice which failed to gain weight on HFD. Pang et. al. studied a different strain of Gal3 KO mice, and also reported a modest degree of obesity in the KOs compared to WT(Pang et al., 2013). However, in this case, the weight gain in both groups was also substantially less than one usually sees after 12 weeks upon 60% HFD in male C57BL/6 mice, with final body weights of about 38g compared to 45-50g. These investigators reported that the Gal3 KOs displayed glucose intolerance but also found that systemic antibiotic treatment, which normally improves glucose tolerance, as it did in their WT animals, had no effect on the KOs. Based on this, they attributed their phenotype, at least in part, to unspecified changes in the gut microbiome. A paper by Darrow et. al. showed increased glucose levels in Gal3 KOs compared to WT on HFD. However, there was no significant difference in the basal glucose levels in the same experiment. Moreover, there were no differences in insulin sensitivity between the groups and plasma insulin levels were lower in the Gal3 KOs, suggesting beta cell dysfunction as a cause of hyperglycemia. We did not detect evidence for beta cell dysfunction in our studies, nor was this reported in earlier papers using these mice (Darrow and Shohet, 2015). Baek et. al., using the same strain of Gal3 KO mice as we have used, reported a lean phenotype in the Gal3 KOs on HFD, opposite from the first two papers mentioned above (Baek et al., 2015). This was associated with reduced inflammation and improved metabolism, as in our studies. There are two additional papers reporting results on female Gal3 KO mice (Iacobini et al., 2011; Iacobini et al., 2009). These papers are focused on either NASH or atherosclerosis, with one showing that the Gal3 KO mice were more susceptible to HFD-induced NASH, whereas the other showed that the KO mice were partially protected from atherosclerosis. Both papers found that WT and KO mice gained the same amount of weight on HFD, and most relevant to the current studies, both papers also showed that on HFD, the Gal3 KO mice had better glucose tolerance compared to WT. Although we cannot reconcile all of the conflicting data of the above-mentioned reports, we recognize that technical differences, breeding and housing changes, and microbiome differences could all contribute. However, we believe that the results reported in the current studies are quite comprehensive with multiple different measures indicating metabolic improvement in Gal3 KOs. This concept is supported by our findings of metabolic deterioration with Gal3 treatment or overexpression and metabolic benefit with administration of a Gal3 inhibitor.
Our current studies report a marked improvement in obesity-associated inflammation, glucose tolerance and systemic insulin sensitivity in the Gal3 KOs on HFD, as well as in aged lean 8 month-old chow diet-fed animals (Figures 1 and S1). Importantly, we consistently observed no differences in body weight gain between WT and Gal3 KO mice on either HFD or chow diet over many different cohorts. We also demonstrated direct in vitro effects of Gal3 to decrease insulin signaling in adipocytes, hepatocytes, and myocytes and provide a cellular mechanism for this effect. Finally, we were able to reproduce the metabolic phenotype by BMT studies with Gal3 KO donor bone marrow, indicating that BM-derived immune cells are largely responsible for the phenotype. Together, our studies are fairly comprehensive, including both genetic and pharmacologic loss and gain of function studies, all providing internally consistent results. Overall, we conclude that Gal3 is a hematopoietic cell-secreted factor which can directly cause decreased insulin signaling and insulin resistance and, at the same time, promote adipose tissue inflammation. This suggests the idea that inhibition of Gal3 could be an anti-diabetic therapeutic avenue to achieve improved insulin sensitivity and glucose tolerance.
STAR METHODS
Detailed methods are provided in the online version of this paper and include the following:
KEY RESOURCES TABLE
CONTACT FOR REAGENT AND RESOURCE SHARING
- EXPERIMENTAL MODEL AND SUBJECT DETAILS
- Mice
- Clinical Samples
- Cell Lines and Primary Hepatocytes
- METHOD DETAILS
- ITTs, GTTs, and hyperinsulinemic-euglycemic clamp study
- SVCs isolation, and FACS analysis
- Immunohistochemistry
- In vitro chemotaxis assay.
- In vivo monocyte tracking.
- RNA isolation and qPCR.
- Western blot analysis.
- Immunoprecipitation assay.
- Glucose uptake in L6 muscle cells and human iPSC-derived muscle cells
- Conditioned media (CM) collection from IP-Macs and glucose uptake assay in L6 myocytes.
- Glucose output assay in mouse hepatocytes.
- Gal3 treatment in NC fed mice.
- Ad-Gal3 treatment.
- LPS measurement.
- Glut4 translocation experiments.
- Insulin binding experiments.
- Lipid contents measurement in the liver of Gal3−/− mice on HFD.
- Clodronate treatment.
- Bone marrow transplantation.
QUANTIFICATION AND STATISTICAL ANALYSIS
DATA AND SOFTWARE AVAILABILITY
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for reagents may be directed to the corresponding author Pingping Li (Institute of Materia Medica, Chinese Academy of Medical Science & Peking Union Medical College).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Fu Tong Liu from University of California Davis for providing the Gal3 KO mice. We also thank Michael Meehl, Dmitri Pissarnitski, Payal Sheth, and Amanda Babbs for reagent generation, compound synthesis, and mass spectrometry support. This study was funded in part by grants to J M O (DK033651, DK074868, DK-063491, DK-09062), and a grant from Merck Inc.
Footnotes
AUTHOR CONTRIBUTIONS
P. L. conceived this idea, designed the studies and performed most of the experiments; S. L. performed Co-IP, W.B. and glucose uptake assay; M. L. performed the virus work; D. Y. O., and J. W. performed chemotaxis and FACS analysis; G. B. assisted with glucose uptake and gluconeogenesis assay; T. I. performed the Glut4 translocation experiment; A. M. J. assisted with GTTs and ITTs; D. S. provided the human samples; Z. S., C. B., L. K., S. H., X. L., and O. O assisted with collecting tissues and gene expression measurements; S. M. W. measured the lipids content in liver; W. Y. performed the clodronate study; S. I., M. L., and M. B. performed the glucose uptake in IPSC. P. L. and J. M. O. analyzed, interpreted data, supervised the project and co–wrote the manuscript.
REFERENCES
- Ahmad N, Gabius HJ, Andre S, Kaltner H, Sabesan S, Roy R, Liu B, Macaluso F, Brewer CF. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J Biol Chem. 2004;279:10841–10847. doi: 10.1074/jbc.M312834200. [DOI] [PubMed] [Google Scholar]
- Baek JH, Kim SJ, Kang HG, Lee HW, Kim JH, Hwang KA, Song J, Chun KH. Galectin-3 activates PPARgamma and supports white adipose tissue formation and high-fat diet-induced obesity. Endocrinology. 2015;156:147–156. doi: 10.1210/en.2014-1374. [DOI] [PubMed] [Google Scholar]
- Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Feizi T, Gitt MA, Hirabayashi J, Hughes C, Kasai K, et al. Galectins: a family of animal beta-galactoside-binding lectins. Cell. 1994a;76:597–598. doi: 10.1016/0092-8674(94)90498-7. [DOI] [PubMed] [Google Scholar]
- Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994b;269:20807–20810. [PubMed] [Google Scholar]
- Birkenfeld AL, Shulman GI. Non alcoholic fatty liver disease, hepatic insulin resistance and type 2 diabetes. Hepatology. 2013 doi: 10.1002/hep.26672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow AL, Shohet RV. Galectin-3 deficiency exacerbates hyperglycemia and the endothelial response to diabetes. Cardiovascular diabetology. 2015;14:73. doi: 10.1186/s12933-015-0230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donath MY, Dalmas E, Sauter NS, Boni-Schnetzler M. Inflammation in obesity and diabetes: islet dysfunction and therapeutic opportunity. Cell metabolism. 2013;17:860–872. doi: 10.1016/j.cmet.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Feng B, Jiao P, Nie Y, Kim T, Jun D, van Rooijen N, Yang Z, Xu H. Clodronate liposomes improve metabolic profile and reduce visceral adipose macrophage content in diet-induced obese mice. PloS one. 2011;6:e24358. doi: 10.1371/journal.pone.0024358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Simpson JL, Zhang J, Gibson PG. Galectin-3: its role in asthma and potential as an anti-inflammatory target. Respiratory research. 2013;14:136. doi: 10.1186/1465-9921-14-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffner S, Taegtmeyer H. Epidemic obesity and the metabolic syndrome. Circulation. 2003;108:1541–1545. doi: 10.1161/01.CIR.0000088845.17586.EC. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- Iacobini C, Menini S, Ricci C, Blasetti Fantauzzi C, Scipioni A, Salvi L, Cordone S, Delucchi F, Serino M, Federici M, et al. Galectin-3 ablation protects mice from diet-induced NASH: a major scavenging role for galectin-3 in liver. Journal of hepatology. 2011;54:975–983. doi: 10.1016/j.jhep.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Iacobini C, Menini S, Ricci C, Scipioni A, Sansoni V, Cordone S, Taurino M, Serino M, Marano G, Federici M, et al. Accelerated lipid-induced atherogenesis in galectin-3-deficient mice: role of lipoxidation via receptor-mediated mechanisms. Arterioscler Thromb Vasc Biol. 2009;29:831–836. doi: 10.1161/ATVBAHA.109.186791. [DOI] [PubMed] [Google Scholar]
- Imamura T, Vollenweider P, Egawa K, Clodi M, Ishibashi K, Nakashima N, Ugi S, Adams JW, Brown JH, Olefsky JM. G alpha-q/11 protein plays a key role in insulin-induced glucose transport in 3T3-L1 adipocytes. Mol Cell Biol. 1999;19:6765–6774. doi: 10.1128/mcb.19.10.6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager J, Gremeaux T, Cormont M, Le Marchand-Brustel Y, Tanti JF. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology. 2007;148:241–251. doi: 10.1210/en.2006-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo HG, Goedegebuure PS, Sadanaga N, Nagoshi M, von Bernstorff W, Eberlein TJ. Expression and function of galectin-3, a beta-galactoside-binding protein in activated T lymphocytes. Journal of leukocyte biology. 2001;69:555–564. [PubMed] [Google Scholar]
- Lackey DE, Olefsky JM. Regulation of metabolism by the innate immune system. Nat Rev Endocrinol. 2016;12:15–28. doi: 10.1038/nrendo.2015.189. [DOI] [PubMed] [Google Scholar]
- Lanthier N, Molendi-Coste O, Horsmans Y, van Rooijen N, Cani PD, Leclercq IA. Kupffer cell activation is a causal factor for hepatic insulin resistance. Am J Physiol Gastrointest Liver Physiol. 2009;298:G107–116. doi: 10.1152/ajpgi.00391.2009. [DOI] [PubMed] [Google Scholar]
- Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. The New England journal of medicine. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- Li LC, Li J, Gao J. Functions of galectin-3 and its role in fibrotic diseases. The Journal of pharmacology and experimental therapeutics. 2014;351:336–343. doi: 10.1124/jpet.114.218370. [DOI] [PubMed] [Google Scholar]
- Li P, Lu M, Nguyen MT, Bae EJ, Chapman J, Feng D, Hawkins M, Pessin JE, Sears DD, Nguyen AK, et al. Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. The Journal of biological chemistry. 2010;285:15333–15345. doi: 10.1074/jbc.M110.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Oh da Y, Bandyopadhyay G, Lagakos WS, Talukdar S, Osborn O, Johnson A, Chung H, Mayoral R, Maris M, et al. LTB4 promotes insulin resistance in obese mice by acting on macrophages, hepatocytes and myocytes. Nat Med. 2015;21:239–247. doi: 10.1038/nm.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu FT, Hsu DK, Zuberi RI, Kuwabara I, Chi EY, Henderson WR., Jr. Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. The American journal of pathology. 1995;147:1016–1028. [PMC free article] [PubMed] [Google Scholar]
- Liu FT, Rabinovich GA. Galectins: regulators of acute and chronic inflammation. Annals of the New York Academy of Sciences. 2010;1183:158–182. doi: 10.1111/j.1749-6632.2009.05131.x. [DOI] [PubMed] [Google Scholar]
- Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- Pang J, Rhodes DH, Pini M, Akasheh RT, Castellanos KJ, Cabay RJ, Cooper D, Perretti M, Fantuzzi G. Increased adiposity, dysregulated glucose metabolism and systemic inflammation in Galectin-3 KO mice. PloS one. 2013;8:e57915. doi: 10.1371/journal.pone.0057915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell metabolism. 2008;8:301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pejnovic NN, Pantic JM, Jovanovic IP, Radosavljevic GD, Milovanovic MZ, Nikolic IG, Zdravkovic NS, Djukic AL, Arsenijevic NN, Lukic ML. Galectin-3 deficiency accelerates high-fat diet-induced obesity and amplifies inflammation in adipose tissue and pancreatic islets. Diabetes. 2013;62:1932–1944. doi: 10.2337/db12-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillon NJ, Bilan PJ, Fink LN, Klip A. Cross-talk between skeletal muscle and immune cells: muscle-derived mediators and metabolic implications. American journal of physiology Endocrinology and metabolism. 2013;304:E453–465. doi: 10.1152/ajpendo.00553.2012. [DOI] [PubMed] [Google Scholar]
- Reaven GM. The insulin resistance syndrome: definition and dietary approaches to treatment. Annu Rev Nutr. 2005;25:391–406. doi: 10.1146/annurev.nutr.24.012003.132155. [DOI] [PubMed] [Google Scholar]
- Salameh BA, Cumpstey I, Sundin A, Leffler H, Nilsson UJ. 1H-1,2,3-triazol-1-yl thiodigalactoside derivatives as high affinity galectin-3 inhibitors. Bioorg Med Chem. 2010;18:5367–5378. doi: 10.1016/j.bmc.2010.05.040. [DOI] [PubMed] [Google Scholar]
- Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. The Journal of biological chemistry. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- Sano H, Hsu DK, Yu L, Apgar JR, Kuwabara I, Yamanaka T, Hirashima M, Liu FT. Human galectin-3 is a novel chemoattractant for monocytes and macrophages. J Immunol. 2000;165:2156–2164. doi: 10.4049/jimmunol.165.4.2156. [DOI] [PubMed] [Google Scholar]
- Sato S, Hughes RC. Regulation of secretion and surface expression of Mac-2, a galactoside-binding protein of macrophages. J Biol Chem. 1994;269:4424–4430. [PubMed] [Google Scholar]
- Sato S, Ouellet N, Pelletier I, Simard M, Rancourt A, Bergeron MG. Role of galectin-3 as an adhesion molecule for neutrophil extravasation during streptococcal pneumonia. J Immunol. 2002;168:1813–1822. doi: 10.4049/jimmunol.168.4.1813. [DOI] [PubMed] [Google Scholar]
- Stanley TL, Zanni MV, Johnsen S, Rasheed S, Makimura H, Lee H, Khor VK, Ahima RS, Grinspoon SK. TNF-alpha antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. The Journal of clinical endocrinology and metabolism. 2011;96:E146–150. doi: 10.1210/jc.2010-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider P, Martin SS, Haruta T, Morris AJ, Nelson JG, Cormont M, Le Marchand-Brustel Y, Rose DW, Olefsky JM. The small guanosine triphosphate-binding protein Rab4 is involved in insulin-induced GLUT4 translocation and actin filament rearrangement in 3T3-L1 cells. Endocrinology. 1997;138:4941–4949. doi: 10.1210/endo.138.11.5493. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang RY, Rabinovich GA, Liu FT. Galectins: structure, function and therapeutic potential. Expert reviews in molecular medicine. 2008;10:e17. doi: 10.1017/S1462399408000719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.