Abstract
Background
Transbronchial biopsy (TBB) using radial endobronchial ultrasound with a guide sheath (REBUS-GS) has improved the diagnosis of peripheral pulmonary lesions (PPLs). Because of the high cost of the GS, REBUS with distance (REBUS-D) has certain advantages. The aim of this study was to compare the diagnostic yield of the REBUS-GS and REBUS-D by thin bronchoscopy for PPLs.
Methods
Patients with PPLs were enrolled in a prospective randomized crossover study from August 2014 and July 2015. Once the lesion was localized, TBB using REBUS-GS and TBB using REBUS-D were performed sequentially in a randomized order in each patient. Each patient received four to five transbronchial biopsies with REBUS-GS as well as four to five transbronchial biopsies with REBUS-D. All brushing was performed through GS.
Results
A total of 54 patients were enrolled in this study. After excluding seven participants with PPLs that were not detected by REBUS, a total of 47 subjects underwent REBUS-TBB. The diagnostic yield of REBUS-GS-TBB and REBUS-D-TBB was 72.2% (39/54) and 75.9% (41/54) respectively (P=0.625). Moreover, there was no statistically significant difference in diagnostic yield between REBUS-GS and REBUS-D in different lobe lesions and lesion sizes. Two cases of adenocarcinoma were only diagnosed with REBUS-GS-TBB. Two cases of tuberculosis, one case of mucosa-associated lymphoid tissue lymphoma (MALT) and one case of adenocarcinoma were only diagnosed by REBUS-D-TBB. The mean biopsy time after visualization of PPLs for REBUS-GS-TBB and REBUS-D-TBB were 5.17±2.34 and 7.36±3.18 min (P=0.00053).
Conclusions
Using thin bronchoscopy, the diagnostic yield for PPLs with REBUS-D-TBB is not inferior to the yield with REBUS-GS-TBB. The diagnosis rate of small subpleural lesions with REBUS-D is lower than the rate with REBUS-GS. Although it is associated with shorter operation time and less bleeding, REBUS-GS has a higher cost and sometimes leads to check failure due to small specimens and the impact of the bronchoscope curvature.
Keywords: Radial endobronchial ultrasound (REBUS), peripheral pulmonary lesions (PPLs), transbronchial biopsy (TBB), thin bronchoscopy, guide sheath (GS)
Introduction
The diagnosis of peripheral pulmonary lesions (PPLs) is clinically challenging compared to central lesions. Transbronchial biopsy (TBB) with fluoroscopy guidance is no longer recommended because of its low diagnostic yield (1). Radial probe endobronchial ultrasound (REBUS) is a valuable technique that has increased the diagnostic yield for PPLs (2). Nevertheless, REBUS using a guide sheath (GS) could further improve the diagnostic accuracy (3). The results of a meta-analysis showed that the diagnostic yield for PPLs was 73.2% with REBUS-GS (4).
However, one disadvantage is that the cost of the GS is high, which would lead to increased healthcare expenses. A K-201 kit (Olympus Ltd., Tokyo, Japan) equipped with a GS, biopsy forceps, and a brush costs 1,700 RMB, whereas disposable forceps and a brush cost 320 RMB. In order to reduce healthcare costs, Chung et al. first proposed a possible alternative to REBUS-GS, REBUS with distance (REBUS-D) for PPLs (5). The insertion depth of the biopsy forceps is determined by measuring the distance between the detected lesion and the orifice of the bronchus or outer orifice of the working channel of the bronchoscope (5-7). According to Huang, REBUS-D-TBB with thin bronchoscope for PPLs leads to a higher diagnostic yield (73%) than with a therapeutic bronchoscope (53%) (7,8). Other reports support this contention (9-11). Our previous work showed that the diagnostic sensitivity was 65% for PPLs with REBUS-D by thin bronchoscopy (10). Therefore, applying thin bronchoscopy to REBUS-D-TBB for PPLs has certain advantages, such as a high diagnostic yield and low-cost.
However, no researchers have directly compared the two methods. Accordingly, the aim of the current study was to investigate the difference in the diagnostic yield of the REBUS-GS-TBB and REBUS-D-TBB with thin bronchoscope for PPLs.
Methods
Subjects
A prospective randomized crossover trial involving endobronchial ultrasound for the diagnosis of PPLs was carried out at the Third Affiliated Hospital of Soochow University from August 2014 to July 2015. The enrollment criteria were as follows: (I) patients with PPLs defined as abnormal growth shown via CT scans; and (II) age ≥18 years. The exclusion criteria were as follows: (I) age <18 years; (II) pure ground-glass opacities; (III) lesion diameter <10 mm; and (IV) patients with contraindications for bronchoscopy, such as active ischemic heart disease. Informed consent was obtained from all subjects. This study was approved by the ethics committee of the Third Affiliated Hospital of Soochow University (No. 2014077).
Procedures
All procedures were performed by three experienced pulmonologists using a BF-P260F flexible bronchoscope (Olympus Ltd., Tokyo, Japan) with a 2.0 mm working channel and a 4.0 mm outer diameter, an endoscopic ultrasound system (EU-ME1, Olympus Ltd., Tokyo, Japan) and an ultrasound processor (MAJ-935, Olympus Ltd., Tokyo, Japan). The radial EBUS miniature probe measured 1.4 mm in diameter (UM-S20-17s, Olympus Ltd., Tokyo, Japan). The GS had a diameter of 1.95 mm. The biopsy forceps had a diameter of 1.5 mm (K-201, Olympus Ltd., Tokyo, Japan). Disposable forceps with a 1.8-mm outer diameter were used during REBUS-D-TBB. The operation on each patient was completed by the same pulmonologist.
The chest CT scan images were reviewed before bronchoscopy. The size of each PPL was measured at its largest diameter on axial lung window settings. The location and distance of each PPL from the costal and visceral plural were also recorded.
All bronchoscopies were conducted under local anesthesia with 2% lidocaine. After completing the inspection of the central airways endoscopically, the operator maneuvered the bronchoscope to the suspected bronchi as far as possible with knowledge of prior CT scans. Then, the EBUS probe was inserted into the suspected bronchi to detect the PPLs. Once the lesion was localized, REBUS-GS-TBB and REBUS-D-TBB were performed in randomized order. The REBUS-GS-TBB and REBUS-D-TBB procedures were performed as previously described in other publications (3,5).
A brief introduction to the REBUS-D-TBB is as follows: when the lesion was detected by EBUS, the probe was marked at the outer orifice of the working channel of the bronchoscope before being withdrawn until the end of the probe reached the visible orifice of the target bronchus. The distance measured between the marker and the outer orifice of the working channel was the inserted depth of the forceps from the orifice of the corresponding bronchus (Figure 1). Every patient sequentially received four to five TBBs with REBUS-GS as well as four to five TBBs with REBUS-D. All brushing was performed through the GS.
Figure 1.

Measuring the distance between the marker and the outer orifice of the working channel.
The length of time required to complete the biopsy and address complications was documented. The duration of REBUS detection was limited to 20 minutes. Patients were excluded from the study if the lesion were not identified within the allotted amount of time. Fluoroscopy was not used during the procedures. Previous researchers have reported that a chest radiograph is not necessary within 2–4 hours after the procedure, but we performed one in order to identify any iatrogenic pneumothorax (12).
The pathologists were blinded to the biopsy order. A positive histological diagnosis meant either proof of malignancy or a defined benign pathology, such as granuloma or pneumonia. If the TBB yielded a nondiagnostic result, such as fibrosis or chronic inflammation, the patient was referred for a CT-guided transthoracic needle biopsy, surgery, or follow up for radiological improvement after being treated accordingly.
Study endpoints
The primary endpoint was to investigate the difference in the diagnostic yield of the REBUS-GS-TBB and REBUS-D-TBB with thin bronchoscope for PPLs. A secondary endpoint was to calculate the mean biopsy time associated with the two methods.
Statistical analysis
The data were analyzed using IBM SPSS Statistics 23 (IBM Corporation, New York, USA). McNemar’s chi-square test was utilized to evaluate the frequency data. Paired t-tests were used to compare the means of the independent variables. A P value of <0.05 was considered statistically significant. The study was considered non-inferior if the diagnosis rate associated with REBUS-D-TBB was <10% of that associated with REBUS-GS-TBB.
Results
A total of 54 subjects with PPLs were enrolled. Excluding 7 who had undetectable lesions, 47 subjects were included in the randomized crossover study. The trial flow diagram is shown in Figure 2. The mean age of the 54 subjects (35 men, 19 women) was 60.85±9.12 years. The mean size of the PPLs was 30.2±13.5 mm. The mean distance from the pleura was 9.5±8.3 mm.
Figure 2.
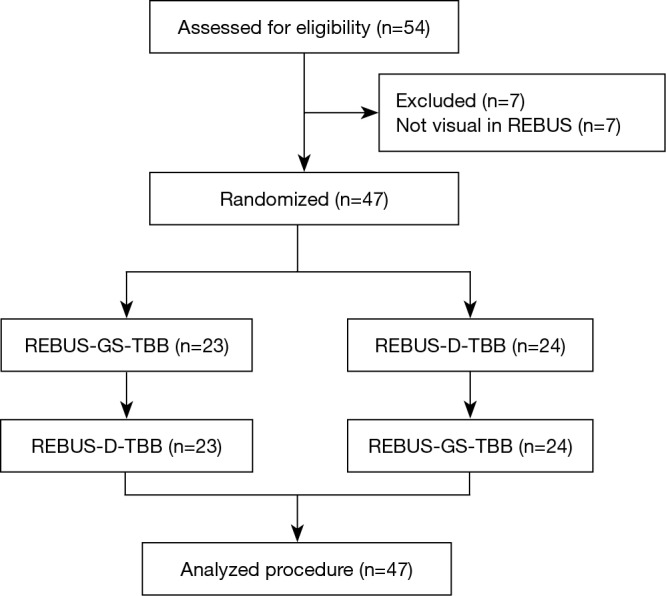
Trial flow of subject allocation to the two groups. REBUS-GS, radial endobronchial ultrasound with a guide sheath; REBUS-D, REBUS with distance; TBB, transbronchial biopsy.
All subjects with PPLs were biopsied sequentially by REBUS-GS-TBB and REBUS-D-TBB in randomized order. Final diagnoses of targeted lesions are shown in Table 1. The diagnostic yield by REBUS-GS and REBUS-D was 72.2% (39/54) and 75.9% (41/54), respectively. The difference in the diagnosis rate between the two methods was −3.7%. In a total of 47 subjects by visual REBUS, the diagnostic yield first with GS and second with GS was 87.0% (20/23) and 91.7% (21/24), respectively. The difference in the diagnosis rate between the two methods was −4.7%.
Table 1. Final diagnoses of targeted PPL.
| Diagnosis | N | REBUS visual | Diagnosed by REBUS-GS | Diagnosed by REBUS-D | P value |
|---|---|---|---|---|---|
| Malignant | 38 | 34 | 30 (78.9%) | 30 (78.9%) | 1 |
| Adenocarcinoma | 31 | 29 | 26 | 25 | |
| Squamous | 4 | 2 | 2 | 2 | |
| Adenosquamous | 2 | 2 | 2 | 2 | |
| MALT | 1 | 1 | 0 | 1 | |
| Benign | 16 | 13 | 9 (56.3%) | 11 (68.8%) | 0.5 |
| Abscess | 10 | 8 | 6 | 6 | |
| TB | 4 | 4 | 2 | 4 | |
| Mucormycosis | 1 | 1 | 1 | 1 | |
| Atypical adenoma | 1 | 0 | 0 | 0 | |
| Total | 54 | 47 | 39 (72.2%) | 41 (75.9%) | 0.625 |
PPL, peripheral pulmonary lesion; MALT, mucosa-associated lymphoid tissue lymphoma; TB, tuberculosis; REBUS-GS, radial endobronchial ultrasound with a guide sheath; REBUS-D, REBUS with distance.
Four cases were not diagnosed by bronchoscopy but were diagnosed by surgery or had lesions that disappeared after anti-inflammatory treatment. The diagnostic yields by REBUS-GS and REBUS-D in different lobe lesions are shown in Table 2. The influence of lesion size on diagnostic yield is shown in Table 3.
Table 2. Diagnostic yield (REBUS-GS vs. REBUS-D) in different lobe lesions.
| Lobe | N | REBUS visual | Diagnosed by REBUS-GS (%) | Diagnosed by REBUS-D (%) | P value |
|---|---|---|---|---|---|
| Right upper | 18 | 16 | 12 (66.7) | 14 (77.8) | 0.456 |
| Right middle | 3 | 3 | 3 (100.0) | 3 (100.0) | – |
| Right lower | 8 | 6 | 3 (37.5) | 4 (50.0) | – |
| Left upper | 15 | 12 | 11 (73.3) | 11 (73.3) | 1 |
| Left lower | 10 | 10 | 10 (100.0) | 9 (90.0) | 0.819 |
REBUS-GS, radial endobronchial ultrasound with a guide sheath; REBUS-D, REBUS with distance.
Table 3. Diagnostic yield (REBUS-GS vs. REBUS-D) in different lesion sizes.
| Lesion size | N | REBUS visual | Diagnosed by REBUS-GS | Diagnosed by REBUS-D | P value |
|---|---|---|---|---|---|
| Malignant (mm) | 38 | 34 | 30 | 30 | |
| >30 | 13 | 13 | 12 (92.3%) | 13 (100%) | 1 |
| 20–30 | 16 | 14 | 12 (75.0%) | 12 (75.0%) | 1 |
| <20 | 9 | 7 | 6 (66.7%) | 5 (55.6%) | 0.414 |
| Benign (mm) | 16 | 13 | 9 | 11 | |
| >30 | 8 | 8 | 5 (62.5%) | 7 (87.5%) | 0.564 |
| 20–30 | 5 | 4 | 3 (60%) | 3 (60%) | – |
| <20 | 3 | 1 | 1 (33.3%) | 1 (33.3%) | – |
REBUS-GS, radial endobronchial ultrasound with a guide sheath; REBUS-D, REBUS with distance.
In six cases, only one method yielded a positive diagnosis (Table 4). The two cases diagnosed only by REBUS-GS-TBB were adenocarcinomas. The diameters of these two lesions were 20 and 16 mm. The distances from the pleura were 5 and 5.7 mm. Of the four cases with positive diagnosis only by EBUS-D-TBB, two cases involved tuberculosis and one case involved mucosa-associated lymphoid tissue lymphoma (MALT) that could not be confirmed by pathology due to the small samples. Another lesion that ultimately was confirmed as adenocarcinoma located in the right upper apical lung and affixed to the pleura was determined to be a negative diagnosis by EBUS-GS-TBB. The details from this case are as follows: male, 79 years old, a 25-mm nodule in the right upper apical lung. After visualization by REBUS, REBUS-D-TBB was completed successfully, whereas REBUS-GS-TBB failed because the bronchoscope with the GS could not reach the target bronchus.
Table 4. Analysis of the six cases diagnosed by only one method.
| Method | Diagnosis | Position | Size (mm) | Distance from pleura (mm) |
|---|---|---|---|---|
| REBUS-GS + REBUS-D − | Adenocarcinoma | Left upper posterior | 20 | 5.0 |
| Adenocarcinoma | Left lower anteromedial | 16 | 5.7 | |
| REBUS-GS − REBUS-D + | MALT | Right lower superior | 40 | 4.0 |
| Adenocarcinoma | Right upper apical | 25 | 0 | |
| TB | Left upper anterior | 31 | 17.0 | |
| TB | Right upper apical | 32 | 7.7 |
MALT, mucosa-associated lymphoid tissue lymphoma; TB, tuberculosis; REBUS-GS, radial endobronchial ultrasound with a guide sheath; REBUS-D, REBUS with distance.
After visualization of the lesions, the length of biopsy time for REBUS-GS-TBB and REBUS-D-TBB was 5.17±2.34 vs. 7.36±3.18 min (P=0.00053) (Figure 3). More than 50 mL of bleeding was observed in five subjects in the REBUS-D-TBB group and no subjects in the REBUS-GS-TBB group. The bleeding was improved after local application of norepinephrine and intravenous drip of vasopressin. Pneumothorax and pulmonary infection were not encountered in this study.
Figure 3.
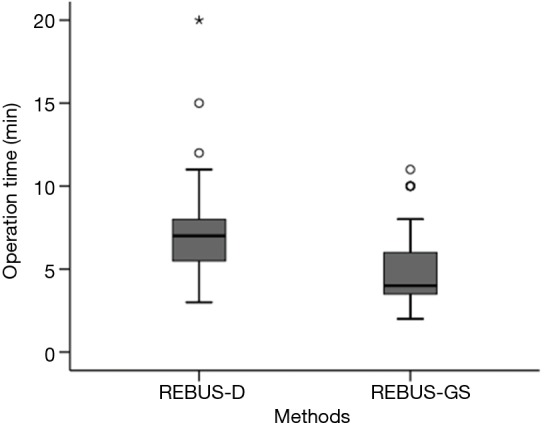
The operation time after visualization of PPLs for REBUS-GS-TBB and REBUS-D-TBB (P=0.00053). PPLs, peripheral pulmonary lesions; REBUS-GS, radial endobronchial ultrasound with a guide sheath; REBUS-D, REBUS with distance; TBB, transbronchial biopsy.
Discussion
This purpose of this prospective randomized crossover trial was to compare the diagnostic yield of the REBUS-GS and REBUS-D for PPLs. The results suggest that both methods showed a good level of safety. Additionally, non-inferiority was observed in diagnostic yield, regardless of the order of operating sequence. Moreover, there was no statistically significant difference in diagnostic yield between the two methods in different lobe lesions and lesion sizes.
The diagnostic yield with REBUS-GS (72.2%) was comparable to the diagnostic yields reported in other studies (63.9–72.5%) (13-16). The diagnostic yield for PPLs with REBUS-D by thin bronchoscope (75.9% in total, 78.9% for MT) in the present study was higher than that the diagnostic yields reported in previous studies with therapeutic bronchoscopy (53% in total, 73% for MT) (6,7). The flexible bronchoscope (BF-P260F) with a 2.0-mm working channel and a 4.0-mm outer diameter that was used could reach the distal end of 4–5 grade bronchi. Consequently, the ultrasonic probe was able to reach the lesion more easily. Furthermore, the thin bronchoscope could partially replace the GS functions to improve biopsy accuracy. In addition, the percentage of subjects with a lesion diameter smaller than 20 mm was only 22.2% (12/54) in our study. These factors may have contributed to the higher diagnostic yield in the EBUS-D group.
Although there was no statistically significant difference in the diagnostic yield of REBUS-GS and REBUS-D, the cases diagnosed by the two methods were not completely consistent. The diameters of the two lesions diagnosed by REBUS-GS only were less than 20 mm. Additionally, the distances from the pleura were less than 10 mm. Whereas the thin bronchoscope can reach the distal bronchial, it may still be diverted to non-lesion sites when the biopsy forceps reenter. Thus, for diagnosing small lesions located in subpleural lung regions, REBUS-GS-TBB may be more reliable.
A lesion with a diameter of 25 mm located in the right upper apical lung and affixed to the pleura was diagnosed by REBUS-D-TBB only because the bronchoscope with GS could not reach the target bronchus. The bronchoscope with the ultrasonic probe covered by GS might be less flexible and less able to reach the small angled branch of the peripheral bronchial tree.
For tuberculosis, pneumonia, and MALT, due to the small biopsy forceps with a diameter of 1.5 mm in the GS, the tissue samples obtained by REBUS-GS-TBB are not sufficient for pathological confirmation.
A larger sample could be acquired with REBUS-D compared to REBUS-GS. Therefore, REBUS-D has more advantages in the diagnosis of lesions that require larger samples for pathological diagnosis. Further research is needed to determine whether the diagnostic yield for benign lesions and MALT with REBUS-GS would be enhanced by increasing the number of the biopsy specimens.
After visualization of the lesions, the mean biopsy time for REBUS-GS-TBB was shorter than that for REBUS-D-TBB (P=0.00053). During REBUS-D-TBB, the operator has to clean up the image and measure the distance at each biopsy. In contrast, given that the biopsy site is relatively fixed with GS, REBUS-GS-TBB saves time.
In a recent study, it was reported that the complication rate of REBUS-GS procedures was 0.8% for pneumothorax and 0.5% for pulmonary infection with no significant hemorrhage (17). In this study, pneumothorax and pulmonary infection did not occur. Bleeding greater than 50 mL was observed in five subjects in the REBUS-D group and no subjects in the REBUS-GS group. Less bleeding in the REBUS-GS group may have been due to local pressure effects of the GS and the small biopsy sample.
There are several limitations in our study. First, it was performed at a single institute. In addition, owing to the difficulties in recruiting subjects, we did not have sufficient sample size, especially in terms of lesions with a diameter smaller than 20 mm. However, some potentially useful information has been provided. For instance, 4–5 block specimens may not be enough when with REBUS-GS by thin bronchoscopy for the diagnosis of peripheral pulmonary benign lesions, and the number of biopsies need to be increased. Moreover, every subject had to endure two guided biopsy methods in this randomized crossover study so that the results may have had some bias.
Conclusions
In conclusion, the results of our study suggest that REBUS-D-TBB with thin bronchoscope is useful for diagnosing PPLs and leads to large healthcare cost savings (1,380 RMB) compared to REBUS-GS-TBB. The difference in the diagnostic yield of the two modalities was not statistically significant. Although it is associated with shorter operation time and less bleeding, REBUS-GS has a higher cost and sometimes leads to check failure due to small specimens and the impact of the bronchoscope curvature. However, the diagnosis rate of small subpleural lesions with EBUS-D-TBB is perhaps lower than that of EBUS-GS. Further study is needed to identify which groups of patients can benefit most while still being cost-effective.
Acknowledgements
We thank all the physicians and endoscopy suite personnel in the Third Affiliated Hospital of Soochow University and recruitment of subjects.
Ethical Statement: This study was approved by the ethics committee of the Third Affiliated Hospital of Soochow University (No. 2014077). Informed consent was obtained from all subjects.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Rivera MP, Mehta AC, American College of Chest Physicians. Initial diagnosis of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:131S-148S. [DOI] [PubMed] [Google Scholar]
- 2.Kuo CH, Lin SM, Lee KY, et al. Endobronchial ultrasound-guided transbronchial biopsy and brushing: a comparative evaluation for the diagnosis of peripheral pulmonary lesions. Eur J Cardiothorac Surg 2014;45:894-8. 10.1093/ejcts/ezt472 [DOI] [PubMed] [Google Scholar]
- 3.Kurimoto N, Miyazawa T, Okimasa S, et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest 2004;126:959-65. 10.1378/chest.126.3.959 [DOI] [PubMed] [Google Scholar]
- 4.Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012;142:385-93. 10.1378/chest.11-1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung YH, Lie CH, Chao TY, et al. Endobronchial ultrasonography with distance for peripheral pulmonary lesions. Respir Med 2007;101:738-45. 10.1016/j.rmed.2006.08.014 [DOI] [PubMed] [Google Scholar]
- 6.Fuso L, Varone F, Magnini D, et al. Role of ultrasound-guided transbronchial biopsy in the diagnosis of peripheral pulmonary lesions. Lung Cancer 2013;81:60-4. 10.1016/j.lungcan.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 7.Huang CT, Ho CC, Tsai YJ, et al. Factors influencing visibility and diagnostic yield of transbronchial biopsy using endobronchial ultrasound in peripheral pulmonary lesions. Respirology 2009;14:859-64. 10.1111/j.1440-1843.2009.01585.x [DOI] [PubMed] [Google Scholar]
- 8.Huang CT, Tsai YJ, Liao WY, et al. Endobronchial ultrasound-guided transbronchial biopsy of peripheral pulmonary lesions: how many specimens are necessary? Respiration 2012;84:128-34. 10.1159/000339412 [DOI] [PubMed] [Google Scholar]
- 9.Zaric B, Perin B, Stojsic V, et al. Detection of premalignant bronchial lesions can be significantly improved by combination of advanced bronchoscopic imaging techniques. Ann Thorac Med 2013;8:93-8. 10.4103/1817-1737.109820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang S, Zhou J, Zhang Q, et al. Endobronchial ultrasonography with distance by thin bronchoscopy in diagnosing peripheral pulmonary lesions. Zhonghua Jie He He Hu Xi Za Zhi 2015;38:566-9. [PubMed] [Google Scholar]
- 11.Oki M, Saka H, Kitagawa C, et al. Endobronchial ultrasound-guided transbronchial biopsy using novel thin bronchoscope for diagnosis of peripheral pulmonary lesions. J Thorac Oncol 2009;4:1274-7. 10.1097/JTO.0b013e3181b623e1 [DOI] [PubMed] [Google Scholar]
- 12.Izbicki G, Shitrit D, Yarmolovsky A, et al. Is routine chest radiography after transbronchial biopsy necessary?: A prospective study of 350 cases. Chest 2006;129:1561-4. 10.1378/chest.129.6.1561 [DOI] [PubMed] [Google Scholar]
- 13.Minezawa T, Okamura T, Yatsuya H, et al. Bronchus sign on thin-section computed tomography is a powerful predictive factor for successful transbronchial biopsy using endobronchial ultrasound with a guide sheath for small peripheral lung lesions: a retrospective observational study. BMC Med Imaging 2015;15:21. 10.1186/s12880-015-0060-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chavez C, Sasada S, Izumo T, et al. Endobronchial ultrasound with a guide sheath for small malignant pulmonary nodules: a retrospective comparison between central and peripheral locations. J Thorac Dis 2015;7:596-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durakovic A, Andersen H, Christiansen A, et al. Retrospective analysis of radial EBUS outcome for the diagnosis of peripheral pulmonary lesion: sensitivity and complications. Eur Clin Respir J 2015;2:28947. 10.3402/ecrj.v2.28947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamada N, Yamazaki K, Kurimoto N, et al. Factors related to diagnostic yield of transbronchial biopsy using endobronchial ultrasonography with a guide sheath in small peripheral pulmonary lesions. Chest 2007;132:603-8. 10.1378/chest.07-0637 [DOI] [PubMed] [Google Scholar]
- 17.Hayama M, Izumo T, Matsumoto Y, et al. Complications with Endobronchial Ultrasound with a Guide Sheath for the Diagnosis of Peripheral Pulmonary Lesions. Respiration 2015;90:129-35. 10.1159/000431383 [DOI] [PubMed] [Google Scholar]


