Abstract
Background
This study aimed to determine the effects of smoke bomb-induced acute inhalation injury on pulmonary function at different stages of lung injury.
Methods
We performed pulmonary function tests (PFTs) in 15 patients with acute inhalation injury from days 3 to 180 after smoke inhalation. We measured the trace element zinc in whole blood on days 4 and 17, and correlations of zinc levels with PFTs were performed.
Results
In the acute stage of lung injury (day 3), 3 of 11 patients with mild symptoms had normal pulmonary function and 8 patients with restrictive ventilatory dysfunction and reduced diffusing capacity. Some patients also had mild obstructive ventilatory dysfunction (5 patients) and a decline in small airway function (6 patients). For patients with severe symptoms, PFT results showed moderate to severe restrictive ventilatory dysfunction and reduced diffusing capacity. PaCO2 was significantly higher (P=0.047) in patients with reduced small airway function compared with those with normal small airway function. Whole blood zinc levels in the convalescence stage (day 17) were significantly lower than those in the acute stage (day 4). Zinc in the acute stage was negatively correlated with DLCO/VA on days 3, 10, and 46 (r=−0.633, −0.676, and −0.675 respectively, P<0.05).
Conclusions
Smoke inhalation injury mainly causes restrictive ventilatory dysfunction and reduced diffusing capacity, and causes mild obstructive ventilatory dysfunction and small airway function decline in some patients. Zinc is negatively correlated with DLCO/VA. Zinc levels may be able to predict prognosis and indicate the degree of lung injury.
Keywords: Zinc chloride, smoke inhalation injury, pulmonary function test
Introduction
Smoke bombs are widely used in military drills, firefighter training, and on the battlefield as obscurants. After combustion, smoke bombs release fumes that may contain zinc chloride, zinc oxide, hydrochloric acid, hexachloroethane, calcium chloride, aluminum, and other chemical ingredients. Zinc chloride is the main toxic factor that leads to inhalation injury.
The mean diameter of smoke particles is approximately 2 to 3 µm (1). Particles of this size are large enough to be captured using protective masks, such as high efficiency particulate air filter respirators. However, if not filtered, the smoke particles are small enough to penetrate down to the alveolus deep in the lungs and easily damage the alveolus. This causes opportunistic infections of the lungs and secondary injury, even inducing acute respiratory distress syndrome (ARDS) (1-6).
Some studies have suggested that inhalation of smoke from smoke bombs in a confined space causes interstitial and alveolar edema, proliferation of fibroblasts, an increase in collagen in the interstitium and alveolus in the early phase, and pulmonary fibrosis in the late phase, which may lead to death (3,4). Smoke inhalation injury has a complex pathogenesis and there is no specific treatment. The collective effect of inhaled toxic gases and particles, hypoxia, and thermal environment can rapidly cause damage of the respiratory system. This causes increased permeability of lung capillaries, decreased lung compliance, and bronchospasm, resulting in a reduction in pulmonary function.
In this study, we examined the effects of smoke bomb-induced acute airway and lung injury on pulmonary function of 15 soldiers in China, and changes in pulmonary function at different stages.
Methods
Patients
We studied 15 soldiers who had acute airway injury and were sent to our hospital for treatment on November 2014. These patients were all men, aged 18–24 years, with a mean age of 21.07±1.62 (mean ± SD) years. They had been exposed to dense fumes for approximately 1 minute in a confined space following an accidental smoke bomb explosion during a military drill. They initially experienced chest distress, tachypnea, and coughing with black sputum. Some patients had dizziness, runny nose, nausea, and vomiting. Seven to twenty-eight hours after smoke inhalation, patients were sent to the emergency department for symptomatic treatment. They were transferred to the Department of Pulmonary and Critical Care Medicine. Their clinical diagnosis was inhalation lung injury. As part of their initial evaluation, pulmonary function tests (PFTs), high-resolution computed tomography scans, blood gas analysis, bronchoscopy, and an assay of the trace element zinc in whole blood were performed. After rehabilitation, patients were followed up with outpatient services, and PFTs, high-resolution computed tomography scans, and blood gas analysis were performed.
This study was conducted in accordance with the amended Declaration of Helsinki. We obtained informed consent for the study from the patients and approved by the ethics committee of the Chinese PLA General Hospital (S2014-011-01).
PFT
PFTs were undertaken to determine the degree of functional impairment and were performed in the pulmonary function laboratory of our hospital. We used standard procedures and complied with the American Thoracic Society and European Respiratory Society guide published in 2005 (7-10). Pulmonary ventilation, lung capacity, and gas exchange were measured with a spirometer (MasterScreen Body; Jaeger, Wurzburg, Germany). Diffusing capacity of the lungs was determined by the single-breath carbon monoxide technique. The measurement results were analyzed by computer software (Master Lab Manager V5.31.0 software; Jaeger). Each test was repeated three times or more, and all spirometric values were expressed as a percentage of the predicted values. The main test items included: forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), FEV1/FVC, peak expiratory flow (PEF), maximal mid expiratory flow (MMEF75/25), residual volume (RV), functional residual capacity (FRC), total lung capacity (TLC), RV/TLC, diffusing capacity for carbon monoxide of the lungs (DLCO), alveolar ventilation, and diffusing capacity for carbon monoxide per liter of alveolar volume (DLCO/VA).
Measurement of zinc
The main pathogenic ingredient of smoke bombs is zinc chloride. We measured zinc levels in the whole blood of each patient 4 days after smoke inhalation (acute stage, n=15), and in some of the patients who had severe lung injury 17 days after smoke inhalation (convalescence stage, n=4). The test sample was anticoagulated venous blood (3 mL) and the anticoagulant was heparin. The detection method was atomic absorption spectrometry. The equipment is BH5100S atomic absorption spectrometer (Bohui Innovation Technology. Co., Ltd., China).
Statistical analysis
SPSS 17.0 software was used for statistical analysis. The comparison of blood gas analysis results between normal MMEF75/25 group and abnormal MMEF75/25 group were analyzed using the independent samples t-test. The paired-sample t test was used to compare zinc levels in the acute and convalescence stages. The Pearson correlation test was used to correlate PFT results with zinc levels. Measurement data are expressed as the mean ± standard deviation (mean ± SD). Differences were considered significant when the P value was <0.05.
Results
A total of 11 patients were diagnosed with inhalation lung injury, with a relatively stable condition with mild symptoms. Another four patients with severe symptoms were diagnosed with severe lung injury and multiple organ damage (one patient with ARDS). These patients were admitted to the respiratory intensive care unit. Chest CT revealed ground-glass opacity of four patients with severe symptoms, and the remaining patients were normal. Patients were treated with anti-infectious agents, antidote (Dimercaptopropane sodium sulfonate), liver protectants (Polyene Phosphatidyl choline and Reduced Glutathione), gastric acid inhibitors (Omeprazole sodium), hormones (Methylprednisolone sodium succinate) and Ulinastatin to inhibit the inflammatory response. Critically ill patients were provided continuous renal replacement therapy, plasmapheresis, bronchoscopy, and bronchoalveolar lavage. After treatment, 14 patients in the convalescence stage were hospitalized for 7–44 days, for a mean of 16.86±13.06 days. One critically ill patients with ARDS, progression of the disease through the stage of acute lung injury, Multiple organ dysfunction and progressive pulmonary fibrosis. We did endotracheal intubation, mechanical ventilation mode was synchronized intermittent mandatory ventilation (SIMV) + pressure support (prone position ventilation), the patient with ARDS and secondary pulmonary fibrosis died on the 19th day of hospitalization because of multiple organ failure.
PFT
Except for one patient with severe lung injury, all of the patients underwent PFTs. Eleven patients with mild symptoms were administered PFTs on the 3rd to 46th days after the accident. Most of the patients had tests on days 3 (n=11), 6 (n=11), 10 (n=10), and 46 (n=8). The PFT results of patients with mild symptoms are shown in Table 1. On the 3rd day after smoke inhalation (acute phase), Three patients (27.3%) had completely normal lung function, while eight patients (72.7%) had abnormal function. A total of 18.2% of the patients with TLC and FVC <80% predicted values; 27.3% with FEV1 <80%; 45.5% with FEV1/FVC <80% (but more than 70%); 27.3% with peak expiratory flow <80%; 63.6% with MMEF75/25 <80%; 54.5% had small airway function decline; 27.3% with RV/TLC >35%; 45.5% with DLCO <80%; 9% with DLCO/VA <80%. These findings indicated that most patients mainly had restrictive ventilatory dysfunction and reduced diffusing capacity in the acute stage of smoke inhalation. Furthermore, small airway dysfunction was found in most patients. Thereafter, PFT results of all of the patients progressively improved (Figure 1). On the 46th day after smoke inhalation, only one patient had mild reduced diffusing capacity, and 27.3% patients had small airway dysfunction.
Table 1. PFT results of patients with mild symptoms.
| PFT | d3 | d6 | d10 | d46 |
|---|---|---|---|---|
| FVC | 77.79±19.42 | 80.91±17.64 | 96.06±7.32 | 96.30±10.37 |
| FEV1 | 77.58±20.53 | 84.52±19.48 | 100.62±10.61 | 100.42±11.32 |
| FEV1/FVC | 84.79±8.72 | 88.69±9.01 | 88.71±7.19 | 88.56±6.074 |
| PEF | 85.65±19.69 | 90.80±16.00 | 110.86±18.38 | 103.95±12.68 |
| MMEF75/25 | 72.55±28.37 | 88.97±31.34 | 100.01±27.18 | 98.96±22.10 |
| RV | 105.87±18.89 | 120.02±30.44 | 111.66±20.45 | 103.84±26.39 |
| TLC | 81.94±10.21 | 83.41±10.84 | 92.87±6.72 | 93.94±9.95 |
| RV/TLC | 30.79±7.33 | 34.02±9.17 | 27.95±4.14 | 25.82±5.53 |
| FRC | 95.52±13.37 | 108.75±21.15 | 107.17±16.08 | 103.25±20.08 |
| DLCO/VA | 96.19±18.55 | 92.71±15.96 | 93.65±12.19 | 99.91±13.80 |
| DLCO | 77.51±19.71 | 76.19±18.46 | 84.90±11.51 | 91.30±13.34 |
FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; PEF, peak expiratory flow; MMEF75/25, maximal mid expiratory flow; RV, residual volume; TLC, total lung capacity; FRC, functional residual capacity; DLCO/VA, diffusing capacity for carbon monoxide per liter of alveolar volume; DLCO, diffusing capacity for carbon monoxide of the lungs. Data are presented as percentage of the predicted value (except for the FEV1/FVC ratio). (d 3: n=11, d 6: n=11, d 10: n=10, d 46: n=8).
Figure 1.
PFT Changes on days 3, 6, 10, and 46 after smoke inhalation of patients with mild symptoms (patients 1 to 11). PFT results showed that patients mainly had restrictive ventilatory dysfunction and reduced diffusing capacity in the acute stage of smoke inhalation. Thereafter, the PFT results of all of the patients progressively improved (P<0.05). On day 46, one patient had mild reduced diffusing capacity. Data are presented as the percentage of the predicted value (d 3: n=11, d 6: n=11, d 10: n=10, d 46: n=8).
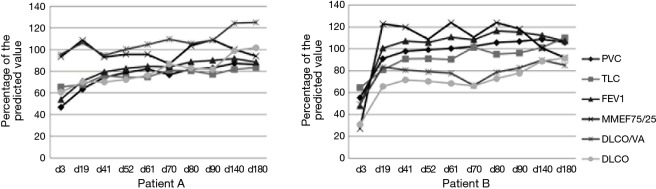
Among 4 patients with severe lung injury and other organ damage, one of them died without being administered a PFT. One patient was only administered infrequent PFTs because of his severe symptoms. Another two patients (patient A and patient B) had a longer period of hospitalization and follow-up than the other patients. These two patients were administered PFTs on days 3, 19, 41, 52, 61, 70, 80, 90, 140, and 180 after smoke inhalation. The PFT results showed that on the 3rd day after smoke inhalation, these two patients had moderate to severe restrictive ventilatory dysfunction and reduced diffusing capacity. Which FEV1 was 54% and 48%; FVC was 46.9% and 55.3%; FEV1/FVC was 98% and 73%; RV/TLC was 36% and 31%; DLCO was 60.7% and 30.9%. One patient had small airway dysfunction. Thereafter, the PFT results of these two patients progressively improved. On the 140th day of follow-up, the results of these two patients’ PFTs returned to normal (Figure 2).
Figure 2.
PFT Changes on days 3 to 180 after smoke inhalation of patients with severe symptoms (patients A and B). On day 3, the patients had moderate to severe restrictive ventilatory dysfunction and reduced diffusing capacity. Thereafter, the PFT results progressively improved. On the 140th day of follow-up, PFT results returned to normal. Data are presented as the percentage of the predicted value.
In the first 3 days after smoke inhalation, a total of 13 patients underwent PFTs. Eight of these patients demonstrated a decline in MMEF75/25 while five patients showed normal values. The patients were divided into two groups: one group had normal MMEF75/25 values and the other group had abnormal MMEF75/25 values. Blood gas analysis showed that the partial pressure of carbon dioxide (PaCO2) was significantly higher in the MMEF75/25 abnormal group than in the MMEF75/25 normal group (46.54±2.41 vs. 43.60±2.49 mmHg, P=0.047).
Correlation of zinc levels with PFT results
All of the patients’ zinc levels in whole blood were measured in the acute phase (day 4, n=15). The mean blood zinc level was 104.28+17.40 mol/L. Four patients had their blood zinc levels measured a second time, and mean zinc levels were 98.93+18.27 mol/L in the acute stage and 80.93+17.85 mol/L in the convalescence stage. Zinc levels in the convalescence stage were significantly lower than those in the acute stage (P=0.032, Figure 3).
Figure 3.

Zinc levels in the acute stage (day 4) and the convalescence stage (day 17) of four critically ill patients. In 4 patients who had zinc levels measured in the acute and convalescence stages, zinc levels in the convalescence stage were significantly lower than those in the acute stag (P=0.032, n=4).
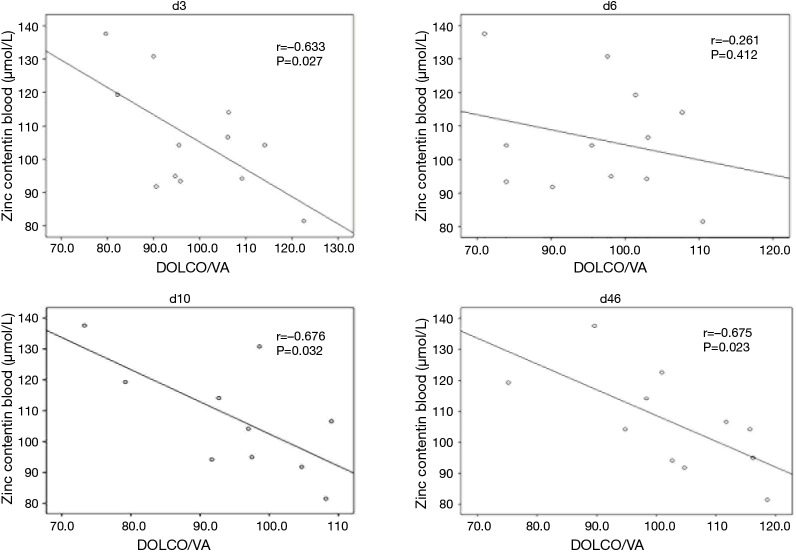
Whole blood zinc levels in the acute stage (day 4) were negatively correlated with DLCO/VA on days 3, 10, and 46 (r=−0.633, −0.676, and −0.675, respectively; P=0.027, 0.032 and 0.023, respectively), but there was no significant correlation on day 6 (r=−0.261, P=0.412). Whole blood zinc levels in the acute stage were not correlated with FVC, TLC, FEV1, DLCO, and zinc levels in the convalescence stage. A scatterplot of zinc levels in the acute stage and DLCO/VA is shown in Figure 4.
Figure 4.
Scatterplot of zinc levels in the acute stage (day 4) and DLCO/VA on days 3, 6, 10, and 46. Zinc (day 4) levels were negatively correlated with DLCO/VA on days 3, 10, and 46, but there was no significant correlation on day 6 (d 3: n=12; d 6: n=12; d 10: n=10; d 46: n=11).
Discussion
As early as 1945, Evans reported the first zinc chloride smoke poisoning incident, which occurred during the Second World War. In this case, 70 soldiers were exposed to zinc chloride smoke, causing lung injury. Ultimately, ten of these soldiers died (11). The smoke bomb is still used to cause acute airway injury and acute lung injury. Even though it is uncommon, smoke bombs can be fatal. After combustion, smoke bombs release fumes that may contain zinc chloride, zinc oxide, hydrochloric acid, hexachloroethane, calcium chloride, and aluminum. If these fumes diffuse in an open, ventilated, and high-humidity environment, smoke bombs have little effect on the human body (12). However, if fumes from smoke bombs diffuse in a relatively closed environment, even just 1–2 minutes of inhalation is dangerous to the human body. Smoke inhalation injury is mainly caused by zinc chloride. Zinc chloride is strongly corrosive and can rapidly cause respiratory mucosal damage. Substantial inhalation results in coughing, chest tightness, hoarseness, tachypnea, dyspnea, and fever (3,4,12). Exposure to high concentrations of zinc chloride, especially in a confined space, may produce ARDS, and possibly death (3,4).
Clinical, radiographic, and pathological findings, as well as treatment in patients who are exposed to this smoke, have been described previously (1-6,12). However to date, the description of pulmonary function findings has been limited to a small number of cases (1,2,5), and there is a lack of knowledge regarding long-term effects on pulmonary function. Therefore, in the current study, patients with smoke bomb-induced acute inhalation injury underwent investigation of pulmonary function, and PFTs were performed from 3 to 180 days after smoke inhalation.
Lung injury caused by smoke inhalation affects a patient’s lung function because normal lung function depends on integrity of the chest wall, improvement in respiratory muscle, an open airway, normal lung tissue structure, good lung compliance, and other factors. After smoke inhalation, the synergistic action of toxic ingredients and heat factors can cause a series of changes in pathophysiological processes, such as inflammation reactions, increased pulmonary capillary permeability, increased bronchial blood flow, bronchospasm, and a decrease in compliance of the respiratory system. These abnormalities of lung parenchyma and interstitial tissue cause abnormal lung function.
Our study showed that, in the acute stage of lung injury, 27.3% of patients with mild symptoms had completely normal pulmonary function. Additionally, 72.7% of patients had varying degrees of decline in lung function, mainly declining in FVC, TLC, FEV1, and DLCO, small airway dysfunction, and mild obstructive ventilatory dysfunction. A total of 18.2% of patients showed restrictive ventilatory dysfunction and 45.5% of patients experienced decreased diffusion capacity. For patients with severe symptoms who were diagnosed with ARDS, PFT results showed moderate to severe restrictive ventilatory dysfunction and reduced diffusing capacity, and one patient had small airway dysfunction. Thereafter, PFT results of all patients progressively improved. In mild cases, most patients had almost normal PFT results on the 46th day after smoke inhalation. For severe cases, patients had a longer follow-up, and at 140 days after smoke inhalation, PFT results returned to normal.
These results suggested that smoke inhalation injury affects lung function mainly by restrictive ventilatory dysfunction and reduced diffusing capacity, which are consistent with previous literature (5). In some patients, we also observed mild obstructive ventilatory dysfunction and a decline in small airway function, which were not mentioned in previous clinical studies. Our study showed that PaCO2 was significantly higher in the MMEF75/25 abnormal group than in the MMEF75/25 normal group. However, for some patients with mild lung injuries, there may be no significant change in lung function or function may decline but remain within the normal range. If smoke inhalation injury is complicated by multiple organ damage, there is a greater effect on lung function, and a longer recovery period is required. In such cases, patients need 5 months to fully recover. Some studies have shown that patients with severe lung injury caused by zinc chloride fumes still show restrictive ventilatory dysfunction after 6 months of smoke inhalation (1). In the current study, we did not observe this situation.
DLCO/VA is the ratio of carbon monoxide diffusing capacity and alveolar volume, and is the diffusing capacity of per lung volume unit. DLCO/VA is relatively more important than DLCO. Our study showed that whole blood zinc levels in the convalescence stage were significantly lower than those in the acute stage. Additionally, zinc levels in the acute stage were negative correlated with DLCO/VA in the acute and convalescence stages. This finding suggests that whole blood zinc levels may reliably predict the severity of zinc chloride smoke inhalation injury and prognosis. Additionally, the degree of lung injury may be related to the inhalation time and the inhaled dose of zinc chloride.
While the main pathogenic ingredient of smoke bomb-induced inhalation injury is zinc chloride, there are likely to be other toxic components. In our study, 3 patients showed benzoic acid in serum (0.1–0.2 µg/mL), but no hexachloroethane, trichloropropane, or other volatile toxic gas components were detected.
Changes in biochemistry and pathophysiology of smoke inhalation lung injury are complicated and not fully understood. Treating patients whose conditions are complicated by ARDS is difficult. A good understanding of the pathophysiological process in acute smoke inhalation injury is required. PFTs are useful for patients with inhalation lung injury, especially for follow-up surveys. Observing changes in pulmonary function helps physicians understand the pathophysiology and is helpful for determining effective methods of treatment. Identifying effective prognostic factors in the early stage of inhalation lung injury is useful for rehabilitation of patients.
In this study, we observed the characteristics of pulmonary function in patients with smoke bomb-induced lung injury. For the first time, we found a correlation between pulmonary function and the trace element zinc in whole blood in a relatively large series of patients with zinc chloride smoke inhalation injury. Our findings show the danger of inhalation of zinc chloride smoke, and severe cases can lead to lung injury and even death. Although some of the surviving patients had transient decreases in lung capacity and reduced diffusing capacity, they recovered after a few months. However, whether there are long-term effects on lung function in these patients is still unknown, and further follow-up observations are required. Our findings should be useful for the future treatment of smoke inhalation injury.
Conclusions
We performed a study to show the effects of smoke bomb-induced acute inhalation injury on pulmonary function at different stages of lung injury. Fifteen patients with acute inhalation injury were evaluated from 3 to 180 days after smoke inhalation. We found that the main problems caused by smoke inhalation injury were restrictive ventilatory dysfunction and reduced diffusing capacity; some patients developed mild obstructive ventilatory dysfunction and small airway function decline. This study is the first to confirm a relationship, specifically a negative correlation, between the zinc concentration and pulmonary function in patients with zinc chloride-induced smoke inhalation injury. Zinc levels may be able to predict prognosis and indicate the degree of lung injury. Our findings will be useful for both the treatment of smoke inhalation injury and as baseline information for future research in this field.
Acknowledgements
This work was supported by the Clinical research funding of Chinese People’s Liberation Army General Hospital. Furthermore, we are grateful for HAO Feng-Ying, Wang Xiao-Qing and other technicians in Pulmonary Function Testing Room of Chinese People’s Liberation Army General Hospital for their contribution to this study.
Ethical Statement: The name of the ethics committee: ethics committee of the Chinese PLA General Hospital [S2014-011-01]. This study was conducted in accordance with the amended Declaration of Helsinki. We obtained informed consent for the study from the patients and approved by this study was conducted in accordance with the amended Declaration of Helsinki. We obtained informed consent for the study from the patients and approved by the ethics committee of the Chinese PLA General Hospital.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Huang KL, Chen CW, Chu SJ, et al. Systemic inflammation caused by white smoke inhalation in a combat exercise. Chest 2008;133:722-8. 10.1378/chest.07-2076 [DOI] [PubMed] [Google Scholar]
- 2.Chian CF, Wu CP, Chen CW, et al. Acute respiratory distress syndrome after zinc chloride inhalation: survival after extracorporeal life support and corticosteroid treatment. Am J Crit Care 2010;19:86-90. 10.4037/ajcc2009908 [DOI] [PubMed] [Google Scholar]
- 3.Homma S, Jones R, Qvist J, et al. Pulmonary vascular lesions in the adult respiratory distress syndrome caused by inhalation of zinc chloride smoke: a morphometric study. Hum Pathol 1992;23:45-50. 10.1016/0046-8177(92)90010-Z [DOI] [PubMed] [Google Scholar]
- 4.Pettilä V, Takkunen O, Tukiainen P. Zinc chloride smoke inhalation: a rare cause of severe acute respiratory distress syndrome. Intensive Care Med 2000;26:215-7. 10.1007/s001340050049 [DOI] [PubMed] [Google Scholar]
- 5.Hsu HH, Tzao C, Chang WC, et al. Zinc chloride (smoke bomb) inhalation lung injury: clinical presentations, high-resolution CT findings, and pulmonary function test results. Chest 2005;127:2064-71. 10.1378/chest.127.6.2064 [DOI] [PubMed] [Google Scholar]
- 6.Gil F, Pla A, Hernández AF, et al. A fatal case following exposure to zinc chloride and hexachloroethane from a smoke bomb in a fire simulation at a school. Clin Toxicol (Phila) 2008;46:563-5. 10.1080/15563650701610890 [DOI] [PubMed] [Google Scholar]
- 7.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 8.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J 2005;26:511-22. 10.1183/09031936.05.00035005 [DOI] [PubMed] [Google Scholar]
- 9.Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005;26:720-35. 10.1183/09031936.05.00034905 [DOI] [PubMed] [Google Scholar]
- 10.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948-68. 10.1183/09031936.05.00035205 [DOI] [PubMed] [Google Scholar]
- 11.Evans EH. Casualties following exposure to zinc chloride smoke. Lancet 1945;2:368-70. 10.1016/S0140-6736(45)91239-6 [DOI] [Google Scholar]
- 12.Matarese SL, Matthews JI. Zinc chloride (smoke bomb) inhalational lung injury. Chest 1986;89:308-9. 10.1378/chest.89.2.308 [DOI] [PubMed] [Google Scholar]