Abstract
Sequestration of CO2, either from gas mixtures or directly from air (direct air capture), is a technological goal important to large-scale industrial processes such as gas purification and the mitigation of carbon emissions. Previously, we investigated five porous materials, three porous metal–organic materials (MOMs), a benchmark inorganic material, Zeolite 13X and a chemisorbent, TEPA-SBA-15, for their ability to adsorb CO2 directly from air and from simulated flue-gas. In this contribution, a further 10 physisorbent materials that exhibit strong interactions with CO2 have been evaluated by temperature-programmed desorption for their potential utility in carbon capture applications: four hybrid ultramicroporous materials, SIFSIX-3-Cu, DICRO-3-Ni-i, SIFSIX-2-Cu-i and MOOFOUR-1-Ni; five microporous MOMs, DMOF-1, ZIF-8, MIL-101, UiO-66 and UiO-66-NH2; an ultramicroporous MOM, Ni-4-PyC. The performance of these MOMs was found to be negatively impacted by moisture. Overall, we demonstrate that the incorporation of strong electrostatics from inorganic moieties combined with ultramicropores offers improved CO2 capture performance from even moist gas mixtures but not enough to compete with chemisorbents.
This article is part of the themed issue ‘Coordination polymers and metal–organic frameworks: materials by design’.
Keywords: adsorption, ultramicroporous, physisorption, temperature-programmed desorption
1. Introduction
Anthropogenic emissions of carbon dioxide (CO2) are accepted as a significant risk to global climate. Atmospheric CO2 concentration has surpassed 400 ppm on several occasions since 2013, which represents an increase of over 100 ppm since pre-industrial revolution levels [1]. At the 2015 United Nations Climate Change Conference, the 196 parties in attendance signed an agreement calling for zero net anthropogenic greenhouse gas emissions to be reached during the second half of the twenty-first century [2]. There are two pathways currently being considered for the reduction of CO2 emissions: (i) CO2 removal from CO2-rich post-combustion industrial point sources (i.e. flue-gas capture) and (ii) the removal of CO2 from the atmosphere via direct air capture (DAC) [3]. Although addressing the increase in global CO2 concentrations presents a scientific and technological challenge of the highest order [4], it also presents an opportunity because DAC becomes more viable at higher CO2 levels and CO2 is a useful commodity. Carbon capture and storage technologies focus on capturing and storing CO2. Carbon capture and utilization looks to exploit the large volumes of CO2 produced by industrial practices for use in other applications [5]. For example, CO2 is currently used as a feed gas in the chemical industry for the production of various alcohols [6], dimethyl ether [7], biodiesel [8] and polymers [9]. These large-scale processes are suited to the high volumes of CO2 associated with post-combustion CO2 capture. However, other niche applications such as the use of CO2 gas in greenhouses to encourage photosynthesis or in algae farms to promote biofuels production may be more applicable to smaller scale CO2 capture via DAC technologies. DAC may also be feasible for mitigating emissions from mobile sources and, if recycling costs are minimal, might represent an approach to the introduction of new carbon negative technologies. The catch is that DAC is handicapped by the relative availability of CO2 in the atmosphere (0.0004 atm versus 0.15 atm in post-combustion capture) and, in the case of physisorption, by competition with other gases and vapours, such as N2 and H2O [10]. DAC is therefore much more challenging to physisorbents than post-combustion CO2 capture, but it may be practical if an adsorbent offers optimum uptake, appropriate CO2 selectivity over N2 and H2O and facile recyclability [11].
At present, DAC systems typically employ solid supported amine-based adsorbents, wherein amine functional groups are tethered to the surface of cellulose [12,13], porous polymer networks [14,15] and porous silica materials [10,16–19]. Chemisorption of CO2 is feasible but in order for chemisorbents to be recycled one must reverse the chemical reaction that captures CO2. Therefore, although chemisorbents achieve moderate CO2 adsorption capacities (0.5–3.6 mmol g−1) for DAC, they also typically require elevated temperatures for sorbent regeneration (more than 100°C). It has also been found that gas constituents such as NOx, SOx and CO2 itself can negatively impact amine-modified solids by poisoning the chemisorbent and deactivating the amine adsorption sites [20–22]. Furthermore, amine-modified materials are sometimes subject to thermal and oxidative degradation [23,24]. DAC using physisorbent materials has been much less studied, presumably due to the lack of suitable candidate materials. In principle, advanced sorbents that capture CO2 through highly selective physisorption offer great promise, because they require much less energy for recycling. Unfortunately, existing classes of physisorbent materials do not meet the requirements for DAC, mainly high CO2 selectivity over N2 (SCN) and H2O (SCW), which are major constituents of air.
Recently, we reported a systematic study of DAC performance for a number of benchmark physisorbents [10]; prototypal metal–organic materials (MOMs) HKUST-1 and Mg-MOF-74, a zeolite, Zeolite 13X, and a hybrid ultramicroporous material (HUM) [25], SIFSIX-3-Ni. A highly adsorbent amine-modified chemisorbent material, TEPA-SBA-15, was included in the study for comparison. All four physisorbent materials were observed to exhibit a dramatic decrease in performance with respect to CO2 uptake in the presence of water vapour. SIFSIX-3-Ni exhibited the highest CO2 uptake from DAC of the physisorbent materials at 4.07 l CO2 kg−1 adsorbent. While the chemisorbent TEPA-SBA-15 was unaffected by water vapour, its energetics and recyclability were not as favourable as those of SIFSIX-3-Ni. In this contribution, we examine the performance of 10 additional benchmark physisorbent materials for capturing CO2 from flue-gas and DAC using a combination of temperature-programmed desorption (TPD), thermogravimetric analysis (TGA) and mass spectrometry (MS). SIFSIX-3-Cu [26], DICRO-3-Ni-i [27], MOOFOUR-1-Ni [28], SIFSIX-2-Cu-i [26], Ni-4-PyC [29], ZIF-8 [30], DMOF-1 [31], UiO-66 [32], UiO-66-NH2 [33] and MIL-101 [34] were evaluated for their performance with respect to DAC and five other CO2-rich gas mixtures. The 10 physisorbents studied herein represent two classes of MOMs that have been widely studied for carbon capture (figure 1).
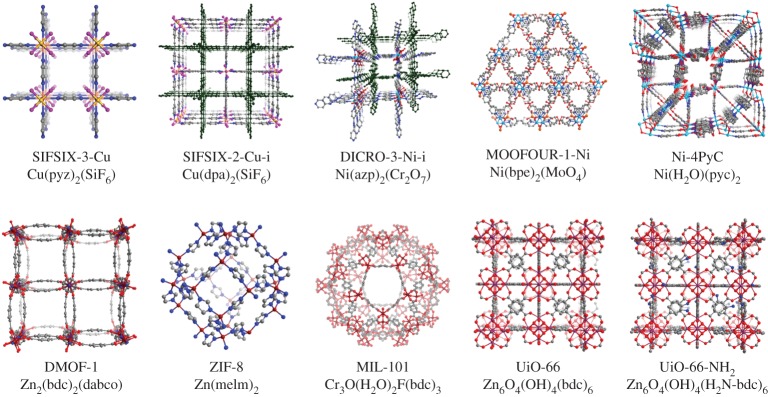
Figure 1.
Ten physisorbent materials were evaluated in this study. Elements C, O, N, Si, F, Cu, Cr, Ni, Mo, Zn and Zr are represented by grey, red, blue, yellow, pink, salmon, dark red, sky blue, orange, brown and purple, respectively; H atoms have been omitted for clarity. The dark green net represents the second interpenetrated network in SIFSIX-2-Cu-i and DICRO-3-Ni-i.
The rich structural and functional diversity of MOMs means that they can be tuned for specific purifications and separations of gas mixtures [35]. SIFSIX-3-Cu [26], SIFSIX-2-Cu-i [26], DICRO-3-Ni-i [27] and MOOFOUR-1-Ni [28] are hybrid ultramicroporous materials (HUMs) [11,25,26,28,36], which exhibit ultramicropores (less than 0.7 nm) and comprise metal cation nodes linked by two types of linkers: neutral organic ligands and anionic inorganic pillars. The use of an appropriately charged inorganic pillar means that the resulting network is uncharged and creates a relatively high electrostatic contribution that, when combined with tight binding sites, enables strong interactions between adsorbent and adsorbate (large Qst) and ultra-high selectivity for polarizable gases such as CO2 versus less polarizable gases such as N2. ZIF-8 [30], DMOF-1 [31], UiO-66 [32], UiO-66-NH2 [33] and MIL-101 [34] are prototypal examples of physisorbent MOMs, also known as metal–organic frameworks, MOFs [37,38], or porous coordination polymers, PCPs [39–41]. Ni-4-PyC [29] is a recently reported example of an ultramicroporous MOM and, being built from a single small ligand; Ni-4-PyC exhibits similar pore dimensions to HUMs (0.35 and 0.48 nm). However, there is a reduced electrostatic contribution due to the lack of an inorganic pillar. These sorbents were synthesized following the literature methods (see the electronic supplementary material). Each of the sorbents was characterized via powder X-ray diffraction (PXRD; electronic supplementary material, figures S3–S12) to verify phase purity. Sorbents were then subjected to solvent exchange and activation using published procedures; details for the exchange process and activation protocols are given in the electronic supplementary material. After activation, each sorbent was subjected to sorption experiments to verify a match with their reported apparent surface area, uptake capacity and isosteric enthalpy of adsorption for CO2 (Isotherms; electronic supplementary material, figures S13–S22).
The CO2 adsorption performance of each physisorbent was evaluated using pristine, activated samples exposed to a specific gas mixture for a prescribed time period before being subjected to TPD. In a typical TPD experiment, a sample was placed in a quartz reactor cell positioned within a tube furnace. This cell was heated to a temperature that promotes expulsion of guest molecules from the host in the presence of He carrier gas. The exhaust gas was continuously monitored using a mass spectrometer. These experiments provide the identity and relative quantity of gases and vapours desorbed by the sample as a function of temperature, or, if temperature is held constant, as a function of time. They also afford an understanding of the energy required for recycling the adsorbent. In short, TPD experiments address relative CO2/H2O uptake and afford at least a qualitative indication of the ease with which the sorbent can be recycled. In conjunction with the DAC experiments where the material was exposed to laboratory atmosphere, the 10 physisorbents were also subjected to TPD–TGA experiments in which each material was exposed to five additional gas mixtures following a protocol previously established [10]. Each of the five gas mixtures was selected to address a different aspect of the sorbent's performance with respect to CO2 sorption. Data from the DAC and TPD–TGA experiments on the 10 adsorbents, as well as the five previously studied materials [10], are presented in table 1 (for full dataset of results, see the electronic supplementary material).
Table 1.
TPD coupled with mass spectrometry.
| DAC (1 atm; 49% RH) |
moist CO2 (0.15 atm; 75% RH)b |
dry CO2 (0.15 atm) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| sorbent | CO2 | H2O | CO2 l kg−1 (l l−1) | SCW | CO2 | H2O | CO2 l kg −1 (l l−1) | SCW | CO2 | CO2 l kg−1 (l l−1) |
| SIFSIX-3-Nia | <8% | >92% | 4.07 | 5.43 | 62% | 38% | 38.69 | 0.27 | 100% | 55.49 |
| (8.0) | (93) | (6.55) | (76) | (46) | (62.29) | (109) | (89.34) | |||
| HKUST-1a | 1% | 99% | 1.07 | 0.63 | 8.5% | >91% | 6.52 | 0.02 | 100% | 35.64 |
| (2.1) | (178) | (0.95) | (12.8) | (137) | (5.78) | (70) | (31.61) | |||
| Mg-MOF-74a | <4% | >96% | 3.21 | 2.60 | 51% | 49% | 34.62 | 0.17 | 100% | 119.64 |
| (6.3) | (171) | (2.94) | (68) | (65) | (31.71) | (235) | (109.59) | |||
| Zeolite 13Xa | 1% | 99% | 0.76 | 0.63 | 22% | 78% | 13.39 | 0.05 | 100% | 71.27 |
| (1.5) | (146) | (0.99) | (26.3) | (93) | (17.54) | (140) | (93.36) | |||
| TEPA-SBA-15a | 93% | 7% | 80.44 | 830 | 92% | 8% | 66.33 | 1.92 | 100% | 77.38 |
| (158) | (12) | n.a.c | (130.3) | (11) | n.a.c | (152) | n.a.c | |||
| SIFSIX-3-Cu | 13.8% | >86% | 7.18 | 10.03 | 65% | 35% | 51.42 | 0.31 | 100% | 60.58 |
| (14.1) | (88) | (11.55) | (101) | (54) | (82.68) | (119) | (97.41) | |||
| DICRO-3-Ni-i | >2% | <97% | 0.97 | 1.47 | <53% | >47% | 9.77 | 0.19 | 100% | 12.17 |
| (1.9) | (80) | (1.40) | (19.2) | (17,2) | (14.08) | (23.9) | (17.54) | |||
| SIFSIX-2-Cu-i | 1% | 99% | <1.00 | 0.63 | 59.5% | 40.5% | 9.21 | 0.24 | 100% | 15.78 |
| (1.6) | (155) | (1.247) | (18.1) | (12.3) | (11.48) | (31) | (19.68) | |||
| MOOFOUR-1-Ni | >5% | <95% | 1.27 | 3.29 | 60% | 40% | 19.85 | 0.25 | 100% | 29.53 |
| (2.5) | (49) | (1.60) | (39) | (26) | (25.01) | (58) | (37.21) | |||
| Ni-4-PyC | 2% | 98% | 1.68 | 1.28 | 61% | 39% | 7.94 | 0.26 | 100% | 13.24 |
| (3.3) | (154) | (1.74) | (15.6) | (10.1) | (8.20) | (26) | (13.68) | |||
| DMOF-1 | >2% | <98% | 1.00 | 1.28 | 56% | 44% | 4.94 | 0.21 | 100% | 8.81 |
| (1.3) | (56) | (0.83) | (9.7) | (7.7) | (4.08) | (17.3) | (7.28) | |||
| ZIF-8 | 23% | 77% | 1.20 | 18.67 | 75% | 25% | 1.27 | 0.50 | 100% | 0.92 |
| (2.3) | (7.6) | (1.10) | (2.5) | (>1) | (1.16) | (1.8) | (0.84) | |||
| MIL-101 | <1% | >99% | <1.00 | <0.63 | 40% | 60% | 5.70 | 0.11 | 100% | 16.85 |
| (<1.0) | (95) | (0.62) | (11.2) | (16.8) | (3.53) | (33.1) | (10.45) | |||
| UiO-66 | <1% | >99% | 0.36 | <0.63 | 29.6% | 70.4% | 4.28 | 0.07 | 100% | 16.19 |
| (0.7) | (195) | (1.45) | (8.4) | (19.6) | (5.30) | (31.8) | (20.04) | |||
| UiO-66-NH2 | <2% | >98% | 5.70 | 1.28 | 46.7% | 53.3% | 26.12 | 0.15 | 100% | 30.04 |
| (11.2) | (237) | (7.38) | (51.3) | (58.5) | (33.83) | (59) | (38.90) | |||
aPreviously reported adsorbents [10].
bWater saturated gas feeds were obtained by bubbling each pure gas through deionized water.
cDensity for TEPA-SBA-15 is indeterminable. Mass of analyte in mg g−1.
2. Results and discussion
In terms of DAC from laboratory atmosphere, SIFSIX-3-Cu exhibits the highest gravimetric uptake of CO2 (7.18 l CO2 kg−1) of the 10 physisorbents examined during this study, outperforming SIFSIX-3-Ni, the top physisorbent from our previous study. SIFSIX-3-Cu was previously found to exhibit a high Qst (56 kJ mol−1) and high CO2 uptake at low partial pressure (1.24 mmol g−1) during single-component CO2 adsorption experiments [11]. SIFSIX-3-Cu exhibits a higher Qst and gravimetric uptake at 400 ppm than SIFSIX-3-Ni (50.8 kJ mol−1, 1.10 mmol g−1 respectively), which explains why the DAC uptake of CO2 for SIFSIX-3-Cu (7.18 l CO2 kg−1) is larger than that of SIFSIX-3-Ni (4.07 l CO2 kg−1). The ultramicroporous pore channel and high electrostatic contribution of the inorganic 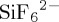 pillar is key to the DAC performance of the SIFSIX-3-M compounds [26]. However, whereas pristine SIFSIX-3-Cu performs best in terms of DAC performance, it was found to be inherently unstable when exposed to elevated temperature and humidity (40°C, 75% RH). Indeed, the PXRD pattern was completely changed (electronic supplementary material, figure S100) and surface area lost (electronic supplementary material, figure S101).
pillar is key to the DAC performance of the SIFSIX-3-M compounds [26]. However, whereas pristine SIFSIX-3-Cu performs best in terms of DAC performance, it was found to be inherently unstable when exposed to elevated temperature and humidity (40°C, 75% RH). Indeed, the PXRD pattern was completely changed (electronic supplementary material, figure S100) and surface area lost (electronic supplementary material, figure S101).
UiO-66-NH2 was observed to exhibit the next highest gravimetric uptake of CO2 (5.7 l CO2 kg−1) of the 10 physisorbents examined during this study. The ‘decoration’ of the terephthalic acid linker with an amine group to form UiO-66-NH2 has a significant effect on the CO2 uptake by this adsorbent compared with the parent material, UiO-66 (DAC < 1.0 l CO2 kg−1), under all adsorption conditions. The reason UiO-66-NH2 outperforms UiO-66 is again correlated with isosteric enthalpy of adsorption (Qst). UiO-66 and UiO-66-NH2 exhibit Qst values of 25.5 kJ mol−1 and 35.1 kJ mol−1, respectively [42,43]. High Qst values are reflective of stronger adsorbate/adsorbent interactions, but they also require higher regeneration energies during the desorption process to liberate the adsorbed CO2 [44]. The improvement in CO2 adsorption and increase in enthalpy of adsorption of UiO-66-NH2 over UiO-66 is attributed to the addition of the highly polar amine group; this in turn increases the affinity of UiO-66-NH2 towards polarizable gases such as CO2 [43,45]. The addition of highly polar ligands also leads to a considerable enhancement of CO2/N2 selectivity (SCN) [46,47]. The affinity of UiO-66-NH2 towards CO2
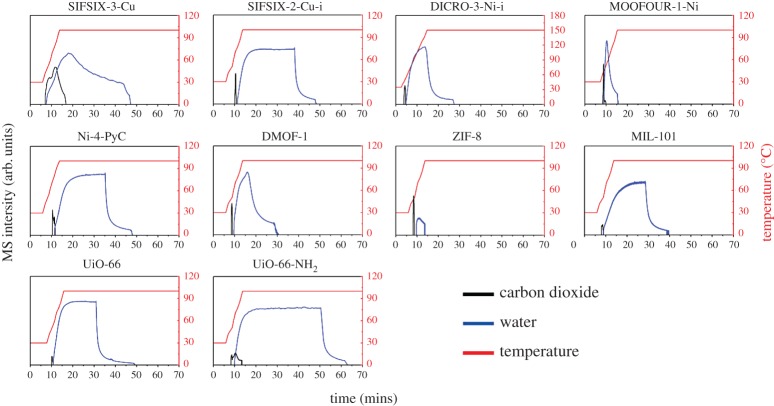
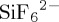 has previously been observed to increase with an increase in the amine density [48]. This phenomenon also has been observed in other amine functionalized MOMs [49–53]. The increase in the amount of CO2 adsorbed may also be as a result of a quasi-chemisorption interaction between CO2 and the functional amino group in UiO-66-NH2, whereby CO2 interacts with the amine to form anhydrous carbamates in the absence of H2O or bicarbonate species under moist conditions as observed in other amine functionalized porous materials [54]. This increased affinity towards CO2 also improves SCW compared with that of the parent UiO-66 material and is supported by TPD experiments, which estimate the relative ease of regeneration of the sorbent. The DAC plot in figure 2 reveals that UiO-66-NH2 requires notably more energy and time to liberate CO2 compared with UiO-66 and the other physisorbents studied herein.
has previously been observed to increase with an increase in the amine density [48]. This phenomenon also has been observed in other amine functionalized MOMs [49–53]. The increase in the amount of CO2 adsorbed may also be as a result of a quasi-chemisorption interaction between CO2 and the functional amino group in UiO-66-NH2, whereby CO2 interacts with the amine to form anhydrous carbamates in the absence of H2O or bicarbonate species under moist conditions as observed in other amine functionalized porous materials [54]. This increased affinity towards CO2 also improves SCW compared with that of the parent UiO-66 material and is supported by TPD experiments, which estimate the relative ease of regeneration of the sorbent. The DAC plot in figure 2 reveals that UiO-66-NH2 requires notably more energy and time to liberate CO2 compared with UiO-66 and the other physisorbents studied herein.
Figure 2.
TPD plots for DAC for the 10 sorbents studied. The red curve depicts the temperature profile used for desorption. The MS signal for CO2 and H2O are given by the black and blue curves, respectively.
Comparing SIFSIX-3-Cu and Ni-4-PyC provides insight into the relative impact of two key aspects of HUMs: the presence of ultramicropores and the strong electrostatics by use of inorganic pillars. The use of ultramicropores alone has been shown to significantly enhance the selective adsorption of H2 over CO2 [55] or CO2 over other gases [17,56,57] via size-selective exclusion. However, size exclusion requires very precise and uniform pore size, which is difficult to design and has only been observed in a few instances. While both adsorbents exhibit similar pore dimensions (3.5 and 4.8 Å for Ni-4-PyC and 3.5 Å for SIFSIX-3-Cu), Ni-4-PyC lacks inorganic pillars (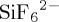 anions), which results in a reduced electrostatic contribution and Qst values (34 versus 56 kJ mol−1) [29]. This, in turn, results in lower CO2 uptake at very low partial pressures such as those in the atmosphere [26]. Ni-4-PyC exhibits lower CO2 uptake (1.68 l CO2 kg−1) from the laboratory atmosphere compared with SIFSIX-3-Cu (7.18 l CO2 kg−1), which further suggests that electrostatics plays an important role in the performance of physisorbent materials in terms of SCN and SCW and that pore size alone does not determine the adsorption performance of ultramicroporous materials.
anions), which results in a reduced electrostatic contribution and Qst values (34 versus 56 kJ mol−1) [29]. This, in turn, results in lower CO2 uptake at very low partial pressures such as those in the atmosphere [26]. Ni-4-PyC exhibits lower CO2 uptake (1.68 l CO2 kg−1) from the laboratory atmosphere compared with SIFSIX-3-Cu (7.18 l CO2 kg−1), which further suggests that electrostatics plays an important role in the performance of physisorbent materials in terms of SCN and SCW and that pore size alone does not determine the adsorption performance of ultramicroporous materials.
SIFSIX-2-Cu-i and MOOFOUR-1-Ni performed only marginally better than the non-HUM physisorbents. While MOOFOUR-1-Ni (1.27 l CO2 kg−1) has a very high Qst (56 kJ mol−1), its larger pores (ca 7 Å) are borderline supermicroporous [36]. Comparing SIFSIX-2-Cu-i (less than 1.0 l CO2 kg−1) with SIFSIX-3-Cu, we see the former has a somewhat larger pore size (ca 5 Å), but also a lower density of inorganic pillars than the latter due to the interpenetrated nature of this compound. These two factors combine to create a lower Qst (31.9 kJ mol−1) for SIFSIX-2-Cu-i and presumably account for the decreased DAC performance. Of the non-HUM physisorbents in this study, UiO-66-NH2 aside, ZIF-8 performed the best, adsorbing 1.2 l CO2 kg−1 from the laboratory atmosphere. DMOF-1, MIL-101 and UiO-66 all adsorbed less than 1.0 l CO2 kg−1 under these conditions.
When the materials were exposed to simulated flue-gas (moist 0.15 atm CO2/0.85 atm N2), SIFSIX-3-Cu was again the top performer of the 10 adsorbents studied herein in terms of gravimetric CO2 uptake, adsorbing 51.42 l CO2 kg−1. This is comparable to SIFSIX-3-Ni and Mg-MOF-74, which were previously found to adsorb 38.69 l CO2 kg−1 and 34.62 l CO2 kg−1, respectively [10]. UiO-66-NH2 (26.12 l CO2 kg−1), MOOFOUR-1-Ni (19.85 l CO2 kg−1) and Ni-4-PyC (12.57 l CO2 kg−1) were the next best materials under simulated flue-gas conditions.
The addition of moisture to the gas stream significantly impacts CO2 uptake by the physisorbent materials studied herein. The gravimetric CO2 uptake from moist simulated flue-gas was reduced by up to 62% when compared with dry flue-gas results obtained for the 10 physisorbents examined in this study. The presence of water vapour in CO2 containing gas streams can have a detrimental effect on physisorbent materials both in terms of CO2 adsorption performance [10,58] and overall stability of the adsorbent [59–62]. The selectivity of sorbent materials for CO2 over H2O (SCW) is an important aspect in determining the suitability of sorbent materials for CO2 capture via post-combustion and DAC methods. From the current study, ZIF-8 performed best in terms of SCW for both DAC (SCW ∼ 18.7) and moist simulated flue-gas (SCW ∼ 0.5). ZIF frameworks are inherently hydrophobic as long as the imidazolate linkers do not contain hydrophilic functional groups [63–65]. Despite the high SCW for ZIF-8, it exhibits low overall gravimetric CO2 uptake for both DAC (1.2 l CO2 kg−1) and simulated flue-gas (1.27 l CO2 kg−1). SIFSIX-3-Cu was the next best physisorbent in terms of CO2/H2O selectivity (DAC SCW ∼ 10.03), which is almost double that of our previous benchmark physisorbent, SIFSIX-3-Ni (DAC SCW ∼ 5.43).
The regeneration performance of the 10 materials studied in this contribution was also examined using TPD experiments (figure 2). The results of these experiments correlates well with the isosteric enthalpy of adsorption (Qst) determined from pure CO2 adsorption isotherms. However, while pure gas isotherms can be used as an indicator of a material's likely ability to selectively adsorb CO2 over competing gases such as N2, TPD studies are necessary to examine the adsorption performance of MOMs when exposed to specific adsorption conditions such as atmosphere and simulated flue-gas conditions. The results of TPD experiments illustrate that water competition is a significant issue when carrying out adsorption studies under humid conditions on physisorbent materials. Consequently, chemisorbents are still the current benchmark materials for CO2 capture via DAC, with CO2 uptakes of up to 80.44 l CO2 kg−1 reported in previous studies [10,12,16]. TEPA-SBA-15 exhibits the highest SCW under all adsorption conditions. However, as mentioned previously, chemisorbent materials can suffer from a high-energy penalty in terms of sorbent regeneration.
3. Conclusion
Capture of CO2 either from flue-gas or directly from air presents a challenge but also an opportunity to play a significant role in tackling greenhouse gases such as CO2 over the coming century. In this contribution, we examine the use of benchmark MOMs for their potential use in CO2 adsorption processes under humid conditions, particularly DAC and moist-simulated flue-gas. Competition with water vapour was found to significantly reduce the CO2 adsorption performance of the physisorbent materials compared with anhydrous conditions. However, there was quite a wide range in performance, with both pore size and pore chemistry affecting the performance of physisorbents studied herein. Humid conditions exacerbated the situation and even wider ranges of uptakes and selectivity were observed. The functionalization of organic ligands with hydrophobic decoration, such as methyl groups in the case of ZIF-8, may be an approach that could be used to improve SCW of physisorbents. However, our results indicate that increased electrostatics generated by inorganic pillars in HUMs or grafted amines are most effective at improving Qst and overall CO2 adsorption performance. In conclusion, competition with water vapour is a significant challenge for implementation of physisorbent materials in CO2 capture, either from DAC or from flue-gas. Control of pore size and pore chemistry through crystal engineering may be a successful strategy to improve CO2 capture performance even in the presence of water vapour and must be further addressed if physisorbents are to compete with chemisorbents in terms of uptake. However, the best physisorbents studied herein were found to be much easier to recycle than the benchmark chemisorbent TEPA-15-SBA, suggesting that faster and less energy intensive recycling of physisorbents could compensate for the lower uptake values.
Supplementary Material
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
D.G.M., J.J.P. and M.J.Z. designed project; A.B. performed ligand synthesis; K.J.C., A.K., H.S.S., R.S. and D.G.M. performed MOMs synthesis; K.J.C., J.J.P. and M.L. performed sorption data collection and analysis; D.G.M. and T.C. performed temperature-programmed desorption; all authors contributed in manuscript writing and gave final approval for publication; M.J.Z. and T.C. performed project supervision.
Competing interests
We have no competing interests.
Funding
M.J.Z., J.J.P., D.G.M., H.S.S., A.B., A.K., R.S., M.L. and K.J.C. are funded by the Science Foundation Ireland (SFI award 13/RP/B2549). T.C. is funded through Science Foundation Ireland for the Solar Research Cluster (SRC) programme (07/SRC/B1160).
References
- 1.Monastersky R. 2013. Global carbon dioxide levels near worrisome milestone. Nature 497, 13–14. ( 10.1038/497013a) [DOI] [PubMed] [Google Scholar]
- 2.UNFCCC. 2015. Adoption of the Paris agreement. Paris: United Nations; FCCC/CP/2015/L.9/Rev.1 [Google Scholar]
- 3.Goeppert A, Czaun M, Surya Prakash GK, Olah GA. 2012. Air as the renewable carbon source of the future: an overview of CO2 capture from the atmosphere. Energ. Environ. Sci. 5, 7833–7853. ( 10.1039/C2EE21586A) [DOI] [Google Scholar]
- 4.Kitagawa S. 2015. Porous materials and the age of gas. Angew. Chem. Int. Ed. 54, 10 686–10 687. ( 10.1002/anie.201503835) [DOI] [PubMed] [Google Scholar]
- 5.Markewitz P, Kuckshinrichs W, Leitner W, Linssen J, Zapp P, Bongartz R, Schreiber A, Muller TE. 2012. Worldwide innovations in the development of carbon capture technologies and the utilization of CO2. Energ. Environ. Sci. 5, 7281–7305. ( 10.1039/C2EE03403D) [DOI] [Google Scholar]
- 6.Centi G, Perathoner S. 2009. Opportunities and prospects in the chemical recycling of carbon dioxide to fuels. Catal. Today 148, 191–205. ( 10.1016/j.cattod.2009.07.075) [DOI] [Google Scholar]
- 7.Olah GA, Goeppert A, Prakash GKS. 2009. Chemical recycling of carbon dioxide to methanol and dimethyl ether: from greenhouse gas to renewable, environmentally carbon neutral fuels and synthetic hydrocarbons. J. Organic Chem. 74, 487–498. ( 10.1021/jo801260f) [DOI] [PubMed] [Google Scholar]
- 8.Schenk PM, Thomas-Hall SR, Stephens E, Marx UC, Mussgnug JH, Posten C, Kruse O, Hankamer B. 2008. Second generation biofuels: high-efficiency microalgae for biodiesel production. BioEnergy Res. 1, 20–43. ( 10.1007/s12155-008-9008-8) [DOI] [Google Scholar]
- 9.Qin Y, Sheng X, Liu S, Ren G, Wang X, Wang F. 2015. Recent advances in carbon dioxide based copolymers. J. CO2 Utilization 11, 3–9. ( 10.1016/j.jcou.2014.10.003) [DOI] [Google Scholar]
- 10.Kumar A, Madden DG, Lusi M, Chen K-J, Daniels EA, Curtin T, Perry JJ, Zaworotko MJ. 2015. Direct air capture of CO2 by physisorbent materials. Angew. Chem. Int. Ed. 54, 14 372–14 377. ( 10.1002/anie.201506952) [DOI] [PubMed] [Google Scholar]
- 11.Shekhah O, Belmabkhout Y, Chen Z, Guillerm V, Cairns A, Adil K, Eddaoudi M. 2014. Made-to-order metal-organic frameworks for trace carbon dioxide removal and air capture. Nat. Commun. 5, 4228 ( 10.1038/ncomms5228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sehaqui H, Gálvez ME, Becatinni V, CHENG Ng Y, Steinfeld A, Zimmermann T, Tingaut P. 2015. Fast and reversible direct CO2 capture from Air onto all-polymer nanofibrillated cellulose-polyethylenimine foams. Environ. Sci. Technol. 49, 3167–3174. ( 10.1021/es504396v) [DOI] [PubMed] [Google Scholar]
- 13.Gebald C, Wurzbacher JA, Tingaut P, Steinfeld A. 2013. Stability of amine-functionalized cellulose during temperature-vacuum-swing cycling for CO2 capture from air. Environ. Sci. Technol. 47, 10 063–10 070. ( 10.1021/es401731p) [DOI] [PubMed] [Google Scholar]
- 14.Lu W, Sculley JP, Yuan D, Krishna R, Zhou H-C. 2013. Carbon dioxide capture from air using amine-grafted porous polymer networks. J. Phys. Chem. C. 117, 4057−4061. ( 10.1021/jp311512q) [DOI] [Google Scholar]
- 15.Wang J, Wang M, Li W, Qiao W, Long D, Ling L. 2015. Application of polyethylenimine-impregnated solid adsorbents for direct capture of low-concentration CO2. AIChE 61, 972–980. ( 10.1002/aic.14679) [DOI] [Google Scholar]
- 16.Alkhabbaz MA, Bollini P, Foo GS, Sievers C, Jones CW. 2014. Important roles of enthalpic and entropic contributions to CO2 capture from simulated flue gas and ambient air using mesoporous silica grafted amines. J. Am. Chem. Soc. 136, 13 170–13 173. ( 10.1021/ja507655x) [DOI] [PubMed] [Google Scholar]
- 17.Goeppert A, Zhang H, Czaun M, May RB, Surya Prakash GK, Olah GA, Narayanan SR. 2014. Easily regenerable solid adsorbents based on polyamines for carbon dioxide capture from the air. ChemSusChem. 7, 1386–1397. ( 10.1002/cssc.201301114) [DOI] [PubMed] [Google Scholar]
- 18.Kulkarni AR, Sholl DS. 2012. Analysis of equilibrium-based TSA processes for direct capture of CO2 from air. Ind. Eng. Chem. Res. 51, 8631–8645. ( 10.1021/ie300691c) [DOI] [Google Scholar]
- 19.Chaikittisilp W, Lunn JD, Shantz DF, Jones CW. 2011. Poly(L-lysine) brush–mesoporous silica hybrid material as a biomolecule-based adsorbent for CO2 capture from simulated flue gas and air. Chem. Euro. J. 17, 10 556–10 561. ( 10.1002/chem.201101480) [DOI] [PubMed] [Google Scholar]
- 20.Hallenbeck AP, Kitchin JR. 2013. Effects of O2 and SO2 on the capture capacity of a primary-amine based polymeric CO2 sorbent. Ind. Eng. Chem. Res. 52, 10 788–10 794. ( 10.1021/ie400582a) [DOI] [Google Scholar]
- 21.Sayari A, Heydari-Gorji A, Yang Y. 2012. CO2-induced degradation of amine-containing adsorbents: reaction products and pathways. J. Am. Chem. Soc. 134, 13 834–13 842. ( 10.1021/ja304888a) [DOI] [PubMed] [Google Scholar]
- 22.Sayari A, Belmabkhout Y, Da'na E. 2012. CO2 deactivation of supported amines: does the nature of amine matter? Langmuir 28, 4241–4247. ( 10.1021/la204667v) [DOI] [PubMed] [Google Scholar]
- 23.Heydari-Gorji A, Sayari A. 2012. Thermal, oxidative, and CO2-induced degradation of supported polyethylenimine adsorbents. Ind. Eng. Chem. Res. 51, 6887–6894. ( 10.1021/ie3003446) [DOI] [Google Scholar]
- 24.Heydari-Gorji A, Belmabkhout Y, Sayari A. 2011. Degradation of amine-supported CO2 adsorbents in the presence of oxygen-containing gases. Micropor. Mesopor. Mater. 145, 146–149. ( 10.1016/j.micromeso.2011.05.010) [DOI] [Google Scholar]
- 25.Scott HS, Bajpai A, Chen K-J, Pham T, Space B, Perry JJ, Zaworotko MJ. 2015. Novel mode of 2-fold interpenetration observed in a primitive cubic network of formula [Ni(1,2-bis(4-pyridyl)acetylene)2(Cr2O7)]n. Chem. Commun. 51, 14 832–14 835. ( 10.1039/c5cc05866) [DOI] [PubMed] [Google Scholar]
- 26.Nugent P, et al. 2013. Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation. Nature 495, 80–84. ( 10.1038/nature11893) [DOI] [PubMed] [Google Scholar]
- 27.Scott HS, et al. 2016. Crystal engineering of a family of hybrid ultramicroporous materials based upon interpenetration and dichromate linkers. Chem. Sci. 7, 5470–5476. ( 10.1039/C6SC01385F) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohamed MH, Elsaidi SK, Wojtas L, Pham T, Forrest KA, Tudor B, Space B, Zaworotko MJ. 2012. Highly selective CO2 uptake in uninodal 6-connected ‘mmo’ nets based upon MO42– (M=Cr, Mo) pillars. J. Am. Chem. Soc. 134, 19 556–19 559. ( 10.1021/ja309452y) [DOI] [PubMed] [Google Scholar]
- 29.Nandi S, De Luna P, Daff TD, Rother J, Liu M, Buchanan W, Hawari AI, Woo TK, Vaidhyanathan R. 2015. A single-ligand ultra-microporous MOF for precombustion CO2 capture and hydrogen purification. Sci. Adv. 1, e1500421 ( 10.1126/sciadv.1500421) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park KS, Ni Z, Xe PA, Choi JY, Huang R, Uribe-Romo FJ, Chae HK, O'Keeffe M, Yaghi O. 2006. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl Acad. Sci. USA 103, 10 186–10 191. ( 10.1073/pnas.0602439103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Tanabe KK, Cohen SM. 2009. Accessing postsynthetic modification in a series of metal-organic frameworks and the influence of framework topology on reactivity. Inorg. Chem. 48, 296–306. ( 10.1021/ic801837t) [DOI] [PubMed] [Google Scholar]
- 32.Cavka JH, Jakobsen S, Olsbye U, Guillou N, Lamberti C, Bordiga S, Lillerud KP. 2008. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 130, 13 850–13 851. ( 10.1021/ja8057953) [DOI] [PubMed] [Google Scholar]
- 33.Kandiah M, et al. 2010. Synthesis and stability of tagged UiO-66 Zr-MOFs. Chem.Mater. 22, 6632–6640. ( 10.1021/cm102601v) [DOI] [Google Scholar]
- 34.Férey G, Mellot-Draznieks C, Serre C, Millange F, Dutour J, Surblé S, Margiolaki I. 2005. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 309, 2040–2042. ( 10.1126/science.1116275) [DOI] [PubMed] [Google Scholar]
- 35.Sapchenko SA, Dybtsev DN, Samsonenko DG, Belosludov RV, Belosludov VR, Kawazoe Y, Schroder M, Fedin VP. 2015. Selective gas adsorption in microporous metal-organic frameworks incorporating urotropine basic sites: an experimental and theoretical study. Chem. Commun. 51, 13 918–13 921. (10.1039doi:/C5CC05779E) [DOI] [PubMed] [Google Scholar]
- 36.Mohamed MH, Elsaidi SK, Pham T, Forrest KA, Tudor B, Wojtas L, Space B, Zaworotko MJ. 2013. Pillar substitution modulates CO2 affinity in ‘mmo’ topology networks. Chem. Commun. 49, 9809–9811. ( 10.1039/C3CC44745F) [DOI] [PubMed] [Google Scholar]
- 37.Farrusseng D. 2011. Metal-organic frameworks: applications from catalysis to gas storage, pp. 269–308. Weinheim, Germany: Wiley-VCH. [Google Scholar]
- 38.MacGillivray LR. 2010. Metal-organic frameworks: design and applications. Hoboken, NJ: Wiley. [Google Scholar]
- 39.Foo ML, Matsuda R, Kitagawa S. 2014. Functional hybrid porous coordination polymers. Chem. Mater. 26, 310–322. ( 10.1021/cm402136z) [DOI] [Google Scholar]
- 40.Kitagawa S, Uemura K. 2005. Dynamic porous properties of coordination polymers inspired by hydrogen bonds. Chem. Soc. Rev. 34, 109–119. ( 10.1039/b313997m) [DOI] [PubMed] [Google Scholar]
- 41.Kitagawa S, Kitaura R, Noro S. 2004. Functional porous coordination polymers. ACIE 43, 2334–2375. ( 10.1002/anie.200300610) [DOI] [PubMed] [Google Scholar]
- 42.Huang Y, Qin W, Li Z, Li Y. 2012. Enhanced stability and CO2 affinity of a UiO-66 type metal-organic framework decorated with dimethyl groups. Dalton Trans. 41, 9283–9285. ( 10.1039/C2DT30950E) [DOI] [PubMed] [Google Scholar]
- 43.Cmarik GE, Kim M, Cohen SM, Walton KS. 2012. Tuning the adsorption properties of UiO-66 via ligand functionalization. Langmuir 28, 15 606–15 613. ( 10.1021/la3035352) [DOI] [PubMed] [Google Scholar]
- 44.Jones CW. 2011. CO2 capture from dilute gases as a component of modern global carbon management. Annu. Rev. Chem. Biomol. Eng. 2, 31–52. ( 10.1146/annurev-chembioeng-061010-114252) [DOI] [PubMed] [Google Scholar]
- 45.Yang W, et al. 2012. Selective CO2 uptake and inverse CO2/C2H2 selectivity in a dynamic bifunctional metal-organic framework. Chem. Sci. 3, 2993–2999. ( 10.1039/C2SC20443F) [DOI] [Google Scholar]
- 46.Bae Y-S, Farha OK, Hupp JT, Snurr RQ. 2009. Enhancement of CO2/N2 selectivity in a metal-organic framework by cavity modification. J. Mater. Chem. 19, 2131–2134. ( 10.1039/B900390H) [DOI] [Google Scholar]
- 47.Deria P, Li S, Zhang H, Snurr RQ, Hupp JT, Farha OK. 2015. A MOF platform for incorporation of complementary organic motifs for CO2 binding. Chem. Commun. 51, 12 478–12 481. ( 10.1039/C5CC04808G) [DOI] [PubMed] [Google Scholar]
- 48.Ethiraj J, Albanese E, Civalleri B, Vitillo JG, Bonino F, Chavan S, Shearer G, Lillerud KP, Bordiga S. 2014. Carbon dioxide adsorption in amine-functionalized mixed-ligand metal–organic frameworks of UiO-66 topology. ChemSusChem. 7, 3382–3388. ( 10.1002/cssc.201402694) [DOI] [PubMed] [Google Scholar]
- 49.Maity DK, Halder A, Bhattacharya B, Das A, Ghoshal D. 2016. Selective CO2 Adsorption by nitro functionalized metal organic frameworks. Cryst. Growth. Des. 16, 1162–1167. ( 10.1021/acs.cgd.5b01686) [DOI] [Google Scholar]
- 50.Zhao Y, et al. 2011. Enhancing gas adsorption and separation capacity through ligand functionalization of microporous metal–organic framework structures. Chem. Euro. J. 17, 5101–5109. ( 10.1002/chem.201002818) [DOI] [PubMed] [Google Scholar]
- 51.Stavitski E, Pidko EA, Couck S, Remy T, Hensen EJM, Weckhuysen BM, Denayer J, Gascon J, Kapteijn F. 2011. Complexity behind CO2 Capture on NH2-MIL-53(Al). Langmuir 27, 3970–3976. ( 10.1021/la1045207) [DOI] [PubMed] [Google Scholar]
- 52.Sumida K, Rogow DL, Mason JA, McDonald TM, Bloch ED, Herm ZR, Bae T-H, Long JR. 2012. Carbon dioxide capture in metal–organic frameworks. Chem. Rev. 112, 724–781. ( 10.1021/cr2003272). [DOI] [PubMed] [Google Scholar]
- 53.Vitillo JG, Savonnet M, Ricchiardi G, Bordiga S. 2011. Tailoring metal–organic frameworks for CO2 capture: the amino effect. ChemSusChem. 4, 1281–1290. ( 10.1002/cssc.201000458) [DOI] [PubMed] [Google Scholar]
- 54.Zhang H, Goeppert A, Prakash GKS, Olah G. 2015. Applicability of linear polyethylenimine supported on nano-silica for the adsorption of CO2 from various sources including dry air. RSC Adv. 5, 52 550–52 562. ( 10.1039/C5RA05428A) [DOI] [Google Scholar]
- 55.Peng Y, Li YS, Ban YJ, Jin H, Jiao WM, Liu XL, Yang WS. 2014. Metal-organic framework nanosheets as building blocks for molecular sieving membranes. Science 346, 1356–1359. ( 10.1126/science.1254227) [DOI] [PubMed] [Google Scholar]
- 56.Dybtsev DN, Chun H, Yoon SH, Kim D, Kim K. 2004. Microporous manganese formate: a simple metal-organic porous material with high framework stability and highly selective gas sorption properties. J. Am. Chem. Soc. 126, 32–33. ( 10.1021/ja038678c) [DOI] [PubMed] [Google Scholar]
- 57.McCormick LJ, Duyker SG, Thornton AW, Hawes CS, Hill MR, Peterson VK, Batten SR, Turner DR. 2014. Ultramicroporous MOF with high concentration of vacant CuII sites. Chem. Mater. 26, 4640–4646. ( 10.1021/cm502189c) [DOI] [Google Scholar]
- 58.Mason JA, McDonald TM, Bae T-H, Bachman JE, Sumida K, Dutton JJ, Kaye SS, Long JR. 2015. Application of a high-throughput analyzer in evaluating solid adsorbents for post-combustion carbon capture via multicomponent adsorption of CO2, N2, and H2O. J. Am. Chem. Soc. 137, 4787–4803. ( 10.1021/jacs.5b00838) [DOI] [PubMed] [Google Scholar]
- 59.Jasuja H, Jiao Y, Burtch NC, Huang Y-G, Walton KS. 2014. Synthesis of cobalt-, nickel-, copper-, and zinc-based, water-stable, pillared metal–organic frameworks. Langmuir. 30, 14 300–14 307. ( 10.1021/la503269f) [DOI] [PubMed] [Google Scholar]
- 60.Burtch NC, Jasuja H, Walton KS. 2014. Water stability and adsorption in metal–organic frameworks. Chem. Rev. 114, 10 575–10 612. ( 10.1021/cr5002589) [DOI] [PubMed] [Google Scholar]
- 61.Schoenecker PM, Carson CG, Jasuja H, Flemming CJJ, Walton KS. 2012. Effect of water adsorption on retention of structure and surface area of metal–organic frameworks. Ind. Eng. Chem. Res. 51, 6513–6519. ( 10.1021/ie202325p) [DOI] [Google Scholar]
- 62.Canivet J, Fateeva A, Guo Y, Coasne B, Farrusseng D. 2014. Water adsorption in MOFs: fundamentals and applications. Chem. Soc. Rev. 43, 5594–5617. ( 10.1039/c4cs00078a) [DOI] [PubMed] [Google Scholar]
- 63.Zhang K, Lively RP, Zhang C, Chance RR, Koros WJ, Sholl DS, Nair S. 2013. Exploring the framework hydrophobicity and flexibility of ZIF-8: From biofuel recovery to hydrocarbon separations. J. Phys. Chem. Lett. 4, 3618–3622. ( 10.1021/jz402019d) [DOI] [Google Scholar]
- 64.Zhang K, Lively RP, Zhang C, Koros WJ, Chance RR. 2013. Investigating the intrinsic ethanol/water separation capability of ZIF-8: an adsorption and diffusion study. J. Phys. Chem. C. 117, 7214–7225. ( 10.1021/jp401548b) [DOI] [Google Scholar]
- 65.Zhang K, Lively RP, Dose ME, Brown AJ, Zhang C, Chung J, Nair S, Koros WJ, Chance RR. 2013. Alcohol and water adsorption in zeolitic imidazolate frameworks. Chem. Commun. 49, 3245–3247. ( 10.1039/C3CC39116G) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.