Abstract
Background
Microwave ablation (MWA) of lung tumors is a new approach for local tumor control. The purpose of this retrospective study was to evaluate the preliminary results of safety and efficacy of MWA with a dynamic frequency range (902–928 MHz) and power (10–32 W) for local tumor control of thoracic malignancies.
Methods
From December 1, 2013 to February 1, 2016, there were total 32 lung tumors among 15 patients (7 men, 8 women, age range 43–82 years, mean 57.8±11.1 years of age) receiving MWA of thoracic neoplasms, including lung adenocarcinoma (n=5), metastatic colorectal cancer (n=7), invasive thymoma (n=1), metastatic uterine leiomyosarcoma (n=1), and metastatic ampullary carcinoma (n=1). Mean tumor size was 13.5 mm (range, 3.0–32.0 mm). The mean sequential ablation during each MWA was 2.3±1.1 times (range, 1–5 times). The outcomes of ablation were evaluated by follow-up computed tomography (CT) scans and the complications were assessed by medical records and CT scan after ablation.
Results
The mean follow-up interval of each tumor was 446.8 days (range, 196–902 days). Local tumor recurrence was found in 5 of the 32 tumors resulting in a local control rate 84.4%. No MWA-related mortality was noted. After MWA, the incidence of pneumothorax was 37.5% (12/32). Only one patient with pneumothorax required air evacuation. Third-degree skin burn adjacent to the entry site occurred in one patient and required debridement and closure with flap.
Conclusions
After appropriate patient selection, MWA with a dynamic frequency range (902–928 MHz) and power (10–32 W) is an effective and safe procedure for local tumor control of recurrent and metastatic lung tumors.
Keywords: Lung neoplasms; microwaves; tomography, X-ray computed
Introduction
Local recurrence of lung cancer or metastatic lung tumor from extrapulmonary malignancies is commonly encountered. Surgical lung metastasectomy is a safe and potentially curative procedure (1). Unfortunately, patients with comorbidities having recurrent or metastasic lung cancers might not be suitable for surgical tumor resection (1). Stereotactic radiation therapy (SBRT) has been demonstrated being an effective alternative treatment without significant complications in medically impaired patients who were not amenable to surgery (2). However, patient selection for SBRT is important to achieve better results (2). Although surgical metastasectomy is the first choice of treatment in the prior report, some patients are not willing for surgery due to their personal reasons or medical conditions. There are different alternative treatments which could be considered in these patients with metastatic lung disease or local tumor recurrence, including chemotherapy (3), target therapy (4), radiotherapy (2), and tumor thermal ablation (5). Recently, thermal ablation of pulmonary malignancies is a rapidly growing technique because of its minimally invasive approach and preservation of normal tissue (5,6).
Radiofrequency ablation (RFA) is the most widely used technique for the ablation of solid organs (7). By using RFA, Dupuy et al. reported that the local tumor recurrence-free rate in patients with stage IA medically inoperable non-small cell lung cancer (NSCLC) was 68.9% at 1 year and 59.8% at 2 years but was worse if the tumor size is larger than 2 cm (8). Theoretically, MWA in lung shows advantages over RFA (9) because microwaves are less susceptible to heat sinks and able to penetrate deeper into low-conductivity tissue such as lung. There were limited reports of using MWA tumor ablation. By using MWA, Lu et al. reported the recurrence-free survival rates for NSCLC patients in 1, 2, and 3 years were 72.9%, 50.0%, and 27.1% (10). A retrospective study by Egashira et al. using high-energy MWA (140 W, 2,450 MHz) for pulmonary tumors reported that local tumor progression was found only in two of 87 tumors during 15-month median follow-up interval (11). Liu et al. demonstrated that percutaneous 915 MHz MWA with cooled-shaft antennae could achieve a high technique effectiveness rate compared with 2,450 MHz MWA under equal energy delivery in large hepatocellular carcinoma (12). The purpose of our study was to evaluate the results of safety and efficacy of MWA with a dynamic frequency range (902–928 MHz) and power (10–32 W) for local tumor control of thoracic malignancies.
Methods
Study design
This retrospective study was conducted in a single tertiary referral center. From December 1, 2013 to February 1, 2016, there were 21 patients with 39 lung tumors received MWA. After excluding 6 patients who had follow-up CT studies less than 6 months, there were total 15 patients with 32 tumors undergoing MWA enrolled in this study. The inclusion criteria were as follows: (I) patients with pulmonary malignant tumors, including primary NSCLC, NSCLC with recurrent pulmonary metastasis, and metastatic pulmonary tumors; (II) patients with medically inoperable tumors or patients unwilling to receive surgery; (III) no CT evidence of tumor infiltration or invasion in the mediastinum or chest wall; (IV) no CT evidence of tumor encasement of the central thoracic vessels such as aorta, superior vena cava or central pulmonary arteries. The exclusion criteria of this study were: (I) patients with medically uncorrectable coagulopathy: platelet count <80×109/L and an international normalized ratio >1.5; (II) pregnant women; (III) patients without follow-up CT studies longer than 6 months after MWA of lung tumors. The study protocol was approved by the Committee of Research Ethics of National Taiwan University Hospital (201608061RINA) and was conducted in accordance with the Helsinki Declaration of 1964 (revised 2008). All patients had informed consent for MWA before the procedure after a thorough explanation.
CT guided percutaneous microwave ablation (MWA)
MWA was performed under CT guidance (LightSpeed, GE Healthcare, Milwaukee, Wisconsin) using low dose exposure and thin slice protocol (1.25 mm thickness, 1.3 pitch, 0.7 sec/rotation, 120 kV, 50 mA) to monitor the needle position during whole procedure. Before MWA, a recent chest CT without and with contrast enhancement was used to plan proper patient positioning and needle entrance. After positioning the patients, an unenhanced CT scan of the target tumor was performed to decide the most appropriate route of antenna probe insertion and the size of probe to cover adequate tumor margin. For covering adequate tumor margin, a 14G large antenna probe would be used in the tumor larger than 30 mm in greatest diameter while a 16G antenna probe in the lesion less than 30 mm. After local anesthesia with 2% lidocaine, an antenna probe (Avecure 16G probe 16-15-LH-15 or 14G probe 14-15-LH-35, Medwaves, San Diego, CA, USA) was advanced to the target tumor under step-by-step CT guidance. Multiple sequential ablations were performed if the tumor could not be covered by only one ablation session according to the size, location and geometry. The patients were sedated using midazolam hydrochloride (0.010–0.035 mg/kg) and fentanyl citrate (1 mcg/kg) for pain relief in the procedure. The oxygen saturation, blood pressure, and heart rate were monitored during the whole procedure of MWA. Skin protection using continuous cold saline gauze (4–8 °C) around the needle entrance was performed in all patients to avoid high temperature skin injury or burn.
The microwave generator (Avecure MWG881, Medwaves, San Diego, CA, USA) with a dynamic frequency range (902–928 MHz) and power (10–32 W) was used (Figure 1). The generator automatically adjusted the frequency and power to maintain a target temperature around 110 °C at the microwave antenna tip. Sequential multiple ablations in differential locations of the tumor were performed for complete coverage of tumor margin (13). After completing MWA of the targeted lesion, an immediate CT scan was performed to determinate the efficacy of MWA if the tumor was surrounded by a halo of ground glass opacity (GGO) indicating the margin of tumor ablation zone (14) (Figure 2) and to find out whether any WMA-related complications such as pneumothorax, pulmonary hemorrhage or any other soft tissue injury occurred.
Figure 1.

MedWaves microwave ablation system consisting of (A) generator (Avecure MWG881, Medwaves, San Diego, CA, USA); (B) 14G antenna probe (Avecure 14-15-LH-35, Medwaves, San Diego, CA, USA).
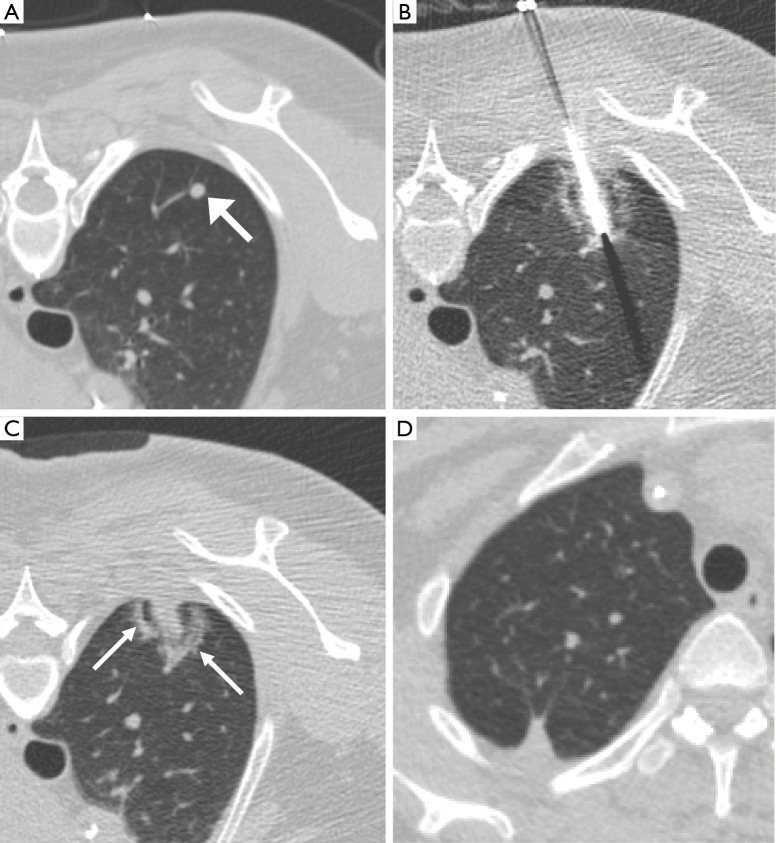
Figure 2.
Image feature of the halo ground glass opacity. (A) A 46-year-old woman with colorectal cancer has a new solid 5 mm metastatic tumor at the posterior aspect of the right upper lobe (arrow) before MWA; (B) the antenna is placed through the center of the targeted nodule and shows a surrounding halo ground glass opacity during the procedure; (C) after removal of the antenna, there is an apparent hypodense track inside the tumor in addition to the halo; (D) follow up non-contrast CT one year after MWA shows focal fibrosis and disappearance of the metastatic nodule, indicating complete tumor ablation.
Follow-up examinations and complications
Most patients had a CT scan the day after MWA as the baseline images. All patients underwent follow-up CT scans 1 month later and about every 3–6 months. Local tumor recurrence was defined as enlargement of the tumor compared with baseline images after six months of the procedure or persistent hot spot in positron-emission tomography (PET) after the procedure. MWA-related complications were assessed by medical records and CT images after MWA. The adverse events were classified according to the Common Terminology Criteria for Adverse Events version 4.03 (CTCAE) from grade 1 (mild) to grade 5 (death related to adverse events) (15). Major complications were defined as those procedures resulting in further intervention, hospitalization, and death, e.g., CTCAE grade 3–5 adverse events.
Statistical analysis
Group comparison between patients with recurrent tumor (MWA failed group) and patients with complete tumor ablation (MWA successful group) was made by using Student t-test.
Results
The demographic information of the enrolled 32 lung tumors among 15 patients (7 men, 8 women, age range 43–82 years of age, mean 57.8±11.1 years) is shown in Table 1. The mean follow-up interval of each tumor was 446.8±196.2 days (range, 196–902 days). The clinical diagnoses of the 15 patients included colorectal cancer (n=7), lung adenocarcinoma (n=5), invasive thymoma (n=1), metastatic uterine leiomyosarcoma (n=1), and metastatic ampullary carcinoma (n=1). The tumor size was 13.5±8.6 mm (median 12.5 mm, range 3.0–32.0 mm). Among these tumors, 12 tumors were <10 mm, 13 tumors were 10–20 mm, and 7 tumors were >20 mm. The locations of the tumors were right upper lobe (n=10), right middle lobe (n=3), right lower lobe (n=9), left upper lobe (n=3), and left lower lobe (n=7). Ten of 32 tumors were located at the central portion of lung (distance from pleura larger than 30 mm) while 6 tumors were adjacent to pleura (distance from pleura less than 10 mm).
Table 1. Demographic information of 15 patients with 32 lung tumors receiving microwave ablation (MWA) of pulmonary tumors.
| Parameter | Value |
|---|---|
| Age (years) | |
| Mean | 57.8±11.1 |
| Range | 43–82 |
| Sex | |
| Male | 7 |
| Female | 8 |
| Clinical diagnosis | |
| Colorectal cancer | 7ǂ |
| Lung adenocarcinoma | 5ǂ |
| Invasive thymoma | 1ǂ |
| Uterine leiomyosarcoma | 1ǂ |
| Ampullary carcinoma | 1ǂ |
| Number of tumors | 32 |
| Tumor size (mm) | |
| Mean | 13.5±8.6 |
| Range | 3.0–32.0 |
| <10 | 12* |
| 10–20 | 13* |
| >20 | 7* |
| Location of the tumors | |
| Right upper lobe | 10* |
| Right middle lobe | 3* |
| Right lower lobe | 9* |
| Left upper lobe | 3* |
| Left lower lobe | 7* |
| Distance from pleura (mm) | |
| <10 | 6* |
| 10–30 | 16* |
| >30 | 10* |
| Follow-up interval of each tumor (days) | |
| Mean | 446.8±196.2 |
| Range | 196–902 |
ǂ, number of patients; *, number of tumors.
For achieving adequate tumor coverage in MWA, the mean ablation time was 646.4±445.8 seconds (median 553.5 seconds, range 120–1,690 seconds) according to the theoretical thermal distribution and the mean sequential ablations during each MWA was 2.3±1.1 times (range, 1–5 times). The mean highest ablation temperature was 105.2±11.7 °C (range, 55–110 °C). The mean energy delivery of each tumor was 10.1±6.2 KJ (range, 2.3–26.5 KJ) according to the tumor size and tumor geometry.
During their admission, some patients (n=11) showed asymptomatic pneumothorax which were classified as grade 1 adverse event (asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated). One patient with moderate pneumothorax received air evacuation immediately after the procedure (grade 2 moderate adverse event without prolonged hospitalization). Five patients had asymptomatic pulmonary hemorrhage after MWA (grade 1 adverse event). No patient had hemoptysis and fever. One patient received debridement of the skin due to third-degree burn injury adjacent to the entry site (grade 3 adverse event). None of them had more severe adverse event classified as grade 4 and 5 (Table 2).
Table 2. MWA-related complications in 32 pulmonary malignant tumor.
| Complication* | N (%) |
|---|---|
| Pneumothorax | 12 (37.5) |
| Mild (grade 1) | 11 (34.4) |
| Moderate (grade 2) | 1 (3.1) |
| Pulmonary hemorrhage (mild, grade 1) | 5 (15.6) |
| Hemoptysis | 0 (0) |
| Fever | 0 (0) |
| Burn injury (grade 3) | 1 (3.1) |
| Death | 0 (0) |
*, classified according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. MWA, microwave ablation.
Among 32 tumors, 5 tumors among 5 patients including colorectal cancer (n=2), lung adenocarcinoma (n=1), uterine leiomyosarcoma (n=1), and ampullary carcinoma (n=1) showed tumor recurrence in follow-up CT scans (Table 3). Mean tumor size before MWA was 19.2±8.3 mm (range, 9.0–32.0 mm). There was no statistical significance of tumor size between successfully ablated (n=27) and local recurrent (n=5) tumors (P=0.11). Three of five recurrent tumors after MWA were located at the central portion of lung while two were adjacent to pleura. In this study, the overall local tumor control rate was 84.4% (27/32).
Table 3. Characteristics of five local recurrent tumors after MWA.
| Parameter | Value |
|---|---|
| Original tumor size (mm) | |
| Mean | 19.2±8.3 |
| Range | 9.0–32.0 |
| <10 | 1* |
| 10–20 | 3* |
| >20 | 1* |
| Clinical diagnosis | |
| Colorectal cancer | 2* |
| Lung adenocarcinoma | 1* |
| Uterine leiomyosarcoma | 1* |
| Ampullary carcinoma | 1* |
| Distance from pleura (mm) | |
| <10 | 2* |
| 10–30 | 0* |
| >30 | 3* |
*, number of tumors. MWA, microwave ablation.
Discussion
In this study, we demonstrated the efficacy of MWA using a dynamic frequency range (902–928 MHz) and power (10–32 W) for recurrent and metastastic lung tumors. After appropriate selection of patients, this procedure is a safe and effective procedure for local tumor control. The local tumor control rate was 84.4%, similar to success rate (67–78.26%) in previous studies (6,10,14). Tumor larger than 3 cm in diameter was significantly more likely to have recurrent disease at the ablation site in the study by Wolf et al. (14). Lu et al. demonstrated that the mass larger than 4 cm had local progression after MWA (10). But in the study by Egashira et al., there is no correlation between local tumor progression and the tumor size (11). In our study, no statistical significance of the tumor size was noted between successfully ablated and local recurrent tumors. However, our sample size was too small to show the significance. About ablation time, high-energy MWA had a shorter ablation time in ex vivo study, compared with the similar system as this study (16). Egashira et al. also reported a median ablation time of 2 minutes in the 12 mm median tumor size (11). The median ablation time was 553.5 seconds (mean 646.4±445.8 seconds) in this study, longer than the high-energy MWA study but similar with the low-energy MWA study (mean 15±5 minutes) (6).
In this study, none of our patients had life-threatening adverse events. The most common complication was pneumothorax and the incidence was 37.5% (12/32). Other studies showed the similar results and the incidence was 8.5–39% (6,10,14). One major complication was burn injury adjacent to the entry site during our earlier stage of using this technique because of insufficient skin protection. The incidence was 3.1% (1/32), similar with the other studies (0.8% and 3%) (6,14). Liang et al. reported that the use of noncooled-shaft probe caused skin burn requiring surgery in malignant liver tumors (17). There was no skin burn requiring surgery by the use of cooled-shaft probe. However, in the previous study by Wolf et al., one patient had third-degree skin burn requiring debridement by using the cooled-shaft probe. The incidence of skin burn was 3%, similar with our study using noncooled-shaft probe (3.1%). Currently, no large series study in pulmonary MWA had the same sample size as the study by Liang et al. Further large-scale investigation of complications in pulmonary MWA is considered.
This study had some limitations. Because our study is a single-center, retrospective study, some biases may affect the selection of cases. A prospective, randomized-controlled study is considered in further investigation. Our sample size is not larger enough and shows no statistical significance in some comparisons, such as tumor size between successfully ablated and local recurrent cases. Although mean follow-up interval was more than one year (446.8±196.2 days) in this study, local recurrence still occurred in two and three years later according to previous studies (8,10). Further observation is needed for its long-term effect.
In conclusion, our study suggests that MWA with a dynamic frequency range and power is an effective and safe procedure for local tumor control of recurrent and metastatic lung tumors. A large sample size, prospective, randomized-controlled study is considered in further investigation.
Acknowledgements
None.
Ethical Statement: The study protocol was approved by the Committee of Research Ethics of National Taiwan University Hospital (201608061RINA) and was conducted in accordance with the Helsinki Declaration of 1964 (revised 2008). All patients had informed consent for MWA before the procedure after a thorough explanation.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. 10.1016/S0022-5223(97)70397-0 [DOI] [PubMed] [Google Scholar]
- 2.Wulf J, Haedinger U, Oppitz U, et al. Stereotactic radiotherapy for primary lung cancer and pulmonary metastases: a noninvasive treatment approach in medically inoperable patients. Int J Radiat Oncol Biol Phys 2004;60:186-96. 10.1016/j.ijrobp.2004.02.060 [DOI] [PubMed] [Google Scholar]
- 3.Alberti W, Anderson G, Bartolucci A, et al. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ 1995;311:899-909. 10.1136/bmj.311.7010.899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. 10.1016/S1470-2045(11)70184-X [DOI] [PubMed] [Google Scholar]
- 5.Dupuy DE. Image-guided thermal ablation of lung malignancies. Radiology 2011;260:633-55. 10.1148/radiol.11091126 [DOI] [PubMed] [Google Scholar]
- 6.Vogl TJ, Naguib NN, Gruber-Rouh T, et al. Microwave ablation therapy: clinical utility in treatment of pulmonary metastases. Radiology 2011;261:643-51. 10.1148/radiol.11101643 [DOI] [PubMed] [Google Scholar]
- 7.Ni Y, Mulier S, Miao Y, et al. A review of the general aspects of radiofrequency ablation. Abdom Imaging 2005;30:381-400. 10.1007/s00261-004-0253-9 [DOI] [PubMed] [Google Scholar]
- 8.Dupuy DE, Fernando HC, Hillman S, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients: Results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial. Cancer 2015;121:3491-8. 10.1002/cncr.29507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol 2009;38:135-43. 10.1067/j.cpradiol.2007.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu Q, Cao W, Huang L, Wan Y, et al. CT-guided percutaneous microwave ablation of pulmonary malignancies: Results in 69 cases. World J Surg Oncol 2012;10:80. 10.1186/1477-7819-10-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egashira Y, Singh S, Bandula S, et al. Percutaneous High-Energy Microwave Ablation for the Treatment of Pulmonary Tumors: A Retrospective Single-Center Experience. J Vasc Interv Radiol 2016;27:474-9. 10.1016/j.jvir.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 12.Liu FY, Yu XL, Liang P, et al. Comparison of percutaneous 915 MHz microwave ablation and 2450 MHz microwave ablation in large hepatocellular carcinoma. Int J Hyperthermia 2010;26:448-55. 10.3109/02656731003717574 [DOI] [PubMed] [Google Scholar]
- 13.Wright AS, Lee FT, Jr, Mahvi DM. Hepatic microwave ablation with multiple antennae results in synergistically larger zones of coagulation necrosis. Ann Surg Oncol 2003;10:275-83. 10.1245/ASO.2003.03.045 [DOI] [PubMed] [Google Scholar]
- 14.Wolf FJ, Grand DJ, Machan JT, et al. Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology 2008;247:871-9. 10.1148/radiol.2473070996 [DOI] [PubMed] [Google Scholar]
- 15.National Cancer Institute, National Institutes of Health, US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Published: May 28, 2009; Revised version 4.03 June 14, 2010. Available online: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf (Accessed July 31, 2016).
- 16.Hoffmann R, Rempp H, Erhard L, et al. Comparison of four microwave ablation devices: an experimental study in ex vivo bovine liver. Radiology 2013;268:89-97. 10.1148/radiol.13121127 [DOI] [PubMed] [Google Scholar]
- 17.Liang P, Wang Y, Yu X, et al. Malignant liver tumors: treatment with percutaneous microwave ablation--complications among cohort of 1136 patients. Radiology 2009;251:933-40. 10.1148/radiol.2513081740 [DOI] [PubMed] [Google Scholar]