Abstract
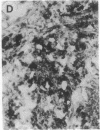
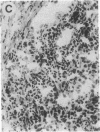
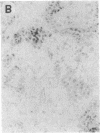
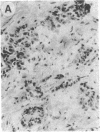
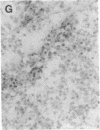
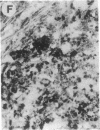
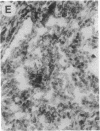
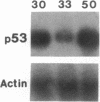
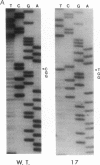
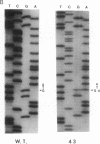
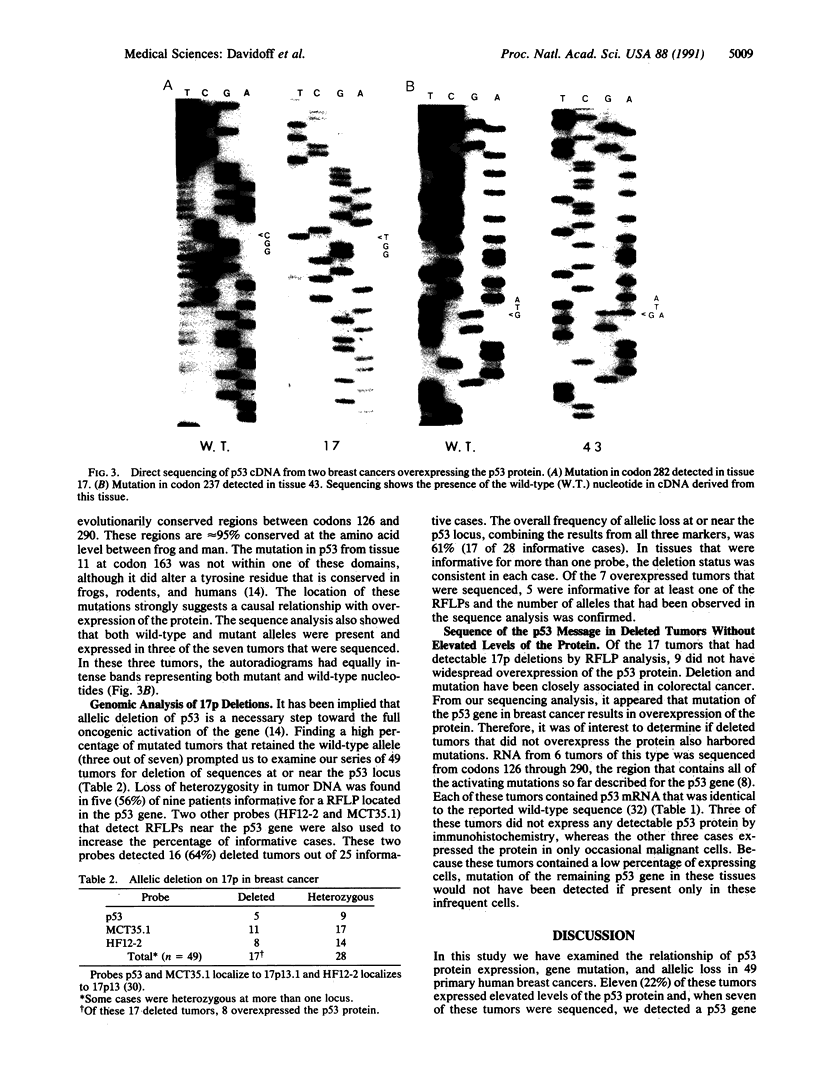
Overexpression of an activated form of the p53 protein may be involved in neoplastic transformation. We found widespread overexpression of p53 by immunohistochemical staining in 11 (22%) of 49 primary invasive human breast cancers. Northern blot analysis showed that this overexpression was not due to an increase in the steady-state level of p53 mRNA. The p53 gene was directly sequenced in 7 of these tumors with elevated levels of the protein and, in each case, a mutation that altered the coding sequence for p53 was found in a highly conserved region of the gene. Whereas 4 of these tumors contained only a mutant p53 allele, the other 3 tumors exhibited coding sequences from both a mutant and a wild-type allele. p53 mutations have previously been correlated with allelic loss of part of chromosome 17p that contains the p53 locus. Examination of all 49 breast tumors revealed a 61% frequency of deletion at or near the p53 locus. However, the presence of allelic deletion did not correlate with overexpression of the protein. Six tumors that were deleted but did not express high levels of the protein were sequenced and all retained a wild-type p53 allele. In this series of human breast cancers, overexpression of the p53 protein, not allelic loss on chromosome 17p, was always associated with mutation of the p53 gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks L., Matlashewski G., Crawford L. Isolation of human-p53-specific monoclonal antibodies and their use in the studies of human p53 expression. Eur J Biochem. 1986 Sep 15;159(3):529–534. doi: 10.1111/j.1432-1033.1986.tb09919.x. [DOI] [PubMed] [Google Scholar]
- Bartek J., Iggo R., Gannon J., Lane D. P. Genetic and immunochemical analysis of mutant p53 in human breast cancer cell lines. Oncogene. 1990 Jun;5(6):893–899. [PubMed] [Google Scholar]
- Buchman V. L., Chumakov P. M., Ninkina N. N., Samarina O. P., Georgiev G. P. A variation in the structure of the protein-coding region of the human p53 gene. Gene. 1988 Oct 30;70(2):245–252. doi: 10.1016/0378-1119(88)90196-5. [DOI] [PubMed] [Google Scholar]
- Cattoretti G., Rilke F., Andreola S., D'Amato L., Delia D. P53 expression in breast cancer. Int J Cancer. 1988 Feb 15;41(2):178–183. doi: 10.1002/ijc.2910410204. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coles C., Thompson A. M., Elder P. A., Cohen B. B., Mackenzie I. M., Cranston G., Chetty U., Mackay J., Macdonald M., Nakamura Y. Evidence implicating at least two genes on chromosome 17p in breast carcinogenesis. Lancet. 1990 Sep 29;336(8718):761–763. doi: 10.1016/0140-6736(90)93236-i. [DOI] [PubMed] [Google Scholar]
- Devilee P., van den Broek M., Kuipers-Dijkshoorn N., Kolluri R., Khan P. M., Pearson P. L., Cornelisse C. J. At least four different chromosomal regions are involved in loss of heterozygosity in human breast carcinoma. Genomics. 1989 Oct;5(3):554–560. doi: 10.1016/0888-7543(89)90023-2. [DOI] [PubMed] [Google Scholar]
- Eliyahu D., Goldfinger N., Pinhasi-Kimhi O., Shaulsky G., Skurnik Y., Arai N., Rotter V., Oren M. Meth A fibrosarcoma cells express two transforming mutant p53 species. Oncogene. 1988 Sep;3(3):313–321. [PubMed] [Google Scholar]
- Eliyahu D., Michalovitz D., Eliyahu S., Pinhasi-Kimhi O., Oren M. Wild-type p53 can inhibit oncogene-mediated focus formation. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8763–8767. doi: 10.1073/pnas.86.22.8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Levine A. J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989 Jun 30;57(7):1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Tan T. H., Eliyahu D., Oren M., Levine A. J. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol Cell Biol. 1988 Feb;8(2):531–539. doi: 10.1128/mcb.8.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon J. V., Greaves R., Iggo R., Lane D. P. Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J. 1990 May;9(5):1595–1602. doi: 10.1002/j.1460-2075.1990.tb08279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds P. W., Finlay C. A., Quartin R. S., Baker S. J., Fearon E. R., Vogelstein B., Levine A. J. Mutant p53 DNA clones from human colon carcinomas cooperate with ras in transforming primary rat cells: a comparison of the "hot spot" mutant phenotypes. Cell Growth Differ. 1990 Dec;1(12):571–580. [PubMed] [Google Scholar]
- Hinds P., Finlay C., Levine A. J. Mutation is required to activate the p53 gene for cooperation with the ras oncogene and transformation. J Virol. 1989 Feb;63(2):739–746. doi: 10.1128/jvi.63.2.739-746.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo R., Gatter K., Bartek J., Lane D., Harris A. L. Increased expression of mutant forms of p53 oncogene in primary lung cancer. Lancet. 1990 Mar 24;335(8691):675–679. doi: 10.1016/0140-6736(90)90801-b. [DOI] [PubMed] [Google Scholar]
- Jenkins J. R., Rudge K., Chumakov P., Currie G. A. The cellular oncogene p53 can be activated by mutagenesis. 1985 Oct 31-Nov 6Nature. 317(6040):816–818. doi: 10.1038/317816a0. [DOI] [PubMed] [Google Scholar]
- Lamb P., Crawford L. Characterization of the human p53 gene. Mol Cell Biol. 1986 May;6(5):1379–1385. doi: 10.1128/mcb.6.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay J., Steel C. M., Elder P. A., Forrest A. P., Evans H. J. Allele loss on short arm of chromosome 17 in breast cancers. Lancet. 1988 Dec 17;2(8625):1384–1385. doi: 10.1016/s0140-6736(88)90584-3. [DOI] [PubMed] [Google Scholar]
- Marks J. R., Lin J., Hinds P., Miller D., Levine A. J. Cellular gene expression in papillomas of the choroid plexus from transgenic mice that express the simian virus 40 large T antigen. J Virol. 1989 Feb;63(2):790–797. doi: 10.1128/jvi.63.2.790-797.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer W. E., Avignolo C., Baserga R. Role of the p53 protein in cell proliferation as studied by microinjection of monoclonal antibodies. Mol Cell Biol. 1984 Feb;4(2):276–281. doi: 10.1128/mcb.4.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen T., Cavenee W. K. Suppressors of the malignant phenotype. Cell Growth Differ. 1990 Apr;1(4):201–207. [PubMed] [Google Scholar]
- Milner J., Milner S. SV40-53K antigen: a possible role for 53K in normal cells. Virology. 1981 Jul 30;112(2):785–788. doi: 10.1016/0042-6822(81)90327-5. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Lathrop M., O'Connell P., Leppert M., Barker D., Wright E., Skolnick M., Kondoleon S., Litt M., Lalouel J. M. A mapped set of DNA markers for human chromosome 17. Genomics. 1988 May;2(4):302–309. doi: 10.1016/0888-7543(88)90018-3. [DOI] [PubMed] [Google Scholar]
- Nigro J. M., Baker S. J., Preisinger A. C., Jessup J. M., Hostetter R., Cleary K., Bigner S. H., Davidson N., Baylin S., Devilee P. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989 Dec 7;342(6250):705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Oren M., Maltzman W., Levine A. J. Post-translational regulation of the 54K cellular tumor antigen in normal and transformed cells. Mol Cell Biol. 1981 Feb;1(2):101–110. doi: 10.1128/mcb.1.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich N. C., Levine A. J. Growth regulation of a cellular tumour antigen, p53, in nontransformed cells. Nature. 1984 Mar 8;308(5955):199–201. doi: 10.1038/308199a0. [DOI] [PubMed] [Google Scholar]
- Reich N. C., Oren M., Levine A. J. Two distinct mechanisms regulate the levels of a cellular tumor antigen, p53. Mol Cell Biol. 1983 Dec;3(12):2143–2150. doi: 10.1128/mcb.3.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogel A., Popliker M., Webb C. G., Oren M. p53 cellular tumor antigen: analysis of mRNA levels in normal adult tissues, embryos, and tumors. Mol Cell Biol. 1985 Oct;5(10):2851–2855. doi: 10.1128/mcb.5.10.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovinski B., Benchimol S. Immortalization of rat embryo fibroblasts by the cellular p53 oncogene. Oncogene. 1988 May;2(5):445–452. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sato T., Tanigami A., Yamakawa K., Akiyama F., Kasumi F., Sakamoto G., Nakamura Y. Allelotype of breast cancer: cumulative allele losses promote tumor progression in primary breast cancer. Cancer Res. 1990 Nov 15;50(22):7184–7189. [PubMed] [Google Scholar]
- Shohat O., Greenberg M., Reisman D., Oren M., Rotter V. Inhibition of cell growth mediated by plasmids encoding p53 anti-sense. Oncogene. 1987;1(3):277–283. [PubMed] [Google Scholar]
- Soussi T., Caron de Fromentel C., Méchali M., May P., Kress M. Cloning and characterization of a cDNA from Xenopus laevis coding for a protein homologous to human and murine p53. Oncogene. 1987 Mar;1(1):71–78. [PubMed] [Google Scholar]
- Tan T. H., Wallis J., Levine A. J. Identification of the p53 protein domain involved in formation of the simian virus 40 large T-antigen-p53 protein complex. J Virol. 1986 Sep;59(3):574–583. doi: 10.1128/jvi.59.3.574-583.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]