Abstract
Thoracoscopic pulmonary segmentectomy is technically meticulous due to the complicated anatomical variations of segmental bronchus and vessels. Currently three dimensional-computed tomography angiography (3D-CTA) could only meet the simple requirements of segmentectomy. Our center developed a software for reconstruction, “deepinsight”, which could effectively solve some key technical problems. Preoperative three-dimensional computed tomography bronchography and angiography (3D-CTBA) could reveal the anatomical structures and improve the accuracy of operation. Preoperative simulation on the three-dimensional image is helpful for surgery planning, which includes nodule location, identification of the targeted vessels, bronchus and surgical margin, revealing of anatomical variations, and planning of surgical approach. With the assistance of 3D navigation, during the surgical procedure all the targeted structures could be divided accurately, the intersegmental veins could be preserved, the targeted parenchyma could be en bloc removed, and the surgical margin could be ensured. Our center has developed a method to separate pulmonary segments from the lobe based on cone-shaped principle, and we named it “Cone-shaped Segmentectomy”. This technique covers precise identification and dissection of segmental bronchus, vessels and intersegmental demarcation, which ultimately achieve a completely anatomical segmentectomy.
Keywords: Three-dimensional computed tomography, bronchography and angiography, thoracoscopy, pulmonary segmentectomy
In recent years pulmonary segmentectomy has been widely used for early stage non-small cell lung cancer (NSCLC). Compared with lobectomy, segmentectomy is associated with equivalent oncologic outcomes in stage IA (T1aN0M0) NSCLC (1-3), with the advantage of preserving pulmonary function and reducing postoperative complications (4,5). The common segmentectomies reported previously were lingular, left upper division, superior, and basical segmentectomy (6,7). The technique of this series of segmentectomies is similar to lobectomy. Most other types of segmentectomies are technically more sophisticated than lobectomy (8,9), due to the complicated anatomical variations of segmental bronchus and vessels. The technical difficulties of thoracoscopic anatomical segmentectomy includes confirmation of the nodule locations, identification of the targeted structures, preservation of the intersegmental veins, division of the intersegmental demarcation and ensurance of the surgical margin.
Three-dimensional computed tomography bronchography and angiography (3D-CTBA) could demonstrate the segmental structures, reveal the anatomical variations and locate the pulmonary nodule (10-12). Preoperative simulation on 3D images is beneficial to remove the targeted segments accurately, reduce the surgical difficulty and shorten the learning curve. Reviewing related literature and summarizing our own experiences, this paper evaluated the utility of 3D-CTBA for thoracoscopic segmentectomy and subsegmentectomy.
Location of pulmonary nodules
During operation, it is difficult to palpate and locate the pulmonary small nodule due to the ground glass opacity (GGO) composition and its deep location within the parenchyma of the lung. Currently, there are various methods of pulmonary nodules localization, such as CT-guided Hookwire, Spring coil, Methylene blue (13), and lung surface anatomic marks localization, which can effectively locate the tiny pulmonary nodule during operation. For pulmonary wedge resection and lobectomy, these methods are simple and practical.
However, the above methods are not able to completely solve the problem of the segmental ascription of the nodules, especially when the nodule is on border of the adjacent segments, which is difficult to localize by using common two-dimensional CT (Figure 1). Besides, because of many segmental anatomic variations, the dominant pulmonary segment often appears, and the volume is significantly greater than the normal segment. According to the conventional anatomic view, it is easy to misjudge the nodule location in the pulmonary segment. In the absence of accurate nodule localization in the pulmonary segment, premature segmentectomy may lead to inaccurate segmental resection, and, even in some cases, it could not be distinguished that a segmentectomy or a subsegmentectomy was performed.
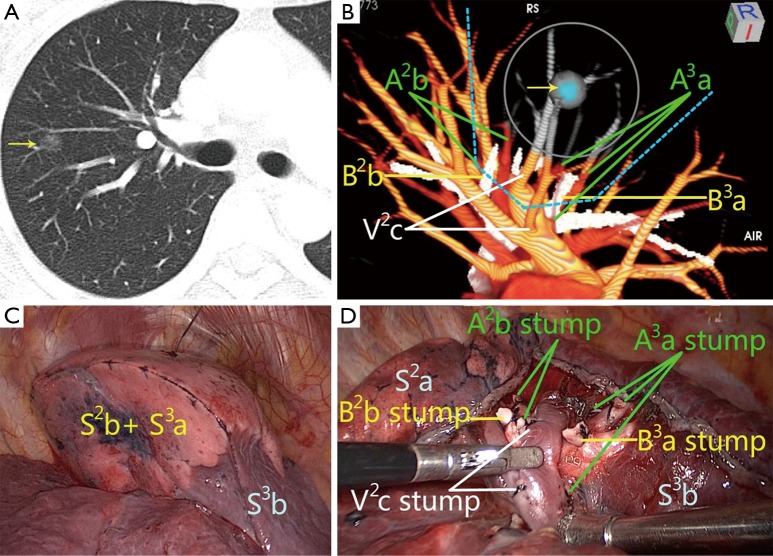
Figure 1.
Illustration of a combined subsegmentectomy (CSS) of right S2b + S3a under the guidance of 3D-CTBA images. (A) CT image revealed a mixed ground glass nodule (yellow arrow), 10 mm in diameter, in the right upper lobe; (B) 3D image from the right posterior inferior view revealed the primary lesion (yellow arrow) located in between S2b and S3a. The grey area denotes the safety margin. A simulated operation on a 3D image demonstrated that a CSS with sufficient margin was possible. The cone-shaped blue dotted line represented the intersubsegmental demarcation of the CSS. There were two targeted bronchi (B2b and B3a), five targeted arteries (A2b and A3a), and two targeted veins (V2c); (C) the intersubsegmental demarcation was identified by the modified inflated-deflated line and divided using electrocautery and endoscopic staplers; (D) view of the hilum after S2b + S3a removal showed the stumps of targeted bronchi and vessels. Postoperative pathological findings confirmed the diagnosis of minimally invasive adenocarcinoma (MIA). The surgical margin width was greater than 20 mm.
On 3D reconstructed images, which segment that the nodule is located in can be determined according to the positional relationship between the nodule and the vessels and bronchus (Figure 1B). The nodule location also can be identified intuitively and accurately based on the relationship between the nodule and the reconstructed segmental border.
Identification of surgical margins and segmental resection approach
The operative indications of pulmonary segmentectomy, in combination with NCCN guideline for NSCLC (Version 1.2016) (14), as follows: (I) poor pulmonary reserve or other major comorbidity that contraindicates lobectomy; (II) peripheral nodule, ≤2 cm with at least one of the following: (i) pure adenocarcinoma in situ histology; (ii) nodule has ≥50% ground-glass appearance on CT; and (iii) radiologic surveillance conforms a long doubling time (≥400 days). Other indications include undetermined or benign nodules and isolated metastatic lung tumors deep in the lung that were unreachable by wedge resection. In cases of malignancy, the surgical margins should be more than 2 cm or greater than the size of the nodule.
Preoperative segmentectomy simulation using 3D images enables to identify safe surgical margins, and then minimize the anatomic resection of the targeted segment under the premise of meeting oncological requirements. A safe spherical margin is virtualized by using 3D-CTBA to reconstruct pulmonary nodule, 2 cm nodule edge or nodule diameter (Figure 1B) (11). All of the bronchus and vessels that involved by the spherical area are regarded as the bronchus and vessels of the targeted segment, which are needed to be dissected during operation. Operators observe the anatomic structures belonging to which pulmonary segment or segments, and then perform the corresponding segmentectomies, such as subsegmentectomy, subsegmentectomies, single segmentectomy, extended segmentectomy or combined segmentectomy and subsegmentectomy.
Currently, the effect of the vessel reconstruction by using three dimensional-computed tomography angiography (3D-CTA) is getting better, but there are more factors influencing the effect of the bronchus reconstruction, and the interface between the pulmonary segments is difficult to reconstruct. Now, several centers are exploring the reconstruction of the interface between the pulmonary segments, and have a preliminary implementation (12).
Identification of the targeted segmental anatomic structures and 3D printing
After using the above method to determine the nodules location, identify a safe margin and plan a surgical approach, according to the anatomic character of pulmonary bronchus and arteries, the bronchus and arteries of the targeted segment are identified and made out on 3D images (Figure 1B) (11,15,16). The segmental vein that runs on the surfaces of the intersegments and collects venous blood of the adjacent segments is the natural boundary of the segments, which should be preserved during operation. Wrong division of the main intersegmental vein can cause postoperative hemoptysis. On 3D images, we could identify which intersegmental vein should be preserved and which intrasegmental veins could be divided (16), measure the closest distance between the nodule and the intersegmental vein, determine whether the targeted segment is cut adequately or not according to the requirement of the safe margins, and make the surgical plan. Researchers from both China and overseas have reported that 3D printing technology has been applied to guide segmentectomy. By inputting 3D-CTBA reconstruction data to the computer, 3D printers can produce pulmonary vessels and bronchus models using multiple printing materials (17). Thanks to these vivid models, researchers could uncover the relationship between nodules and surrounding anatomic structures, and at the same time identify the target segmental vessels, bronchus and intersegmental veins. At present, 3D printers and printing materials are easy to obtain and not too expensive, but its practicability of simulating and guiding the operation is not better than 3D images. Currently, virtual reality (VR) technology has developed rapidly, its effect is far better than that of 3D printing.
Preoperative confirmation of anatomic variations
Thoracoscopic pulmonary segmentectomy is technically more meticulous than lobectomy due to the complicated anatomic variations of segmental bronchus and vessels, which have greater influences on a segmentectomy than on a lobectomy. For example, the common arterial variation of the left upper lobe is that the linguar artery arising from the pars anterior. When left upper division or anterior segmentectomy is performed, it is most likely that the variant lingular segmental artery is mistaken for the anterior segmental artery. Another example is the variation of the right upper lobe vein: V1a empties into V2. When posterior segmentectomy is performed, wrong division of the variant V1a leads to venous infarction of the reserved apical subsegment.
Preoperative 3D images can reveal clearly the segmental anatomic structure and the variations of bronchus and vessels (18). If the anatomic variations are found prior to surgery and identified carefully during operation, the accuracy and security of operation will be improved.
Planning of surgical approach
During the segmentectomy, finding a proper surgical approach and dissecting the segmental portal structure sequentially can significantly improve the efficiency of segmentectomy and are especially important for subsegmentectomy. The most superficial anatomic structures related to the targeted segment are usually found as markers on 3D images prior to operation. For example, the superficial intersegmental veins are usually used as markers in the resection of right or left anterior segment or subsegment. For another example, in the resection of apico-posterior segment or subsegment of the left upper lobe when there are no superficial intersegmental veins are referred, the identification and division of the targeted artery can be used as the first step in operation. With the guidance of preoperative 3D images, there is no need to dissect the whole hilar structures to identify targeted bronchus and vessels. Based on the anatomic relationship of the targeted segment on 3D images, the targeted bronchus, arteries, and intrasegmental veins are dissected and divided sequentially, and the intersegmental veins are preserved. Preoperative planning of surgical approach using 3D images can reduce the surgical complexity, and improve the accuracy of the operation.
Thoracoscopic pulmonary segmentectomy under the guidance of 3D images
The essence of pulmonary segmentectomy is the anatomic resection of the targeted segment, including separate resection of the targeted bronchus, artery and intrasegmental veins, preservation of intersegmental veins, and anatomic dissection of intersegmental border, and maximum preservation of the retained segment in full accordance with the oncological requirements.
Currently, the general concept of anatomic segmentectomy is only confined to the anatomic resection of the targeted bronchus and artery (19), which has no guiding significance for the preservation of the intersegmental veins and anatomic dissection of intersegmental border. The anatomic relationship between pulmonary segment and lobe is that each lobe is assembled by several irregular cone-shaped segments which tip is the opening of segmental bronchus, and pulmonary veins invade the intersegmental planes and further travel on the surfaces of the intersubsegments, interlobules and interacini (20). Our center has developed a method to separate pulmonary segments from the lobe based on cone-shaped principle, and named it “Cone-shaped Segmentectomy”, which achieves the complete anatomic resection of pulmonary segment under the guidance of 3D images, including accurate dissection of the targeted bronchus, arteries and intrasegmental veins, and preservation of the intersegmental veins (Figure 2). Our pilot study reveals that cone-shaped segmentectomy is safe and feasible—it can ensure safe surgical margins, reduce the overlap squeezing of the margin tissues, and maintain the original geometrical shape of the retained segments.
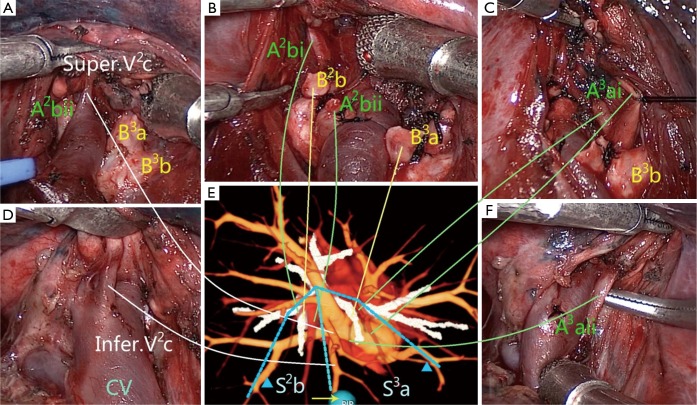
Figure 2.
Illustration of the surgical procedure with the assistance of 3D image navigation. The sequence of the surgical procedure was from (A-D,F). The dissection begun at the central vein, and all following dissections were kept going along it. (E) 3D image from the right superior view revealed the anatomic structures of the right upper lobe. The targeted subsegments were S2b and S3a, the targeted arteries were A2bi, A2bii, A3ai and A3aii, the targeted bronchi were B2b and B3a, and the targeted veins were inferior V2c and superior V2c. The veins marked with blue triangles were the intersubsegmental veins, which were the marks of intersubsegmental demarcation and should be preserved. All solid lines reflected the one-to-one correspondence between the actual and virtual anatomic structures.
Limitations of 3D-CTBA for surgical guidance
Even if pulmonary anatomy can be observed clearly via 3D-CTBA reconstruction of bronchus and vessels, preoperative imaging is still not fully identical to intra-operative actual anatomy, and sometimes it is difficult to be one to one correspondence. The lung with preoperative chest CT detection is inflated and in normal position, but the operative side lung in thoracoscopic surgery is collapsed and retracted, thus the traveling path of the segmental vessels and bronchus under the two different conditions are sort of distinct, which needs the accumulation of experience to identify accurately. The reconstruction of bronchus is closely related to the patients’ pulmonary parenchyma and the bronchus inflation degree, the one with poor contrast has a poor effect on the bronchus reconstruction, which causes the reduction in the surgical guiding significance.
Conclusions
3D-CTBA reconstruction technology is developing constantly and advancing gradually, the existing reconstruction software can meet the primary requirements of the operation, but there is still insufficience, remains to be further improved. Thoracoscopic pulmonary segmentectomy is becoming mature gradually and is applied widely, and the complete anatomic resection is the inevitable trend. The guidance of 3D reconstructed images enables to achieve the accurate segmentectomy, reduce the surgical complications and difficulties, and shorten the learning curve. For the treatment of small malignant lung nodules, the guidance of 3D reconstructed images not only enables to achieve a safe surgical margin but also can minimize the anatomic resection of the lung tissues, which is good for ensuring the oncology efficacy and retaining more healthy tissues. The theory named “Cone-shaped Segmentectomy” is the concentrated reflection of the entire anatomic segmentectomy.
Acknowledgements
Funding: This study was supported by the Jiangsu Provincial Six Talent Peaks Foundation (to L Chen) (No. WSW-028) and Major Program of Science and Technology Foundation of Jiangsu Province (to Q Zhu) (No. BE2016790).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. 10.1016/j.jtcvs.2006.02.063 [DOI] [PubMed] [Google Scholar]
- 2.Shapiro M, Weiser TS, Wisnivesky JP, et al. Thoracoscopic segmentectomy compares favorably with thoracoscopic lobectomy for patients with small stage I lung cancer. J Thorac Cardiovasc Surg 2009;137:1388-93. 10.1016/j.jtcvs.2009.02.009 [DOI] [PubMed] [Google Scholar]
- 3.Zhong C, Fang W, Mao T, et al. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy for small-sized stage IA lung cancer. Ann Thorac Surg 2012;94:362-7. 10.1016/j.athoracsur.2012.04.047 [DOI] [PubMed] [Google Scholar]
- 4.Wisnivesky JP, Henschke CI, Swanson S, et al. Limited resection for the treatment of patients with stage IA lung cancer. Ann Surg 2010;251:550-4. 10.1097/SLA.0b013e3181c0e5f3 [DOI] [PubMed] [Google Scholar]
- 5.Harada H, Okada M, Sakamoto T, et al. Functional advantage after radical segmentectomy versus lobectomy for lung cancer. Ann Thorac Surg 2005;80:2041-5. 10.1016/j.athoracsur.2005.06.010 [DOI] [PubMed] [Google Scholar]
- 6.Yang CF, D'Amico TA. Thoracoscopic segmentectomy for lung cancer. Ann Thorac Surg 2012;94:668-81. 10.1016/j.athoracsur.2012.03.080 [DOI] [PubMed] [Google Scholar]
- 7.Okada M, Tsutani Y, Ikeda T, et al. Radical hybrid video-assisted thoracic segmentectomy: long-term results of minimally invasive anatomical sublobar resection for treating lung cancer. Interact Cardiovasc Thorac Surg 2012;14:5-11. 10.1093/icvts/ivr065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamashita S, Tokuishi K, Anami K, et al. Thoracoscopic segmentectomy for T1 classification of non-small cell lung cancer: a single center experience. Eur J Cardiothorac Surg 2012;42:83-8. 10.1093/ejcts/ezr254 [DOI] [PubMed] [Google Scholar]
- 9.Gossot D, Ramos R, Brian E, et al. A totally thoracoscopic approach for pulmonary anatomic segmentectomies. Interact Cardiovasc Thorac Surg 2011;12:529-32. 10.1510/icvts.2010.257493 [DOI] [PubMed] [Google Scholar]
- 10.Eguchi T, Takasuna K, Kitazawa A, et al. Three-dimensional imaging navigation during a lung segmentectomy using an iPad. Eur J Cardiothorac Surg 2012;41:893-7. 10.1093/ejcts/ezr127 [DOI] [PubMed] [Google Scholar]
- 11.Iwano S, Yokoi K, Taniguchi T, et al. Planning of segmentectomy using three-dimensional computed tomography angiography with a virtual safety margin: technique and initial experience. Lung Cancer 2013;81:410-5. 10.1016/j.lungcan.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 12.Chan EG, Landreneau JR, Schuchert MJ, et al. Preoperative (3-dimensional) computed tomography lung reconstruction before anatomic segmentectomy or lobectomy for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2015;150:523-8. 10.1016/j.jtcvs.2015.06.051 [DOI] [PubMed] [Google Scholar]
- 13.Wicky S, Mayor B, Schnyder P. Methylene blue localizations of pulmonary nodules under CT-guidance: a new procedure used before thoracoscopic resections. Int Surg 1997;82:15-7. [PubMed] [Google Scholar]
- 14.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Non-Small Cell Lung Cancer (Version 2. 2016). Available online: http://www.nccn.org/patients
- 15.Nakamoto K, Omori K, Nezu K, et al. Superselective segmentectomy for deep and small pulmonary nodules under the guidance of three-dimensional reconstructed computed tomographic angiography. Ann Thorac Surg 2010;89:877-83. 10.1016/j.athoracsur.2009.11.037 [DOI] [PubMed] [Google Scholar]
- 16.Oizumi H, Kanauchi N, Kato H, et al. Anatomic thoracoscopic pulmonary segmentectomy under 3-dimensional multidetector computed tomography simulation: a report of 52 consecutive cases. J Thorac Cardiovasc Surg 2011;141:678-82. 10.1016/j.jtcvs.2010.08.027 [DOI] [PubMed] [Google Scholar]
- 17.Akiba T, Nakada T, Inagaki T. Simulation of the fissureless technique for thoracoscopic segmentectomy using rapid prototyping. Ann Thorac Cardiovasc Surg 2015;21:84-6. 10.5761/atcs.nm.13-00322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakashima S, Watanabe A, Ogura K, et al. Advantages of preoperative three-dimensional contrast-enhanced computed tomography for anomalous pulmonary artery in video-assisted thoracoscopic segmentectomy. Eur J Cardiothorac Surg 2010;38:388. 10.1016/j.ejcts.2010.02.037 [DOI] [PubMed] [Google Scholar]
- 19.Atkins BZ, Harpole DH, Jr, Mangum JH, et al. Pulmonary segmentectomy by thoracotomy or thoracoscopy: reduced hospital length of stay with a minimally-invasive approach. Ann Thorac Surg 2007;84:1107-12; discussion 1112-3. 10.1016/j.athoracsur.2007.05.013 [DOI] [PubMed] [Google Scholar]
- 20.Yamashita H. Roentgenologic anatomy of the lung. Tokyo: Igaku-Shoin, 1978:70-94. [Google Scholar]