Abstract
Background
Malaria infections are often genetically diverse, potentially leading to competition between co-infecting strains. Such competition is of key importance in the spread of drug resistance.
Methods
The effects of drug treatment on within-host competition were studied using the rodent malaria Plasmodium chabaudi. Mice were infected simultaneously with a drug-resistant and a drug-sensitive clone and were then either drug-treated or left untreated. Transmission was assessed by feeding mice to Anopheles stephensi mosquitoes.
Results
In the absence of drugs, the sensitive clone competitively suppressed the resistant clone; this resulted in lower asexual parasite densities and also reduced transmission to the mosquito vector. Drug treatment, however, allowed the resistant clone to fill the ecological space emptied by the removal of the sensitive clone, allowing it to transmit as well as it would have done in the absence of competition.
Conclusion
These results show that under drug pressure, resistant strains can have two advantages: (1) they survive better than sensitive strains and (2) they can exploit the opportunities presented by the removal of their competitors. When mixed infections are common, such effects could increase the spread of drug resistance.
Background
Malaria infections often consist of more than one parasite genotype [1-3]. Humans represent ecological niches for co-infecting malaria parasites, with shared predators (immune responses) and limited resources, so that competition between co-infecting malaria strains is likely to be intense [4]. Such competition could strongly affect the relative transmission of newly arisen drug-resistant strains, and thus the spread of drug resistance [5].
Resistant and sensitive strains will co-occur in the same host both when de novo mutations arise, and when hosts acquire resistant and sensitive strains from one mosquito bite simultaneously or from different mosquito bites. In the absence of drug treatment, the transmission success of the resistant strain will depend on its intrinsic fitness and competitive ability. However, if drug treatment does occur, the resistant strain has two potential fitness advantages. First, it will better survive the drug than the sensitive strain. Second, treatment can remove drug-sensitive competitors, thus freeing up ecological space for the resistant strains to occupy; this would increase the relative transmission of the drug-resistant strain. This second effect, well recognized in theory, has the potential to greatly enhance the rate of spread of drug resistance in a population [5]. However, there is no direct experimental evidence that removal of competitors by drug treatment does enhance the transmission of drug-resistant parasites. This paper reports the first direct experimental demonstration that competitive release of drug-resistant strains can occur following drug treatment.
Competition between drug-sensitive and -resistant malaria clones was studied using the rodent malaria Plasmodium chabaudi. This parasite is commonly used as a model for human malaria [6], and has been extensively used to study drug resistance [7]. In the absence of drugs, the drug-resistant clone is competitively suppressed by a drug-sensitive clone [8]. Here, competition between the two strains in drug-treated and untreated mice is compared.
Methods
Two genetically distinct Plasmodium chabaudi chabaudi clones were used: an AS clone resistant to the antifolate drug pyrimethamine [9], and AJ, a sensitive clone. These clones will be referred to as R (for resistant) and S (for sensitive) from hereon. Hosts were eight weeks old CBA/Ca inbred female mice (Ann Walker, University of Edinburgh; Harlan, England). Two experiments were performed. In the first, two groups of five mice were infected with 106 R parasites, and two groups with 106 R + 106 S parasites, as described elsewhere [8]. One group from each of these two infection types was drug-treated within three hours of inoculation and again on days 1, 2 and 3 PI (post-infection), using an oral administration of 8 mg pyrimethamine per kg mouse body weight.
Asexual parasite densities and gametocyte densities – the latter being the transmission stages to the mosquito – were monitored using microscopic examination of thin blood smears and determination of red blood cell densities using flow cytometry (Beckman Coulter), as described elsewhere [8]. Real-time quantitative PCR was used to distinguish and quantify R and S parasites in mixed infections [8,10]. This protocol cannot distinguish between asexual parasites and gametocytes, but real-time PCR data were used as estimates of asexual densities, because gametocyte densities were 2–3 orders of magnitude lower than asexual densities and thus a negligible component of overall parasite numbers. For each infection, two phases were distinguished: the acute phase, involving the first wave of parasites, and the chronic phase, beginning when parasite numbers began to recover after the collapse of that first wave around day 12. All parasites had disappeared below detectable levels after 50 days.
In the second experiment, two groups of nine mice were infected as above with either R parasites or R+S parasites. The subsequent transmission success of clone R was assayed by allowing batches of 30 starved Anopheles stephensi mosquitoes to feed on 3 mice from each group on each of days 7, 14, and 21 PI, as described elsewhere [e.g. [11]]. Eight days after the feeds, mosquitoes were dissected, and DNA extracted from midguts carrying oocysts. Real-time quantitative PCR was subsequently used to determine the prevalence of clone R in these mosquitoes.
All procedures were regulated and carried out under the British Home Office Animals (Scientific Procedures) Act 1986.
Results
Two untreated mice infected with R+S parasites died on days 10 and 11 PI respectively, and were excluded from the analysis.
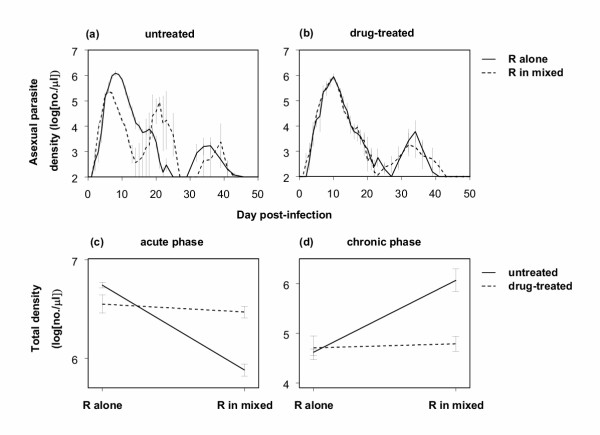
In untreated mice, there were far fewer R parasites during the acute phase in mixed infections with clone S than in R-only infections (Figures 1a,1c). However, in drug-treated mice, where S parasites were entirely removed by pyrimethamine (none of the PCR reactions performed were positive for clone S), there were as many R parasites in mixed infections as there were in R-only infections (Figures 1b,1c; Drug treatment × Alone/Mixed interaction: F1,14 = 14.4, p = 0.002). Thus, R parasites were competitively suppressed in mixed infections in untreated mice, but this suppression was negated when mice were treated with pyrimethamine, which effectively removed S parasites.
Figure 1.
Log asexual parasite densities of the resistant clone R over time in untreated (a) and drug-treated (b) mice infected with R alone or a mixture of R+S clones, and total numbers of R parasites produced over the acute (c) and chronic phases (d). All data points (mean ± 1 s.e.m.) are based on 5 replicate mice, except for mixed infections in untreated mice in (a) (4 mice on day 11 and 3 mice from day 12 onwards) and (c) and (d) (3 mice). As the limit of detection was 100 parasites per μl blood, y-axes in (a) and (b) start at 2.
During the chronic phase, clone R was more numerous in untreated mice in mixed infections than in single-clone infections (due to the parasite peak around day 21; Figures 1a,1d). Thus, in untreated mice in the chronic phase, clone R did not suffer from competition, and actually benefited from the presence of clone S (facilitation). In drug-treated mice, however, R parasites were similarly numerous in mixed- and single- clone infections (Figures 1b,1d; Drug treatment × Alone/Mixed interaction: F1,14 = 13.8, p = 0.002).
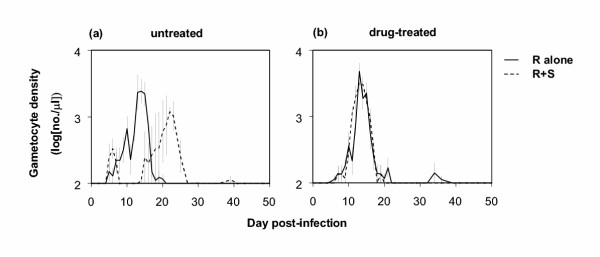
The large peak of R parasites in the chronic phase in the untreated mixed infections around day 21 (Figure 1a) coincided with a large peak of gametocytes, the transmissible stages of the parasite (Figure 2a). This was in contrast with single-clone infections of R in untreated mice, and infections in drug-treated mice, where gametocytes were mainly produced around day 14 (Figures 2a,2b). Overall, gametocyte numbers were the same for all four infection types (p > 0.05 for both Drug treatment and Alone/Mixed). Whether clone R really suffered from competitive suppression by clone S in untreated mice thus depends on how many of the gametocytes around day 21 were of the R genotype, and on how transmissible they were.
Figure 2.
Log gametocyte densities over time (mean ± 1 s.e.m.) for untreated (a) and drug-treated (b) mice. In (a) gametocyte densities for mixed R+S infections reflect overall R+S gametocytes, as the PCR assay could not distinguish between these (see text); in (b) all gametocytes are produced by clone R, as clone S was cleared from mixed infections. All data points are based on 5 replicate mice, except for mixed infections in untreated mice in (a): 4 mice on day 11 and 3 mice from day 12 onwards. As the limit of detection was 100 gametocytes per μl blood, y-axes start at 2.
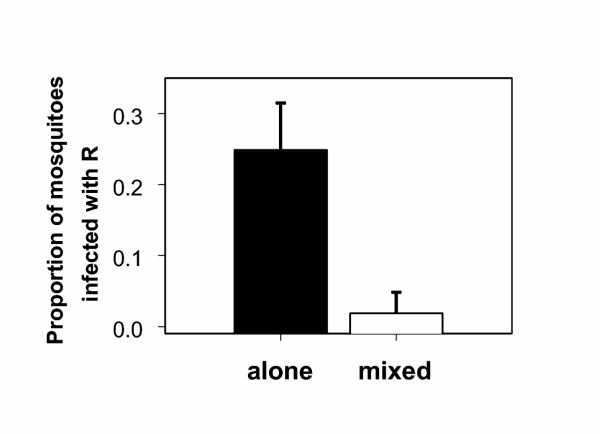
The second experiment assessed transmission to mosquitoes on days 7, 14 and 21 PI. It was found that the resistant clone R infected far fewer mosquitoes from mixed infections than from single infections (figure 3; Alone/Mixed: p = 0.002), indicating that transmissibility of gametocytes produced around day 21 was low, probably as a result of transmission-blocking immunity [12]. Thus, the competitive suppression of the resistant clone in untreated infections translated into reduced transmission success.
Figure 3.
Proportions of mosquitoes infected with the resistant clone R (mean and 95% confidence interval); mosquitoes fed either on mice infected with clone R alone or mice infected with a mixture of clones R and S. Means are based on 9 mice (3 on day 7, 3 on day 14 and 3 on day 21 PI) from which totals of 205 (R alone) and 216 (mixed R+S) mosquitoes took a blood meal. Infection with clone R was assessed by real-time PCR.
Discussion
These results show that drug treatment of malaria infections can severely affect ecological interactions between co-infecting strains. The drug-resistant clone was competitively suppressed by the drug-sensitive clone in untreated mice, in terms of both within-host growth and transmission to the mosquito vector. However, drug treatment removed that competitive suppression, and allowed the resistant clone to fill the ecological space emptied, giving it a substantial and additional fitness benefit in addition to the simple survival advantage conferred by resistance. Thus, under drug pressure, resistant strains can have two advantages: they survive better than sensitive strains and they can exploit the opportunities presented by the removal of their competitors, thereby increasing their relative transmission. Competition was studied between two unrelated clones, and thus did not reflect the situation in which a resistant clone arose de novo [13], but it seems likely that the competitive release following drug therapy would also apply there.
Competitive release following drug treatment will greatly enhance the spread of drug resistance [5]. Also, with only the resistant strain left in the host, the probability of outbreeding is reduced, thus reducing the chances of meiotic recombination destroying multi-locus resistance [14]. In combination, these two processes could enhance the spread of drug resistance, especially in areas with high numbers of strains per infection [5].
Of course, this is an argument for judicious use of drugs, not their non-use. Clearance of drug-sensitive strains from mixed infections might enhance the spread of drug resistance, but this has to be offset against the short-term public health benefits, such as reducing overall malaria prevalence. In these experiments, the drug-sensitive clone was also the more virulent clone [8], and when it was cleared from mixed infections by drug treatment, mice were less sick, in that they lost less weight and became less anaemic (results not shown).
In this experiment, mice were drug-treated before symptoms occurred, resulting in competitive release. This situation perhaps best mimics the case of prophylactic drug use, or what might occur to new co-infections in high transmission areas where drug use is common. A battery of more complex experiments will be necessary to determine if competitive release occurs when treatment follows symptoms, and when drugs are used to treat semi-immune individuals. The facilitation observed in chronic infections (Figures 1a,1d) suggests the situation might be very complex.
Within-host competition in P. chabaudi is now firmly established [8,15,16]. Evidence for competition between co-infecting genotypes in human malaria infections is necessarily indirect, but consistent with this [4]. In older children and adults, for example, parasite densities do not increase with increasing numbers of clones, thus indicating that parasite clones are not regulated independently [17]. Given this, and the high frequency of mixed infections in human malaria [1-3,18] often consisting of both resistant and sensitive genotypes [19], and the fact that genetic diversity can be altered by antimalaria prophylaxis [20], it is highly likely that competitive release of drug resistance also occurs in human malaria. Indeed, a recent study has already implicated release of within-host competition as a key-factor in the spread of drug resistance in Uganda [21].
Authors' contributions
JCdR and RC designed and performed the first experiment, while JCdR and ASB performed the second experiment. JCdR analysed the results and drafted the manuscript. ASB developed the real-time PCR assays for analysis of parasite populations inside mosquitoes. AFR assisted in designing both experiments and writing the manuscript. All authors read and approved of the final version of the manuscript.
Competing interests
None declared.
Acknowledgments
Acknowledgements
D. Walliker is thanked for assistance with drug-treating mice, B. Chan for technical assistance, R. Carter and S. Cheesman for fruitful discussion and development of real-time PCR assays, M. Guinnee for comments on an earlier manuscript, and the staff of the March animal house for excellent husbandry. The study was funded by the Wellcome Trust and the BBSRC, and JCdR was supported by the Darwin Trust of Edinburgh.
Contributor Information
Jacobus C de Roode, Email: j.de.roode@ed.ac.uk.
Richard Culleton, Email: richard.culleton@ed.ac.uk.
Andrew S Bell, Email: andrew.bell@ed.ac.uk.
Andrew F Read, Email: a.read@ed.ac.uk.
References
- Snewin VA, Herrera M, Sanchez G, Scherf A, Langsley G, Herrera S. Polymorphism of the alleles of the merozoite surface-antigens MSA1 and MSA2 in Plasmodium falciparum wild isolates from Colombia. Mol Biochem Parasitol. 1991;49:265–276. doi: 10.1016/0166-6851(91)90070-M. [DOI] [PubMed] [Google Scholar]
- Daubersies P, Sallenave-Sales S, Magne S, Trape J-F, Contamin H, Fandeur T, Rogier C, Mercereau-Puijalon O, Druilhe P. Rapid turnover of Plasmodium falciparum populations in asymptomatic individuals living in a high transmission area. Am J Trop Med Hyg. 1996;54:18–26. doi: 10.4269/ajtmh.1996.54.18. [DOI] [PubMed] [Google Scholar]
- Babiker HA, Ranford-Cartwright LC, Walliker D. Genetic structure and dynamics of Plasmodium falciparum infections in the Kilombero region of Tanzania. Trans R Soc Trop Med Hyg. 1999;93:11–14. doi: 10.1016/s0035-9203(99)90321-8. [DOI] [PubMed] [Google Scholar]
- Read AF, Taylor LH. The ecology of genetically diverse infections. Science. 2001;292:1099–1102. doi: 10.1126/science.1059410. [DOI] [PubMed] [Google Scholar]
- Hastings IM, D'Alessandro U. Modelling a predictable disaster: the rise and spread of drug-resistant malaria. Parasitol Today. 2000;16:340–347. doi: 10.1016/S0169-4758(00)01707-5. [DOI] [PubMed] [Google Scholar]
- Cox FEG. Major models in malaria research: rodent. In: Wernsdorfer WH, McGregor I, editor. Malaria: Principles and Practice of Malariology. Edinburgh: Churchill Livingstone; 1988. pp. 1503–1543. [Google Scholar]
- Carlton JM, Hayton K, Cravo PV, Walliker D. Of mice and malaria mutants: unravelling the genetics of drug resistance using rodent malaria models. Trends Parasitol. 2001;17:236–242. doi: 10.1016/S1471-4922(01)01899-2. [DOI] [PubMed] [Google Scholar]
- De Roode JC, Culleton R, Cheesman SJ, Carter R, Read AF. Host heterogeneity is a determinant of competitive exclusion or coexistence in genetically diverse malaria infections. Proc R Soc Lond B Biol Sci. 2004;271:1073–1080. doi: 10.1098/rspb.2004.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walliker D, Carter R, Sanderson A. Genetic studies on Plasmodium chabaudi: recombination between enzyme markers. Parasitology. 1975;70:19–24. doi: 10.1017/s0031182000048824. [DOI] [PubMed] [Google Scholar]
- Cheesman SJ, de Roode JC, Read AF, Carter R. Real-time quantitative PCR for analysis of genetically mixed infections of malaria parasites: technique validation and applications. Mol Biochem Parasitol. 2003;131:83–91. doi: 10.1016/S0166-6851(03)00195-6. [DOI] [PubMed] [Google Scholar]
- Ferguson HM, Mackinnon MJ, Chan BH, Read AF. Mosquito mortality and the evolution of malaria virulence. Evolution. 2003;57:2792–2804. doi: 10.1111/j.0014-3820.2003.tb01521.x. [DOI] [PubMed] [Google Scholar]
- Naotunne TDeS, Rathnayake KD, Jayasinghe A, Carter R, Mendis KN. Plasmodium cynomolgi: serum-mediated blocking and enhancement of infectivity to mosquitoes during infections in the natural host, Macaca sinica. Exp Parasitol. 1990;71:305–313. doi: 10.1016/0014-4894(90)90035-B. [DOI] [PubMed] [Google Scholar]
- White NJ, Pongtavornpinyo W. The de novo selection of drug-resistant malaria parasites. Proc R Soc Lond B Biol Sci. 2003;270:545–554. doi: 10.1098/rspb.2002.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye C, Williams BG. Multigenic drug resistance among inbred malaria parasites. Proc R Soc Lond B Biol Sci. 1997;264:61–67. doi: 10.1098/rspb.1997.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor LH, Walliker D, Read AF. Mixed-genotype infections of malaria parasites: within-host dynamics and transmission success of competing clones. Proc R Soc Lond B Biol Sci. 1997;264:927–935. doi: 10.1098/rspb.1997.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snounou G, Bourne T, Jarra W, Viriyakosol S, Wood JC, Brown KN. Assessment of parasite population dynamics in mixed infections of rodent plasmodia. Parasitology. 1992;105:363–374. doi: 10.1017/s0031182000074539. [DOI] [PubMed] [Google Scholar]
- Smith T, Beck HP, Kitua A, Mwankusye S, Felger I, Fraser-Hurt N, Irion A, Alonso P, Teuscher T, Tanner M. Age dependence of the multiplicity of Plasmodium falciparum infections and of other malariological indices in an area of high endemicity. Trans R Soc Trop Med Hyg. 1999;93:15–20. doi: 10.1016/s0035-9203(99)90322-x. [DOI] [PubMed] [Google Scholar]
- Felger I, Smith T, Edoh D, Kitua A, Alonso P, Tanner M, Beck HP. Multiple Plasmodium falciparum infections in Tanzanian infants. Trans R Soc Trop Med Hyg. 1999;93:29–34. doi: 10.1016/s0035-9203(99)90324-3. [DOI] [PubMed] [Google Scholar]
- Babiker HA, Creasey AM, Bayoumi RAL, Walliker D, Arnot DE. Genetic diversity of Plasmodium falciparum in a village in Eastern Sudan. 2. Drug-resistance, molecular karyotypes and the mdr1 genotype of recent isolates. Trans R Soc Trop Med Hyg. 1991;85:578–583. doi: 10.1016/0035-9203(91)90349-4. [DOI] [PubMed] [Google Scholar]
- Beck HP, Felger I, Vounatsou P, Hirt R, Tanner M, Alonso P, Menendez C. Effect of iron supplementation and malaria prophylaxis in infants on Plasmodium falciparum genotypes and multiplicity of infection. Trans R Soc Trop Med Hyg. 1999;93:41–45. doi: 10.1016/s0035-9203(99)90326-7. [DOI] [PubMed] [Google Scholar]
- Talisuna AO, Langi P, Mutabingwa TK, Van Marck E, Speybroeck N, Egwang TG, Watkins WW, Hastings IM, D'Alessandro U. Intensity of transmission and spread of gene mutations linked to chloroquine and sulphadoxine-pyrimethamine resistance in falciparum malaria. Int J Parasitol. 2003;33:1051–1058. doi: 10.1016/S0020-7519(03)00156-5. [DOI] [PubMed] [Google Scholar]