Key Points
In chorea-acanthocytosis, spiculated red cells are characterized by heightened Lyn kinase activity and dysregulated autophagy.
Regulation of protein turnover by autophagy plays a key role in erythropoiesis and red cell integrity.
Abstract
Chorea-acanthocytosis is one of the hereditary neurodegenerative disorders known as the neuroacanthocytoses. Chorea-acanthocytosis is characterized by circulating acanthocytes deficient in chorein, a protein of unknown function. We report here for the first time that chorea-acanthocytosis red cells are characterized by impaired autophagy, with cytoplasmic accumulation of active Lyn and of autophagy-related proteins Ulk1 and Atg7. In chorea-acanthocytosis erythrocytes, active Lyn is sequestered by HSP90-70 to form high-molecular-weight complexes that stabilize and protect Lyn from its proteasomal degradation, contributing to toxic Lyn accumulation. An interplay between accumulation of active Lyn and autophagy was found in chorea-acanthocytosis based on Lyn coimmunoprecipitation with Ulk1 and Atg7 and on the presence of Ulk1 in Lyn-containing high-molecular-weight complexes. In addition, chorein associated with Atg7 in healthy but not in chorea-acanthocytosis erythrocytes. Electron microscopy detected multivesicular bodies and membrane remnants only in circulating chorea-acanthocytosis red cells. In addition, reticulocyte-enriched chorea-acanthocytosis red cell fractions exhibited delayed clearance of mitochondria and lysosomes, further supporting the impairment of authophagic flux. Because autophagy is also important in erythropoiesis, we studied in vitro CD34+-derived erythroid precursors. In chorea-acanthocytosis, we found (1) dyserythropoiesis; (2) increased active Lyn; (3) accumulation of a marker of autophagic flux and autolysososme degradation; (4) accumlation of Lamp1, a lysosmal membrane protein, and LAMP1-positive aggregates; and (5) reduced clearance of lysosomes and mitochondria. Our results uncover in chorea-acanthocytosis erythroid cells an association between accumulation of active Lyn and impaired autophagy, suggesting a link between chorein and autophagic vesicle trafficking in erythroid maturation.
Introduction
Circulating acanthocytes (spiculated red cells) are a clinical hallmark of chorea-acanthocytosis, one of several hereditary neurodegenerative disorders known as neuroacanthocytosis syndromes (NAs).1,2 Chorea-acanthocytosis is associated with mutations in the VSP13A gene encoding VSP13A/chorein,2,3 a protein implicated by cultured cell expression studies in protein trafficking.4-6 However, despite progress toward elucidation of the molecular defects responsible for chorea-acanthocytosis, the functional contribution of chorein deficiency to chorea-acanthocytosis remains unclear.2,3
Chorein is present in normal mature erythrocytes, but it is partially or completely absent in chorea-acanthocytosis red cells.1,7,8 Studies comparing the human VPS13A gene product, chorein, with homologs in other organisms such as yeast and Dictyostelium discoideum suggest possible involvement of chorein in trafficking of transmembrane proteins or in vesicular sorting systems directing cargo to the prevacuolar compartment (comparable to the human late endosome).1 A possible link between chorein and autophagic processes has been suggested by the recent report of perturbed autophagic flux with impaired accumulation of cytosolic microtubule-associated protein light chain (LC3) in VPS13a-deficient Dictyostelium discoideum and HeLa cells.9 Chorein was recently linked to mitochondrial clearance in yeast, supporting a role of chorein in vesicle trafficking and autophagic processes.10 Overexpression of chorein in cell lines (MRC5, CO7, and HEK) leads to its vesicular accumulation, supporting the involvement of chorein in cell vesicle trafficking.11 Similar data were recently reported for Vps13C-expressing HeLa cells.12
The current lack of information regarding chorein structure impedes understanding of its role in red cell homeostasis. We recently reported that chorea-acanthocytosis erythrocytes exhibit abnormal phosphorylation of the integral membrane protein and spectrin-based cytoskeletal anchor, band 3, by the Src family tyrosine kinase (SFK) Lyn.13,14 In addition, the observation of acanthocytes in genetically engineered mice expressing constitutively active Lyn15 supports the importance of Lyn signaling in the dysregulation of protein–protein interactions underlying the membrane–cytoskeletal network that generates abnormally shaped erythrocytes.
Here, we uncover in chorea-acanthocytosis erythroid cells an association between accumulation of active Lyn and impaired autophagic flux, suggesting a link between chorein and autophagic vesicle trafficking during erythroid maturation. Although chorea-acanthocytosis is a rare disease, it represents an interesting model to explore and characterize new mechanisms involved in red cell proteostasis and erythroid maturation processes and may be useful in understanding other hematologic disorders characterized by altered in autophagy such as β-thalassemia.16,17
Materials and methods
Design of the study
We studied 16 patients diagnosed with chorea-acanthocytosis based on clinical neurologic manifestations and confirmation by chorein immunoblot analysis and/or detection of VPS13A mutations18 (Table 1). Details are reported in supplemental Materials and methods, available on the Blood Web site.
Table 1.
Demographic and molecular data of control subjects and patients with ChAc
| Sex | Age (years) | Molecular defect | Wb analysis for chorein | |
|---|---|---|---|---|
| Controls (n = 28) | 18 F/10 M | 45.9 ± 5.3 | ND | ND |
| ChAc 1 | F | 35 | Intron 3: c.188-5T>G (splice site); mutation on other allele unknown | ND |
| ChAc 2 | F | 34 | ND | Chorein absent |
| ChAc 3 | F | 30 | Exon 37: c.4286G>C;p.A1428P; intron 55: c.7806G>A;p.P2602P (splice site) | Chorein absent |
| ChAc 4 | F | 40 | Exon 34: c.3889C>T; p.R1297X; exon 36: c.4216del ; p.V1406CfsX20 | ND |
| ChAc 5 | M | 47 | Intron 22: c.2288+2T>C; (splice-site mut); Intron 61: c.8472-1G>C; (splice-site mut) | ND |
| ChAc 6 | F | 56 | Exon 13: c.1115del; p.K372SfsX2 (homozygous) | ND |
| ChAc 7 | M | 32 | ND | Chorein absent |
| ChAc 8 | F | 38 | Intron 6: c.495+5G>A (splice-site mut) exon 40: c.4903_4906del ; p.K1635VfsX6 | ND |
| ChAc 9 | F | 40 | ND | Chorein absent |
| ChAc 10 | M | 23 | c.6059 delC | Chorein absent |
| ChAc 11 | M | 28 | c.6059 delC | Chorein absent |
| ChAc 12 | F | 45 | c.1208_1211del, c.7867C>T | Chorein absent |
| ChAc 13 | M | 53 | c.237del, c.9429_9432del | Chorein absent |
| ChAc14 | M | 34 | Heterozygous c. early stop codon mutation | ND |
| ChAc 15 | M | 29 | deletions of exons 8 and 9 (>500 bp); deletion of exon 13 (>400 bp) | Chorein absent |
| ChAc 16 | F | 35 | c.9065_9066delAG; c.1207C>T | Chorein absent |
ChAc, chorea-acanthocytosis; F, female; M, male; ND, not determined; Wb, western blot.
Red cell membrane, cytosol preparation, immunoblot analysis, and immunoprecipitation assays
Erythrocytes were isolated from whole blood after removal of white blood cells and platelets as previously described.14,19-21 Red cell membrane (ghost) and cytosol fractions were obtained as previously reported.22-24 Immunoblot23,25 and immunoprecipitation (IP)14,26-28 were carried out as previously reported (see supplemental Materials and methods for details). When required, the IP assays were carried out as required in the absence or presence of the GST-Lyn/SH3 domain or of the inhibitors PP2 or geldanamycin (GA).29
Phosphorylation of erythrocyte membranes
Red cell ghosts (3 μg protein) were in vitro phosphorylated as previously reported.22,23,25,28,30 Syk-phosphorylated membranes were used as substrates, as described in De Franceschi et al.14
In vitro kinase assay
Generation of potassium iodide–stripped, inside-out vesicles (KIOVs)
Details are reported in supplemental Materials and methods.23,31
Fractionation by centrifugation on glycerol gradient
Cytosolic proteins from healthy and chorea-acanthocytosis red cells were loaded on a linear glycerol (10-40%) gradient as described in Trentin et al.32 The tubes were centrifuged 18 hours at 100 000g in a SW60Ti rotor (Beckman) at 4°C, and 18 fractions were collected from the top of each tube.32
Computational structural modeling and protein–protein docking of Hsp90 and Lyn tyrosine kinase domain and in silico amino acid sequence analyses
Details are reported in supplemental Materials and methods.14,33-38
Electron microscopy
Details are reported in supplemental Materials and methods.
Cell culture and functional analysis
Peripheral blood from adult normal volunteers and from chorea-acanthocytosis patients was collected. We analyzed 10 erythroid cultures from the peripheral blood of different normal subjects and 8 erythroid cultures from 4 chorea-acanthocytosis patients (see supplemental Materials and methods for details).22,39,40
Immunoblot analysis of human erythroid precursors
Details on immunoblot analysis39 are reported in the supplemental Materials and methods.22,39,40
Immunofluorescence assay for LAMP1 and MitoTrack and LisoTrack analysis on erythroid precursors and reticulocyte-enriched fraction
Details are provided in supplemental Materials and methods.41
Statistical analysis
The 2-way analysis of variance algorithm for repeated measures was used for data analysis. Differences of P < .05 were considered significant.
Results
Lyn hyperactivation weakens band 3-ankyrin association in chorea-acanthocytosis red cells
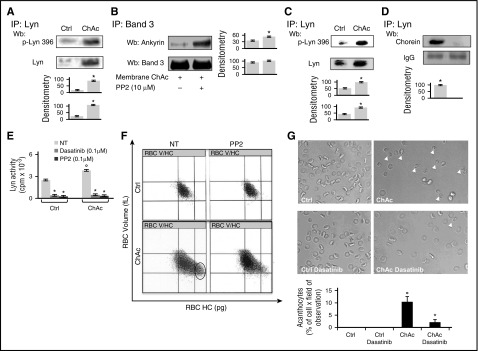
We first confirmed in a larger number of chorea-acanthocytosis patients the increased abundance of active Lyn associated with the red cell membrane (Figure 1A; supplemental Figure 1A).14 We then evaluated whether tyrosine (Tyr-) phosphorylated band 3 might affect its interaction with ankyrin in chorea-acanthocytosis. We phosphorylated control red cell membrane with exogenous Syk in the presence or absence of exogenous Lyn (supplemental Figure 1B). As shown in supplemental Figure 1B, the amount of ankyrin coimmunoprecipitated with band 3 progressively decreased as a function of increasing band 3 pTyr content and reached its minimum only after sequential phosphorylation of control red cell membranes by Syk and Lyn. Because we previously showed in chorea-acanthocytosis erythrocytes that Lyn-mediated Tyr phosphorylation of band 3 is independent from its primary phosphorylation by Syk, we immunoprecipitated band 3 from chorea-acanthocytosis red cells and observed a low level of coprecipitated ankyrin (Figure 1B). Incubation with Lyn inhibitor PP2 markedly increased ankyrin coprecipitation, suggesting that abnormal activation of Lyn affects the association between band 3 and ankyrin in chorea-acanthocytosis erythrocytes (Figure 1B). To further test this hypothesis, we generated inside-out vesicles stripped of their spectrin-based cytoskeleton (potassium iodide [KI]–stripped, inside-out vesicles [KIOVs]). We found that the amount of ankyrin retained by band 3 in KIOV exposed to increasing ionic strength was markedly reduced in chorea-acanthocytosis KIOV compared with normal KIOV (supplemental Figure 1C). These data, combined with our previous observations,14 suggest that in chorea-acanthocytosis, the abnormally activated Lyn weakens band 3–based multiprotein complexes anchoring the membrane to the skeleton with generation of acanthocytes. We then explored whether the “toxic” accumulation of membrane-associated Lyn was paralleled by accumulation of active Lyn in cytosol from chorea-acanthocytosis red cells.
Figure 1.
Chorea-acanthocytosis (ChAc) red cells show weakening of band-3 ankyrin interactions and increased cytosolic active Lyn. (A) (Upper) Total Lyn was immunoprecipitated from detergent-solubilized red cells membranes of healthy (Ctrl, control) and ChAc subjects and detected with antibody to active Lyn (phospho-Lyn 396) or total Lyn (Wb, western blot). The representative experiment shown is 1 of 16 similar experiments, each with red cells from an individual ChAc subject and each with similar results. A colloidal Coomassie-stained twin gel is shown in supplemental Figure 1. (Lower) Densitometric analysis of the immunoblots; data are shown as means ± standard deviation (SD: n = 16; *P < .05 compared with healthy red cells). (B) Band 3 was immunoprecipitated (IP) from detergent-solubilized membranes of ChAc red cells previously incubated without or with the inhibitor PP2 (10 μM). The IPs were subjected to western blot (Wb) analysis with anti-ankyrin antibody and with anti–band 3 antibody. The figure is representative of 3 independent experiments with similar results. Densitometric analysis of the immunoblot bands is shown at right. Data are shown as means ± SD (n = 6; *P < .05 compared with control untreated ChAc red cells). (C) (Upper) Total Lyn was immunoprecipitated from red cell cytosol fraction of healthy (Ctrl, control) and ChAc subjects and detected with antibody to active Lyn (phospho-Lyn 396) or antibody to total Lyn. The experiment shown is representative of 15 such experiments, each from an individual ChAc subject and each with similar results. Colloidal Coomassie-stained twin gel is shown in supplemental Figure 1D. (Lower) Densitometric analysis of the immunoblots; data are shown as means ± SD (n = 15; P < .05 compared with control erythrocytes). (D) (Upper) Total Lyn was immunoprecipitated from red cell cytosol fraction of healthy (Ctrl, control) and ChAc individuals and subjected to immunoblot detection of chorein. The experiment shown is representative of 3 such experiments, each from an individual ChAc subject and each with similar results. (Lower) Densitometric analysis of the immunoblots; data are shown as means ± SD (n = 3; *P < .05 compared with control erythrocytes). (E) Lyn kinase activity from cytosols of control or ChAc red cells was detected using Src-specific CDC2 peptide substrate (6-20) in the absence or presence of Src family-specific inhibitors dasatinib and PP2 (n = 6; *P < .05 compared with untreated red cells). (F) Red cell distribution histograms generated for red cells volume and cell hemoglobin concentration (RBC-HC) of red cells from healthy control (Ctrl) and patients with ChAc without and with PP2 treatment to inhibit Lyn activity. One experiment representative of 7 with similar results is shown. Related values for CHCM and HDW are shown in supplemental Figure 1E. (G) (Upper) Morphology of red cells from healthy and ChAc subjects with and without dasatinib (0.1 μM as in Figure 1D). Cells were imaged at 100× original magnification using a Panfluor objective with 1.30 numeric aperture on a Nikon Eclipse DS-5M camera and processed with Digital Slide (DS-L1) Nikon. One experiment representative of 7 with similar results is shown. (Lower) Quantitation of acanthocytes by brightfield microscopic analysis on ChAc red cells treated with or without dasatinib (0.1 μM). Data from 50 visual fields were collected by 2 blinded researchers. Results are presented as means ± SD (n = 6; °P < .002 compared with healthy red cells; *P < .05 compared with vehicle-treated ChAc red cells).
Elevated levels of active Lyn are distributed across a broad range of high-molecular-weight complexes in the cytosol of chorea-acanthocytosis erythrocytes
Cystosol from chorea-acanthocytosis red cells showed higher levels of activated Lyn than healthy controls (Figure 1C; supplemental Figure 1D). In addition, we found that chorein coimmunoprecipitated with Lyn in healthy red cells but not in chorea-acanthocytosis erythrocytes (Figure 1D). We then evaluated the effect of either PP2 or dasatinib, a synthetic pharmacologic SFK inhibitor, on Lyn activity. As shown in Figure 1E, we found a significant reduction in Lyn activity in healthy and chorea-acanthocytosis erythrocytes by both PP2 and dasatinib. To evaluate the impact of Lyn inhibition on chorea-acanthocytosis red cell phenotype, we fractionated red cells as a function of cell hemoglobin content and cell volume (V/HC), revealing a dense cell fraction present only in chorea-acanthocytosis subjects. Thus, the increased cell hemoglobin concentration mean (CHCM) and hemoglobin distribution width (HDW) in chorea-acanthocytosis might serve as a marker of dense fraction of red cell–containing acanthocytes.42,43 As shown in Figure 1F, PP2 significantly reduced the fraction of dense red cells in chorea-acanthocytosis (left; blue circle) as supported by significant reductions in CHCM and HDW observed only in chorea-acanthocytosis erythrocytes (supplemental Figure 1E). Dasatinib reversed acanthocytes to almost normal red cell morphology, indicating the key role of abnormally activated Lyn in the chorea-acanthocytosis hematologic phenotype (Figure 1G). No changes were observed in dasatinib-treated healthy red cells (Figure 1G).
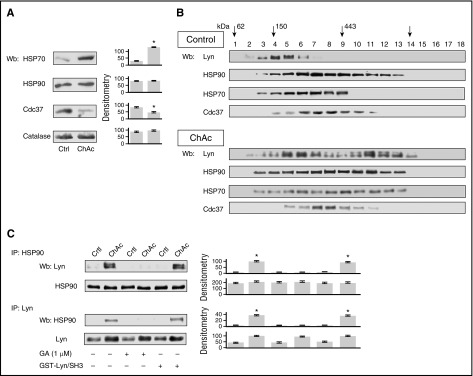
Studies in other cell models have shown that the fate of SFK is under strict control of the HSP90/HSP70 chaperone machinery.44-47 Here, we found reduced expression of Cdc37 and increased expression of HSP70 in the cytosolic fraction of chorea-acanthocytosis red cells, compared with control erythrocytes, whereas HSP90 levels were similar in both cell types (Figure 2A). Because reticulocyte levels were similar in control and chorea-acanthocytosis subjects (data not shown, see also ref. 1), the differences in Cdc37 and HSP70 expression are unlikely related to cell age, but rather to the chorea-acanthocytosis phenotype. Western blot analysis of red cell cytosol fractions from glycerol gradient fractionation revealed a peak of Lyn abundance in complexes of ∼250 kDa in normal red cells. In contrast, chorea-acanthocytosis erythrocytes exhibited a bimodal distribution of Lyn, with peaks at both ∼250 and ∼500 kDa, the latter suggesting presence of an aberrant complex (Figure 2B; supplemental Figure 2A). To evaluate the possible role of chaperone components in this complex, the same glycerol gradient fractions were probed with antibodies directed against HSP90, HSP70, and Cdc37. Whereas HSP90 and Cdc37 profiles were minimally if at all changed in chorea-acanthocytosis red cell cytosol, the chorea-acanthocytosis–associated change in Lyn profile was paralleled by similar substantial changes in the HSP70 profile, suggesting participation of HSP70 in formation of the aberrant Lyn complex (Figure 2B; supplemental Figure 2B). To test this hypothesis, we first evaluated the formation of a high-molecular-weight complex in the presence of GA, a specific HSP 90 inhibitor.
Figure 2.
In ChAc red cells, increased active Lyn is present in cytosol fractions coelutes with HSP90/HSP70 chaperone components and requires active heat shock protein HSPs. (A) Western blot (Wb) analysis of HSP70, HSP90, and Cdc37 in cytosolic fractions from red cells of healthy (Crtl) and ChAc subjects. Catalase served as protein loading control. Densitometric analysis (arbitrary units) of the immunoblot bands similar to those shown are presented at right: the data are shown as means ± SD (n = 5; *P < .01 compared with healthy erythrocytes). (B) Cytosol from control or ChAc red cells was loaded onto a linear glycerol gradient (10-40%) and centrifuged 18 hours at 100 000g in an SW60Ti rotor (Beckman Coulter) at 4°C. Eighteen fractions (200 μL each) were collected from above and analyzed by immunoblotting with antibodies to Lyn, HSP90, HSP70, and Cdc37. Arrows mark the glycerol gradient molecular mass standards: glutamate dehydrogenase (62 kDa), alcohol dehydrogenase (150 kDa), apoferritin (443 kDa), and thyroglobulin (669 kDa). The experiment shown is representative of 6 such experiments, each from an individual healthy or ChAc subject. Densitometric analysis is reported in supplemental Figure 2A. Data on HSP70 are shown as supplemental Figure 2B. (C) Healthy (Ctrl, control) or ChAc red cells were treated for 30 minutes at 4°C in the absence or presence of 1 μM geldanamycin (GA) or 0.1 μM GST/SH3-Lyn, and cytosol was further subjected to immunoprecipitation by anti-HSP90 and anti-Lyn antibodies and assayed for Lyn and HSP90, respectively, by western blot analysis (see also supplemental Figure 2B for HSP70).
GA inhibits Lyn binding to HSP90 and HSP70 in a manner independent of Lyn-SH3 phosphorylation state
We found Lyn coimmunoprecipitated with HSP90 and HSP70 only in chorea-acanthocytosis red cells (Figure 2C; supplemental Figure 2B), whereas it was undetectable in HSP90 immunoprecipitates from GA-treated chorea-acanthocytosis red cell lysates (Figure 2C). In agreement, HSP90 and HSP70 were undetectable in Lyn immunoprecipitates from GA-treated chorea-acanthocytosis red cell lysates. These findings clearly suggest that active HSP90 and HSP70 are required for binding Lyn. This conclusion is supported by the absence of high molecular complex formation in lysates from both healthy and chorea-acanthocytosis red cells incubated with GA (supplemental Figure 6B). To evaluate the role of Lyn's SH3 domain in the interaction with HSP90 or HSP70, we performed competition assays using recombinant Lyn SH3 domain.14,29,32 The Lyn SH3 domain serves as an interaction domain for protein partners and as a regulatory domain for Lyn kinase.14,29,32 Addition of GST-Lyn/SH3 domain failed to disrupt the interaction between Lyn and HSP90 or HSP70 (Figure 2C; supplemental Figure 2B), confirming previous data in other cell models that highlighted the central role of the Lyn catalytic domain in binding HSP90.32 These data together suggest that active Lyn in chorea-acanthocytosis erythrocytes is sequestered by chaperone machinery for either proteasomal degradation or rescue from degradation.
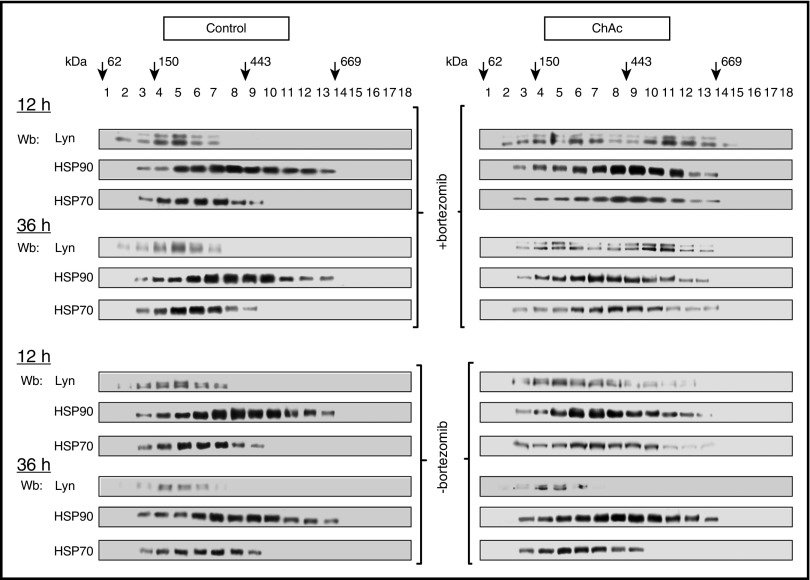
Bortezomib protects Lyn from proteasomal degradation in chorea-acanthocytosis red cells
The modeled structure of the complex of HSP90 and Lyn48-53 was consistent with the hypothesis of reduced Lyn accessibility to the proteasome system, resulting in Lyn accumulation to chorea-acanthocytosis red cells (supplemental Figure 3). Because previous studies have shown that SFK degradation mainly involves proteasome system,44-46,54 we evaluated the effects of bortezomib, a selective inhibitor of 20S proteasome.44-46,54 In chorea-acanthocytosis red cells, bortezomib prevented degradation of Lyn associated with HSP90/HSP70 in high-molecular-weight complexes (Figure 3; supplemental Figures 4 and 5). In the absence of bortezomib, the amount of Lyn associated with HSP90/HSP70 in high-molecular-weight complexes decreased progressively with increasing incubation time (Figure 3; supplemental Figure 5). These findings suggest that chaperonin machinery in chorea-acanthocytosis red cells monitors Lyn to stabilize and prevent its toxic effects related to delayed protesomal degradation.
Figure 3.
Lyn associated with chaperone machinery is longer protected from proteasome degradation in ChAc red cells than in healthy erythrocytes. Cytosol from control or ChAc red cells were incubated in the presence (upper 4 panels) or absence of the 20S proteosome inhibitor bortezomib (20 µM; lower 4 panels) for 12 or 36 hours and subsequently subjected to the separation procedure described in Figure 2B. Steady-state patterns of high-molecular-weight complexes from healthy and chorea-acanthocytosis cells at time zero (0h) is shown in supplemental Figure 4. Eighteen gradients were collected from the top and analyzed by immunoblotting with antibodies to Lyn, HSP90, and HSP70. Arrows represent glycerol gradient molecular mass standards: glutamate dehydrogenase (62 kDa), alcohol dehydrogenase (150 kDa), apoferritin (443 kDa), and thyroglobulin (669 kDa). The experiment shown is representative of 8 such experiments, each from an individual healthy or ChAc subject. Densitometric analysis is shown in supplemental Figure 5.
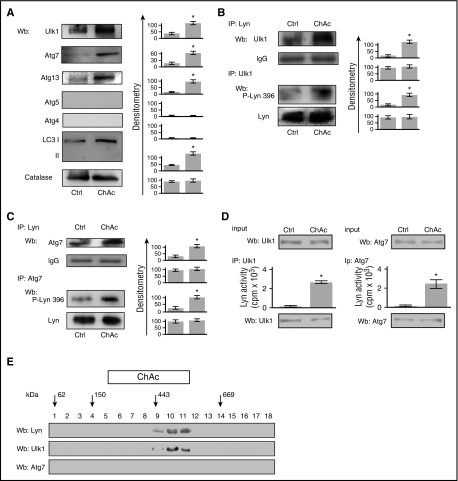
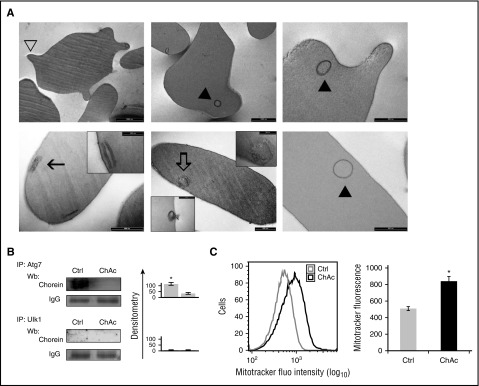
Chorea-acanthocytosis erythrocytes show accumulation of autophagy-related proteins Ulk1 and Atg7 in complex with active Lyn
Accumulation of active Src protected from proteasomal degradation has been shown to be linked with autophagy in other cell models.55-57 As shown in Figure 4A, Ulk1, Atg7, Atg13, Rab5, and the cytosolic form of LC3 (LC3I) accumulated to higher levels in chorea-acanthocytosis than in healthy red cells, whereas Atg4 and Atg5 were undetectable in both healthy and chorea-acanthocytosis erythrocytes. Based on these findings, we sought candidate Src phosphorylation motifs in the sequence of Ulk1, Atg7, and Atg13. We found stringent-threshold SFK consensus sequence in (1) Ulk1 (Tyr295 for Src) and (2) Agt7 (Tyr602 for Src and Tyr184, Tyr401, and Tyr657 for Lyn). Indeed, we found increased Tyr-phsphorylation state of Atg7 but not of Ulk1 (supplemental Figure 6A). We then evaluated the possible association of Ulk1 and Atg7 with Lyn, and we found that both Ulk1 and Atg7 were present at higher levels in Lyn immunoprecipitates from chorea-acanthocytosis red cells than in healthy controls (Figure 4B-C, upper). To validate this observation, we immunoprecipitated Ulk1 and Atg7 from normal and diseased red cells and immunoblotted them with antibody to active Lyn. As shown in Figure 4B-C (lower), levels of active Lyn (P-Lyn396) coimmunoprecipitated with Ulk1 and with Atg7 were higher from chorea-acanthocytosis red cell cytosols than from healthy controls. To verify the increased activity of Lyn associated with either Ulk1 or Atg7, we immunoprecipitated equal amount of proteins and then carried out the assay for Lyn activity. As shown in Figure 4D, we found greatly increased Lyn kinase activity in both Ulk1 and Atg7 immunoprecipitates from chorea-acanthocytosis red cells compared with those from healthy controls.
Figure 4.
ChAc red cells showed accumulation of autophagy-related proteins, which associated with active Lyn. (A) Western blot (Wb) analysis of Ulk1 (Atg1), Atg7, Atg13, Atg4, Atg5, and LC3 in cytosolic fractions from red cells of healthy (Crtl) and ChAc subjects. Catalase was used as protein loading control. Densitometric analyses of the immunoblot bands similar to those shown are presented at right. Data are shown as means ± SD (n = 6; *P < .01 compared with healthy erythrocytes). (B-C) (Upper) Total Lyn was immunoprecipitated from red cell cytosol fraction of healthy (Ctrl, control) and ChAc subjects and detected with antibody to either (B) Ulk1 or (C) Atg7. (Lower) (B) Ulk1 or (C) Atg7 was immunoprecipitated from red cell cytosol fraction of healthy (Ctrl, control) and ChAc subjects and detected either with antibody to active Lyn (phospho-Lyn 396) or to total Lyn. The experiment shown is representative of 6 such experiments, each from an individual ChAc subject and each with similar results. IgG was used as loading control. Data are shown as means ± SD (n = 6; *P < .01 compared with healthy erythrocytes). (D) Immunoprecipitates containing equal amounts of Ulk1 (left) or Atg7 (right) were immunoprecipitated from red cell cytosol fraction of healthy (Ctrl, control) and ChAc subjects, and Lyn kinase activity was detected using Src-specific cdc2 peptide substrate (6-20). Data are shown as means ± SD (n = 6; *P < .01 compared with healthy erythrocytes). (E) Lyn complex from ChAc red cell cytosol was prepared as in Figure 2B, and fractions 10 to 12 were collected and resubmitted to a further centrifugation step on a glycerol gradient. Aliquots of gradient fractions underwent western blot analysis for Lyn, Ulk1, and Atg7. Arrows represent glycerol gradient molecular mass standards: glutamate dehydrogenase (62 kDa), alcohol dehydrogenase (150 kDa), apoferritin (443 kDa), and thyroglobulin (669 kDa). The experiment shown is representative of 8 such experiments, each from an individual ChAc subjects (see also supplemental Figure 5A for the effects of GA on high-molecular-weight complex formation).
Because a previous study showed that Ulk1 requires the HSP90-cdc37 complex to initiate autophagy,58 we evaluated the presence of Ulk1 in the high-molecular-mass complexes of chorea-acanthocytosis erythrocyes. Ulk1 but not Atg7 was present together with Lyn in the high-molecular-weight cytosolic complex only in chorea-acanthocytosis (Figure 4E) but not in healthy red cells (data not shown). Treatment with GA prevented the formation of Lyn-containing high-molecular-weight complexes together with Ulk1 (supplemental Figure 6B). These data indicate the functional connection between active Lyn and Atg proteins in chorea-acanthocytosis, suggesting possible impairment of autophagy in chorein-deficient red cells.
Circulating chorea-acanthocytosis erythrocytes accumulate multivesicular bodies, consistent with impaired autophagy
The accumulation of Ulk1 and Atg758-61 in chorea-acanthocytosis red cells prompted the hypothesis that chorein functions in protein trafficking to the autophagosome. We therefore searched in the chorein primary sequence for candidate LC3-interacting region (LIR) motifs proposed to target autophagy substrates.62 Alignment of the chorein sequence with the W/F/YxxL/I/V consensus identified 9 putatitive LIR sites distributed throughout the protein. Although identification of LIR motifs is not itself indicative of LIR binding propensity, the presence of serine or threonine N-terminally flanked by a negative residue is considered a crucial determinant of a functional LIR.63 Based on these findings, we then explored the possible interplay between chorein and autophagy in chorea-acanthocytosis. Electron microscopic (EM) analysis revealed multivesicular bodies close to the surface membrane or in the process of extrusion as well as ring-shaped intracellular membranes and double membrane fragments (Figure 5A). These cell constituents were present in both acanthocytic and normally shaped chorea-acanthocytosis red cells but were not detected in erythrocytes from healthy controls. In addition, chorein was coimmunoprecipitated with Atg7 from healthy but not from chorea-acanthocytosis red cells, directly linking chorein to the physiologic autophagic machinery (Figure 5B). Previous studies have shown that autophagy plays a key role in vesicle trafficking and mitochondrial clearance during late stage of erythropoiesis and in reticulocytes.16,41,64 Thus, we used MitoTracker and LysoTracker to follow mitochondrial and lysososmal clearance in the reticulocyte-enriched red cells fraction from healthy and chorea-acanthocytosis subjects incubated for 3 hours in a plasma-like medium.41,64 As shown in Figure 5C, we found delayed mitochondria (supplemental Figure 6C) and lysosomal clearance (supplemental Figure 6D) in chorea-acanthocytosis red cells compared with those from healthy subjects. These data collectively support a perturbation of autophagic flux in chorein-deficient erythroid cells.
Figure 5.
ChAc red cells show accumulation of multivesicular bodies and delayed mitochondrial clearance. (A) ChAc red cells of normal shape and spiculated acanthocytes (open arrowhead) are both found in peripheral blood of ChAc patients (upper left; bar, 2 μm). Ring-shaped membrane structures (solid arrowhead) are found in both acanthocytes (upper center and right panel; bar, 1 and 0.5 μm, respectively) and in ChAc red cells of normal shape (lower right panel; bar, 0.5 μm); remnants of individual and paired double membrane (lower left panel, higher original magnification in inset; bar, 0.5 and 0.2 μm, respectively) are also visible. In ChAc red cell, multivesicular bodies are found close to the cell membrane (lower center panel; higher original magnification in upper inset; bar, 0.5 and 0.2 μm, respectively) and may be in the process of being extruded from the cell (lower center panel, lower inset; bar, 0.5 μm). (B) Ulk1 and Atg7 were individually immunoprecipitated from red cell cytosol of healthy (Ctrl, control) and ChAc subjects and then subjected to immunoblot with anti-chorein antibody. The experimental results shown are representative of 6 similar experiments, each from an individual ChAc subject. IgG was used as loading control. Data are shown as means ± SD (n = 6; *P < .01 compared with ChAc subjects). (C) (Left) Flow cytometry of reticulocyte-enriched red cell fractions from healthy (Crtl, control) and ChAc subjects intravitally stained with MitoTrack. One representative of 3 independent experiments with similar results. (Right) Data are shown as means ± SD (n = 3; *P < .05 compared with healthy fractioned red cells).
Dyserythropoiesis associated with accumulation of vesicles, organellar remnants, and active Lyn characterized chorea-acanthocytosis
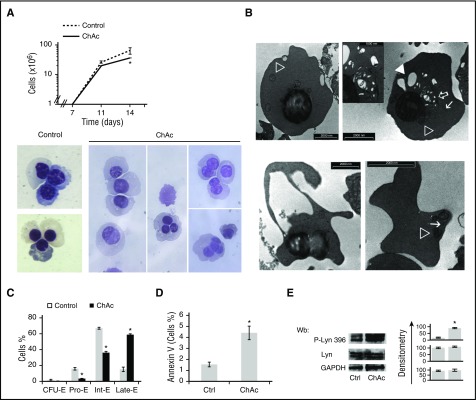
To explore the possible impact of impaired autophagy in chorea-acanthocytosis during erythroid maturation, we studied erythropoiesis using CD34+ cells from healthy and chorea-acanthocytosis subjects. As shown in Figure 6A, culture day 14 cell production was significantly reduced in chorea-acanthocytosis compared with healthy controls, corresponding to the late stage of erythropoiesis. Morphologic analyses revealed signs of dyserythropoiesis characterized by bi- and trinucleated cells, hypocondensated nuclei, and greater accumulation of vesicles compared with healthy cells (Figure 6A, lower; see supplemental Figure 6E for quantitation and supplemental Figure 7A for primary FACS data). Electron microscopy of chorea-acanthocytosis erythroid precursors at 14 days of culture showed (1) erythroblasts with pyknotic nuclei, containing variable amounts of cytoplasmic constituents including mitochondria, small and large vesicular elements, and profiles of endoplasmic reticulum (Figure 6B, upper); and (2) cells apparently in the process of losing large portions of cytoplasm and differentiated, acanthocyte-like red cell–containing organellar remnants (Figure 6B, lower). The chorea-acanthocytosis maturation profile at culture day 14 revealed decreased pro-erythroblasts and intermediate erythroblasts associated with increased late erythroblasts compared with healthy controls. This was associated with increased apoptosis of chorea-acanthocytosis erythroblasts compared with healthy controls (Figure 6C-D) and accumulation of active Lyn compared with healthy cells (Figure 6D). These data indicate the presence of dyserythropoiesis associated with accumulation of vesicles and organellar remnants in chorein-deficient erythroblasts.
Figure 6.
ChAc diserythropoiesis, remnant intracellular vesicles, and accumulation of active Lyn during erythroid maturation. (A) (Upper) Cell proliferation of erythroid precursors derived by in vitro liquid culture of CD34+ cells isolated from peripheral blood of normal (control cells) subjects and ChAc subjects (n = 6). Data are presented as means ± SD; *P < .05 compared with control cells. Percentage decrease in cell number was 53.2 ± 2.9% (n = 6). (Lower) Morphology of culture day 14 erythroid precursors from healthy controls and ChAc subjects. Cytospins were stained with May-Grunwald-Giemsa. Cells were imaged under oil at 100× original magnification using a Panfluor objective with 1.30 numeric aperture on a Nikon Eclipse DS-5M camera and processed with Digital Slide (DS-L1) Nikon. Images representative of 6 separate experiment at 14 days of culture. Quantification of bi-trinucleated cells is reported in supplemental Figure 5C. (B) Electron microscopy of ChAc erythroid precursors at 14 days of culture. (Upper) Hemoglobinized cells of roundish shape filled exhibit pyknotic nucleus and cytoplasmic organellar remnants, including mitochondria (open arrowhead), profiles of endoplasmic reticulum (arrow), and small (open arrow) and large (solid arrowhead) vesicles. Scale bar, 2 μm. Remnants at higher original magnification are shown in the inset; bar, 1 μm. (Lower left) Small erythroid cell, a portion of cytoplasm is apparently detaching from the cell body: bar, 2 μm. (Lower right) A mature, acanthocytoid erythrocyte shows organelle remnants in the cytoplasm (mitochondrion, open arrowhead, endoplasmic reticulum, arrow); bar, 2 μm. (C) Cytofluorimetric maturation analysis of day 14 erythroid precursors (see also supplemental Materials and methods for gating strategy). This cyto-fluorimetric strategy allows the identification of the following homogenous cell populations: pro-erythroblasts (Pro-E), basophilic erythroblasts corresponding to intermediate erythroblasts (Int-E), and polychromatic and orthochromatic erythroblasts as late erythroblasts (Late-E). Data are expressed as percentages shown as means ± SD (n = 4). (D) Amount of annexin-V–positive cells at 14 days of culture in healthy controls and ChAc subjects. Data are expressed as means ± SD (*P < .05 compared with healthy controls, n = 4). (E) Immunoblot analysis of phospho-Lyn and total Lyn in erythroblasts from healthy (control, Ctrl) subjects and ChAc subjects at 14 days of culture. GADPH was used as loading control. One representative blot from 6 with similar results is presented. Densitometric analysis is presented on the right; data are shown as means ± SD (n = 6; *P < .01 compared with healthy controls).
Chorea-acanthocytosis showed impaired autophagy in late-stage erythropoiesis
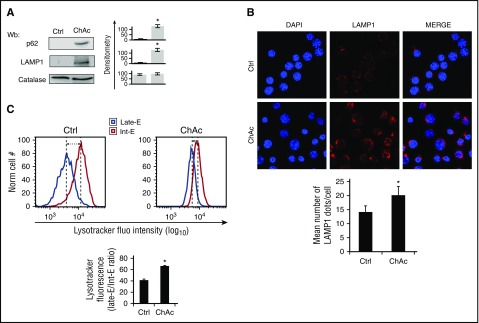
Because erythroid maturation requires efficient autophagic flux, we evaluated expression of lysosmal-associated membrane protein LAMP1 and p62, a key autophagy component of inclusion bodies and autophagosomes that recognizes damaged proteins.65,66 As shown in Figure 7A, chorea-acanthocytosis erythroblasts accumulated LAMP1 and p62 to higher levels than did healthy cells, consistent with impaired autophagy. Immunofluorescent analysis revealed more numerous LAMP1 punctae in chorea-acanthocytosis erythroblasts than in healthy cells (Figure 7B; supplemental Figure 7B). LysoTracker staining confirmed that chorea-acanthocytosis erythroblasts exhibited greater retention of lysossomes than healthy cells (Figure 7C). Reduced mitochondrial clearance in chorea-acanthocytosis erythroblasts was also confirmed by MitoTracker staining, in agreement with findings in chorea-acanthocytosis reticulocyte enriched red cell fractions (supplemental Figure 7C). These data collectively indicate an impairment of autophagic flux in chorea-acanthocytosis erythroid cells, resulting in dyserythropoiesis, delayed clearance of mitochondria and lysososmes, and accumulation of autophagy proteins and active Lyn in chorea-acanthocytosis cells.
Figure 7.
In late-phase erythropoiesis, ChAc erythroblasts exhibit impaired autophagy with delayed clearance of lysosomes. (A) Western blot analysis of p62 and LAMP1 in day 14 erythroblasts cultured from healthy (Crtl) and ChAc subjects. Catalase was used as protein loading control. Densitometric analysis of the immunoblot bands are presented at right with data as means ± SD (n = 6; *P < .01 compared with controls). (B) (Upper) LAMP1 immunostained cytospin preparations of day 14 erythroblasts from healthy (Crtl) and ChAc subjects counterstained with DAPI. (Lower) The puncta mean fluorescence was measured using Image J software. Data are presented as means ± SD (n = 4); *P < .05 compared with healthy cells. (C) (Upper) Flow cytometry of LysoTracker-stained day 14 erythroblasts from healthy (Crtl) and ChAc subjects (late-E, late-erythroblasts; Int-E, intermediate erythroblasts). One representative experiment of 4 separate experiments with similar results. (Lower) Data are shown as means ± SD (n = 4; *P < .05 compared with healthy cells).
Discussion
In this paper, we show for the first time that chorea-acanthocytosis red cells accumulate activated Lyn and autophagy-related proteins to deleterious levels, resulting in retention of membrane remnants and multivesicular bodies. These findings were associated with delayed clearance of mitochodria and lysosome in chorea-acanthocytosis reticulocyte-enriched fractions, indicating impaired autophagy with accumulation of toxic Lyn in chorein-deficient erythroid cells. We also presented the first evidence of the importance of chorein in late-stage erythropoiesis, influencing both autophagy and lysosomal clearance.
In chorea-acanthocytosis erythrocytes, increased levels of active Lyn were not restricted to membrane fractions as in control red cells. Membrane-associated hyperactivated Lyn phosphorylated band 3, weakening the membrane’s interaction with the ankyrin-based multiprotein complex (Figure 1). This finding parallels our previous report showing the effects of Lyn-dependent tyrosine phosphorylation on the interaction of band 3 with β-adducin in chorea-acanthocytosis erythrocytes. Taken together, our data strengthen the proposed role of hyperactive Lyn in altering plasma membrane attachment of multiple cytoskeletal proteins and leading to generation of acanthocytes in chorea-acanthocytosis. Remarkably, the inhibition of Lyn by either PP2 or dasatinib largely reversed the chorea-acanthocytosis hematologic phenotype (Figure 1F-G). In addition, we found that Lyn coimmunoprecipitaed with chorein in healthy erythrocytes. Collectively, these data suggest a perturbation of proteostasis in chorea-acanthocytosis red cells, resulting in accumulation of active Lyn. Indeed, cytosolic active Lyn is stabilized in 500-kDa multiprotein cytosolic complexes that include HSP70 and 90. The presence of HSP70 in high-molecular-weight complexes only in chorea-acanthocytosis red cells most likely reflects to its role as an HSP promoting proteasomal protein degradation.46 These data are consistent with evidence from other cell models, in which levels of active SFKs are strictly monitored by HSPs.45,54 Indeed, the interaction of HSP90 with Fgr and Lck detected in rabbit reticulocytes has suggested a role for HSP in the metabolic fate of erythroid SFKs.67,68 Moreover, treatment with HSP90 inhibitor GA blocked HSP90 interaction with active Lyn by a mechanism independent of the Lyn-SH3 domain, indicating the involvement of HSP90 in monitoring the active form of Lyn.44 In addition, GA also prevented the formation of Lyn-containing high-molecular-weight complexes in chorea-acanthocytosis erythrocytes, further supporting the functional interaction between HSPs and active Lyn in chorea-acanthocytosis (supplemental Figure 5B).
Because the proteasome system is the major route of degradation for HSP client proteins in many cell types,46 we evaluated the impact of the proteasome inhibitor bortezomib on Lyn fate in both healthy and chorea-acanthocytosis red cells. Although bortezomib prevented Lyn degradation in both chorea-acanthocytosis and healthy erythrocytes, Lyn degradation in chorea-acanthocytosis erythrocytes was slower than in cells, favoring Lyn accumulation in chorea-acanthocytosis. Thus, perturbation of intracellular signaling associated with accumulation of active Lyn in chorea-acanthocytosis erythrocytes can generate acanthocytes and might be a sign of impaired quality control mechanisms for cellular protein. This is in agreement with the reported reduction in haptoglobin levels in chorea-acanthocytosis patients,3 suggesting that abnormal Lyn-mediated signal transduction might impact in vivo red cell homeostasis in chorea-acanthocytosis subjects.
Studies in other cell models have shown that accumulation of SFKs might be related to perturbation of protein quality control related to autophagy.56,57,69 Indeed, chorea-acanthocytosis red cells revealed accumulation of autophagy-related proteins Ulk1, Atg7, Atg13, and cytosolic LC3 compared with healthy erythrocytes. A substantial proportion of active Lyn was also largely associated with Ulk1 and Atg7 in chorea-acanthocytosis, but not in healthy red cells. It is of note that the Atg7 Tyr phosphorylation state but not Ulk1 was also increased in chorea-acanthocytosis red cells. Collectively, these findings may reflect functional regulation of Ulk1 and Atg7 through Tyr phosphorylation of their consensus SFK phosphorylation sites or, alternatively, could reflect the ability of Lyn association with Ulk1 and Atg7 to promote their trafficking to the autophagosome, as previously reported for Src in a different cell model.56 The observation of chorein coimmunoprecipitation with Atg7 only in healthy erythrocytes links chorein to autophagy. We therefore propose that that chorein may function as an autophagic partner for active Lyn, preventing the accumulation of active Lyn and the perturbation of intracellular signaling. Indeed, the EM evidence of membrane remnants and multivesicular bodies only in chorea-acanthocytosis red cells of both acanthocytic and nonacanthocytic morphologies further supports the proposed link between chorein and autophagy and the involvement of chorein in erythroid maturation processes. Previous studies have shown that mitochondrial clearance is part of autophagy during the erythroid maturation processes and involves Ulk1 with the HSP90–cdc37 complex, as well as with Atg7.16,41 Chorea-acanthocytosis red cells exhibited accumulation of Ulk1 and Atg7 in concert with delayed mitochondrial and lysosomal clearance in reticulocyte-enriched red cell fractions, supporting the proposed contribution of chorein to autophagic flux. It is of note that Atg4, which has been linked to fusion of autophagosomes to lysosomes during erythropoiesis,70 has not been found in chorea-acanthocytosis red cells, suggesting that the absence of chorein may affect initiation of autophagy related to Ulk1- or Atg-7–linked formation of autophagosomes.16,41 Indeed, chorea-acanthocytosis erythroid precursors exhibit dyserythropoiesis associated with EM-documented retention of vesicles, mitochondria, and profiles of endoplasmic reticulum, along with accumulation of active Lyn (Figure 6). In addition, the accumulation of p62, delivering proteins to autophagosomes,71 and of lysosomal membrane protein LAMP1,66 further links the absence of chorein with impaired autophagy and accumulation of proteins such as Lyn that require controlled degradation. Indeed, late-stage chorea-acanthocytosis erythroblasts exhibited delayed clearance of mitochondria and lysosomes, indicating deficient autophagy during erythroid maturation in chorea-acanthocytosis (supplemental Figure 7). Although mitochondrial clearance during erythroid maturation is mainly mediated by autophagy, our data do not exclude the involvement of alternative autophagy-independent pathway(s) for mitochondrial degradation.72
In conclusion, we show for the first time that chorea-acanthocytosis red cells exhibit accumulation of toxic active Lyn accompanied by accumulation of autophagy-related proteins, resulting in retention of membrane remnants and multivesicular bodies in chorea-acanthocytosis erythrocytes (supplemental Figure 8). The delayed proteasomal degradation of Lyn via high-molecular-weight complex formation with HSPs and autophagy-related protein Ulk1 represents a novel element of proteostasis in normal and chorea-acanthocytosis red cells. The link between impaired autophagy and chorein deficiency in human erythroid cells enhances our understanding of chorea-acanthocytosis and of chorein's homeostastic role promoting intracellular degradation of cytotoxic and/or damaged proteins and organellar constituents (supplemental Figure 8). The chorea-acanthocytosis–associated impairment of autophagy leads to dyserythropoietic features. The accompanying delayed clearance of mitochondria and lysosomes and the accumulation of active Lyn dysregulates red cell homeostasis and underlies the acanthocytosis of syndromic chorea-acanthocytosis.
Acknowledgments
The authors thank the patients and control subjects for donating their blood and Sara Santos Franco who contributed to some preliminary data on chorea-acanthocytosis erythropoiesis. This paper is dedicated to Glenn Irvine, who strongly supported research on NA disorders and devoted his energies to generating an international research network on NA.
This work was supported by Telethon grant GPP13005 (L.D.F.); an NA advocacy grant (L.D.F.); Fondo Universitario di Ricerca (L.D.F.); Federal Ministry of research and education Germany grant ERARE12-081-European Multidisciplinary Initiative on Neuroacantocytosis (EMINA)-2, under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases (A.H.); and the Doris Duke Charitable Foundation (S.L.A.). Western blot analysis of chorein was performed by G. Kwiatkowski and B. Bader, with financial support of NA advocacy and European Agency–net E-Rare consortium EMINA (BMBF01 GM1003) in the laboratories of H. Kretzschmar/A. Giese (Neuropathology) and A. Danek (Neurology) at Ludwing-Maximilians-Universitat Munich (Germany).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: L.D.F. and A.M.B. designed the experiments and analyzed the data; S.L.A. contributed to study design and the writing of the manuscript; F.L., A. Siciliano, and A.M. performed the experiments; A.K.S. performed the protein structure analysis; M.B. carried out in silico analysis; A.M.B., E.T., and F.Z. performed part of the Lyn analysis, analyzed the data, and contributed in writing the manuscript; D.B. and C.Z. performed electron microscopy and ultrastructural analysis; A.D., R.H.W., A.H., B.B., and A. Storch performed neurological diagnosis and provided blood samples; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lucia De Franceschi, Department of Medicine, University of Verona, AOUI-Verona, Policlinico GB Rossi, P.Le L. Scuro 10, 37134 Verona, Italy; e-mail: lucia.defranceschi@univr.it.
References
- 1.De Franceschi L, Bosman GJ, Mohandas N. Abnormal red cell features associated with hereditary neurodegenerative disorders: the neuroacanthocytosis syndromes. Curr Opin Hematol. 2014;21(3):201-209. [DOI] [PubMed] [Google Scholar]
- 2.Jung HH, Danek A, Walker RH. Neuroacanthocytosis syndromes. Orphanet J Rare Dis. 2011;6:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dobson-Stone C, Danek A, Rampoldi L, et al. . Mutational spectrum of the CHAC gene in patients with chorea-acanthocytosis. Eur J Hum Genet. 2002;10(11):773-781. [DOI] [PubMed] [Google Scholar]
- 4.Shiokawa N, Nakamura M, Sameshima M, et al. . Chorein, the protein responsible for chorea-acanthocytosis, interacts with β-adducin and β-actin. Biochem Biophys Res Commun. 2013;441(1):96-101. [DOI] [PubMed] [Google Scholar]
- 5.Brickner JH, Fuller RS. SOI1 encodes a novel, conserved protein that promotes TGN-endosomal cycling of Kex2p and other membrane proteins by modulating the function of two TGN localization signals. J Cell Biol. 1997;139(1):23-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samaranayake HS, Cowan AE, Klobutcher LA. Vacuolar protein sorting protein 13A, TtVPS13A, localizes to the tetrahymena thermophila phagosome membrane and is required for efficient phagocytosis. Eukaryot Cell. 2011;10(9):1207-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cluitmans JC, Tomelleri C, Yapici Z, et al. . Abnormal red cell structure and function in neuroacanthocytosis. PLoS One. 2015;10(5):e0125580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olivieri O, De Franceschi L, Bordin L, et al. . Increased membrane protein phosphorylation and anion transport activity in chorea-acanthocytosis. Haematologica. 1997;82(6):648-653. [PubMed] [Google Scholar]
- 9.Muñoz-Braceras S, Calvo R, Escalante R. TipC and the chorea-acanthocytosis protein VPS13A regulate autophagy in Dictyostelium and human HeLa cells. Autophagy. 2015;11(6):918-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JS, Thorsness MK, Policastro R, et al. . Yeast Vps13 promotes mitochondrial function and is localized at membrane contact sites. Mol Biol Cell. 2016;27(15):2435-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Velayos-Baeza ALC, Dobson-Stone C, Monaco AP. The function of chorein. In: Walker RHSS, Danek A, eds. Neuro-Acanthocytosis Syndromes II. Berlin: Springer-Verlag; 2008:87-105. [Google Scholar]
- 12.Yang RY, Xue H, Yu L, Velayos-Baeza A, Monaco AP, Liu FT. Identification of VPS13C as a Galectin-12-Binding Protein That Regulates Galectin-12 Protein Stability and Adipogenesis. PLoS One. 2016;11(4):e0153534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Franceschi L, Scardoni G, Tomelleri C, et al. . Computational identification of phospho-tyrosine sub-networks related to acanthocyte generation in neuroacanthocytosis. PLoS One. 2012;7(2):e31015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Franceschi L, Tomelleri C, Matte A, et al. . Erythrocyte membrane changes of chorea-acanthocytosis are the result of altered Lyn kinase activity. Blood. 2011;118(20):5652-5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slavova-Azmanova NS, Kucera N, Satiaputra J, et al. . Gain-of-function Lyn induces anemia: appropriate Lyn activity is essential for normal erythropoiesis and Epo receptor signaling. Blood. 2013;122(2):262-271. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Wu K, Xiao X, et al. . Autophagy as a regulatory component of erythropoiesis. Int J Mol Sci. 2015;16(2):4083-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lithanatudom P, Wannatung T, Leecharoenkiat A, Svasti S, Fucharoen S, Smith DR. Enhanced activation of autophagy in β-thalassemia/Hb E erythroblasts during erythropoiesis. Ann Hematol. 2011;90(7):747-758. [DOI] [PubMed] [Google Scholar]
- 18.Velayos Baeza A, Dobson-Stone C, Rampoldi L, et al. . Chorea-acanthocytosis. In: Pagon RA, Adam MP, Ardinger HH, et al., eds. GeneReviews. Seattle, WA: University of Washington, Seattle; 1993. [Google Scholar]
- 19.De Franceschi L, Brugnara C, Rouyer-Fessard P, Jouault H, Beuzard Y. Formation of dense erythrocytes in SAD mice exposed to chronic hypoxia: evaluation of different therapeutic regimens and of a combination of oral clotrimazole and magnesium therapies. Blood. 1999;94(12):4307-4313. [PubMed] [Google Scholar]
- 20.Andolfo I, Alper SL, Delaunay J, et al. . Missense mutations in the ABCB6 transporter cause dominant familial pseudohyperkalemia. Am J Hematol. 2013;88(1):66-72. [DOI] [PubMed] [Google Scholar]
- 21.De Franceschi L, Olivieri O, Miraglia del Giudice E, et al. . Membrane cation and anion transport activities in erythrocytes of hereditary spherocytosis: effects of different membrane protein defects. Am J Hematol. 1997;55(3):121-128. [DOI] [PubMed] [Google Scholar]
- 22.Matté A, Pantaleo A, Ferru E, et al. . The novel role of peroxiredoxin-2 in red cell membrane protein homeostasis and senescence. Free Radic Biol Med. 2014;76:80-88. [DOI] [PubMed] [Google Scholar]
- 23.Matte A, Bertoldi M, Mohandas N, et al. . Membrane association of peroxiredoxin-2 in red cells is mediated by the N-terminal cytoplasmic domain of band 3. Free Radic Biol Med. 2013;55:27-35. [DOI] [PubMed] [Google Scholar]
- 24.Matte A, Low PS, Turrini F, et al. . Peroxiredoxin-2 expression is increased in beta-thalassemic mouse red cells but is displaced from the membrane as a marker of oxidative stress. Free Radic Biol Med. 2010;49(3):457-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iolascon A, De Falco L, Borgese F, et al. . A novel erythroid anion exchange variant (Gly796Arg) of hereditary stomatocytosis associated with dyserythropoiesis. Haematologica. 2009;94(8):1049-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Franceschi L, Fumagalli L, Olivieri O, Corrocher R, Lowell CA, Berton G. Deficiency of Src family kinases Fgr and Hck results in activation of erythrocyte K/Cl cotransport. J Clin Invest. 1997;99(2):220-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perrotta S, Borriello A, Scaloni A, et al. . The N-terminal 11 amino acids of human erythrocyte band 3 are critical for aldolase binding and protein phosphorylation: implications for band 3 function. Blood. 2005;106(13):4359-4366. [DOI] [PubMed] [Google Scholar]
- 28.De Franceschi L, Turrini F, del Giudice EM, et al. . Decreased band 3 anion transport activity and band 3 clusterization in congenital dyserythropoietic anemia type II. Exp Hematol. 1998;26(9):869-873. [PubMed] [Google Scholar]
- 29.Zonta F, Pagano MA, Trentin L, et al. . Lyn-mediated procaspase 8 dimerization blocks apoptotic signaling in B-cell chronic lymphocytic leukemia. Blood. 2014;123(6):875-883. [DOI] [PubMed] [Google Scholar]
- 30.Lupo F, Russo R, Iolascon A, et al. . Protease inhibitors-based therapy induces acquired spherocytic-like anaemia and ineffective erythropoiesis in chronic hepatitis C virus patients. Liver Int. 2016;36(1):49-58. [DOI] [PubMed] [Google Scholar]
- 31.Blanc L, Salomao M, Guo X, An X, Gratzer W, Mohandas N. Control of erythrocyte membrane-skeletal cohesion by the spectrin-membrane linkage. Biochemistry. 2010;49(21):4516-4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trentin L, Frasson M, Donella-Deana A, et al. . Geldanamycin-induced Lyn dissociation from aberrant Hsp90-stabilized cytosolic complex is an early event in apoptotic mechanisms in B-chronic lymphocytic leukemia. Blood. 2008;112(12):4665-4674. [DOI] [PubMed] [Google Scholar]
- 33.Blacklock K, Verkhivker GM. Differential modulation of functional dynamics and allosteric interactions in the Hsp90-cochaperone complexes with p23 and Aha1: a computational study. PLoS One. 2013;8(8):e71936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hagn F, Thamm C, Scheibel T, Kessler H. pH-dependent dimerization and salt-dependent stabilization of the N-terminal domain of spider dragline silk--implications for fiber formation. Angew Chem Int Ed Engl. 2011;50(1):310-313. [DOI] [PubMed] [Google Scholar]
- 35.Wu S, Zhang Y. LOMETS: a local meta-threading-server for protein structure prediction. Nucleic Acids Res. 2007;35(10):3375-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu S, Zhang Y. MUSTER: Improving protein sequence profile-profile alignments by using multiple sources of structure information. Proteins. 2008;72(2):547-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sali A, Potterton L, Yuan F, van Vlijmen H, Karplus M. Evaluation of comparative protein modeling by MODELLER. Proteins. 1995;23(3):318-326. [DOI] [PubMed] [Google Scholar]
- 38.Pettersen EF, Goddard TD, Huang CC, et al. . UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605-1612. [DOI] [PubMed] [Google Scholar]
- 39.Franco SS, De Falco L, Ghaffari S, et al. . Resveratrol accelerates erythroid maturation by activation of FoxO3 and ameliorates anemia in beta-thalassemic mice. Haematologica. 2014;99(2):267-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matte A, De Falco L, Iolascon A, et al. . The interplay between peroxiredoxin-2 and nrf2 is important in limiting oxidative mediated dysfunction in beta-thalassemic erythropoiesis. Antioxid Redox Signal. 2015;23(16):1284-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J, Randall MS, Loyd MR, et al. . Mitochondrial clearance is regulated by Atg7-dependent and -independent mechanisms during reticulocyte maturation. Blood. 2009;114(1):157-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Billett HH, Fabry ME, Nagel RL. Hemoglobin distribution width: a rapid assessment of dense red cells in the steady state and during painful crisis in sickle cell anemia. J Lab Clin Med. 1988;112(3):339-344. [PubMed] [Google Scholar]
- 43.Kalish BT, Matte A, Andolfo I, et al. . Dietary ω-3 fatty acids protect against vasculopathy in a transgenic mouse model of sickle cell disease. Haematologica. 2015;100(7):870-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scholz GM, Hartson SD, Cartledge K, Volk L, Matts RL, Dunn AR. The molecular chaperone Hsp90 is required for signal transduction by wild-type Hck and maintenance of its constitutively active counterpart. Cell Growth Differ. 2001;12(8):409-417. [PubMed] [Google Scholar]
- 45.Giannini A, Bijlmakers MJ. Regulation of the Src family kinase Lck by Hsp90 and ubiquitination. Mol Cell Biol. 2004;24(13):5667-5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pratt WB, Gestwicki JE, Osawa Y, Lieberman AP. Targeting Hsp90/Hsp70-based protein quality control for treatment of adult onset neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 2015;55:353-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith JR, de Billy E, Hobbs S, et al. . Restricting direct interaction of CDC37 with HSP90 does not compromise chaperoning of client proteins. Oncogene. 2015;34(1):15-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kozakov D, Beglov D, Bohnuud T, et al. . How good is automated protein docking? Proteins. 2013;81(12):2159-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kozakov D, Brenke R, Comeau SR, Vajda S. PIPER: an FFT-based protein docking program with pairwise potentials. Proteins. 2006;65(2):392-406. [DOI] [PubMed] [Google Scholar]
- 50.Comeau SR, Gatchell DW, Vajda S, Camacho CJ. ClusPro: an automated docking and discrimination method for the prediction of protein complexes. Bioinformatics. 2004;20(1):45-50. [DOI] [PubMed] [Google Scholar]
- 51.Comeau SR, Gatchell DW, Vajda S, Camacho CJ. ClusPro: a fully automated algorithm for protein-protein docking. Nucleic Acids Res. 2004;32(Web Server issue):W96-W99. [DOI] [PMC free article] [PubMed]
- 52.Tovchigrechko A, Vakser IA. GRAMM-X public web server for protein-protein docking. Nucleic Acids Res. 2006;34(Web Server issue):W310-W314. [DOI] [PMC free article] [PubMed]
- 53.Pierce BG, Wiehe K, Hwang H, Kim BH, Vreven T, Weng Z. ZDOCK server: interactive docking prediction of protein-protein complexes and symmetric multimers. Bioinformatics. 2014;30(12):1771-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bijlmakers MJ, Marsh M. Hsp90 is essential for the synthesis and subsequent membrane association, but not the maintenance, of the Src-kinase p56(lck). Mol Biol Cell. 2000;11(5):1585-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pal R, Palmieri M, Loehr JA, et al. . Src-dependent impairment of autophagy by oxidative stress in a mouse model of Duchenne muscular dystrophy. Nat Commun. 2014;5:4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sandilands E, Serrels B, Wilkinson S, Frame MC. Src-dependent autophagic degradation of Ret in FAK-signalling-defective cancer cells. EMBO Rep. 2012;13(8):733-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4(6):470-480. [DOI] [PubMed] [Google Scholar]
- 58.Joo JH, Dorsey FC, Joshi A, et al. . Hsp90-Cdc37 chaperone complex regulates Ulk1- and Atg13-mediated mitophagy. Mol Cell. 2011;43(4):572-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okamoto K. Organellophagy: eliminating cellular building blocks via selective autophagy. J Cell Biol. 2014;205(4):435-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mariño G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol. 2014;15(2):81-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiong J. Atg7 in development and disease: panacea or Pandora’s Box? Protein Cell. 2015;6(10):722-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Birgisdottir AB, Lamark T, Johansen T. The LIR motif - crucial for selective autophagy. J Cell Sci. 2013;126(Pt 15):3237-3247. [DOI] [PubMed] [Google Scholar]
- 63.Wild P, McEwan DG, Dikic I. The LC3 interactome at a glance. J Cell Sci. 2014;127(Pt 1):3-9. [DOI] [PubMed] [Google Scholar]
- 64.Honda S, Arakawa S, Nishida Y, Yamaguchi H, Ishii E, Shimizu S. Ulk1-mediated Atg5-independent macroautophagy mediates elimination of mitochondria from embryonic reticulocytes. Nat Commun. 2014;5:4004. [DOI] [PubMed] [Google Scholar]
- 65.Karagöz GE, Duarte AM, Akoury E, et al. . Hsp90-Tau complex reveals molecular basis for specificity in chaperone action. Cell. 2014;156(5):963-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Appelqvist H, Wäster P, Kågedal K, Öllinger K. The lysosome: from waste bag to potential therapeutic target. J Mol Cell Biol. 2013;5(4):214-226. [DOI] [PubMed] [Google Scholar]
- 67.Hartson SD, Ottinger EA, Huang W, Barany G, Burn P, Matts RL. Modular folding and evidence for phosphorylation-induced stabilization of an hsp90-dependent kinase. J Biol Chem. 1998;273(14):8475-8482. [DOI] [PubMed] [Google Scholar]
- 68.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci USA. 1994;91(18):8324-8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu L, Feng D, Chen G, et al. . Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat Cell Biol. 2012;14(2):177-185. [DOI] [PubMed] [Google Scholar]
- 70.Betin VM, Singleton BK, Parsons SF, Anstee DJ, Lane JD. Autophagy facilitates organelle clearance during differentiation of human erythroblasts: evidence for a role for ATG4 paralogs during autophagosome maturation. Autophagy. 2013;9(6):881-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem Sci. 2011;36(1):30-38. [DOI] [PubMed] [Google Scholar]
- 72.Mortensen M, Ferguson DJ, Simon AK. Mitochondrial clearance by autophagy in developing erythrocytes: clearly important, but just how much so? Cell Cycle. 2010;9(10):1901-1906. [DOI] [PubMed] [Google Scholar]