Abstract
Mouse models of the transcriptional modulator Methyl-CpG-Binding Protein 2 (MeCP2) have advanced our understanding of Rett syndrome (RTT). RTT is a ‘prototypical’ neurodevelopmental disorder with many clinical features overlapping with other intellectual and developmental disabilities (IDD). Therapeutic interventions for RTT may therefore have broader applications. However, the reliance on the laboratory mouse to identify viable therapies for the human condition may present challenges in translating findings from the bench to the clinic. In addition, the need to identify outcome measures in well-chosen animal models is critical for preclinical trials. Here, we report that a novel Mecp2 rat model displays high face validity for modelling psychomotor regression of a learned skill, a deficit that has not been shown in Mecp2 mice. Juvenile play, a behavioural feature that is uniquely present in rats and not mice, is also impaired in female Mecp2 rats. Finally, we demonstrate that evaluating the molecular consequences of the loss of MeCP2 in both mouse and rat may result in higher predictive validity with respect to transcriptional changes in the human RTT brain. These data underscore the similarities and differences caused by the loss of MeCP2 among divergent rodent species which may have important implications for the treatment of individuals with disease-causing MECP2 mutations. Taken together, these findings demonstrate that the Mecp2 rat model is a complementary tool with unique features for the study of RTT and highlight the potential benefit of cross-species analyses in identifying potential disease-relevant preclinical outcome measures.
Introduction
Rett syndrome (RTT, MIM 312750) is an X-linked neurodevelopmental disorder caused by mutations in MECP2 (1). Typical RTT is characterized by a period of apparently normal development followed by the loss of acquired skills and a striking pattern of disease onset and progression (2,3). In addition, disease-causing MECP2 mutations and genomic abnormalities have been reported in a wide range of disorders such as autism (4–6), schizophrenia (7–9), intellectual disability (10–12), developmental delay, obsessive compulsive disorder, attention deficit-hyperactivity disorder (13), and MECP2 duplication syndrome (14–16), and have also been reported in individuals with mild cognitive and behavioural impairments (17–24). Thus, the true impact of MECP2 mutations may be wider than is often appreciated, as suggested by work identifying common variants of MECP2 as potential risk factors for autism and autism spectrum disorders (ASD) (25). Because of the overlapping disease features of RTT with other intellectual disabilities and autism spectrum disorders (IDD/ASD), therapeutic interventions that may help RTT phenotypes may also prove useful for the treatment of many neurodevelopmental and neurological conditions.
Recent studies in animal models have demonstrated that RTT may indeed be reversible; most notably, many features of the disorder are normalized in mouse models following re-expression of the Mecp2 gene (26). Phenotypic deficits are also induced following deletion of the gene in adult mice, arguing that the disease is the result of functional abnormalities of neurons and/or neural circuits rather than abnormal development (27). Although many promising therapeutic leads (28–33) have emerged, the field must proceed with caution. Historically, the vast majority of experimental therapies for neurological disorders failed to translate successfully from animal models to human disease therapies for reasons such as sub-optimal animal models, poor study design, and the lack of rigorous evaluations (34–37).
Given these challenges and potential limitations of genetic mouse models (37–39), additional genetic mammalian model systems may provide powerful insight into alterations that are not always readily captured using the mouse, and importantly, may also be necessary for identifying needed preclinical measures. Rats provide an alternative rodent model and are currently the translational model system of choice for validation of therapeutic strategies (40). In addition, rats display sophisticated social and cognitive behaviours (41,42), some of which are not present in mice (43). The purpose of our study was to therefore determine the extent to which constitutive loss of MeCP2 in female rats recapitulates behavioural features of RTT. In addition, by studying the molecular consequences of loss of MeCP2 in a second mammalian rodent species, we may identify evolutionarily conserved alterations that may have better disease relevance to RTT pathogenesis.
Results
The Mecp2 zinc-finger nuclease rat is a viable model for studies of MeCP2 loss-of-function
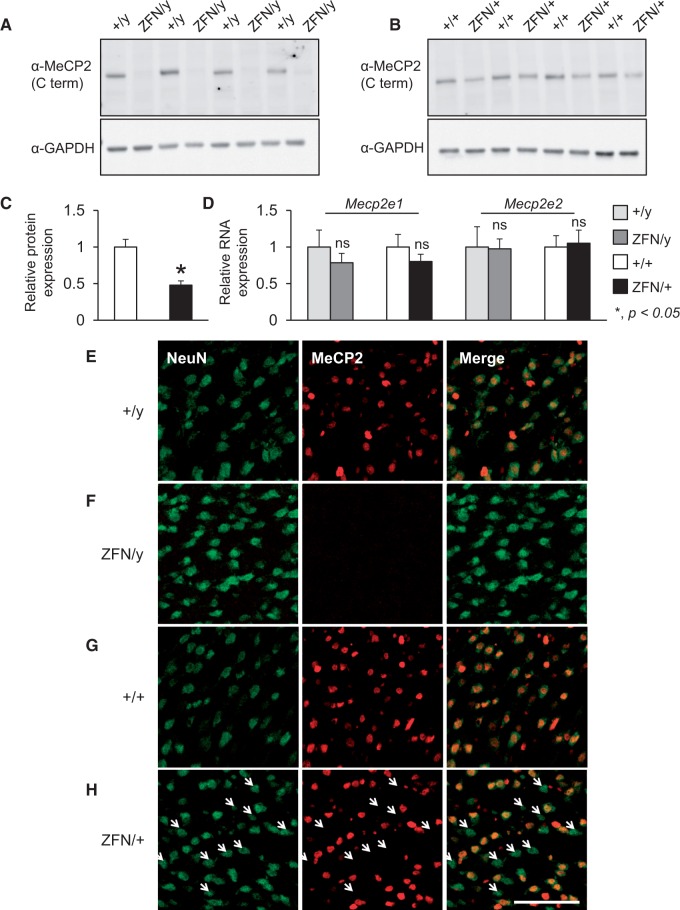
Mecp2 rats were generated using a zinc-finger nuclease (ZFN) strategy (44) that resulted in a 71 base pair deletion within exon 4 and is predicted to result in an early stop codon at amino acid 245 (Supplementary Material, Fig. S1). Western blot analysis of forebrain tissue using a C-terminal anti-MeCP2 antibody demonstrated a complete loss of full-length MeCP2 in male rats lacking Mecp2 (Mecp2ZFN/y) and an approximate 50% reduction in MeCP2 levels in female rats lacking one copy of Mecp2 (Mecp2ZFN/+) (Figure 1A–C; Supplementary Material, Fig. S2A, B). However, quantification of Mecp2e1 and Mecp2e2 RNA isoforms by QPCR showed normal expression at the transcript level (Figure 1D), suggesting that modification of the endogenous rat Mecp2 locus resulted in post-transcriptional loss of MeCP2 protein. Alterations in protein levels were confirmed by immunofluorescence (IF) staining of cortical tissue. Male Mecp2ZFN/y rats showed a complete absence of MeCP2 in NeuN-positive cells as expected in male rats carrying only the mutant copy of Mecp2 (Figure 1E,F). In contrast, female Mecp2ZFN/+ rats demonstrated a heterogeneous pattern of MeCP2 expression as expected in females carrying one mutant and one normal copy of Mecp2 (Figure 1G,H). In addition, quantification of MeCP2 staining in cortical tissue from female Mecp2ZFN/+ rats also showed an approximate 50% reduction in MeCP2 IF signal intensity with approximately half of NeuN-positive cells expressing MeCP2 (Supplementary Material, Fig. S3). Furthermore, although the ZFN-induced deletion is predicted to result in a truncated protein, an N-terminal anti-MeCP2 antibody did not reveal any detectable product by Western blot (Supplementary Material, Fig. S2C) or IF staining (Supplementary Material, Fig. S2D). Together, these data indicate that despite normal Mecp2 transcript levels, the Mecp2 ZFN rat is a viable model to study the consequences of loss of MeCP2 protein.
Figure 1.
The Mecp2 zinc-finger nuclease (ZFN) rat is a viable model to study the consequences of the loss of MeCP2 function. (A–C) Western blot analysis of brain tissue obtained from male Mecp2ZFN/y (ZFN/y) and Mecp2ZFN/+ (+/+) rats compared with corresponding male and female wild-type littermate male animals (n = 4 per genotype per sex). A C-terminal MeCP2 antibody does not detect MeCP2 in male Mecp2ZFN/y rats (A). Female Mecp2ZFN/+rats display an approximate 50% reduction in MeCP2 protein levels (B, C). (D) ZFN targeting of the endogenous rat Mecp2 locus does not alter Mecp2 RNA expression levels of either the Mecp2e1 or Mecp2e2 isoforms in cortical tissue of male or female Mecp2 rats as measured by quantitative real-time RT-PCR (n = 4 per genotype per sex). (E–H) Representative images of immunofluorescence staining of cortical tissue using a C-terminal MeCP2 antibody detects MeCP2 (red) in NeuN-positive cells (green) in male wild-type littermate rats (+/y) (E), but not male Mecp2ZFN/y rats (ZFN/y) (F). Female wild-type littermate rats (+/+) show normal MeCP2 staining (G); in contrast, female Mecp2ZFN/+rats (ZFN/+) show a mosaic pattern of MeCP2 expression (H). Quantification of MeCP2 signal intensity in female Mecp2ZFN/+rats compared with female wild-type littermate rats confirms an approximate 50% reduction in MeCP2 levels, confirming observations by Western blot analysis; MeCP2 is detected in approximately half of NeuN-positive cells (Supplementary Material, Figure S3). White arrows indicate the loss of MeCP2 in NeuN-positive cells, scale bar indicates 100 µm. +/y, male wild-type littermate, +/+, female wild-type littermate; ZFN/y, Mecp2ZFN/y; ZFN/+, Mecp2ZFN/+; *P < 0.05; ns, not significant.
Female Mecp2ZFN/+ rats model regression and juvenile play deficits
To determine the neurobehavioural consequences of loss of MeCP2 in the rat, we used assays that may be well-suited to testing specifically in the laboratory rat, as well as conventional tests of behaviour in rodents. It is noteworthy that the rat was the model organism primarily used for rodent behavioural studies (45), especially learning and memory test paradigms, prior to the advent of genetic engineering that made manipulations of the mouse genome straightforward. As genetic tools in the mouse evolved, assays used for behaviour in the rat were adapted, developed and improved for comparable studies in the mouse (46,47).
Our studies primarily focused on measurements that were either initiated in juvenile female rats and examined longitudinally through young adulthood, or restricted to juvenile ages. Studying young female Mecp2ZFN/+ rats, as opposed to male Mecp2ZFN/y rats, is the sex-appropriate disease model for the evaluation of neurobehavioural deficits of clinical relevance to RTT (48). In addition, we selected juvenile ages, in contrast to the adult time points used in the majority of behavioural studies of Mecp2 mouse models, as the focus of our work was to determine whether female Mecp2ZFN/+ rats display behavioural problems during the early stages of life that would better reflect clinical manifestations that occur in young girls with RTT. Two cohorts of female animals were generated to evaluate potential behavioural impairments: one cohort for modelling psychomotor regression, and a separate cohort for investigating the impact of MeCP2 deficiency on multiple behavioural domains.
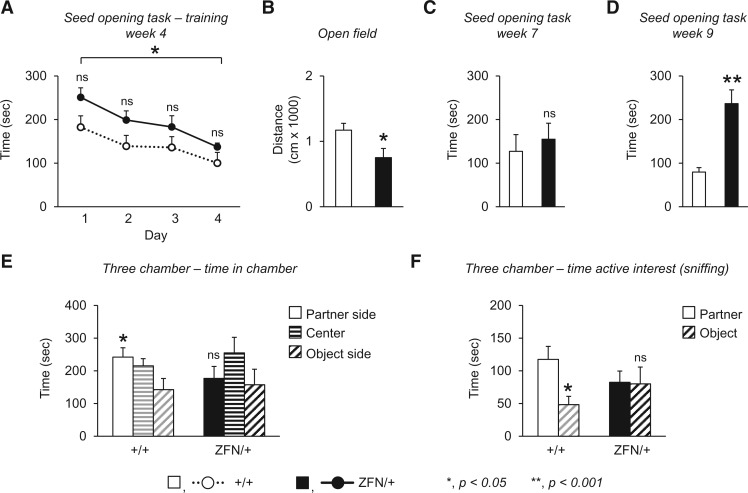
Although MeCP2 deficiency in mice results in broad neurobehavioural impairments (29,48–56), it is unclear whether Mecp2 mouse models display psychomotor regression, a key feature of RTT that is also observed in other IDD/ASD (57). Regression in RTT is clinically defined as the loss of acquired skills such as purposeful hand use or language (57). Mecp2 mouse models show a worsening of specific phenotypes over time, such as generalized exploratory activity in an open arena, or coordination and balance in assays of motor function (49). However, the initial identification and subsequent worsening of a phenotype that model disease progression may not specifically reflect psychomotor regression in RTT. Therefore, we set out to determine whether Mecp2 rats displayed regression of an acquired fine motor skill. In a cohort of animals tested longitudinally, naïve young female Mecp2ZFN/+ rats and wild-type littermates were trained in a seed opening task at 4 weeks of life, and their ability to open the seeds was evaluated through 9 weeks of life. During the training phase, we found that both groups of rats showed a significant improvement, i.e. reduction in the time spent opening seeds, and similar rate of acquiring the ability to open the seeds across the four days of training (Figure 2A). Furthermore, a significant difference in performance on the first day compared to the fourth day of training was observed independent of genotype (Figure 2A). The ability to acquire and perform in this forepaw skill task was therefore normal for young female Mecp2ZFN/+ rats in this cohort of animals tested despite evidence showing reduced generalized exploratory activity in the open field arena (Figure 2B) at the same age of life. To determine whether this acquired skill was maintained over time, rats were tested in a single trial at 7 and at 9 weeks of life. We found that Mecp2ZFN/+ rats and wild-type littermates performed equally well at 7 weeks of life (Figure 2C); however, at 9 weeks of life, Mecp2ZFN/+ rats showed a loss of this learned skill, spending a significantly longer amount of time to open seeds (Figure 2D). Taken together, these data suggest that psychomotor regression can indeed be modelled in female Mecp2ZFN/+ rats.
Figure 2.
Female Mecp2ZFN/+ rats display psychomotor regression and impaired sociability. (A–D) Both female Mecp2ZFN/+rats (ZFN/+) and female wild-type (+/+) littermates were trained in the seed task assay, a test of fine forepaw motor function, at 4 weeks of life, and then evaluated at 7 and 9 weeks of life. Female Mecp2ZFN/+rats and wild-type littermates performed similarly in their ability to open seeds at 4 weeks of life (A), in spite of reduced general activity in the open field at the same time point as observed in a separate cohort of rats (B). At 7 weeks of life, no difference was observed in the same task (C). At 9 weeks of life, Mecp2ZFN/+rats appeared to lose the ability of this learned skill, spending significantly more time to open the seeds (D). (E, F) In the three chamber test, female wild-type littermates show the expected pattern of normal sociability, spending more time in the chamber containing the novel partner compared with the chamber containing the novel object; in contrast, female Mecp2ZFN/+ rats show the conventional definition of impaired sociability (60), spending a comparable time in the chambers containing the novel partner and novel object (E). In addition, female Mecp2ZFN/+rats do not display a difference in time spent actively investigating, i.e. sniffing, the partner or object (F). *P < 0.05; **P < 0.001; ns, not significant, n = 12 per genotype for seed opening task; 9–13 per genotype for three chamber test. A complete statistical summary of behavioural data is provided in Supplementary Material, Table ST12.
To test whether Mecp2 rats can also model additional neurobehavioural features of RTT that have been challenging to study in mice, we evaluated the features of social behaviour in female Mecp2ZFN/+ rats and wild-type littermates during the course of studying multiple behavioural domains in a separate cohort of animals not used for the seed opening task study. Although not among the primary diagnostic symptoms of RTT (58), deficits in sociability are prominent, may persist throughout childhood (59), and have been reported in individuals with disease-causing MECP2 mutations in the absence of a clinical RTT diagnosis (2,17). These clinical findings suggest that impaired social behaviour is an important aspect of MeCP2 disorders. Mice lacking MeCP2, however, demonstrate a reduction in social approach behaviour but do not display the conventional definition of impaired sociability in the three chamber test, an established assay commonly implemented in studies of social behaviour in genetic mouse models (49,60–62). Therefore, we evaluated the performance of juvenile rats in this assay (61) developed for use with mice, and in a social interaction test paradigm that would allow us to take advantage of the rich behavioural repertoire offered by the laboratory rat. In the three chamber test, we found that female wild-type littermate rats exhibit a pattern of normal sociability, spending more time in the chamber side containing the novel conspecific partner compared with the chamber side containing the novel object (Figure 2E); in contrast, female Mecp2ZFN/+ rats display the conventional definition of impaired sociability spending a comparable amount of time in the chamber sides containing the partner and object (Figure 2E). Evaluation of the time spent actively interested, i.e. sniffing, the novel partner or object, confirmed the findings from analyzing the chamber time alone (Figure 2F).
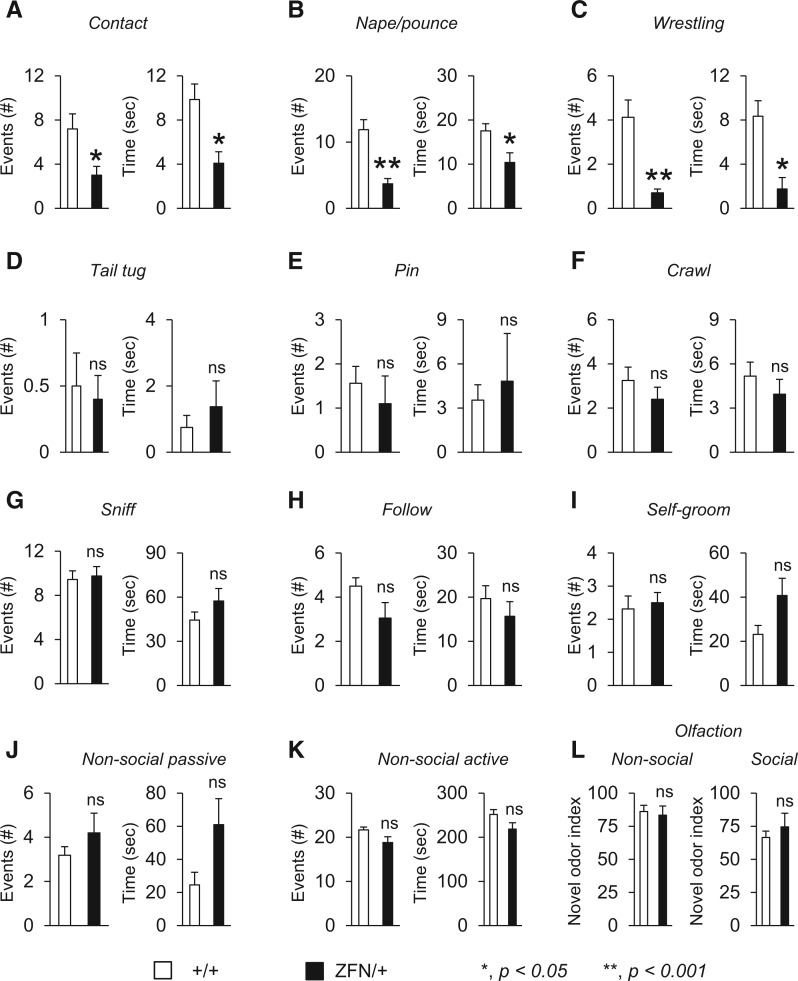
To further refine the nature of the social behaviour deficit of female Mecp2ZFN/+ rats beyond a simple evaluation of indirect social interest, we performed a direct social interaction test that evaluated both general social and juvenile-specific play behaviours (62). Normally, rats in direct contact with each other display social interaction patterns that include age-restricted rough-and-tumble play behaviour (41,43,62,63). We found that juvenile female Mecp2ZFN/+ rats engage their conspecific partners less in both generalized contact and specific juvenile play behaviours compared with female wild-type littermate rats (Figure 3A–C). ‘Contact’ behaviour of female Mecp2ZFN/+ rats, defined as the test subject’s paw touching the conspecific juvenile partner’s body, was reduced in both the number of events observed as well as the duration of the contact behaviour (Figure 3A). Similarly, female Mecp2ZFN/+ rats also displayed a reduction in the number of events and duration of the play behaviours ‘nape/pouncing’ and ‘wrestling’ (Figure 3B,C). In contrast, other play behaviours such as ‘tail tug’, ‘pin’ and ‘crawl’ were normal in comparison to wild-type littermate rats (Figure 3D–F). To ensure the selective deficits in aspects of play behaviour were not due to broad deficits in olfaction, movement, or general activity during the assay, we evaluated sniffing, following/chasing, self-grooming, passive non-social, and active/exploratory non-social behaviours and found that female Mecp2ZFN/+ rats did not show differences in these control parameters in comparison to wild-type littermates (Figure 3G–K). In addition, olfaction in both non-social and social odour comparisons revealed normal novel versus familiar odour discrimination indices (Figure 3L), indicating that the impairments in overall sociability and play behaviour were not due to problems in gross olfaction.
Figure 3.
Juvenile female Mecp2ZFN/+ rats display selective deficits in aspects of play behaviour. (A–C) In a test for direct social interaction, female Mecp2ZFN/+ (ZFN/+) rats in comparison with female wild-type littermates (+/+) showed a reduction in the number of events and duration of activity for general paw contact (A), nape/pounce behaviour (B) and wrestling (C) when paired with a conspecific partner rat of the same sex and age. (D–H) In contrast, other aspects of play behaviour directed at conspecific partner rats such as tail tugging (D), pinning (E), crawling over-and-under (F), sniffing (G) and following (H) were normal. (I–K) As a control, evaluations of self-grooming, non-social passive behaviour and non-social active behaviour such as digging and general exploratory activity demonstrated comparable activity for both female Mecp2ZFN/+ and wild-type littermate rats during the test period. (L) Furthermore, no differences were observed in social odour discrimination, indicating that the loss of MeCP2 in female rats does not alter gross olfaction. *P < 0.05; **P < 0.001; ns, not significant, n = 8–10 per genotype. A complete statistical summary of behavioural data is provided in Supplementary Material, Table ST12.
Juvenile female Mecp2ZFN/+ rats display additional behavioural abnormalities that are either consistent with or different from previous studies of Mecp2 mouse models
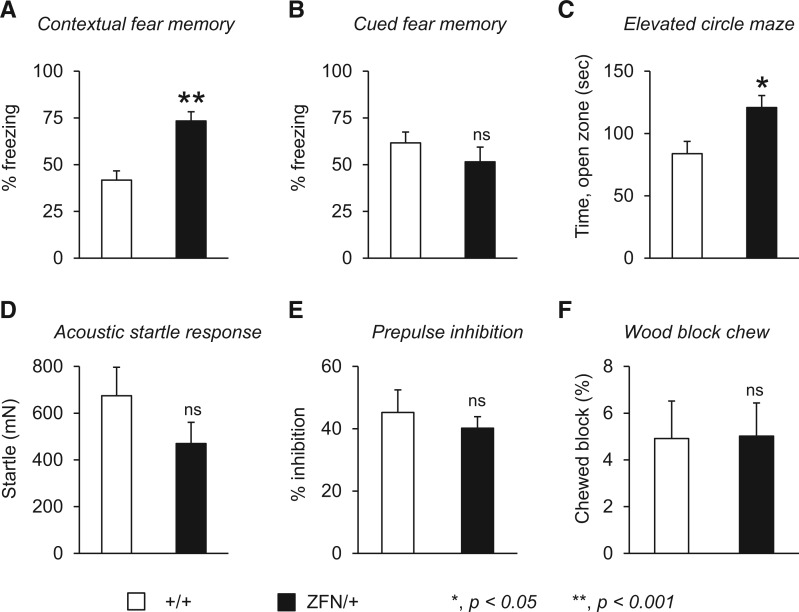
To further identify similarities and/or differences among Mecp2 rat and mouse models, the female animals evaluated for social behaviour were also tested for impairments in anxiety-like behaviour, sensorimotor gating, learning and memory, and perseverative behaviour. Although fear conditioning studies in Mecp2 mouse models have demonstrated reduced contextual fear and normal cued fear memory in adult animals (49,51,53,64), we found that juvenile female Mecp2ZFN/+ rats displayed enhanced contextual fear memory (Figure 4A), yet normal cued fear memory (Figure 4B). Increased anxiety-like behaviour is one potential confound that may contribute to enhanced freezing behaviour; however, juvenile female Mecp2ZFN/+ rats in comparison with wild-type littermate rats displayed reduced anxiety-like behaviour in the elevated circle maze, spending more time in the lit areas of the circle maze compared with wild-type littermates (Figure 4C). Reduced anxiety-like behaviour is consistent with reported studies in Mecp2 mouse models (49,51) and suggests that the enhanced contextual fear memory observed in young female Mecp2ZFN/+ rats is not due to increased anxiety-like behaviour; however, these findings may be potentially confounded by gross motor deficits suggested by the decrease in activity observed in the open field (Figure 2B). Finally, the female juvenile Mecp2ZFN/+ rats performed normally in evaluations of sensorimotor gating such as prepulse inhibition of the acoustic startle response (Figure 4D,E), and in the wood block chew assay, a measurement of perseverative behaviour (62) (Figure 4F). A table summarizing the neurobehavioural phenotypes of female Mecp2ZFN/+ rats in comparison with the onset and nature of phenotypes in female mice harbouring the Mecp2-null mouse alleles (50–53,65) is shown in Table 1.
Figure 4.
Juvenile female Mecp2ZFN/+ rats display alterations in fear memory and anxiety-like behaviour but normal sensorimotor gating and perseverative behaviour. (A, B) Juvenile female Mecp2ZFN/+ rats (ZFN/+) display increased freezing behaviour compared with female wild-type littermates (+/+) when tested for contextual (A) but not cued fear memory (B). (C) Juvenile female Mecp2ZFN/+ rats compared with female wild-type littermates spend more time in the open zones of the elevated circle maze. (D, E) Acoustic startle (D) and prepulse inhibition of the startle response (E) are normal in juvenile female Mecp2ZFN/+ rats. (F) In a test for perseverative behaviour, juvenile female Mecp2ZFN/+ rats and female wild-type littermates do not display differences in chewing a wooden block. *P < 0.05; **P < 0.001; ns, not significant, n = 9–13 per genotype. A complete statistical summary of behavioural data is provided in Supplementary Material, Table ST12.
Table 1.
Comparison of the juvenile neurobehavioural phenotypes in rodent models of Rett syndrome
| Female juvenile RTT rat |
Earliest reported onset in female RTT mice |
||
|---|---|---|---|
| Loss-of-function |
Null |
||
| Behavioural domain | Mecp2 ZFN allele | Mecp2 Tm1.1Bird (deletion exon3-4) allele | Mecp2 Tm1.1Jae (deletion exon3) allele |
| Regression of forepaw use | Normal, 4 & 7 wk; impaired 9 wk | N.E. | N.E. |
| Sociability | Impaired, ∼ 4 wk | Moderate impairment, 12 wk | N.E. |
| Anxiety-like behaviour | Reduced, ∼ 3 wk | Reduced, 5 wk | Reduced, 8 wk |
| Locomotor activity | Reduced, ∼ 4 wk | Reduced, 12 wk | Reduced, 5-6 wk |
| Sensorimotor gating | Normal, ∼ 5 wk | Altered, 7 wk | N.E. |
| Contextual fear memory | Enhanced, 5 wk | Impaired, 8 wk | Normal, 6 wk |
| Cued fear memory | Normal, 5 wk | Normal, 8 wk | Normal, 6 wk |
| Olfaction | Normal, ∼ 6 wk | Normal, 12 wk | N.E. |
| Perseverative behaviour | Normal, 6 wk | N.E. | N.E. |
Summary of the findings from our studies of juvenile female Mecp2ZFN/+ rats compared with published reports on the age of onset and nature of deficits reported in female mice that have either one copy of the Mecp2Tm1.1Bird allele (49,50), or one copy of the Mecp2Tm1.1Jae allele (51–53). wk, week; N.E., not evaluated or reported.
Comparative gene expression studies demonstrate the value of evaluating both Mecp2 rodent models in parallel
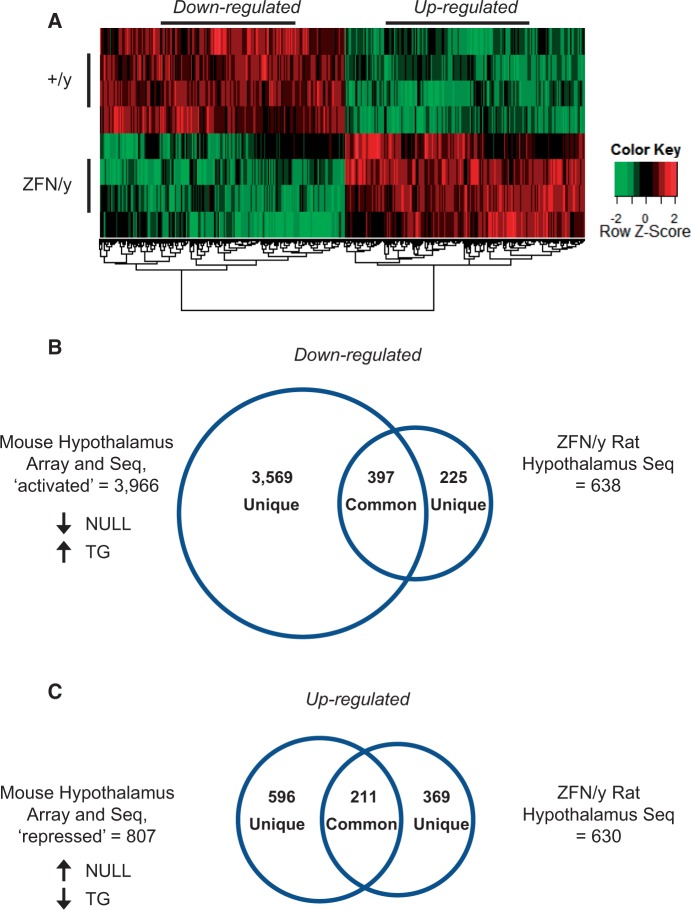
To determine whether the Mecp2 rat model also demonstrates molecular changes that either parallel or differ from those reported in Mecp2 mice, we compared the transcriptomes of Mecp2 rats and mice (66–69). For these studies, we reasoned that using male Mecp2ZFN/y rats completely lacking MeCP2 would provide a direct comparison with previously published data on Mecp2Tm1.1Bird/y mice (66,69), the most commonly used Mecp2-null mouse allele (50). We found that in contrast to the thousands of gene expression alterations previously reported in the hypothalamus of Mecp2Tm1.1Bird/y mice (NULL) using both array- and sequencing-based technologies, RNA-seq analysis of Mecp2ZFN/y rat hypothalamus revealed a total of 1,268 significantly altered gene expression changes (Figure 5A). Similar to previous studies in the mouse, the magnitude of fold changes were not dramatic, with gene expression changes within the range of approximately log2 ± 2 (Figure 5A; Supplementary Material, Tables ST1-8). However, unlike Mecp2 mouse models that showed a disproportionate number of up-regulated compared with down-regulated gene expression changes in the hypothalamus (66,69), Mecp2ZFN/y rats appear to have an approximate equal balance of alterations (638 down-regulated versus 630 up-regulated genes; Figure 5B,C; Table 2).
Figure 5.
Transcriptional profiling of the hypothalamus using RNA-seq highlights the similarities and differences between transcriptional changes in Mecp2 rodent models. (A) Transcriptional profiling of Mecp2ZFN/y (ZFN/y) and wild-type male (+/y) littermate rat hypothalamus reveals an approximate equal distribution of gene expression alterations (638 up-regulated and 630 down-regulated, Bonferroni-adjusted p value (q) < 0.05). The heat map shown displays baseline expression in +/y animals (top row) relative to altered expression in ZFN/y animals (bottom row) with each row indicating a single animal. (B, C) Rat RNA-seq data were compared with existing transcriptional data sets for the hypothalamus of Mecp2Tm1.1Bird/y (NULL) and MECP2-TG (TG) mice. The overlap in the number of genes altered between Mecp2 rats and mice is shown, comparing genes down-regulated in rat to genes decreased in NULL/increased in TG, i.e. ‘activated’, defined as decreased in the absence of MeCP2 and increased in the presence of excess MeCP2’ (66,69) (B), and genes up-regulated in rat to genes increased in NULL/decreased in TG, i.e. ‘repressed’, defined as increased in the absence of MeCP2 and decreased in the presence of excess MeCP2 (66, 69) (C). Within these categories, a fraction of genes changed in the ZFN/y rat were either changed in NULL only, changed in TG but not NULL, or changed in other brain regions such as the cerebellum and hypothalamus as previously reported (67, 68), and these changes were either identical or opposite from NULL and TG findings (Table 2). The number of gene expression alterations that were uniquely altered in the MeCP2 rat and mouse models is also shown (B, C). A complete list of gene expression changes in ZFN/y rats identified by RNAseq and compared with existing mouse gene expression datasets is provided in Supplementary Material, Tables ST1-8, gene ontology terms associated with commonly affected gene expression alterations among Mecp2 rodents and uniquely affected in the Mecp2 ZFN/y rat are provided in Supplementary Material, Tables ST9, ST10.
Table 2.
Summary of gene expression alterations in Mecp2 rat hypothalamus and comparison with findings from Mecp2 mouse hypothalamus
| Total expression changes in ZFN/y Rat HYP (#) | SHARED |
UNIQUE | |||||
|---|---|---|---|---|---|---|---|
| Common to ZFN/y Rat & Mouse NULL/TG HYP |
Changed in ZFN/y Rat & Mouse CER or AMY, not HYP | Unique to ZFN/y Rat HYP | |||||
| Changed in Rat & TG, not NULL | Opposite from NULL or like TG | Opposite from NULL & TG | |||||
| DOWN | 638 | 397 | 29 | 4 | 0 | 16 | 225 |
| UP | 630 | 211 | 32 | 22 | 1 | 50 | 369 |
| Grand total | 1268 | 608 | 61 | 26 | 1 | 66 | 594 |
| Percent of total (%) | 47.95 | 4.81 | 2.05 | 0.08 | 5.21 | 46.85 | |
Table summarizing the total number of gene expression changes identified by RNAseq in the hypothalamus of Mecp2ZFN/y rats (ZFN/y) in comparison with reported findings from either array or RNAseq data in Mecp2 mice (66–69). Expression changes are categorized according to whether they are ‘shared’ between Mecp2 rats and mice from our analysis, or are ‘unique’ changes identified only in ZFN/y rats. The genes among the ‘shared’ category indicate the number of genes that are common to the Mecp2 rat and Mecp2 mouse (NULL) models, unique to the rat model, changed in the rat and the MECP2-TG model (TG), opposite from findings in the NULL or showing an expression difference as observed in the TG, opposite from both NULL or TG, or changed in the rat and in other brain regions of the NULL, but not the hypothalamus.
With respect to the shared gene expression changes between Mecp2 mouse and rat, approximately one-half of expression alterations (47.95%, or 608 genes) were changed in the same direction as reported in Mecp2 mouse models. These genes were previously defined as either ‘repressed’ or ‘activated’ according to opposing expression patterns in the hypothalami of NULL mice compared with mice that overexpress MeCP2 (TG) (66,69) (397 down-regulated and 211 up-regulated genes; Figure 5B,C; Supplementary Materials, Tables ST1,ST2). Among this set of commonly altered genes, some alterations in the rat (4.81%, or 61 genes) overlapped with findings in TG mouse hypothalamus alone (66,69), but were not previously reported as altered in NULL mice (29 down-regulated and 32 up-regulated genes; Table 2, and noted in Supplementary Material, Tables ST1, ST2). In addition, only a small fraction of expression changes in the Mecp2ZFN/y rat (2.05%, or 26 genes) were directionally opposite of observations in NULL mouse hypothalamus or in the same direction as TG mice (66,69) (4 down-regulated and 22 up-regulated genes; Table 2; Supplementary Material, Table ST3), and only one gene showed an expression difference that was opposite of both Mecp2 mouse models (Table 2; Supplementary Material, Table ST4). Importantly, however, was the finding that almost half of the gene expression alterations (46.85%, or 594 genes) were uniquely changed in Mecp2ZFN/y rat hypothalamus (225 down-regulated and 369 up-regulated genes; Table 2; Figure 5B,C; Supplementary Material, Tables ST5,ST6). Finally, a small number of genes altered in Mecp2ZFN/y rat hypothalamus (5.21%, or 66 genes) were not changed in the hypothalamus of either Mecp2 mouse models, but rather reported to be dysregulated in other brain regions such as the cerebellum (67) and amygdala (68) of Mecp2 mice (16 down-regulated and 50 up-regulated genes; Table 2; Supplementary Material, Table ST7,ST8). A complete list of gene ontology terms associated with common and unique gene expression alterations is listed in Supplementary Material, Tables ST9,ST10.
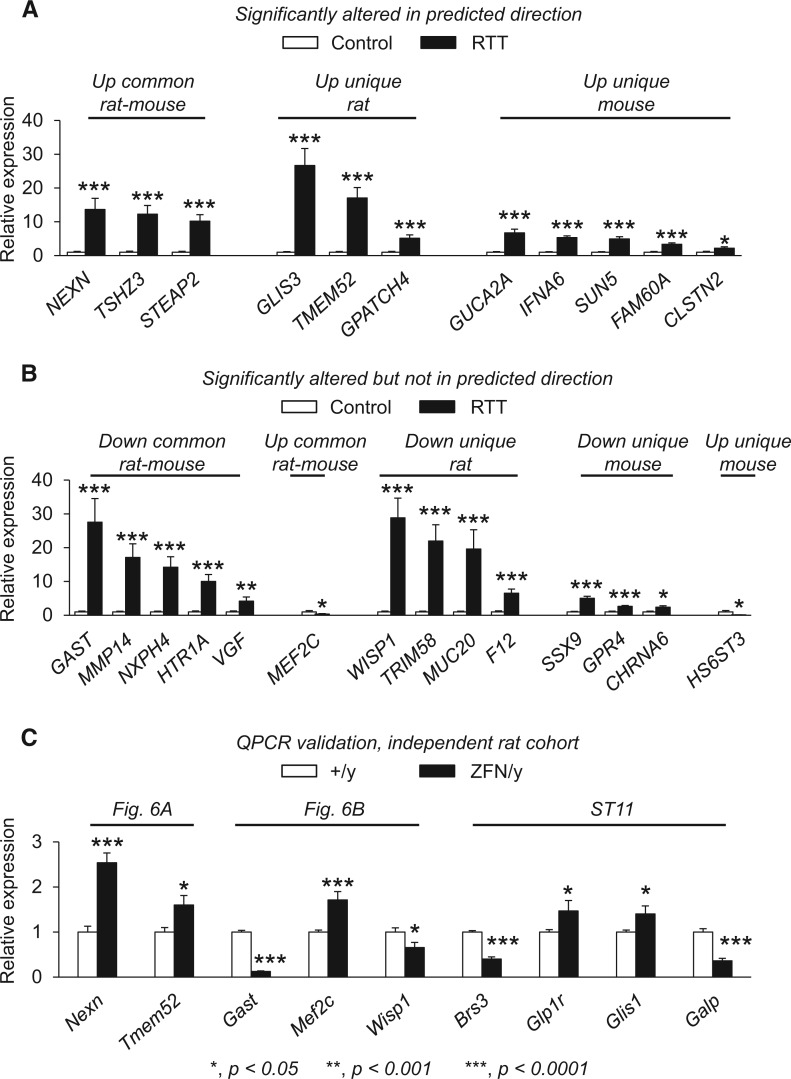
To gain further insight into the potential relevance of these gene expression alterations, we evaluated whether genes either commonly changed in both Mecp2 rat and mouse models (common rat-mouse), uniquely changed in the Mecp2 rat (unique rat), or uniquely changed in the Mecp2 mouse models (66,69) (unique mouse) would be predictive of homologous molecular alterations in post-mortem cortical tissue samples from RTT individuals with identified MECP2 mutations (70). The genes we chose for this analysis were randomly selected from the genes commonly altered in the hypothalamus of Mecp2 rodents (Supplementary Material, Tables ST1,ST2), uniquely altered in the hypothalamus of Mecp2ZFN/y rats (Supplementary Material, Tables ST5,ST6), and uniquely altered in Mecp2 mouse models (66). In comparison with age- and gender-matched controls, we found that of 82 genes tested, a total of 43 genes (∼52% of the total number of genes tested) were altered or undetectable in the RTT brain by QPCR. The remainder of the genes tested (39 genes, or ∼47% of the total number of genes tested) were not significantly altered in RTT brain (33 genes), or expressed in either RTT or control brains (6 genes).
To further outline the similarities and differences among the different categories of gene expression alterations between rat, mouse and human, we found that of the 43 expression alterations, 11 genes showed a significant difference between RTT and control brain tissue samples and were altered in a manner predicted by Mecp2 rodent models. These genes were found to be commonly up-regulated in Mecp2 rat and mouse hypothalamus (‘up common rat-mouse’; NEXN, TSHZ3, and STEAP2), uniquely up-regulated in Mecp2 rat hypothalamus (‘up unique rat’; GLIS3, TMEM52, and GPATCH4), and uniquely up-regulated in Mecp2 mouse hypothalamus (‘up unique mouse’; GUCA2A, IFNA6, SUN5, FAM60A, and CLSTN2) (Figure 6A). In contrast, of the 43 gene expression alterations, 14 genes showed a significant difference between RTT and control brain tissue samples but were altered in a manner that was opposite from the Mecp2 rodent findings. These genes were found to be commonly down-regulated in Mecp2 rat and mouse hypothalamus (‘down common rat-mouse’; GAST, MMP14, NXPH4, HTR1A, and VGF), commonly up-regulated in Mecp2 rat and mouse hypothalamus (‘up common rat-mouse’; MEF2C), uniquely down-regulated in Mecp2 rat hypothalamus (‘down unique rat’; WISP1, TRIM58, MUC20, and F12), uniquely down-regulated in Mecp2 mouse hypothalamus (‘down unique mouse’; SSX9, GPR4, and CHRNA6), and uniquely up-regulated in mouse hypothalamus (HS6ST3) (Figure 6B). Finally, 18 of the 43 gene expression alterations had no significantly measureable expression in RTT brain relative to control brain samples, and these genes were distributed among all categories, with the majority (8 of 18 genes) found in the genes classified as commonly down-regulated in Mecp2 rat and mouse hypothalamus (Supplementary Material, Table ST11). A summary of gene expression data found in RTT brain for each category is provided in Supplementary Material, Table ST11. To ensure that the genes we selected to analyze in human brain tissue samples were indeed significantly altered in male Mecp2ZFN/y rats as indicated by initial RNA-seq findings (Supplementary Material, Tables ST1,2,5,6), we tested the top genes that we found altered in RTT brain in an independent set of male Mecp2ZFN/y and wild-type littermate rats, choosing a single gene within each category (significantly altered in predicted direction, Figure 6A; significantly altered but not in predicted direction, Figure 6B; and undetectable in RTT brain, Supplementary Material, Table ST11). We found that the nine genes we tested were significantly altered in the hypothalami of male Mecp2ZFN/y rats compared with male wild-type littermate animals; thus validating a small subset of the RNA-seq findings from which we based our comparative expression studies (Figure 6C).
Figure 6.
Comparative studies of both Mecp2 rat and mouse models may strengthen the predictive validity of molecular changes that occur due to the loss of MeCP2 function. (A, B) Eight-two genes selected among either the commonly affected alterations between mouse and rat (‘common rat-mouse’), in rat alone (‘unique rat’) or in mouse alone (‘unique mouse)’ were selected for comparative expression studies in human RTT and control brain samples (Brodmann area 9) by QPCR (n = 3 RTT; n = 4 control). Expression level differences relative to controls samples are shown. Several genes within the up-regulated categories of ‘common rat-mouse’, ‘unique rat’ or ‘unique mouse’ were also altered in RTT brain (A). Additional genes were altered in RTT brain relative to controls; however, these were changed in RTT brain in a direction that was opposite from the predicted Mecp2 rodent findings (B). Some genes were detected in control brain samples but were not detected in RTT brain. (C) As an additional control to ensure that the genes selected for this analysis were indeed altered in Mecp2 rat hypothalamus, an independent set of animals were used for QPCR validation studies of the top altered genes within each category. All nine genes analyzed were altered as expected in male Mecp2ZFN/y rats (ZFN/y) compared with wild-type littermate animals (+/y), confirming the initial RNA-seq findings for this subset of genes. Supplementary Material, Table ST11 shows the expression levels of each gene for both human and rat QPCR data.
Discussion
With the development of advanced genome editing technologies, such as the ZFN strategy (44) used to generate the Mecp2 rat model characterized in this study, and even more efficient approaches using CRISPR/Cas gene editing (71–73), generating novel mammalian models of disease beyond the laboratory mouse is feasible. Although some loss-of-function Mecp2 mouse models of RTT have good construct validity and mimic either the human disease mutation itself or the consequence of the human disease-causing mutations (3,48,74), the findings from our work indicate the value of the Mecp2 rat model as a powerful tool with the potential to shape the landscape of future studies focused on both mechanisms of RTT pathophysiology as well as on preclinical efforts to ameliorate disease onset and progression.
In this study, our aim was to test the extent to which the loss of MeCP2 in the rat resulted in behavioural deficits present in RTT. However, to confirm that the rat Mecp2 ZFN allele was appropriate for these studies, we first performed a series of biochemical and molecular evaluations using tissue from both male and female Mecp2 ZFN rats. Unlike the conventional homologous recombination approach used to generate the most widely studied Mecp2 germline null mouse model (50), ZFN targeting of the endogenous Mecp2 locus in the rat did not produce a complete null allele; rather, an allele was created in which normal MeCP2 protein expression was completely abolished yet total abundance of Mecp2 RNA remained unaltered. Interestingly, although this outcome may not initially appear to be desirable, these results from the rat Mecp2 ZFN loss-of-function allele are reminiscent of the findings from single-cell, clonally-derived fibroblast strains from RTT girls with disease-causing MECP2 mutations (75). Indeed, early studies of clonal cell lines from RTT individuals with nonsense mutations showed that MECP2-mutant expressing cell lines lacked MeCP2 protein by Western blot analysis using a C-terminal antibody but had normal transcript levels (75). Moreover, the rat Mecp2 ZFN allele predicted to result in truncated protein did not express any detectable truncation product by either Western blot or antibody staining, similar to observations made with human brain lysates from RTT individuals with truncation mutations (76). In contrast, introducing a late truncating nonsense mutation in the endogenous Mecp2 locus in the mouse does not entirely parallel these findings in the rat or human. For example, the Mecp2-R255X mouse allele (76) resulted in the complete loss of MeCP2 protein and no detectable truncation product, but also caused an approximate 40% reduction in transcript levels. It is noteworthy, however, that the Mecp2-T158A mouse allele that disrupts the methyl-CpG binding ability of the protein resulted in a similar scenario as in the rat Mecp2 ZFN allele; RNA levels were normal despite a loss of protein due to a selective decrease in MeCP2 protein stability (77). Therefore, it is conceivable that humans and rats may share an overlapping post-transcriptional mechanism(s) to regulate the expression of specific mutations in MECP2, such as decreased protein stability or perhaps a block in protein translation, that functions differently in mice harboring similar mutation subtypes. Taken together, with respect to our current confirmatory experiments to assess the validity of the rat allele, our data suggest that the rat Mecp2 ZFN allele has high construct validity that may better reflect the consequences of late-truncating human MECP2 nonsense mutations.
One primary goal of our neurobehavioural analyses was to identify phenotypic deficits that parallel features found in girls and women with RTT, some of which have not been reported or extensively studied in Mecp2 mouse models, such as psychomotor regression and impaired sociability. Although there is significant benefit in studying male Mecp2ZFN/y rats to understand the molecular mechanisms underlying the complete absence of MeCP2, we reasoned that focusing our behavioural evaluations of young female Mecp2ZFN/+ rats would potentially uncover abnormalities in a sex-appropriate animal model that are coincident with the early onset of disease state described in typical RTT.
Psychomotor regression in RTT, defined as the loss of acquired skills such as purposeful hand use, has yet to be well-modelled in Mecp2 mice. Although longitudinal studies have shown the manifestation and subsequent worsening of phenotypes such as reduced motor function in Mecp2 mice (49), and one study of symptomatic 10–12 month old Mecp2-308 females showed deficits in forepaw dexterity at a single time point late in life (78), the seed opening task (79) adapted for this study allowed us to evaluate the extent to which trained, young female Mecp2ZFN/+ rats lose their ability to perform over a period of time through early adulthood. Remarkably, we found that Mecp2ZFN/+ rats performed similarly to wild-type littermates on this task during the training phase at 4 weeks of life, and during the single test trial at 7 weeks of life, suggesting that not only did they acquire and learn the skill over time, but also maintained this learned forepaw ability. However, at 9 weeks of age, female Mecp2ZFN/+ rats lost their ability to perform well on this task, providing evidence of a phenotype reminiscent of psychomotor regression of an acquired forepaw skill. Here, it is important to note the distinction between disease ‘progression’ versus ‘regression’. Although we cannot formally rule out the possibility of an element of disease progression in the longitudinal seed opening test paradigm at this time, our findings appear to model aspects of regression-like behaviour (learning, acquisition, maintenance and eventual loss of the skill). These data suggest female Mecp2ZFN/+ rats demonstrate high face validity for psychomotor regression, an aspect of RTT that not yet been fully appreciated in Mecp2 mouse models to the extent demonstrated in the current study. Similar longitudinal studies using a forepaw training paradigm in the various Mecp2 mouse alleles may provide additional insight into the relationship between Mecp2 mutation subtype, and disease onset and severity in the context of psychomotor regression. However, it remains to be determined whether Mecp2 mice will perform sufficiently well in the seed opening task. Furthermore, because regression in RTT may also coincide with the loss of speech, it may be informative to determine whether the type and nature of ultrasonic vocalizations (USV) emitted by female Mecp2ZFN/+ rats, or other aspects of communication, change over time. Although we did not find differences in the total number of USV emitted by juvenile female Mecp2ZFN/+ rats during direct social interaction tests (data not shown), a recent study of symptomatic 5-11 month old female Mecp2ZFN/+ rats found alterations in speech processing (80), suggesting that communication deficits, in a broader context, may be affected in this model.
Although there is debate about social behaviour abnormalities in RTT (59), impaired social behaviour in RTT that is consistent with the social deficits of clinically defined autism appears to coincide with stages of early disease progression (81). In our social behaviour studies, we found that young female Mecp2ZFN/+ rats displayed both the conventional definition of impaired sociability in an indirect assay, the three chamber test, and selective deficits in aspects of play behaviour. Unlike Mecp2 mice that have only shown a relative reduction in social interest in the three chamber test, female Mecp2ZFN/+ rats indeed show a phenotype that is consistent with findings from other ASD mouse models with impaired sociability. Furthermore, by refining our social behaviour studies to focus on play behaviour (41), an age- and species-specific complex set of behaviours that is only present at a rudimentary level in the mouse (43) and is genuinely present in rats, we found that female Mecp2ZFN/+ rats display juvenile play deficits similar to those observed in other ASD rats such as the Fmr1 (62) and Nlgn3 (62) ZFN models. Despite the potential confound of reduced motor/exploratory activity as may potentially be suggested by the open field data (Figure 2B), measures quantified during the direct social interaction test, such as evaluating the number and duration of ‘following’, ‘self-grooming’ and ‘non-social active’ exploratory behaviours, provided an additional layer of control (Figure 3H,I,K). Normal levels of activity with respect to these control measures in both female Mecp2ZFN/+ and wild-type littermate rats while the animals were actively engaged in this task, as well as normal olfaction with both non-social and social odour cues (Figure 3L), strengthens the interpretation that female Mecp2ZFN/+ rats have impairments in selective aspects of play behaviour. Although the biological pathways regulating play behaviour in these rat models are unclear at this time, these data provide an inroad into understanding the neuroanatomical determinants of sociability in genetically tractable models that have a complex repertoire of behavioural phenotypes. These social behaviour studies together suggest social deficits that manifest early in RTT, especially juvenile play behaviour which cannot be studied well in mice, may be more appropriately modelled in young female rats lacking MeCP2.
In addition to identifying novel phenotypes in the Mecp2 ZFN rat model, we also addressed whether behavioural defects previously reported in Mecp2 mice were also present in the rat. Evaluations of fear conditioning, anxiety-like behaviour, sensorimotor gating and perseverative behaviour revealed changes in juvenile female Mecp2ZFN/+ rats that were either consistent with or different from previous studies of Mecp2 mice. For example, decreased exploratory activity in the open field arena and reduced anxiety-like behaviour in the elevated circle maze were two phenotypes consistent with known Mecp2 mouse behavioural data; however, our findings suggest that these phenotypes emerge earlier in life in female Mecp2ZFN/+ rats compared with Mecp2 mice. In contrast, the juvenile female Mecp2ZFN/+ rats were normal in tests for perseverative behaviour and sensorimotor gating, both of which have been reported to be altered in Mecp2 mice (49,82). This is not entirely unexpected, given the difference in the ages of the animals that were tested in this study in comparison with previous work. Interestingly, juvenile female Mecp2ZFN/+ rats also displayed an increase in freezing in fear conditioning that was suggestive of enhanced contextual fear memory. Because we identified reduced anxiety-like behaviour in female Mecp2ZFN/+ rats, increased anxiety is unlikely a contributor to the increased freezing behaviour after conventional Pavlovian fear conditioning; in contrast, reduced activity observed in the open field may contribute to this phenotype. Reduced contextual fear memory is a hallmark of Mecp2 mouse models; therefore, it remains to be determined whether identical learning and memory phenotypes can be studied in Mecp2 rats. It would be informative to study how our current behavioural findings compare with phenotypes present in adult female Mecp2ZFN/+ rats, as well as how Mecp2 rats and mice of the same age would perform using the identical fear conditioning training protocol. These future studies using female Mecp2ZFN/+ rats may also benefit from evaluating performance in other learning and memory tasks that do not rely on an aversive training stimulus.
Finally, our comparative studies of gene expression alterations in Mecp2 rats and mice and their predictive validity in RTT post-mortem brain tissue revealed several key similarities and differences among the two rodent models. Previous profiling strategies of Mecp2 mice employed microarray and more recently, sequencing-based technologies (66,69). Because strong evidence provided by multiple studies including those led by the Sequencing Quality Control/Microarray Quality Control consortium suggests that there is good concordance across gene expression platforms (83–87), such as array versus sequencing-based approaches, we chose to compare our data to the complete list of genes altered in Mecp2 mice from both array and RNA-seq studies. The ‘union’ of genes expression data, i.e. the total aggregate of all gene expression changes identified in both hypothalamus array (66) and sequencing-based studies (69), from Mecp2 mice allowed us to identify all possible overlapping hypothalamic gene expression changes shared with the Mecp2ZFN/y rats and provide an unbiased reference point that did not rely solely on findings from a single profiling approach. This strategy also ensured that the remaining genes that are not commonly altered within the hypothalamus of Mecp2 rodents are indeed uniquely changed in male Mecp2ZFN/y rat. Interestingly, one notable similarity is that the fold expression changes in both Mecp2 rodents were of the same magnitude (±2 fold, log2 scale). Although this magnitude of fold change has also been reported in other brain regions of Mecp2 mice such as the cerebellum (67) and amygdala (68), as well as the striatum (88), it would be useful to determine whether additional brain regions of the Mecp2 rat are similarly affected. Moreover, although approximately half of the genes identified as altered in male Mecp2ZFN/y rats were commonly altered in Mecp2 mice, the majority of these commonly affected genes were directionally concordant between rat and mouse in terms of up- or down-regulation. Together, these data may indicate that the loss of MeCP2 has strikingly similar effects on gene expression for a subset of genes among divergent rodent species. With respect to differences between gene expression alterations among Mecp2 rodent models, approximately half of the expression changes we identified are unique to the rat; these findings may have important implications in the manifestation of behavioural phenotypes that are present in the Mecp2 rat model.
Lastly, working with the limitations of available human post-mortem tissue, our approach to use Mecp2 rodent data to predict changes in RTT was revealing. By testing shared or unique Mecp2 rodent gene expression changes in RTT and control brains, we found that among the genes altered in RTT brain, the only genes that were predictive of changes in RTT brain were up-regulated in either both Mecp2 rodent models or each rodent model alone. Although additional comparative profiling would be required, these initial data may possibly support the model of MeCP2’s historical role as a transcriptional repressor of genes (89) with specific relevance to human disease pathogenesis. In contrast, the genes that were altered in RTT brain but not in the same direction as predicted from either Mecp2 model appeared to be genes mostly representing the subsets of down-regulated genes. Interestingly, these alterations had the highest relative fold changes in RTT brain. Despite the limited number of genes analyzed by conventional QPCR and the differences in brain region analyzed (rodent hypothalamus compared with human Brodmann area 9 containing the frontal cortex), it is noteworthy we identified concordant gene expression changes in Mecp2 rodent models and RTT brain samples. Our additional validation QPCR studies in an independent set of male Mecp2ZFN/y rats and male wild-type littermate animals confirmed the top altered genes from each category, including those that were not detectable in RTT brain, suggesting that these changes, at least for this small subset of genes, are genuine alterations in the Mecp2 rat model. Taken together, these comparative data strongly suggest that the combination of analyzing both loss-of-function Mecp2 rodent models may provide a significant advantage for identifying transcriptional changes and likely other biochemical and molecular changes that occur in the human RTT brain that may not have been predicted in analyses of either rodent model alone. In the context of recent findings suggesting that MeCP2 may regulate the expression of long genes in a cell-type and brain-region specific manner (90,91), and preferentially bind to DNA at methylated cytosine at CG sites (92) yet also bind to methylated cytosine at non-CG sites (69) as well as nucleosomal sequences with high GC content alone (93), it would be highly informative to pursue similar studies using the Mecp2 rat model to further clarify the role of MeCP2 in the epigenetic regulation of gene expression and its impact on disease features of RTT and related disorders.
In conclusion, our work demonstrates the utility of the rat Mecp2 ZFN allele for identifying molecular alterations and neurobehavioural outcome measures that may be useful for future preclinical studies. In addition, our comparative gene expression studies demonstrate the strength of combining Mecp2 rodent data sets to potentially improve the predictive validity of alterations that occur in RTT, some of which may have greater disease-relevance. Given the perceived shortcomings of using rodent models for preclinical validation studies of therapeutic compounds for use in humans (36,94), and the urgent need for outcome measures to expedite the discovery of successful therapies (48), this work underscores the significant benefits of investigating additional mammalian rodent models of RTT, and provides a framework for studying features provided by the Mecp2 rat model that will complement ongoing mouse studies.
Materials and Methods
Animal husbandry
All research and animal care procedures were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee, and all studies detailing our findings were conducted at Baylor College of Medicine. Rats were maintained on a 12 h light:12 h dark cycle with standard rat chow (Pico Lab Rodent Diet, #5053, Purina USA) and water ad libitum. Animals were generated by mating female Sprague-Dawley rats lacking one copy of functional MeCP2 (Mecp2ZFN/+) with wild-type male Sprague-Dawley rats (SAGE Labs/Horizon, USA) to obtain male rats completely lacking MeCP2 (Mecp2ZFN/y), female Mecp2ZFN/+ rats and wild-type littermate male and female rats. Rats were housed three animals per cage, and randomized and blinded for subsequent experiments described below. Genotyping was performed in accordance with the manufacturer’s protocol (SAGE Labs/Horizon, USA) using the following primers to identify animals that harbour the ZFN allele: forward, 5’-GCAGC ATCA GAAGGTGTTCA-3’; reverse, 5’-GACCTCAATGCTGACGGTTT-3’. All wild-type animals used in the study were age- and sex-matched littermates of the generated male and female Mecp2 rats; conspecific female wild-type partner rats (purchased from SAGE Labs/Horizon, USA) used in the direct social interaction study as described below, were not littermate animals.
Western blot
Fresh brains were dissected from rat cortex samples (n = 4 biological replicates of each genotype, Mecp2ZFN/y and Mecp2ZFN/+, and respective wild-type littermates, 6 weeks of life). The brains were homogenized in 100 mM Tris, pH 8.0, and 2% SDS with protease inhibitor cocktail (Sigma-Aldrich, USA) in a glass dounce homogenizer with the B handle and rocked at room temperature for at least 1 h to ensure complete cell lysis. Soluble proteins were separated from cell debris by centrifugation at 20,000g for 15 min, and the supernatant was quantified using a colorimetric assay (Pierce BCA Protein Assay Kit; ThermoFisher Scientific, USA). Forty µg of protein per sample were loaded onto a PAGE gel and transferred onto a nitrocellulose membrane. The membrane was blocked in Tris-buffered saline, pH 7.6, and 0.1% Tween 20 with 5% milk. For immunodetection of MeCP2, an antibody raised in rabbit (95), diluted 1:2000 was used; detection of the loading control GAPDH was performed with an antibody raised in mouse (sc-32233; Santa Cruz Biotechnology, Inc.; USA) diluted 1:20,000. Membranes were incubated for 1 h with Cy5-conjugated secondary antibodies against mouse and rabbit diluted 1:4000 (GE Healthcare Life Sciences), imaged using the Cy5 channel (ImageQuant LAS 4000, GE Healthcare Life Sciences) and quantified by densitometry with ImageJ software as previously described (76).
Quantitative real time reverse transcriptase PCR
For QPCR analysis of Mecp2e1 and Mecp2e2 in rat, RNA was extracted from rat cortex samples (n = 4 biological replicates of each genotype, Mecp2ZFN/y and Mecp2ZFN/+, and respective wild-type littermates, 6 weeks of life) using TRizol reagent following the manufacturer’s instructions (ThermoFisher Scientific, USA), and 3 µg RNA was used for cDNA synthesis (iScript cDNA synthesis kit, Bio-rad, USA). QPCR using SsoAdvanced Universal SYBR Green Supermix (Bio-rad, USA) was performed using the standard curve method with rat Mecp2e1- and Mecp2e2-specific (96) and rat Gapdh primers, and expression level of each Mecp2 isoform was quantified relative to wild-type littermate control animals as previously described (68). For QPCR analysis using human RTT and control brain tissue samples (n = 3 RTT, n = 4 control; Brodmann area 9 used in previous reports (70,97)), similar methods were used for RNA extraction and 1 µg of RNA was used for cDNA synthesis. QPCR using SsoAdvanced Universal SYBR Green Supermix (Bio-rad, USA) was performed using 96-well plates seeded with a commercial primer assays for 82 genes of interest and controls (PrimePCR, Bio-rad, USA), and relative expression levels comparing RTT to control brain samples were analyzed using commercially available software according to the manufacturer’s instructions (Bio-rad CFX Manager, Bio-rad, USA). Validation QPCR studies were also performed similarly using commercial primer assays for the 9 genes of interest and control Gapdh (PrimePCR, Bio-rad, USA); the top 9 genes among the different categories as described in the main text were selected on the basis of availability of assays, Glis3 was not available and therefore we analyzed Tmem52 as the next representative gene of the category ‘up unique rat’. Validation studies were conducted using hypothalamus obtained from an independent set of male Mecp2ZFN/y (n = 5) and wild-type littermate rats (n = 4) at approximately 6 weeks of life.
Immunofluorescence staining
Fresh frozen tissue was embedded in optimal cutting temperature medium and cut into 25 μm sections onto slides. Tissue was fixed on the slide with 4% paraformaldehyde in phosphate buffered saline (PBS) for 30 min, washed with PBS, permeabilized with 0.3% Triton-X 100 (PBST) for 15 min, and blocked for 2 h in 10% normal goat serum with 0.3% Triton-X 100 in phosphate buffered saline (blocking buffer). Samples were then incubated with primary antibody in blocking buffer overnight, washed with PBST, incubated in secondary antibody in blocking buffer for 4 h, incubated with 0.2 μg/ml DAPI in PBST, and mounted with coverslips using Prolong Gold Antifade Mounting Medium (ThermoFisher Scientific, USA), with wash steps in PBST before each incubation procedure, and final mount step. Primary antibodies used included Chicken anti-MeCP2 (ABE171 diluted 1:500; Millipore, USA), and Rabbit anti-NeuN (ABN78 diluted 1:500; Millipore, USA). Secondary antibodies used included Alexa Fluor conjugated Goat anti-Chicken and Goat anti-Rabbit antibodies (1:500; Jackson ImmunoResearch, USA). Image acquisition was performed using a Zeiss 710 confocal microscope, and prepared with ImageJ as previously described (98). Single animals of each genotype (Mecp2ZFN/y and Mecp2ZFN/+, and respective wild-type littermates, 6 weeks of life) were used to generate the representative images shown in Figure 1; for further quantification of MeCP2 signal intensity and percentage, additional animals were analyzed as described in Supplementary Materials, Text and Figure S3.
Rat neurobehavioural assays
Previous work revealed that young Sprague-Dawley rats exhibited significantly decreased activity under bright light conditions (62), which could interfere with the outcome of behavioural assays. Therefore, all tests were carried out under dim-lighting conditions between 10 and 25 lux as previously described (62). Starting at approximately 24 days of life, rats were subjected to a behavioural battery consisting of the following assays described below. For each assay, rats were habituated to the test room for at least 30 min (60 dB and lux level as indicated below for each test). Behavioural analysis was performed only with female rats in this study. Two separate cohorts of female rats were generated as described above; one cohort consisting of female Mecp2ZFN/+ (n = 12) and wild-type littermate rats (n = 12) for evaluation in the seed opening task, and one cohort consisting of female Mecp2ZFN/+ (n = 9) and wild-type littermate rats (n = 13) for evaluation in elevated circle maze, three chamber test for sociability, direct social interaction, open field activity, acoustic startle response and prepulse inhibition of the startle response, fear conditioning, olfaction and wood block chew test. The cohort tested for multiple behavioural domains was tested first over a period of 6 months total; approximately 1–1.5 year later, the separate cohort tested longitudinally for the seed opening task was tested for a period spanning approximately 6–7 months. For both cohorts, animals were tested in batches as they became available, with 5–6 animals maximum per group. All animals were tested in a randomized fashion; each experiment was performed by the same individual blinded to genotype, including video scoring. In the cohort of animals tested for direct social interaction, all animals were tested; however, four video files were not recovered and only female Mecp2ZFN/+ (n = 10) and wild-type littermate rats (n = 8) were included in the analysis. All other tests that were performed included all animals in the analysis.
Seed opening task
Psychomotor function related to forepaw usage was evaluated by training rats in their ability to open sunflower seeds as previously described (79,99). At 4 weeks of life, in the evening prior to the first day before food deprivation, sunflower seeds were placed in the home cage of rats that were to be tested to expose them to seeds prior to any testing. Animals were food-deprived overnight prior to each training session (approximately 20 h). On training days, animals were subjected to a training period consisting of a 5 min test session performed once per day for four consecutive days. Test subjects were placed in the centre of the cylinder, and were allowed to manipulate and open five sunflower seeds randomly placed in the cylinder. Test subjects were trained in a transparent plexiglass cylinder (22.8 cm, height; 16.5 cm, diameter). A mirror was placed behind the cylinder to facilitate the viewing and scoring of behaviour. At 7 and 9 weeks of life, tests were performed, one single trial per time point, similarly as described above, including overnight food-deprivation; however, if test subjects spent greater than 5 min to manipulate and open seeds during any given test trial, then 300 s was recorded.
Elevated circle maze
At 24 days of life, anxiety-like behaviour was tested on an elevated, circular platform (100) with 2 closed and 2 open regions (39 cm, height; 60 cm, diameter; 6.3 cm, platform width; 15 cm, closed zone wall height; 46 cm, closed zone wall length). Test subjects were placed in one of the open regions and allowed to freely explore the platform. The time spent in the open and closed regions, and behaviours including grooming and rearing were recorded for 10 min in dim lighting conditions (10 lux). Behavioural scoring was performed in real-time by an experimenter blinded to genotype using a hand-held computer (Psion Observer 3, Noldus, USA).
Three chamber test for sociability
At 25 days of life, rats were tested in an apparatus composed of three chambers (two side chamber and a centre chamber with doorways to both side chambers, all of equal dimensions (42.5 cm, length; 17.5 cm, width; 23 cm height). As a modification of the original apparatus (61) to accommodate the juvenile rats, inverted cups were replaced by creating two small chambers within the side chambers as previously described (62). The two small chambers (10 cm, length; 17.5 cm width; 23 cm height) were created with perforated plexiglass partitions that allowed rats to see, hear and smell across the walls. The evaluation of sociability consisted of two 10 min test sessions, a habitation phase and a test phase performed in dim lighting conditions (15 lux). During the habituation phase, each test subject was placed in the centre chamber and allowed to freely explore the apparatus for 10 min. Test subjects were then allowed to return to the centre chamber and the entrances to the side chambers were blocked with plexiglass walls. Either a conspecific novel wild-type partner rat of the same genetic background, age and sex (purchased from SAGE Labs/Horizon, USA), or a novel object (a light grey LEGO block, 6 cm, length; 6 cm, width; 4 cm, height) was immediately placed behind the plexiglass partitions of the side chambers. The walls obstructing the entrances to the chambers were lifted and the test phase was initiated in which test subjects were allowed to freely investigate all three chambers. The entire test was video recorded, and time spent in each chamber (partner side, centre and object side), as well as time spent actively investigating the wild-type partner or novel object (sniffing at the partition) were scored by an experimenter blinded to genotype using a hand-held computer as previously described Psion Observer 3, Noldus, USA).(62).
Direct social interaction
At 27 days of life, test subjects were single-housed in a clean standard rat cage (40.6 cm, length; 20.3 cm, width; 19 cm, height) with bedding for 3 h prior to testing in dim lighting conditions (10 lux). A novel wild-type partner rat of the same genetic background, age and sex (purchased from SAGE Labs/Horizon, USA) was then placed in the clean cage and animals were allowed to freely engage each other for 10 min. Behaviours were video recorded and the number of events and time spent engaged in specific behaviours were quantified by an experimenter blinded to genotype using a hand-held computer (Psion Observer 3, Noldus, USA). The evaluation of distinct behavioural categories was defined according to previous studies of juvenile play behaviour in rats (41,62,63). Contact behaviour was defined as general contact of the test subject (either female Mecp2ZFN/+ rat or wild-type littermate) with the partner rat, such as test subject paw placed on the partner body or face with no additional behavioural activity such as sniffing. Nape/pounce behaviour was defined as the test subject pouncing on the partner rat with activity from the test subject’s face and/or paws directed towards the nape of the partner rat. Wrestling behaviour was defined as events in which both animals push, paw and/or grab each other. Tail tug behaviour was defined as the test subject pulling and/or tugging the partner rat’s tail. Pin behaviour was defined as the test subject standing directly over the partner rat with its dorsal surface touching the test chamber floor, rendering the partner rat immobile. Crawl behaviour was defined as the test subject crawling over and under the partner rat. Sniff behaviour was defined as the test subject sniffing the partner rat during stationary encounters with no additional movement. Follow behaviour was defined as the test subject actively sniffing and following/chasing the partner rat. Additional measurements of behavioural activity were evaluated as control parameters. Self-grooming behaviour was defined as the test subject self-grooming areas of its body and tail with either one or both paws. Non-social passive behaviour was defined as the test subject neither interacting with the partner rat nor participating in active exploratory behaviours. Non-social active behaviour was defined as the test subject generally exploring their test environment without interacting with the partner rat.
Open field activity
At 29 days of life, activity was assessed by measuring the total distance travelled in a plexiglass arena (40 cm, length; 40 cm, width; 30 cm height) equipped with photobeams (Accuscan Instruments, Columbus, OH, USA) for 15 min as previously described (62). Testing was performed under dim lighting conditions (15 lux).
Prepulse Inhibition of the acoustic startle response
At 32 days of life, acoustic startle response (ASR) and prepulse inhibition of the ASR were assessed using the SR-Lab System (San Diego Instruments, San Diego, CA, USA). The apparatus was composed of a sound attenuating chamber, which contained a cylindrical tube where the test subject was placed during testing. Each animal was acclimated to a background white noise of 70 dB for about 5 min prior to the test session. In order to habituate the animals, 10 startle only stimuli were presented at the beginning of each test session as previously described (62). Each test session consisted of 48 trials (six blocks of eight trial types each presented in a pseudo-random order). Each block had a ‘no stimulus’ trial used to measure baseline movement where no sound was presented, a ‘startle only’ trial composed of a 40 ms, 120d B sound burst, ‘prepulse only’ trials (20 ms prepulses of 74, 78 or 82 dB sound) and ‘prepulse inhibition (PPI)’ trials composed of the presentation of one of the three prepulse sounds 100ms prior to the startle stimulus of 120 dB sound. The inter-trial interval ranged from 10–20 s, and the startle response was recorded every 1 ms for 65 ms following the onset of the startle stimulus. Percent PPI of the ASR was calculated as follows: 100 – [(response to acoustic prepulse plus startle stimulus trial/the startle response alone trial) × 100].
Fear conditioning
At 34 and 35 days of life, animals were subjected to classical fear conditioning as previously described (62). Test subjects were conditioned in a chamber (29.2 cm, length; 24.5 cm, width; 21.5 cm, height) composed of a rectangular box with a metal grid floor (Med Associates Inc., St. Albans, VT, USA). Test subjects were placed in the chamber for 2 min, then exposed to one conditioned stimulus-unconditioned stimulus (CS-US) pairing of a 30 s, 80dB sound (CS) followed by a 2 s, 1 mA foot shock (US). Twenty-four hours later, test subjects were evaluated for both context and cued memory. For contextual fear memory, test subjects were placed in the test chamber for 5 min and the time spent freezing was measured. One hour later, cued fear memory was evaluated. Test subjects were placed in a novel test chamber modified with one white opaque plexiglass sheet placed on the floor of the chamber, and a second white opaque plexiglass sheet placed diagonally to divide the chamber into two compartments. Test subjects were placed in one half of the divided chamber, and a small dish containing vanilla extract was placed in the second half of the chamber. The time spent freezing was measured for 3 min without auditory stimulus, followed by 3 min of continuous auditory stimulus. Time spent freezing for both context and cued fear memory was analyzed using automatic detection software (FreezeFrame version 2.0, Actimetrics, Wilmette, IL, USA) and expressed as a percentage.
Olfaction test
At 40 and 41 days of life, test subjects were evaluated for gross olfaction using non-social and social odours. Testing was performed in an open field chamber as described above in dim lighting conditions (15 lux). The floor of the chamber contained 16 holes that were baited with various odours placed in small plastic dishes (2 cm, length; 2 cm, diameter). The holes allowed test subjects to smell but not touch odour cues. On the first day of testing, test subjects were placed in the chamber containing the first non-social odour cue (cilantro paste) in three of the 16 holes. The time spent sniffing the holes containing the non-social odour cue was recorded for 3 min. One of the three holes containing the non-social odour cue was then replaced with a second, novel non-social odour cue (rosemary), and the time spent sniffing the holes was measured for 3 min. The following day, the test was repeated using social odour cues. Test subjects were placed in the chamber containing the first social odour cue (small, plastic dish with bedding material from the test subject’s home cage) in three of the 16 holes, and the time spent sniffing the holes was measured for 3 min. One of the three holes containing the first social odour cue was then replaced with a second, novel social odour cue (small plastic dish with bedding material from a cage of unfamiliar rats of the same sex), and the time spent sniffing the holes was measured for 3 min. The duration of sniffing at all holes was recorded by an experimenter blinded to genotype using a hand-held computer (Psion Observer 3, Noldus, USA). For both non-social and social tests, the time spent sniffing the novel odour was compared with the familiar odour and expressed as a novel odour index (time spent sniffing novel odour/time spent sniffing familiar odour × 100).
Wood block chew test
At 42 days of life, test subjects were single-housed overnight from 16:00 – 09:00 in a clean standard rat cage with a non-toxic, plain wood block (Aspen wood; 0.4 cm, length; 0.2 cm, width; 0.2 cm, height). Wood block mass was recorded before and after the overnight exposure as a measurement of perseverative-like behaviour as previously described (62).
Statistical analyses of behavioural, western and QPCR data
Statistical analyses of data were performed using a commercially available statistical software package (SPSS, version 23), with the exception of seed opening task data. Data from the elevated zero maze, open field, acoustic startle, prepulse inhibition, wood block chew, fear conditioning, olfaction, direct social interaction, and Western blot and QPCR studies were analyzed using a one way analysis of variance (ANOVA) with genotype as a factor. For testing sociability in the three chamber test, data related to time spent actively investigating the partner or object were analyzed using a one way ANOVA with genotype as a factor. Data related to time spent in each chamber (partner chamber, centre, and object chamber) were analyzed using a one way ANOVA with genotype as a factor; the time spent in the centre compartment is shown for illustrative purposes in the graph but not included in the statistical analysis as previously described (60). Seed opening task was analyzed using standard statistical procedures in GraphPad Prism for linear regression analysis, two-way ANOVA and multiple comparison post-hoc testing with Sidak’s procedure.
RNA sequencing and analysis
RNA extraction (n = 4 biological replicates of each genotype, male Mecp2ZFN/y and male wild-type littermate hypothalamus, 6 weeks of life) was performed as described above and 10 µg prepared for pair-end RNA-Sequencing using Illumina HiSeq 2500. All sequencing was performed by the Genomic and RNA Profiling Core at Baylor College of Medicine. For each sample, about 70 to 90 million of 100bp pair-end reads were generated. The raw reads were aligned to the Rattus norvegicus genome (Ensembl RGSC3.4) using TopHat v1.4.1 (101) with default parameters (-r 400 –p 8). The first 10 base-pairs of each read at the 5’-end were trimmed prior to mapping to a reference genome. The mappability for each sample was all above 85%. To prepare the aligned sequence reads into an expression level for differential gene analysis, we employed the free Python program HTSeq (102) for this purpose. The htseq-count function of HTSeq allowed us to accumulate the number of aligned reads that falls under the exons of the gene (union of all the exons of the gene). These read counts obtained are analogous to the expression level of the gene.
Using the obtained raw counts, differential gene analysis was carried out using the DESeq package in the R environment. DESeq includes functions for us to test for gene expression changes between samples in different conditions by the use of the negative binomial distribution and a shrinkage estimator for the distribution’s variance (103). The nbinomTest function of the package was used to test if a gene is significantly differentially expressed. Benjamini-Hochberg False discovery rate (FDR) adjusted p-values are reported. Heatmap of the differentially expressed genes (FDR < 0.05, at least 1.5 fold-changes) were plotted. The plotted expression (z-scores) for each gene was the expression values normalized at the gene level to have a mean of zero and standard deviation of one. The heatmap was drawn based on the normalized expression data clustered by both gene and samples based on Euclidean distance and complete linkage. Gene ontology analysis was carried out with DAVID (http://david.abcc.ncifcrf.gov/) using the mouse gene symbols. The lists of genes were queried in DAVID in order to obtain the biological processes and molecular functions in which the input gene lists are enriched. The –log10(FDR) of the enriched functions were plotted to indicate the significance of the enrichment of each function. Comparisons to existing Mecp2 mouse gene expression datasets (hypothalamus (66,69), cerebellum (67) and amygdala (68)) were made using lists of altered gene sets as provided in previous studies.
Gene Expression Omnibus Accession Number
Supplementary Material
Supplementary Material is available at HMG online.
Acknowledgements
RS and RP conceived the study. RS, RP, SV, YW, DC, SH designed experiments. RS, SV, SH, CW, YW, SS, DC, MP, SGH, JG, LY, AL, and DY performed experiments. RS, SV, DC, CM and SH analyzed all data except RNAseq data. YW and ZL analyzed and curated RNAseq data and comparative gene expression data. CM, JN, and JL provided significant intellectual contribution. RS and SV wrote the manuscript. All authors reviewed the manuscript in its preparation. We thank Drs. David Nelson and Huda Zoghbi for critical reading of the manuscript, and the Baylor College of Medicine (BCM) Genomic and RNA Profiling Core, and BCM Intellectual and Developmental Disabilities Research Center (IDDRC) Neurovisualization and Neurobehavioural Cores for use of facilities.
Conflict of Interest statement. None declared.
Funding
This work was funded by, the International Rett Syndrome Research Foundation (RS), Autism Speaks (RP), and the U.S. National Institutes of Health Grants DP5OD009134 (RS), R01HD062553 (JN), R01NS081913 (JL), R01HD079442 (YW) and U54HD083082 (BCM IDDRC). Human brain tissues were provided by the Harvard Brain Tissue Resource Center, supported by HHSN-271-2013-00030C. RS is a Texas Children’s Hospital Jan and Dan Duncan Neurological Research Institute Fellow. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funding to pay the Open Access publication charges for this article was provided by the NIH Public Access Policy, Division G, Title II, Section 218 of PL 110-161.
References
- 1.Amir R.E., Van den Veyver I.B., Wan M., Tran C.Q., Francke U., Zoghbi H.Y. (1999) Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet., 23, 185–188. [DOI] [PubMed] [Google Scholar]
- 2.Neul J.L., Zoghbi H.Y. (2004) Rett syndrome: a prototypical neurodevelopmental disorder. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry, 10, 118–128. [DOI] [PubMed] [Google Scholar]
- 3.Samaco R.C., Neul J.L. (2011) Complexities of Rett syndrome and MeCP2. J. Neurosci. Off. J. Soc. Neurosci., 31, 7951–7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piton A., Gauthier J., Hamdan F.F., Lafrenière R.G., Yang Y., Henrion E., Laurent S., Noreau A., Thibodeau P., Karemera L., et al. (2011) Systematic resequencing of X-chromosome synaptic genes in autism spectrum disorder and schizophrenia. Mol. Psychiatry, 16, 867–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carney R.M., Wolpert C.M., Ravan S.A., Shahbazian M., Ashley-Koch A., Cuccaro M.L., Vance J.M., Pericak-Vance M.A. (2003) Identification of MeCP2 mutations in a series of females with autistic disorder. Pediatr. Neurol., 28, 205–211. [DOI] [PubMed] [Google Scholar]
- 6.Lam C.W. (2000) Spectrum of mutations in the MECP2 gene in patients with infantile autism and Rett syndrome. J. Med. Genet., 37, 41e–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCarthy S.E., Gillis J., Kramer M., Lihm J., Yoon S., Berstein Y., Mistry M., Pavlidis P., Solomon R., Ghiban E., et al. (2014) De novo mutations in schizophrenia implicate chromatin remodeling and support a genetic overlap with autism and intellectual disability. Mol. Psychiatry, 19, 652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen D., Lazar G., Couvert P., Desportes V., Lippe D., Mazet P., Héron D. (2002) MECP2 mutation in a boy with language disorder and schizophrenia. Am. J. Psychiatry, 159, 148–149. [DOI] [PubMed] [Google Scholar]
- 9.Shibayama A., Cook E.H., Feng J., Glanzmann C., Yan J., Craddock N., Jones I.R., Goldman D., Heston L.L., Sommer S.S. (2004) MECP2 structural and 3′-UTR variants in schizophrenia, autism and other psychiatric diseases: A possible association with autism. Am. J. Med. Genet. B Neuropsychiatr. Genet., 128B, 50–53. [DOI] [PubMed] [Google Scholar]
- 10.Redin C., Gérard B., Lauer J., Herenger Y., Muller J., Quartier A., Masurel-Paulet A., Willems M., Lesca G., El-Chehadeh S., et al. (2014) Efficient strategy for the molecular diagnosis of intellectual disability using targeted high-throughput sequencing. J. Med. Genet., 51, 724–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schönewolf-Greulich B., Tejada M.I., Stephens K., Hadzsiev K., Gauthier J., Brøndum-Nielsen K., Pfundt R., Ravn K., Maortua H., Gener B., et al. (2016) The MECP2 variant c.925C>T (p.Arg309Trp) causes intellectual disability in both males and females without classic features of Rett syndrome. Clin. Genet., 10.1111/cge.12769. [DOI] [PubMed] [Google Scholar]
- 12.Grozeva D., Carss K., Spasic-Boskovic O., Tejada M.I., Gecz J., Shaw M., Corbett M., Haan E., Thompson E., Friend K., et al. (2015) Targeted Next-Generation Sequencing Analysis of 1,000 Individuals with Intellectual Disability. Hum. Mutat., 36, 1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suter B., Treadwell-Deering D., Zoghbi H.Y., Glaze D.G., Neul J.L. (2014) Brief report: MECP2 mutations in people without Rett syndrome. J. Autism Dev. Disord., 44, 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.del Gaudio D., Fang P., Scaglia F., Ward P.A., Craigen W.J., Glaze D.G., Neul J.L., Patel A., Lee J.A., Irons M., et al. (2006) Increased MECP2 gene copy number as the result of genomic duplication in neurodevelopmentally delayed males. Genet. Med. Off. J. Am. Coll. Med. Genet., 8, 784–792. [DOI] [PubMed] [Google Scholar]
- 15.Friez M.J., Jones J.R., Clarkson K., Lubs H., Abuelo D., Bier J.A.B., Pai S., Simensen R., Williams C., Giampietro P.F., et al. (2006) Recurrent infections, hypotonia, and mental retardation caused by duplication of MECP2 and adjacent region in Xq28. Pediatrics, 118, e1687–e1695. [DOI] [PubMed] [Google Scholar]
- 16.Van Esch H., Bauters M., Ignatius J., Jansen M., Raynaud M., Hollanders K., Lugtenberg D., Bienvenu T., Jensen L.R., Gecz J., et al. (2005) Duplication of the MECP2 region is a frequent cause of severe mental retardation and progressive neurological symptoms in males. Am. J. Hum. Genet., 77, 442–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neul J.L. (2012) The relationship of Rett syndrome and MECP2 disorders to autism. Dialogues Clin. Neurosci., 14, 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanchard N.A., Carvalho C.M.B., Bader P., Thome A., Omo-Griffith L., del Gaudio D., Pehlivan D., Fang P., Schaaf C.P., Ramocki M.B., et al. (2012) A partial MECP2 duplication in a mildly affected adult male: a putative role for the 3’ untranslated region in the MECP2 duplication phenotype. BMC Med. Genet., 13, 71.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zappella M., Meloni I., Longo I., Canitano R., Hayek G., Rosaia L., Mari F., Renieri A. (2003) Study of MECP2 gene in Rett syndrome variants and autistic girls. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. Off. Publ. Int. Soc. Psychiatr. Genet., 119B, 102–107. [DOI] [PubMed] [Google Scholar]
- 20.Huppke P., Maier E.M., Warnke A., Brendel C., Laccone F., Gärtner J. (2006) Very mild cases of Rett syndrome with skewed X inactivation. J. Med. Genet., 43, 814–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerr A.M., Archer H.L., Evans J.C., Prescott R.J., Gibbon F. (2006) People with MECP2 mutation-positive Rett disorder who converse. J. Intellect. Disabil. Res. JIDR, 50, 386–394. [DOI] [PubMed] [Google Scholar]
- 22.Renieri A., Mari F., Mencarelli M.A., Scala E., Ariani F., Longo I., Meloni I., Cevenini G., Pini G., Hayek G., et al. (2009) Diagnostic criteria for the Zappella variant of Rett syndrome (the preserved speech variant). Brain Dev., 31, 208–216. [DOI] [PubMed] [Google Scholar]
- 23.Venkateswaran S., McMillan H.J., Doja A., Humphreys P. (2014) Adolescent onset cognitive regression and neuropsychiatric symptoms associated with the A140V MECP2 mutation. Dev. Med. Child Neurol., 56, 91–94. [DOI] [PubMed] [Google Scholar]
- 24.Adegbola A.A., Gonzales M.L., Chess A., LaSalle J.M., Cox G.F. (2009) A novel hypomorphic MECP2 point mutation is associated with a neuropsychiatric phenotype. Hum. Genet., 124, 615–623. [DOI] [PubMed] [Google Scholar]
- 25.Loat C.S., Curran S., Lewis C.M., Duvall J., Geschwind D., Bolton P., Craig I.W. (2008) Methyl-CpG-binding protein 2 polymorphisms and vulnerability to autism. Genes Brain Behav., 7, 754–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guy J., Gan J., Selfridge J., Cobb S., Bird A. (2007) Reversal of neurological defects in a mouse model of Rett syndrome. Science, 315, 1143–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGraw C.M., Samaco R.C., Zoghbi H.Y. (2011) Adult neural function requires MeCP2. Science, 333, 186.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castro J., Mellios N., Sur M. (2013) Mechanisms and therapeutic challenges in autism spectrum disorders: insights from Rett syndrome. Curr. Opin. Neurol., 26, 154–159. [DOI] [PubMed] [Google Scholar]
- 29.Kron M., Howell C.J., Adams I.T., Ransbottom M., Christian D., Ogier M., Katz D.M. (2012) Brain activity mapping in Mecp2 mutant mice reveals functional deficits in forebrain circuits, including key nodes in the default mode network, that are reversed with ketamine treatment. J. Neurosci. Off. J. Soc. Neurosci., 32, 13860–13872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdala A.P.L., Dutschmann M., Bissonnette J.M., Paton J.F.R. (2010) Correction of respiratory disorders in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. U. S. A, 107, 18208–18213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCauley M.D., Wang T., Mike E., Herrera J., Beavers D.L., Huang T.W., Ward C.S., Skinner S., Percy A.K., Glaze D.G., et al. (2011) Pathogenesis of lethal cardiac arrhythmias in Mecp2 mutant mice: implication for therapy in Rett syndrome. Sci. Transl. Med., 3, 113ra125.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Filippis B., Fabbri A., Simone D., Canese R., Ricceri L., Malchiodi-Albedi F., Laviola G., Fiorentini C. (2012) Modulation of RhoGTPases improves the behavioral phenotype and reverses astrocytic deficits in a mouse model of Rett syndrome. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol., 37, 1152–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nag N., Ward B., Berger-Sweeney J.E. (2009) Nutritional factors in a mouse model of Rett syndrome. Neurosci. Biobehav. Rev., 33, 586–592. [DOI] [PubMed] [Google Scholar]
- 34.Landis S.C., Amara S.G., Asadullah K., Austin C.P., Blumenstein R., Bradley E.W., Crystal R.G., Darnell R.B., Ferrante R.J., Fillit H., et al. (2012) A call for transparent reporting to optimize the predictive value of preclinical research. Nature, 490, 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnabel J. (2008) Neuroscience: Standard model. Nature, 454, 682–685. [DOI] [PubMed] [Google Scholar]
- 36.van der Worp H.B., Howells D.W., Sena E.S., Porritt M.J., Rewell S., O’Collins V., Macleod M.R. (2010) Can animal models of disease reliably inform human studies? PLoS Med., 7, e1000245.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott S., Kranz J.E., Cole J., Lincecum J.M., Thompson K., Kelly N., Bostrom A., Theodoss J., Al-Nakhala B.M., Vieira F.G., et al. (2008) Design, power, and interpretation of studies in the standard murine model of ALS. Amyotroph. Lateral Scler. Off. Publ. World Fed. Neurol. Res. Group Mot. Neuron Dis., 9, 4–15. [DOI] [PubMed] [Google Scholar]
- 38.Chadman K.K., Yang M., Crawley J.N. (2009) Criteria for validating mouse models of psychiatric diseases. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. Off. Publ. Int. Soc. Psychiatr. Genet., 150B, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seok J., Warren H.S., Cuenca A.G., Mindrinos M.N., Baker H.V., Xu W., Richards D.R., McDonald-Smith G.P., Gao H., Hennessy L., et al. (2013) Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. U. S. A., 110, 3507–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wöhr M., Scattoni M.L. (2013) Behavioural methods used in rodent models of autism spectrum disorders: current standards and new developments. Behav. Brain Res., 251, 5–17. [DOI] [PubMed] [Google Scholar]
- 41.Pellis S.M., Pellis V.C. (1998) Play fighting of rats in comparative perspective: a schema for neurobehavioral analyses. Neurosci. Biobehav. Rev., 23, 87–101. [DOI] [PubMed] [Google Scholar]
- 42.Rudy J.W. (2009) Context representations, context functions, and the parahippocampal-hippocampal system. Learn. Mem. Cold Spring Harb. N., 16, 573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pellis S.M., Pasztor T.J. (1999) The developmental onset of a rudimentary form of play fighting in C57 mice. Dev. Psychobiol., 34, 175–182. [PubMed] [Google Scholar]
- 44.Carbery I.D., Ji D., Harrington A., Brown V., Weinstein E.J., Liaw L., Cui X. (2010) Targeted genome modification in mice using zinc-finger nucleases. Genetics, 186, 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whishaw I.Q., Kolb B. (2004) The Behavior of the Laboratory Rat Oxford University Press. [Google Scholar]
- 46.Crawley J.N. (2007) Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. Zurich Switz., 17, 448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crawley J.N., Paylor R. (1997) A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Horm. Behav., 31, 197–211. [DOI] [PubMed] [Google Scholar]
- 48.Katz D.M., Berger-Sweeney J.E., Eubanks J.H., Justice M.J., Neul J.L., Pozzo-Miller L., Blue M.E., Christian D., Crawley J.N., Giustetto M., et al. (2012) Preclinical research in Rett syndrome: setting the foundation for translational success. Dis. Model. Mech., 5, 733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samaco R.C., McGraw C.M., Ward C.S., Sun Y., Neul J.L., Zoghbi H.Y. (2013) Female Mecp2(+/-) mice display robust behavioral deficits on two different genetic backgrounds providing a framework for pre-clinical studies. Hum. Mol. Genet., 22, 96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guy J., Hendrich B., Holmes M., Martin J.E., Bird A. (2001) A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat. Genet., 27, 322–326. [DOI] [PubMed] [Google Scholar]
- 51.Stearns N.A., Schaevitz L.R., Bowling H., Nag N., Berger U.V., Berger-Sweeney J. (2007) Behavioral and anatomical abnormalities in Mecp2 mutant mice: a model for Rett syndrome. Neuroscience, 146, 907–921. [DOI] [PubMed] [Google Scholar]
- 52.Nag N., Berger-Sweeney J.E. (2007) Postnatal dietary choline supplementation alters behavior in a mouse model of Rett syndrome. Neurobiol. Dis., 26, 473–480. [DOI] [PubMed] [Google Scholar]
- 53.Schaevitz L.R., Nicolai R., Lopez C.M., D’Iddio S., Iannoni E., Berger-Sweeney J.E. (2012) Acetyl-L-carnitine improves behavior and dendritic morphology in a mouse model of Rett syndrome. PloS One, 7, e51586.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D’Cruz J.A., Wu C., Zahid T., El-Hayek Y., Zhang L., Eubanks J.H. (2010) Alterations of cortical and hippocampal EEG activity in MeCP2-deficient mice. Neurobiol. Dis., 38, 8–16., [DOI] [PubMed] [Google Scholar]
- 55.Wither R.G., Lang M., Zhang L., Eubanks J.H. (2013) Regional MeCP2 expression levels in the female MeCP2-deficient mouse brain correlate with specific behavioral impairments. Exp. Neurol., 239, 49–59. [DOI] [PubMed] [Google Scholar]
- 56.Wither R.G., Colic S., Wu C., Bardakjian B.L., Zhang L., Eubanks J.H. (2012) Daily rhythmic behaviors and thermoregulatory patterns are disrupted in adult female MeCP2-deficient mice. PloS One, 7, e35396.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Glaze D.G. (2004) Rett syndrome: of girls and mice–lessons for regression in autism. Ment. Retard. Dev. Disabil. Res. Rev., 10, 154–158. [DOI] [PubMed] [Google Scholar]
- 58.Neul J.L., Kaufmann W.E., Glaze D.G., Christodoulou J., Clarke A.J., Bahi-Buisson N., Leonard H., Bailey M.E.S., Schanen N.C., Zappella M., et al. (2010) Rett syndrome: revised diagnostic criteria and nomenclature. Ann. Neurol., 68, 944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaufmann W.E., Tierney E., Rohde C.A., Suarez-Pedraza M.C., Clarke M.A., Salorio C.F., Bibat G., Bukelis I., Naram D., Lanham D.C., et al. (2011) Social impairments in Rett syndrome: characteristics and relationship with clinical severity. J. Intellect. Disabil. Res. JIDR, 10.1111/j.1365-2788.2011.01404.x. [DOI] [PubMed] [Google Scholar]
- 60.Yang M., Silverman J.L., Crawley J.N. (2011) Automated three-chambered social approach task for mice. Curr. Protoc. Neurosci. Editor. Board Jacqueline N Crawley Al, Chapter 8, Unit 8.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moy S.S., Nadler J.J., Perez A., Barbaro R.P., Johns J.M., Magnuson T.R., Piven J., Crawley J.N. (2004) Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav., 3, 287–302. [DOI] [PubMed] [Google Scholar]
- 62.Hamilton S.M., Green J.R., Veeraragavan S., Yuva L., McCoy A., Wu Y., Warren J., Little L., Ji D., Cui X., et al. (2014) Fmr1 and Nlgn3 knockout rats: Novel tools for investigating autism spectrum disorders. Behav. Neurosci., 128, 103–109. [DOI] [PubMed] [Google Scholar]
- 63.Vanderschuren L.J.M.J., Trezza V. (2014) What the laboratory rat has taught us about social play behavior: role in behavioral development and neural mechanisms. Curr. Top. Behav. Neurosci., 16, 189–212. [DOI] [PubMed] [Google Scholar]
- 64.Hao S., Tang B., Wu Z., Ure K., Sun Y., Tao H., Gao Y., Patel A.J., Curry D.J., Samaco R.C., et al. (2015) Forniceal deep brain stimulation rescues hippocampal memory in Rett syndrome mice. Nature, 526, 430–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen R.Z., Akbarian S., Tudor M., Jaenisch R. (2001) Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat. Genet., 27, 327–331. [DOI] [PubMed] [Google Scholar]
- 66.Chahrour M., Jung S.Y., Shaw C., Zhou X., Wong S.T.C., Qin J., Zoghbi H.Y. (2008) MeCP2, a key contributor to neurological disease, activates and represses transcription. Science, 320, 1224–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ben-Shachar S., Chahrour M., Thaller C., Shaw C.A., Zoghbi H.Y. (2009) Mouse models of MeCP2 disorders share gene expression changes in the cerebellum and hypothalamus. Hum. Mol. Genet., 18, 2431–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Samaco R.C., Mandel-Brehm C., McGraw C.M., Shaw C.A., McGill B.E., Zoghbi H.Y. (2012) Crh and Oprm1 mediate anxiety-related behavior and social approach in a mouse model of MECP2 duplication syndrome. Nat. Genet., 44, 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen L., Chen K., Lavery L.A., Baker S.A., Shaw C.A., Li W., Zoghbi H.Y. (2015) MeCP2 binds to non-CG methylated DNA as neurons mature, influencing transcription and the timing of onset for Rett syndrome. Proc. Natl. Acad. Sci. U. S. A, 112, 5509–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yasui D.H., Scoles H.A., Horike S.I., Meguro-Horike M., Dunaway K.W., Schroeder D.I., Lasalle J.M. (2011) 15q11.2-13.3 chromatin analysis reveals epigenetic regulation of CHRNA7 with deficiencies in Rett and autism brain. Hum. Mol. Genet., 20, 4311–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li W., Teng F., Li T., Zhou Q. (2013) Simultaneous generation and germline transmission of multiple gene mutations in rat using CRISPR-Cas systems. Nat. Biotechnol., 31, 684–686. [DOI] [PubMed] [Google Scholar]
- 72.Li D., Qiu Z., Shao Y., Chen Y., Guan Y., Liu M., Li Y., Gao N., Wang L., Lu X., et al. (2013) Heritable gene targeting in the mouse and rat using a CRISPR-Cas system. Nat. Biotechnol., 31, 681–683. [DOI] [PubMed] [Google Scholar]
- 73.Ma Y., Ma J., Zhang X., Chen W., Yu L., Lu Y., Bai L., Shen B., Huang X., Zhang L. (2014) Generation of eGFP and Cre knockin rats by CRISPR/Cas9. Febs J., 281, 3779–3790. [DOI] [PubMed] [Google Scholar]
- 74.Ricceri L., De Filippis B., Laviola G. (2008) Mouse models of Rett syndrome: from behavioural phenotyping to preclinical evaluation of new therapeutic approaches. Behav. Pharmacol., 19, 501–517. [DOI] [PubMed] [Google Scholar]
- 75.Traynor J., Agarwal P., Lazzeroni L., Francke U. (2002) Gene expression patterns vary in clonal cell cultures from Rett syndrome females with eight different MECP2 mutations. BMC Med. Genet., 3, 12.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pitcher M.R., Herrera J.A., Buffington S.A., Kochukov M.Y., Merritt J.K., Fisher A.R., Schanen N.C., Costa-Mattioli M., Neul J.L. (2015) Rett syndrome like phenotypes in the R255X Mecp2 mutant mouse are rescued by MECP2 transgene. Hum. Mol. Genet., 24, 2662–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goffin D., Allen M., Zhang L., Amorim M., Wang I.T.J., Reyes A.R.S., Mercado-Berton A., Ong C., Cohen S., Hu L., et al. (2012) Rett syndrome mutation MeCP2 T158A disrupts DNA binding, protein stability and ERP responses. Nat. Neurosci, 15, 274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Filippis B., Musto M., Altabella L., Romano E., Canese R., Laviola G. (2015) Deficient Purposeful Use of Forepaws in Female Mice Modelling Rett Syndrome. Neural Plast., 2015, 326184.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gonzalez C.L.R., Kolb B. (2003) A comparison of different models of stroke on behaviour and brain morphology. Eur. J. Neurosci., 18, 1950–1962. [DOI] [PubMed] [Google Scholar]
- 80.Engineer C.T., Rahebi K.C., Borland M.S., Buell E.P., Centanni T.M., Fink M.K., Im K.W., Wilson L.G., Kilgard M.P. (2015) Degraded neural and behavioral processing of speech sounds in a rat model of Rett syndrome. Neurobiol. Dis., 83, 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Townend G.S., Bartl-Pokorny K.D., Sigafoos J., Curfs L.M.G., Bölte S., Poustka L., Einspieler C., Marschik P.B. (2015) Comparing social reciprocity in preserved speech variant and typical Rett syndrome during the early years of life. Res. Dev. Disabil., 43–44, 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chao H.T., Chen H., Samaco R.C., Xue M., Chahrour M., Yoo J., Neul J.L., Gong S., Lu H.C., Heintz N., et al. (2010) Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature, 468, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Trost B., Moir C.A., Gillespie Z.E., Kusalik A., Mitchell J.A., Eskiw C.H. (2015) Concordance between RNA-sequencing data and DNA microarray data in transcriptome analysis of proliferative and quiescent fibroblasts. R. Soc. Open Sci., 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wan Y.W., Mach C.M., Allen G.I., Anderson M.L., Liu Z. (2014) On the Reproducibility of TCGA Ovarian Cancer MicroRNA Profiles. Plos One, 9, e87782.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Consortium M.A.Q.C., Shi L., Reid L.H., Jones W.D., Shippy R., Warrington J.A., Baker S.C., Collins P.J., de Longueville F., Kawasaki E.S., et al. (2006) The MicroArray Quality Control (MAQC) project shows inter- and intraplatform reproducibility of gene expression measurements. Nat. Biotechnol, 24, 1151–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu J., Gong B., Wu L., Thakkar S., Hong H., Tong W. (2016) Comprehensive Assessments of RNA-seq by the SEQC Consortium: FDA-Led Efforts Advance Precision Medicine. Pharmaceutics, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.SEQC/MAQC-III Consortium(2014) A comprehensive assessment of RNA-seq accuracy, reproducibility and information content by the Sequencing Quality Control Consortium. Nat. Biotechnol, 32, 903–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao Y.T., Goffin D., Johnson B.S., Zhou Z. (2013) Loss of MeCP2 function is associated with distinct gene expression changes in the striatum. Neurobiol. Dis., 59, 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guy J., Cheval H., Selfridge J., Bird A. (2011) The role of MeCP2 in the brain. Annu. Rev. Cell Dev. Biol., 27, 631–652. [DOI] [PubMed] [Google Scholar]
- 90.Sugino K., Hempel C.M., Okaty B.W., Arnson H.A., Kato S., Dani V.S., Nelson S.B. (2014) Cell-type-specific repression by methyl-CpG-binding protein 2 is biased toward long genes. J. Neurosci. Off. J. Soc. Neurosci., 34, 12877–12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gabel H.W., Kinde B., Stroud H., Gilbert C.S., Harmin D.A., Kastan N.R., Hemberg M., Ebert D.H., Greenberg M.E. (2015) Disruption of DNA-methylation-dependent long gene repression in Rett syndrome. Nature, 522, 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Skene P.J., Illingworth R.S., Webb S., Kerr A.R.W., James K.D., Turner D.J., Andrews R., Bird A.P. (2010) Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol. Cell, 37, 457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rube H.T., Lee W., Hejna M., Chen H., Yasui D.H., Hess J.F., LaSalle J.M., Song J.S., Gong Q. (2016) Sequence features accurately predict genome-wide MeCP2 binding in vivo. Nat. Commun., 7, 11025.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nestler E.J., Hyman S.E. (2010) Animal models of neuropsychiatric disorders. Nat. Neurosci., 13, 1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shahbazian M., Young J., Yuva-Paylor L., Spencer C., Antalffy B., Noebels J., Armstrong D., Paylor R., Zoghbi H. (2002) Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron, 35, 243–254. [DOI] [PubMed] [Google Scholar]
- 96.Cassel S., Carouge D., Gensburger C., Anglard P., Burgun C., Dietrich J.B., Aunis D., Zwiller J. (2006) Fluoxetine and cocaine induce the epigenetic factors MeCP2 and MBD1 in adult rat brain. Mol. Pharmacol., 70, 487–492. [DOI] [PubMed] [Google Scholar]
- 97.Nagarajan R.P., Hogart A.R., Gwye Y., Martin M.R., LaSalle J.M. (2006) Reduced MeCP2 expression is frequent in autism frontal cortex and correlates with aberrant MECP2 promoter methylation. Epigenetics off. J. DNA Methylation Soc., 1, e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ward C.S., Arvide E.M., Huang T.W., Yoo J., Noebels J.L., Neul J.L. (2011) MeCP2 is critical within HoxB1-derived tissues of mice for normal lifespan. J. Neurosci. Off. J. Soc. Neurosci., 31, 10359–10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Whishaw I.Q., Sarna J.R., Pellis S.M. (1998) Evidence for rodent-common and species-typical limb and digit use in eating, derived from a comparative analysis of ten rodent species. Behav. Brain Res., 96, 79–91. [DOI] [PubMed] [Google Scholar]
- 100.Shepherd J.K., Grewal S.S., Fletcher A., Bill D.J., Dourish C.T. (1994) Behavioural and pharmacological characterisation of the elevated ‘zero-maze’ as an animal model of anxiety. Psychopharmacology (Berl.), 116, 56–64. [DOI] [PubMed] [Google Scholar]
- 101.Trapnell C., Pachter L., Salzberg S.L. (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinforma. Oxf. Engl., 25, 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Anders S., Pyl P.T., Huber W. (2015) HTSeq–a Python framework to work with high-throughput sequencing data. Bioinforma. Oxf. Engl., 31, 166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Anders S., Huber W. (2010) Differential expression analysis for sequence count data. Genome Biol., 11, R106.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.