Abstract
Background
Tuberculosis (TB) continues to cause an outsized burden of morbidity and mortality worldwide, still missing efficient and largely accessible diagnostic tools determining an appropriate control of the disease. Serological tests have the potentially to impact TB diagnosis, in particular in extreme clinical settings.
Methods
The diagnostic performances of the TB-XT HEMA EXPRESS (HEMA-EXPRESS) immunochromatographic rapid test for active TB diagnosis, based on use of multiple Mycobacterium tuberculosis (MTB) specific antigens, have been evaluated in a large study multicentre TB case-finding study, in populations with different exposure level to TB. A total of 1,386 subjects were enrolled in the six participating centres in Peru: 290 active-TB and 1,096 unaffected subjects.
Results
The TB prevalence (overall 20.5%) varied between 4.0% and 41.1% in the different study groups. Overall, the HEMA-EXPRESS test had 30.6% sensitivity (range 3.9–77.9%) and 84.6% specificity (range 51.6–97.3%). A significant inverse correlation between test accuracy (overall 73.5%, range 40.4–96.4%) and TB prevalence in the various study populations was observed (Pearson’s r=−0.7985; P=0.05).
Conclusions
HEMA-EXPRESS, is rapid and relatively inexpensive test suitable for routine use in TB diagnosis. In low TB prevalence conditions, test performance appears in line with WHO Target Product Profile for TB diagnostics. Performances appear suboptimal in high TB prevalence settings. Appropriate set-up in operative clinical settings has to be considered for novel serological tests for TB diagnosis, particularly for formats suitable for point-of-care use.
Keywords: Tuberculosis (TB), diagnosis, serology, rapid test, later flow immunochromatography, multicentre study, Latin America
Introduction
Tuberculosis (TB) continues to cause an outsized burden of morbidity and mortality, remaining one of the human infections with the highest prevalence worldwide, with an estimated 8.6 million new cases every year, of whom about 1/3 can transmit the disease (i.e., sputum smear-positive pulmonary TB) (1). Furthermore, the lethal combination of TB with human immunodeficiency virus (HIV) co-infection, the prevalence of co-morbidities (i.e., tobacco smoke and diabetes epidemics among the principals) and the spread of Mycobacterium tuberculosis (MTB) multidrug (MDR) and extensively drug-resistant (XDR) strains is worsening the efficiency of TB control efforts while making TB elimination more difficult to reach (1-4).
The essence of TB control is represented by rapid identification and effective treatment of individuals transmitting the bacillus (1,5-7). Moreover, pauci-bacillary active-TB cases [i.e., Acid Fast Bacilli (AFB)-negative subjects], although less effective in spreading MTB, are difficult to diagnose and might receive empirical treatment until culture and drug susceptibility test results are obtained, thus potentially contributing to MDR/XDR strain diffusion (8,9).
In addition, over 50% of the active-TB cases remain without any laboratory confirmation or with delay in diagnosis for unavailability of (or missing accessibility to) efficient diagnostic tools (1,5).
Rapid MTB culture methods and molecular assays may all play important roles in securing a rapid diagnosis of TB, directing decision making on isolation and treatment of patients, and on screening of contacts (10,11). However, the implementation of these methodologies are not possible in all clinical settings (12,13) and also if portable molecular assays for MTB diagnosis (i.e., Xpert-MTB/RIF) are starting to be available, their relative high cost in absence of appropriate support might limit their impact.
Serological tests for TB have potentially the characteristics to overcome these problems and to be used in extreme settings (5,14). However, serological tests for TB has shown sub-optimal sensitivity and specificity (14), due to the complex (cross-reactive) antigens used that may be recognized by non-specific antibody reaction (15-19). In addition, the low reproducibility and specificity of the lateral flow immunochromatographic test format (particularly in those based on complex MTB antigen largely used in India and other South-East Asian Countries) (20), contributed to the World Health Organization (WHO) policy recommendation not to use them (21). There is room to support the development of novel TB serological tests based on more specific reagents, while defining stringent criteria for developing novel rapid tests (which include immunologically-based ones) (22,23).
In this context, serological tests based on the detection of antibodies against recombinant MTB-complex specific protein antigens, show a variable sensitivity (20% to 85%) with high specificity (5,14,24,25). It is well documented that the combined use of test based on multiple MTB complex antigens can increase sensitivity maintaining a high specificity of serological tests (24,26-28).
In line with these considerations and the WHO indication for TB serological tests, we performed a multicentre study to evaluate a lateral flow immunochromatographic assay for TB diagnosis based on multiple MTB-specific antigens under different clinical settings and MTB exposure levels.
We defined the diagnostic performances of this rapid test for active TB diagnosis based on a large study, in clinical setting conditions with the effective “ex vivo” evaluation of the tested samples, more than an “in vitro” evaluation on serum-bank bank samples.
The study belongs to the ALAT (Asociación Latinoamericana de Tórax) and ERS (European Respiratory Society) SinTB project focused on eliminating TB in Latin America.
Methods
Study design and study populations
A multicentre TB case finding study has been performed between May and August 2015 to assess specificity and sensitivity of the rapid 123 TB-XT HEMA EXPRESS test under different clinical settings and in populations with different exposure level to TB. To this end, subjects were enrolled in different areas and defined for being active TB patients or TB unaffected subjects in six different participating centres in Peru. Demographic characteristics of the study groups and of different exposure levels are reported in Table 1.
Table 1. Characteristics of the study populations in the various enrolment centres.
| Population | Area and characteristic | Group | N | Age (years) | Sex | TB prevalence (%) |
|---|---|---|---|---|---|---|
| 1 | Health Care Workers at “Surquillo TB Health Centre”, Lima. The centre is located in a densely populated urban area with TB high incidence rate | Active TB | 9 | 48±20 | 9 M; 0 F | 4.0 |
| Controls | 213 | 46±18 | 103 M; 110 F | |||
| 2 | Urban area of Huaycan. A marginal urban area with high incidence of TB | Active TB | 60 | 46±17 | 41 M; 19 F | 15.4 |
| Controls | 330 | 45±17 | 157 M; 173 F | |||
| 3 | Workers enrolled in the popular food market Wanchaq. A marginal urban area of the city department of Cusco | Active TB | 51 | 44±15 | 27 M; 24 F | 16.7 |
| Controls | 254 | 43±16 | 126 M; 128 F | |||
| 4 | General population enrolled at Paucartambo, rural area of the Cusco department | Active TB | 15 | 46±21 | 10 M; 5 F | 31.9 |
| Controls | 32 | 43±20 | 14 M; 18 F | |||
| 5 | Workers enrolled in a company located in a middle-income area of Surquillo—medical district of Lima | Active TB | 3 | 59±16 | 0 M; 3 F | 5.4 |
| Controls | 52 | 44±16 | 23 M; 29 F | |||
| 6 | Health care workers of regional hospital Huacho, located in a rural area densely populated with high incidence of TB | Active TB | 152 | 43±16 | 83 M; 69 F | 41.4 |
| Controls | 215 | 45±17 | 91 M; 124 F | |||
| Total | Active TB | 290 | 44±16 | 170 M; 120 F | 20.5 | |
| Controls | 1,096 | 45±17 | 514 M; 582 F |
TB, tuberculosis; M, males; F, females.
A total of 1,386 subjects were enrolled in the six participating centres.
Active TB was identified in 290 enrolled subjects according to Centers for Disease Control and Prevention (CDC)/American Thoracic Society (ATS) criteria (29). All active TB patients were microbiologically confirmed by culture test.
The rest of the enrolled subjects (being culture negative) were considered to be TB unaffected and therefore used as control groups (N=1,096). In addition, individuals with signs and symptoms compatible with TB were excluded accordingly by CDC/ATS criteria.
For each individual recruited into the study data have been collected on a Clinical Data Collection Form. Laboratory personnel performing the serological test was blinded to the status of the study subjects.
The study was approved by the institutional review boards of all participating Clinical Institutions and an informed consent was obtained from each study subject before blood sample collection.
Rapid 123 TB-XT HEMA EXPRESS test
The rapid 123 TB-XT HEMA EXPRESS test (hereafter referred as HEMA-EXPRESS) is a lateral flow immune-chromatographic assay for the detection of IgG antibodies against MTB-complex specific proteins in blood and serum samples. The test allows detection of antibodies directed against the following MTB-complex specific immunodominant protein antigens: Rv2031c (14 kDa protein also known as HspX or alpha-crystalline), Rv0934 (38 kDa protein also known as PstS1 or Phosphate Binding Protein), Rv3875 (6 kDa protein also known as EsxA or Esat-6) and Rv3763 (16 kDa protein also known as LpqH) (30). The test was performed and scored positive or negative according to the manufacturer’s instructions.
Statistical analysis
Data are expressed as the mean ± standard deviation (SD) of the mean or percentage as appropriate. Sensitivity, specificity, accuracy, predicting values and prevalence were calculated as previously indicated (31) Comparison between groups was made by using paired or unpaired Student’s t-test or Chi-square test as appropriate. A P value below 0.05 was considered statistically significant.
Results
TB prevalence and characteristics of the study populations
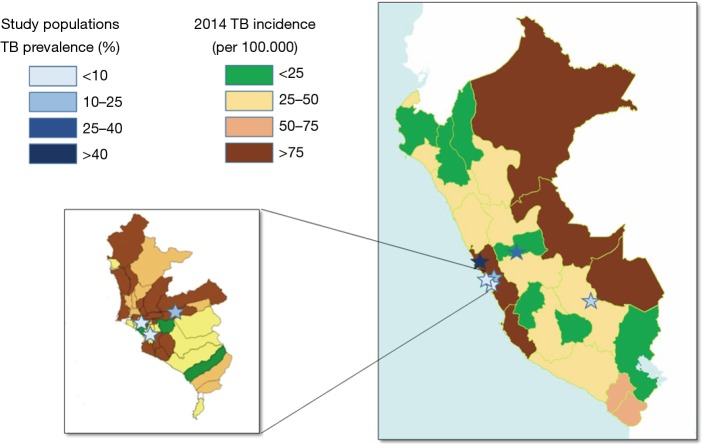
Figure 1 shows the geographic distribution of the study sites with the observed TB prevalence in each evaluated population, in comparison with the official TB incidence registered in the areas where the study was performed. The TB prevalence in the study populations groups varied between 4.0% and 41.1% (overall 20.5%, Table 1). The populations are representative of various situations and settings over the Country according to the reported incidence of TB for the areas (32).
Figure 1.
The geographical distribution in the Republic of Peru of the six study sites is shown. The insert reports a magnification of the province of Lima, capital of Peru, where 3 study sites are present. Site locations (stars) are coloured according to the observed TB prevalence (as indicated in the “prevalence” colour array, see also Table 1). Official incidence of TB in various administrative Departments of Peru is reported by colour gradient (as indicated in the “incidence” colour array), as for the official report of the Ministry of Health of Peru for 2015 (published on February 2016) elaborated on 2014 data. TB, tuberculosis.
In particular, in study population 1 [“Surquillo TB Health Centre”—Health Care Workers (HCWs)], the enrolled population includes doctors, nurses and technicians, at a Ministry of Health facility specialized on TB patients treatment. The facility is located within a crowded urban area presenting a TB incidence 6 times higher than the national average (and 8 times higher than the average for the region of the Americas).
Study population 2 (Huaycan—general population), includes a general population seeking for TB diagnosis and care at the local hospital in Huaycan village (Shanyty town region), located east of Lima, in a poor urban area.
Study population 3 (Wanchaq—popular Market workers), includes workers interested in TB screening of the Cusco Popular Market (general sales of vegetables, fruits and groceries), located in a poor urban area in Wanchaq (a county located at the Cusco downtown).
Study population 4 (Paucartambo—general population) includes a general population interested on TB screening in the only health facility of Paucartambo village. Paucartambo is the poorest rural village located South of Cusco capital.
Study population 5 (Lima—industry workers) includes healthy workers (managers, employers, administrative officers and assistants) of a company located in Lima within an area with the lowest pulmonary TB incidence.
Finally, study population 6 (Huacho—HCW) includes HCW of the “Regional Hospital of Huacho”, northeast of Lima, specialized on TB patients’ treatment. The hospital is located in Huacho, a complex semi-urban area, with poverty, high violence and drugs abuse rate, and lack of sanitation. The area reports the highest pulmonary TB incidence rate in Peru.
Performance of the HEMA-EXPRESS test in the different clinical and TB-exposure settings
Table 2 reports the results of sensitivity, specificity, accuracy and predicting values for the HEMA-EXPRESS rapid test for active TB diagnosis in the different participating centres and study populations.
Table 2. Performance of HEMA-EXPRESS test for active-TB diagnosis in the different study populations.
| Population | TB prevalence (%) | Group | N | HEMA-EXPRESS positives | Sensitivity | Specificity | Accuracy | Positive predicting value | Negative predicting value |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 4.0 | Active TB | 9 | 7 | 77.8 | 97.2 | 96.4 | 53.8 | 99.0 |
| Controls | 213 | 6 | |||||||
| 2 | 15.4 | Active TB | 60 | 35 | 58.3 | 97.3 | 91.3 | 79.5 | 92.8 |
| Controls | 330 | 9 | |||||||
| 3 | 16.7 | Active TB | 51 | 36 | 70.5 | 51.6 | 54.7 | 22.6 | 89.7 |
| Controls | 254 | 123 | |||||||
| 4 | 31.9 | Active TB | 15 | 2 | 13.3 | 53.1 | 40.4 | 11.8 | 56.7 |
| Controls | 32 | 15 | |||||||
| 5 | 5.4 | Active TB | 3 | 2 | 66.6 | 96.1 | 94.5 | 50.0 | 98.0 |
| Controls | 52 | 2 | |||||||
| 6 | 41.4 | Active TB | 152 | 6 | 3.9 | 94.4 | 56.9 | 33.3 | 58.2 |
| Controls | 215 | 12 | |||||||
| Total | 20.5 | Active TB | 290 | 88 | 30.6 | 84.6 | 73.5 | 34.0 | 82.5 |
| Controls | 1,096 | 167 |
HEMA-EXPRESS, rapid 123 TB-XT HEMA EXPRESS test; TB, tuberculosis.
Overall, the HEMA-EXPRESS test had 30.6% sensitivity (range 3.9–77.9%) and 84.6% specificity (range 51.6–97.3%), with lower performances than those proposed by WHO in the high-priority target product profiles (TPP) for new TB diagnostics (22).
However, the analysis of the performance in the different sites (Table 2), indicates that, with lower TB prevalence (study sites 1, 2 and 5), the test performances are more in line with WHO TPPs, with 58.3–77.8% sensitivity (P<0.05 vs. study sites 4 and 6, chi-square test, all comparisons), and 96.1–97.3% specificity (P<0.05 vs. study sites 3 and 4, chi-square test, all comparisons).
In line with the above consideration, a significant inverse correlation was found between test accuracy (overall 73.5%, range 40.4–96.4%) and TB prevalence in the various study populations (Pearson’s r=−0.7985, P=0.05).
Discussion
Despite the major efforts undertaken by WHO and partners, which contributed to reduce TB incidence globally by approximately 2% per annum, much still needs to be do done in order to improve TB control and achieve TB Elimination (2,3,33).
In particular, better diagnostics are necessary, together with other interventions, to approach the pre-elimination phase by 2035 in low-TB incidence countries (2,3).
Due to the current limitation of tests to diagnose active TB (5), serology is still an important alternative (20,25), in particular (as underlined by WHO) for tests based on MTB-specific antigens (21,22).
In this study, we report the performance of a lateral flow immunochromatography assay for active-TB diagnosis based on multiple MTB-specific antigens in various clinical settings for TB case-finding. The overall performances of the test appear sub-optimal if compared to the WHO TPPs for a novel TB test (22). However, the accuracy of the test inversely correlate with the TB prevalence in the study populations. Therefore, in study populations at low TB prevalence, its performance appears to be in line with WHO TPPs indication.
This is suggesting that appropriate set-up of novel serological tests for TB diagnosis should be performed in operative clinical settings, in particular for test formats suitable for point-of-care use in populations with high TB incidence (such as lateral flow immunochromatography assays and other rapid test formats).
The detailed analysis of the study results shows that some of the critical populations were represented by HCW exposed to TB. In this context, in a previous report (34), we found a correlation with exposure level to TB in the workplace, with the development of an antibody response against MTB-specific antigens, including most of the antigens present in the HEMA-EXPRESS test. This suggests that HEMA-EXPRESS test performance in HCWs might be influenced by the specific level of TB exposure, as generally measured in this study by the TB prevalence.
HEMA-EXPRESS test has been previously analysed showing variable performances in sensitivity (29–42%) and specificity (65–80%) (30). The current test version used in this “on site” study, under various clinical settings, appears to over-perform the original test. However, the test variability we have observed in this study might be also due to the relative low reproducibility reported for this test (30).
Serological tests might play a major role in diagnosing AFB-negative patients with signs and symptoms suggesting TB, since their culture results may not be available for 15–40 days after the sample collection.
The data show that with the HEMA-EXPRESS, TB can be diagnosed up to 78% sensitivity and 97% specificity in case-finding settings. In this context, evident differences were identified in the responses to antigens included in the HEMA-EXPRESS test when comparing AFB-negative and AFB-positive TB patients (12-16). Although in the current study we did not rule out the performance of the test with respect to AFB-smear status, the use of multiple antigens in the same test might overcome the differences in performances in AFB-negative and AFB-positive TB patients.
In conclusion, although rapid molecular tests are available for the diagnosis of TB (in particular in AFB-negative patients) the practical diffusion of these tests is still sub-optimal (1). Serological tests, although not as sensitive as the molecular tests, are rapid, relatively inexpensive and suitable for routine use. The use of multiple-antigen based tests using recombinant MTB immunodominant proteins antigens such as in HEMA-EXPRESS, might greatly enhance the power of serological diagnosis of TB for those patients who may, otherwise, receive delayed treatment. However, it is fundamental that novel tests are set-up in appropriate operative clinical settings to avoid significant differences in test performances.
Acknowledgements
The study has been funded by the Peruvian Ministry of Health, TB Prevention and Control Program, at the Primary Health Care Facility of Surquillo (Lima, Peru).
Disclaimer: The authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions and policies of their institutions.
Ethical Statement: The study was approved by the institutional review boards of all participating Clinical Institutions (approval number: MOH-CSSurquillo 12A0-2015) and an informed consent was obtained from each study subject before blood sample collection.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.World Health Organization. Global tuberculosis report 2015. WHO/HTM/TB/2015.22. Geneva: World Health Organization, 2015. [Google Scholar]
- 2.Lönnroth K, Migliori GB, Abubakar I, et al. Towards tuberculosis elimination: an action framework for low-incidence countries. Eur Respir J 2015;45:928-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Ambrosio L, Dara M, Tadolini M, et al. Tuberculosis elimination: theory and practice in Europe. Eur Respir J 2014;43:1410-20. 10.1183/09031936.00198813 [DOI] [PubMed] [Google Scholar]
- 4.Sotgiu G, Tiberi S, D'Ambrosio L, et al. Faster for less: the new "shorter" regimen for multidrug-resistant tuberculosis. Eur Respir J 2016;48:1503-7. 10.1183/13993003.01249-2016 [DOI] [PubMed] [Google Scholar]
- 5.Kunnath-Velayudhan S, Gennaro ML. Immunodiagnosis of tuberculosis: a dynamic view of biomarker discovery. Clin Microbiol Rev 2011;24:792-805. 10.1128/CMR.00014-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tiberi S, D'Ambrosio L, De Lorenzo S, et al. Tuberculosis elimination, patients' lives and rational use of new drugs: revisited. Eur Respir J 2016;47:664-7. 10.1183/13993003.01297-2015 [DOI] [PubMed] [Google Scholar]
- 7.Sotgiu G, Spanevello A, Migliori GB. History of tuberculosis and drug resistance. N Engl J Med 2013;368:88-9. 10.1056/NEJMc1212308 [DOI] [PubMed] [Google Scholar]
- 8.Sotgiu G, D'Ambrosio L, Centis R, et al. TB and M/XDR-TB infection control in European TB reference centres: the Achilles' heel? Eur Respir J 2011;38:1221-3. 10.1183/09031936.00029311 [DOI] [PubMed] [Google Scholar]
- 9.Migliori GB, Sotgiu G, D'Ambrosio L, et al. TB and MDR/XDR-TB in European Union and European Economic Area countries: managed or mismanaged? Eur Respir J 2012;39:619-25. 10.1183/09031936.00170411 [DOI] [PubMed] [Google Scholar]
- 10.Matteelli A, Lönnroth K, Getahun H, et al. Numbers needed to treat to prevent tuberculosis. Eur Respir J 2015;46:1838-9. 10.1183/13993003.01179-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erkens CG, Kamphorst M, Abubakar I, et al. Tuberculosis contact investigation in low prevalence countries: a European consensus. Eur Respir J 2010;36:925-49. 10.1183/09031936.00201609 [DOI] [PubMed] [Google Scholar]
- 12.Weyer K, Mirzayev F, Migliori GB, et al. Rapid molecular TB diagnosis: evidence, policy making and global implementation of Xpert MTB/RIF. Eur Respir J 2013;42:252-71. 10.1183/09031936.00157212 [DOI] [PubMed] [Google Scholar]
- 13.Albert H, Nathavitharana RR, Isaacs C, et al. Development, roll-out and impact of Xpert MTB/RIF for tuberculosis: what lessons have we learnt and how can we do better? Eur Respir J 2016;48:516-25. 10.1183/13993003.00543-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steingart KR, Flores LL, Dendukuri N, et al. Commercial serological tests for the diagnosis of active pulmonary and extrapulmonary tuberculosis: an updated systematic review and meta-analysis. PLoS Med 2011;8:e1001062. 10.1371/journal.pmed.1001062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daniel TM, Debanne SM. The serodiagnosis of tuberculosis and other mycobacterial diseases by enzyme-linked immunosorbent assay. Am Rev Respir Dis 1987;135:1137-51. [DOI] [PubMed] [Google Scholar]
- 16.Cocito CG. Properties of the mycobacterial antigen complex A60 and its applications to the diagnosis and prognosis of tuberculosis. Chest 1991;100:1687-93. 10.1378/chest.100.6.1687 [DOI] [PubMed] [Google Scholar]
- 17.Jackett PS, Bothamley GH, Batra HV, et al. Specificity of antibodies to immunodominant mycobacterial antigens in pulmonary tuberculosis. J Clin Microbiol 1988;26:2313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amicosante M, Paone G, Ameglio F, et al. Antibody repertoire against the A60 antigen complex during the course of pulmonary tuberculosis. Eur Respir J 1993;6:816-22. [PubMed] [Google Scholar]
- 19.Kalish SB, Radin RC, Phair JP, et al. Use of an enzyme-linked immunosorbent assay technique in the differential diagnosis of active pulmonary tuberculosis in humans. J Infect Dis 1983;147:523-30. 10.1093/infdis/147.3.523 [DOI] [PubMed] [Google Scholar]
- 20.Steingart KR, Ramsay A, Dowdy DW, et al. Serological tests for the diagnosis of active tuberculosis: relevance for India. Indian J Med Res 2012;135:695-702. [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization (WHO) report. Commercial serodiagnostic tests for diagnosis of tuberculosis. Policy Statement. 2011. Document WHO/HTM/TB 2011.5. Geneva, World Health Organization 2001. [PubMed]
- 22.World Health Organization (WHO) report. High-priority target product profiles for new tuberculosis diagnostics: report of a consensus meeting. 28-29 April 2014. WHO/HTM/TB 2014.18. Geneva, World Health Organization 2014.
- 23.Migliori GB, Lienhardt C, Weyer K, et al. Ensuring rational introduction and responsible use of new TB tools: outcome of an ERS multisector consultation. Eur Respir J 2014;44:1412-7. 10.1183/09031936.00132114 [DOI] [PubMed] [Google Scholar]
- 24.Bothamley GH. Serological diagnosis of tuberculosis. Eur Respir J Suppl 1995;20:676s-688s. [PubMed] [Google Scholar]
- 25.Pinto LM, Grenier J, Schumacher SG, et al. Immunodiagnosis of tuberculosis: state of the art. Med Princ Pract 2012;21:4-13. 10.1159/000331583 [DOI] [PubMed] [Google Scholar]
- 26.Verbon A, Weverling GJ, Kuijper S, et al. Evaluation of different tests for the serodiagnosis of tuberculosis and the use of likelihood ratios in serology. Am Rev Respir Dis 1993;148:378-84. 10.1164/ajrccm/148.2.378 [DOI] [PubMed] [Google Scholar]
- 27.Bothamley GH, Rudd R, Festenstein F, et al. Clinical value of the measurement of Mycobacterium tuberculosis specific antibody in pulmonary tuberculosis. Thorax 1992;47:270-5. 10.1136/thx.47.4.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amicosante M, Barnini S, Corsini V, et al. Evaluation of a novel tuberculosis complex-specific 34 kDa protein in the serological diagnosis of tuberculosis. Eur Respir J 1995;8:2008-14. 10.1183/09031936.95.08122008 [DOI] [PubMed] [Google Scholar]
- 29.Diagnostic Standards and Classification of Tuberculosis in Adults and Children . This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med 2000;161:1376-95. 10.1164/ajrccm.161.4.16141 [DOI] [PubMed] [Google Scholar]
- 30.WHO. Diagnostics evaluation series, 2. Laboratory-based evaluation of 19 commercially available rapid diagnostic tests for tuberculosis 2008.
- 31.Fletcher RH, Fletcher SW. Clinical epidemiology: the essentials (4th ed). Baltimore, Md.: Lippincott Williams & Wilkins, 2005:45. [Google Scholar]
- 32.Direccion General de Epidemiologia, Ministero de la Salud, Peru. Analisis de la situacion epidemiologica de la tuberculosis en el Peru 2015. February 2016.
- 33.World Health Organization (WHO). Millennium Development Goals (MDGs). Fact sheet N°290. Updated May 2015. Available online: http://www.who.int/mediacentre/factsheets/fs290/en/
- 34.Franchi A, Amicosante M, Rovatti E, et al. Evaluation of a western blot test as a potential screening tool for occupational exposure to Mycobacterium tuberculosis in health care workers. J Occup Environ Med 2000;42:64-8. 10.1097/00043764-200001000-00015 [DOI] [PubMed] [Google Scholar]