Abstract
Background
Although lobectomy is the standard surgical procedure for non-small cell lung cancer (NSCLC), recent studies show favorable outcomes after limited resection in patients with small-sized peripheral tumors. We conducted a randomized controlled trial of such patients to estimate postoperative outcomes and pulmonary function following these surgical techniques.
Methods
Between 2005 and 2008, eligible patients with tumors of 2 cm or less were randomly assigned 1:1 to undergo lobectomy or limited resection; 32 and 33 NSCLC patients in each group, respectively, were analyzed. The primary end points were 5-year overall survival (OS) and disease-free survival (DFS), while the secondary end points were postoperative pulmonary function including forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1).
Results
The 5-year OS rates were 93.8% and 90.9% in the lobectomy and limited resection groups, respectively (P=0.921). The 5-year DFS rates were 93.8% and 90.9% in the lobectomy and limited resection groups, respectively (P=0.714). These rates did not differ significantly between the two resection groups. The median postoperative/preoperative FVC ratios were 84.1% and 90.0% in the lobectomy and limited resection groups, respectively, while the median postoperative/preoperative FEV1 ratios were 81.9% and 89.1%, respectively. Both ratios were significantly higher in the limited resection group (P=0.032 and P=0.005 for FVC and FEV1 ratios, respectively).
Conclusions
A similar outcome, with more preserved postoperative pulmonary function, was observed in patients who underwent limited resection compared to those who underwent lobectomy. Ongoing large-scale multi-institutional prospective randomized trials of lobar versus sublobar resection in patients with small peripheral NSCLCs will hopefully provide definitive information about intentional limited resection of small peripheral tumors.
Keywords: Lung cancer surgery, lung cancer clinical trials, lobectomy, segmentectomy, wedge resection
Introduction
In a prospective randomized study of non-small cell lung cancer (NSCLC) by the Lung Cancer Study Group (LCSG) in the 1980’s, limited resection had a relatively worse postoperative prognosis than lobectomy, as well as an almost 3-fold higher locoregional recurrence rate (1). Since then, lobectomy has been the standard surgical procedure for operable NSCLC patients. However, owing to lung cancers screening programs and the use of refined high-resolution computed tomography (CT) (2,3), smaller lung cancers are detected now than in the past.
Because smaller lesions likely represent an earlier stage and a potentially more curable disease (4), many non-randomized prospective trials of limited resection of small tumors reported favorable postoperative outcomes (5-9). Small tumors with the pathological characteristics of adenocarcinoma in situ (AIS) (10) as indicated by ground-glass opacity (GGO) in CT scans, are considered appropriate for limited resection, especially for wedge resection (5,7,9) although a higher locoregional recurrence rate associated with wedge resection when compared to segmentectomy in small NSCLC patients was reported in previous retrospective studies on one hand (11,12). Moreover, both lobectomy and limited resection produced similar postoperative outcomes in patients with small NSCLCs regardless of their radiological characteristics (6,8,13).
We conducted a multi-institutional randomized controlled trial to estimate overall survival (OS) and disease-free survival (DFS) in patients undergoing lobectomy or limited resection for peripheral NSCLCs ≤2 cm. Here we report the postoperative outcomes and pulmonary function of these patients, and as reference, these factors were compared between patients undergoing two surgical procedures. This would be a pilot study presenting the results of lobectomy vs. limited resection for small-sized peripheral NSCLC prior to ongoing large-scale multi-institutional prospective randomized trials.
Methods
Patients
The patients in this randomized controlled study met the following criteria: (I) histologically or cytologically confirmed NSCLC, or NSCLC suspected on preoperative CT; (II) a tumor diameter of ≤2 cm on preoperative CT [clinical stage T1a according to the 7th edition of the TNM classification (14)]; (III) a tumor located within the outer one-third of the lung field on preoperative CT; (IV) no preoperative radiological evidence of intrathoracic lymph node involvement or distant metastasis (clinical stage N0 M0); (V) 20–75 years of age; and (VI) general condition and respiratory function adequate for lobectomy. Patients with tumors in the middle lobe, previous treatment for primary lung carcinoma, multiple lung carcinomas, or previous treatment for other malignancies within 5 years prior to surgery were excluded. Patients in whom tumors were not diagnosed as NSCLCs by intraoperative or permanent pathological examination were also excluded after randomization. The study protocol was reviewed and approved by the institutional review board at each center (Niigata University: No. 300, Niigata Cancer Center Hospital: No. H16-31, and National Hospital Organization Nishi-Niigata Chuo National Hospital: No. 602); all patients gave written informed consent before enrollment.
All patients underwent preoperative staging via CT and pulmonary function testing. Additional diagnostic testing [brain magnetic resonance imaging (MRI) and bone scintigraphy] was performed at the discretion of the physician, based on the patient’s symptoms and the clinical findings. Positron emission tomography combined with CT was not performed for preoperative staging in any of the patients because it was introduced at the Niigata Cancer Center Hospital in 2010 nor were invasive modalities for mediastinal lymph node staging (such as mediastinoscopy or endobronchial ultrasound-guided transbronchial needle aspiration).
We began enrolling patients in February 2005, and all patients were followed-up for at least 5 years post-resection.
Study design and surgical treatment
This was a randomized controlled multicenter study conducted in four centers in Niigata prefecture belonging to the Niigata Chest Surgery Research Group. It was designed to determine the efficacy of lobectomy and limited resection in NSCLC patients with tumors ≤2 cm in size. Eligible patients were randomly assigned 1:1 to undergo lobectomy or limited resection. The primary end points were 5-year OS and 5-year DFS, while the secondary end point was postoperative pulmonary function assessed as forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1).
The extents of pulmonary resection (segmentectomy or wedge resection) in patients undergoing limited resection were determined by the surgeons’ policies. Intraoperative pathological or cytological examination was performed in patients without preoperative diagnosis of NSCLC; if an NSCLC diagnosis was not obtained, the patient was excluded from the study. Patients were also excluded from the study if malignant pleural effusion, positive pleural lavage cytology, or multiple lung carcinomas, were intraoperatively confirmed. In patients with intraoperatively identified positive lymph nodes, lobectomy was performed regardless of the study group. In the limited resection group, negative surgical margins were intraoperatively confirmed via surgical margin lavage cytology or frozen-section examination. If positive margins were observed, an additional margin was resected. In the lobectomy group, intraoperative pathological examination for lymph nodes was not performed because the surgical procedure was not changed if positive lymph node was intraoperatively identified. In the limited resection group, pathological examination for hilar with/without mediastinal lymph nodes was intraoperatively performed unless the patients had a GGO-dominant tumor on preoperative CT. Regardless of the resection group, hilar lymphadenectomy with mediastinal lymphadenectomy or sampling was performed except for patients who underwent wedge resection. Although the extent of lymphadenectomy was based on the surgeons’ policies, most hilar nodes were examined in all patients to evaluate the potential for curability via surgical treatment.
Patients were seen every 2–3 months after surgery in accordance with the practices of each center. Follow-up evaluation included a physical examination, chest radiography, and measurement of tumor markers. Chest CT was performed at least once a year. If a patient showed any symptoms or signs of recurrence, additional imaging was performed, including CT, brain MRI, and bone scintigraphy. Newly developed lung lesions were deemed recurrent unless they differed histologically from the primary lung carcinoma or exhibited a large GGO component on CT scans. The patients were followed at least for 5 years after surgery, and the postoperative follow-up period was determined by the institutions’ policies.
Statistical analysis
We recorded the baseline characteristics of the two resection groups including age, sex, preoperative serum carcinoembryonic antigen (CEA) level, consolidation/tumor size ratio (C/T ratio) on CT (15), tumor histology (adenocarcinoma or other), lymphadenectomy extent (sampling/hilum, or mediastinum), pathological tumor size, and pathological stage according to the 7th edition of the TNM classification (I or II–III) (11). The distributions of these factors were compared between the two resection groups using the Wilcoxon rank-sum test for continuous factors and Fisher’s exact test for categorical factors.
OS was measured from the date of surgery to the date of death by any cause or last follow-up. DFS was measured from the date of surgery to the date of the first identified recurrence, death by any cause, or last follow-up. Survival curves were constructed using the Kaplan-Meier method, and the log-rank test was used to compare the two procedural groups. Pulmonary function tests (FVC and FEV1) were performed preoperatively and also performed several times during the postoperative follow-up period. The mean FVC and FEV1 values obtained 6 months after surgery were used for analysis of postoperative pulmonary function. Postoperative/preoperative FVC and FEV1 ratios were calculated and compared between the resection groups by using the Wilcoxon rank-sum test.
On univariate analyses, predictors of poor OS and DFS were selected using the log-rank test. Dichotomized data were used only for continuous variables (age, CEA, C/T ratio, and pathological tumor size) during univariate analyses. To clarify the influence of age using univariate models, the patients were divided into two groups according to the median age of 62 years. The cutoff for the normal upper limit of serum CEA was 5 ng/mL. The prognostic values of all factors were assessed via multivariate analysis using the Cox’s proportional hazards model. The forward stepwise regression method was used to identify predictors of poor OS and DFS.
All analyses were performed based on an intention-to-treat principle using IBM SPSS 20.0 (IBM Co., Chicago, IL, USA). A P value <0.05 was considered statistically significant.
Results
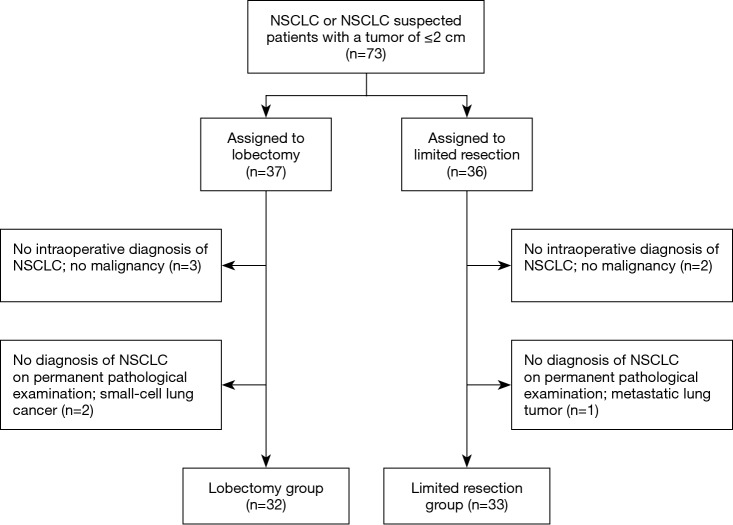
We began enrolling patients in February 2005; the study was terminated in December 2008. A total of 73 patients were enrolled in the study and were randomly assigned to undergo lobectomy or limited resection (Figure 1). Eight patients were excluded for the following reasons: no intraoperative diagnosis of NSCLC (five patients), diagnosis of small-cell lung cancer (two patients), and metastatic lung carcinoma (one patient). Hence, 32 and 33 patients in the lobectomy and limited resection groups were analyzed, respectively. In the limited resection group, 30 patients underwent segmentectomy, 2 underwent wedge resection, and 1 underwent lobectomy because of intraoperatively identified lymph node metastasis. On the permanent pathological examination, two patients and one patient in the lobectomy group, and one in the limited resection group, respectively had positive hilar or mediastinal lymph nodes, respectively. Most baseline characteristics (age, preoperative CEA, C/T ratio on CT, tumor histology, pathological tumor size, and pathological stage) were similar in the two groups (Table 1). There were more male patients and patients who underwent extensive lymphadenectomy in the lobectomy group (P=0.026 and P=0.009, respectively).
Figure 1.
CONSORT-style flowchart of our study. NSCLC, non-small cell lung cancer.
Table 1. Demographic and clinical characteristics for patients in the lobectomy and limited resection groups.
| Characteristics | Lobectomy | Limited resection | P value |
|---|---|---|---|
| All cases (n, %) | 32 (100.0) | 33 (100.0) | – |
| Sex (n, %) | 0.026† | ||
| Male | 20 (63.0) | 11 (33.0) | |
| Female | 12 (37.0) | 22 (67.0) | |
| Age (median, range) (years) | 63 (49.0–75.0) | 62 (42.0–74.0) | 0.264 |
| Preoperative CEA (median, range) (ng/mL) | 1.9 (0.5–6.8) | 2.0 (0.5–23.4) | 0.524 |
| C/T ratio* on CT (median, range) (%) | 93 (0–100.0) | 93 (0–100.0) | 0.578 |
| Tumor histology (n, %) | 0.197 | ||
| Adenocarcinoma | 28 (87.0) | 32 (97.0) | |
| Others | 4 (13.0) | 1 (3.0) | |
| Lymphadenectomy extent (n, %) | 0.009† | ||
| Sampling or hilum | 6 (19.0) | 17 (52.0) | |
| Mediastinum | 26 (81.0) | 16 (48.0) | |
| Pathological tumor size (median, range) (cm) | 1.6 (0.9–2.3) | 1.5 (0.8–3.2) | 0.070 |
| Pathological stage (n, %) | 0.672 | ||
| I | 29 (91.0) | 31 (94.0) | |
| II or III | 3 (9.0) | 2 (6.0) |
*, the maximum diameter of consolidation divided by the maximum tumor diameter; †, indicates significance; CEA, carcinoembryonic antigen; CT, computed tomography.
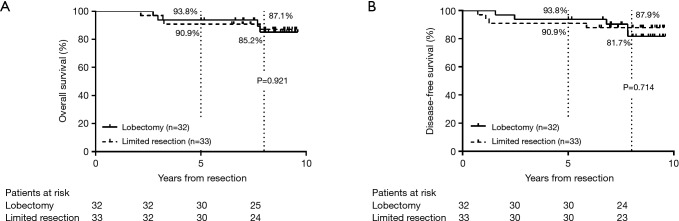
During the postoperative follow-up period, four patients in the lobectomy group and four in the limited resection group developed recurrence; seven of these eight patients died because of cancer relapse. The 5-year and 8-year OS rates in the lobectomy group were 93.8% and 85.2%, respectively, with a median follow-up period of 118 months (Figure 2A). In the limited resection group, the 5-year and 8-year OS rates were 90.9% and 87.1%, respectively, with a median follow-up period of 103 months. The 5-year and 8-year DFS rates in the lobectomy group were 93.8% and 81.7%, respectively with a median follow-up period of 99 months; the 5-year and 8-year DFS rates in the limited resection group were 90.9% and 87.9%, respectively, with a median follow-up period of 103 months (Figure 2B). There were no significant differences in OS and DFS between the two resection groups (P=0.921 and P=0.714, respectively).
Figure 2.
(A) Overall survival (OS) and (B) disease-free survival (DFS) curves of patients in the lobectomy and limited resection groups. (A) The 5-year and 8-year OS rates were 93.8% vs. 90.9%, and 85.2% vs. 87.1% with median follow-up periods of 118 and 103 months in the lobectomy and limited resection groups, respectively (P=0.921); (B) the 5-year and 8-year DFS rates were 93.8% vs. 90.9%, and 81.7% vs. 87.9% with median follow-up periods of 99 and 103 months in the lobectomy and limited resection groups, respectively (P=0.714).
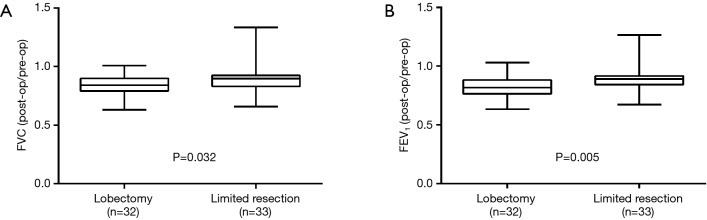
The median postoperative/preoperative FVC ratios were 84.1% and 90.0% in the lobectomy and limited resection groups, respectively (Figure 3A), and the median postoperative/preoperative FEV1 ratios were 81.9% and 89.1% in the lobectomy and limited resection groups, respectively (Figure 3B). Both ratios were significantly higher in the limited resection group (FVC, P=0.032; FEV1, P=0.005).
Figure 3.
(A) Forced vital capacity (FVC) and (B) and forced expiratory volume in 1 second (FEV1) in pulmonary function tests in the lobectomy and limited resection groups. (A) The median postoperative-/preoperative FVC ratios were 84.1% and 90.0% in the lobectomy and limited resection groups, respectively; the ratio was significantly higher in the limited resection group (P=0.032); (B) the median postoperative/preoperative FEV1 ratios were 81.9% and 89.1% in the lobectomy and limited resection groups, respectively; the ratio was significantly higher in the limited resection group (P=0.005).
Univariate analyses identified two significant risk factors each for poor OS (histological type other than adenocarcinoma and pathological stage II–III) and poor DFS (preoperative CEA >5 ng/mL and pathological stage II–III; Table 2). On multivariate analysis, pathological stage II–III was a significant risk factor for both OS and DFS, while histological type other than adenocarcinoma was a significant risk factor for OS (Table 3).
Table 2. Univariate analysis of OS and DFS for 65 patients eligible for the study.
| Variable | No. of patients (%) | 5-yr OS (%) | Univariate P value | 5-yr DFS (%) | Univariate P value |
|---|---|---|---|---|---|
| Group | 0.921 | 0.714 | |||
| Lobectomy | 32 (49.0) | 93.8 | 93.8 | ||
| Limited resection | 33 (51.0) | 90.9 | 90.9 | ||
| Sex | 0.097 | 0.054 | |||
| Male | 31 (48.0) | 90.3 | 90.3 | ||
| Female | 34 (52.0) | 94.1 | 94.1 | ||
| Age (years) | 0.919 | 0.782 | |||
| ≤62 | 33 (52.0) | 93.9 | 93.9 | ||
| >62 | 31 (48.0) | 90.6 | 90.6 | ||
| Preoperative CEA* | 0.141 | 0.012¶ | |||
| Within normal range | 59 (91.0) | 93.2 | 93.2 | ||
| Elevated | 6 (9.0) | 83.3 | 83.3 | ||
| C/T ratio on CT | 0.120 | 0.356 | |||
| ≤50% | 15 (23.0) | – | 100 | ||
| >50% | 50 (77.0) | 90.0 | 90.0 | ||
| Tumor histology | 0.035¶ | 0.069 | |||
| Adenocarcinoma | 60 (92.0) | 93.3 | 93.3 | ||
| Others | 5 (8.0) | 80.0 | 80.0 | ||
| Lymphadenectomy extent | 0.531 | 0.376 | |||
| Sampling or hilum | 23 (35.0) | 91.3 | 91.3 | ||
| Mediastinum | 42 (65.0) | 92.9 | 92.9 | ||
| Pathological tumor size (cm) | 0.422 | 0.378 | |||
| ≤2.0 | 60 (92.0) | 91.7 | 91.7 | ||
| >2.0 | 5 (8.0) | – | – | ||
| Pathological stage | <0.001¶ | 0.001¶ | |||
| I | 60 (92.0) | 95.0 | 95.0 | ||
| II or III | 5 (8.0) | 60.0 | 60.0 |
*, preoperative serum carcinoembryonic antigen level, normal upper limit at 5 ng/mL; ¶, indicates significance. C/T ratio, consolidation/tumor ratio, a maximum diameter of consolidation divided by the maximum tumor diameter; CT, computed tomography; CEA, carcinoembryonic antigen; OS, overall survival; DFS, disease-free survival.
Table 3. Multivariate cox proportional hazard analysis of OS and DFS for 65 patients eligible for the study.
| Variable | OS | DFS | |||||
|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | ||
| Tumor histology | |||||||
| Adenocarcinoma | Reference | — | — | — | — | — | |
| Others | 8.964 | 1.492–53.870 | 0.017 | — | — | — | |
| Pathological stage | |||||||
| I | Reference | — | — | Reference | — | — | |
| II or III | 14.199 | 2.839–71.028 | 0.001 | 7.837 | 1.944–31.598 | 0.004 | |
CI, confidence interval; OS, overall survival; DFS, disease-free survival.
Discussion
Postoperative outcomes appeared to be favorable in patients who underwent both lobectomy and limited resection; moreover, there were no significant differences in OS or DFS between the two groups according to the log-rank test. The type of surgical procedure was not a significant independent prognostic factor of OS or DFS, while the postoperative/preoperative FVC and FEV1 ratios were significantly higher in the limited resection group than in the lobectomy group. Thus, a possibility was suggested that limited resection has a similar postoperative outcome as lobectomy, the current standard surgical procedure, but better preserves pulmonary function in patients with small-sized NSCLCs.
In the 1980s, the LCSG performed the only phase III prospective randomized study of limited resection versus lobectomy in patients with peripheral NSCLCs ≤3 cm (1). Their report, published in 1995, demonstrated unfavorable postoperative prognoses (with an approximately 3-fold increase in the locoregional recurrence rate) in patients who underwent limited resection compared to patients who underwent lobectomy. Moreover, limited resection did not improve perioperative morbidity, mortality, or late postoperative pulmonary function. Thus, the authors of that study deemed lobectomy the surgical procedure of choice, even for patients with small-sized NSCLCs. Consequently, lobectomy has been the standard surgical procedure for operable NSCLC patients. However, smaller and fainter lung cancers are now being detected (2,3), and many prospective trials examined the effects of limited resection on these cancers, as they are thought to be less aggressive than those in the LCSG study (5-9).
Previous studies reported better postoperative outcomes and less pathological invasiveness in NSCLC patients with tumors ≤2 cm than in those with larger tumors (2.1–3 cm) (4,16). Favorable postoperative prognosis was evident even in patients with small tumors undergoing limited resection (17). Based on these results, we conducted the trial described herein; only NSCLCs ≤2 cm in size were examined, in contrast to the LCSG study that included NSCLCs 2.1–3 cm in size (1). In previous prospective non-randomized studies of NSCLCs ≤2 cm, prognosis was similar after limited resection compared to lobectomy (6,8). Thus, our inclusion criteria of tumors ≤2 cm in size may account for the differences between our results and those of the LCSG study.
Another possibility is that differences in tumor subtypes may be responsible. Because all patients in the LCSG study had suspected lung cancer discovered via chest roentgenography (1), tumors with a high proportion of GGO, which can only be detected via CT, would have been overlooked. These tumors have radiologic characteristics consistent with non-invasive or minimally invasive adenocarcinomas (18), and both subtypes represent a distinct group of adenocarcinomas because disease-specific survival is 100% when they are completely resected (19,20). They apparently do not recur after limited resection (5,7,9). A strong correlation has been reported between adenocarcinomas ≤2 cm with ≤25% C/T ratios and adenocarcinomas without pathological involvement or invasion (12). In the current study, ten patients in the limited resection group had tumors with a ≤50% C/T ratio on CT (data not shown). These tumors may therefore be non-invasive or minimally invasive adenocarcinomas, and may thus account in part for the favorable postoperative outcome in the limited resection group.
Differences in the types of pulmonary resection in the limited resection group may also explain the contrast between our results and those of the LCSG study (1). In the LCSG study, 40 of the 122 patients in the limited resection group (32.8%) underwent wedge resection, while only 2 of the 33 patients in the same group (6.1%) underwent wedge resection in our study. In previous retrospective studies that investigated NSCLC patients who underwent limited resection, wedge resection was associated with a high locoregional recurrence rate and poor survival compared to segmentectomy (11,12). In prospective non-randomized studies that reported similar postoperative outcomes for limited resection and lobectomy, only a small percentage of patients (11.5–18.9%) underwent wedge resection in their limited resection groups (6,8). Moreover, in a recent study of large propensity-matched comparison, non-inferior postoperative survival and recurrence rate in patients who underwent segmentectomy were demonstrated in clinical stage I NSCLC (13). Therefore, the unfavorable postoperative survival in the limited resection group of the LCSG study may reflect the relatively high percentage of patients in this group who underwent wedge resection.
Because the adult lung is incapable of regenerating new alveolar septal tissues, postoperative pulmonary function can be theoretically determined by the amount of parenchymal resection (21). Many studies reported preserved pulmonary function after limited resection, compared with lobectomy (8,22,23). In our study, postoperative/preoperative FVC and FEV1 ratios were significantly higher in the limited resection group than in the lobectomy group. In contrast, the LCSG study concluded that limited resection did not improve postoperative pulmonary function because it did not appreciably change FVC and maximum voluntary ventilation (although it did change FEV1) relative to lobectomy (values were compared at baseline and 12–18 months post-resection) (1). To accurately evaluate pulmonary function after pulmonary resection, many factors, such as the time dependence of the alterations, site of resection, severity of pulmonary emphysema, surgical approach, and type of treatment (bronchoplasty or induction chemoradiotherapy), should be considered (21).
This study had some limitations. Although the study was conducted as a randomized controlled trial, the sample size was too small for definitive conclusions. Ongoing large-scale multi-institutional prospective randomized trials of lobar versus sublobar resection in patients with small peripheral NSCLCs [e.g., CALGB 140503 (24) and Japan Clinical Oncology Group 0802/West Japan Oncology Group 4607L (25)] have required a large sample size (target accrual of 1,297 and 1,100 patients, respectively) to investigate non-inferior oncologic outcomes of patients who underwent limited resection. Moreover, the inclusion of a considerable number of patients with non-invasive or minimally invasive adenocarcinomas might be a confounding factor to understand the results of the study. However, we performed this study as a pilot study presenting the results of lobectomy vs. limited resection for small-sized peripheral NSCLC prior to the ongoing large-scale multi-institutional prospective randomized trials, and these large-scale trials will hopefully provide definitive information about intentional limited resection of small peripheral tumors.
Conclusions
In conclusion, we present the results of a randomized, controlled, multi-institutional trial of lobectomy and limited resection in patients with peripheral NSCLCs ≤2 cm. Although the study has a major limitation regarding the small sample size, a similar outcome with more preserved postoperative pulmonary function in patients who underwent limited resection was indicated compared to those who underwent lobectomy.
Acknowledgements
None.
Ethical Statement: The study was approved by institutional review board at each center (Niigata University: No. 300, Niigata Cancer Center Hospital: No. H16-31, and National Hospital Organization Nishi-Niigata Chuo National Hospital: No. 602) and written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. 10.1016/0003-4975(95)00537-U [DOI] [PubMed] [Google Scholar]
- 2.Sobue T, Suzuki T, Naruke T. A case-control study for evaluating lung-cancer screening in Japan. Japanese Lung-Cancer-Screening Research Group. Int J Cancer 1992;50:230-7. 10.1002/ijc.2910500212 [DOI] [PubMed] [Google Scholar]
- 3.Patz EF, Jr, Goodman PC, Bepler G. Screening for lung cancer. N Engl J Med 2000;343:1627-33. 10.1056/NEJM200011303432208 [DOI] [PubMed] [Google Scholar]
- 4.Port JL, Kent MS, Korst RJ, et al. Tumor size predicts survival within stage IA non-small cell lung cancer. Chest 2003;124:1828-33. 10.1378/chest.124.5.1828 [DOI] [PubMed] [Google Scholar]
- 5.Yamato Y, Tsuchida M, Watanabe T, et al. Early results of a prospective study of limited resection for bronchioloalveolar adenocarcinoma of the lung. Ann Thorac Surg 2001;71:971-4. 10.1016/S0003-4975(00)02507-8 [DOI] [PubMed] [Google Scholar]
- 6.Koike T, Yamato Y, Yoshiya K, et al. Intentional limited pulmonary resection for peripheral T1 N0 M0 small-sized lung cancer. J Thorac Cardiovasc Surg 2003;125:924-8. 10.1067/mtc.2003.156 [DOI] [PubMed] [Google Scholar]
- 7.Yoshida J, Nagai K, Yokose T, et al. Limited resection trial for pulmonary ground-glass opacity nodules: fifty-case experience. J Thorac Cardiovasc Surg 2005;129:991-6. 10.1016/j.jtcvs.2004.07.038 [DOI] [PubMed] [Google Scholar]
- 8.Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. 10.1016/j.jtcvs.2006.02.063 [DOI] [PubMed] [Google Scholar]
- 9.Koike T, Togashi K, Shirato T, et al. Limited resection for noninvasive bronchioloalveolar carcinoma diagnosed by intraoperative pathologic examination. Ann Thorac Surg 2009;88:1106-11. 10.1016/j.athoracsur.2009.06.051 [DOI] [PubMed] [Google Scholar]
- 10.Noguchi M, Morikawa A, Kawasaki M, et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer 1995;75:2844-52. [DOI] [PubMed] [Google Scholar]
- 11.El-Sherif A, Fernando HC, Santos R, et al. Margin and local recurrence after sublobar resection of non-small cell lung cancer. Ann Surg Oncol 2007;14:2400-5. 10.1245/s10434-007-9421-9 [DOI] [PubMed] [Google Scholar]
- 12.Koike T, Koike T, Yoshiya K, et al. Risk factor analysis of locoregional recurrence after sublobar resection in patients with clinical stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg 2013;146:372-8. 10.1016/j.jtcvs.2013.02.057 [DOI] [PubMed] [Google Scholar]
- 13.Landreneau RJ, Normolle DP, Christie NA, et al. Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non-small-cell lung cancer: a propensity-matched analysis. J Clin Oncol 2014;32:2449-55. 10.1200/JCO.2013.50.8762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. 10.1097/JTO.0b013e31812f3c1a [DOI] [PubMed] [Google Scholar]
- 15.Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol 2011;6:751-6. 10.1097/JTO.0b013e31821038ab [DOI] [PubMed] [Google Scholar]
- 16.Koike T, Terashima M, Takizawa T, et al. Clinical analysis of small-sized peripheral lung cancer. J Thorac Cardiovasc Surg 1998;115:1015-20. 10.1016/S0022-5223(98)70399-X [DOI] [PubMed] [Google Scholar]
- 17.Okada M, Nishio W, Sakamoto T, et al. Effect of tumor size on prognosis in patients with non-small cell lung cancer: the role of segmentectomy as a type of lesser resection. J Thorac Cardiovasc Surg 2005;129:87-93. 10.1016/j.jtcvs.2004.04.030 [DOI] [PubMed] [Google Scholar]
- 18.Lee KH, Goo JM, Park SJ, et al. Correlation between the size of the solid component on thin-section CT and the invasive component on pathology in small lung adenocarcinomas manifesting as ground-glass nodules. J Thorac Oncol 2014;9:74-82. 10.1097/JTO.0000000000000019 [DOI] [PubMed] [Google Scholar]
- 19.Eguchi T, Kadota K, Park BJ, et al. The new IASLC-ATS-ERS lung adenocarcinoma classification: what the surgeon should know. Semin Thorac Cardiovasc Surg 2014;26:210-22. 10.1053/j.semtcvs.2014.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakagiri T, Sawabata N, Morii E, et al. Evaluation of the new IASLC/ATS/ERS proposed classification of adenocarcinoma based on lepidic pattern in patients with pathological stage IA pulmonary adenocarcinoma. Gen Thorac Cardiovasc Surg 2014;62:671-7. 10.1007/s11748-014-0429-3 [DOI] [PubMed] [Google Scholar]
- 21.Ueda K, Hayashi M, Tanaka N, et al. Long-term pulmonary function after major lung resection. Gen Thorac Cardiovasc Surg 2014;62:24-30. 10.1007/s11748-013-0346-x [DOI] [PubMed] [Google Scholar]
- 22.Takizawa T, Haga M, Yagi N, et al. Pulmonary function after segmentectomy for small peripheral carcinoma of the lung. J Thorac Cardiovasc Surg 1999;118:536-41. 10.1016/S0022-5223(99)70193-5 [DOI] [PubMed] [Google Scholar]
- 23.Keenan RJ, Landreneau RJ, Maley RH, Jr, et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg 2004;78:228-33; discussion 228-33. 10.1016/j.athoracsur.2004.01.024 [DOI] [PubMed] [Google Scholar]
- 24.National Institutes of Health. National Cancer Institute. CALGB-140503. Phase III Randomized Study of Lobectomy Versus Sublobar Resection in Patients With Small Peripheral Stage IA Non-Small Cell Lung Cancer. Available online: http://www.cancer.gov/clinicaltrials/CALGB-140503, accessed Mar 16, 2010.
- 25.Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. 10.1093/jjco/hyp156 [DOI] [PubMed] [Google Scholar]