Abstract
The reaction of ZrCl4 and 2,5-thiophenedicarboxylic acid (H2tdc) in the presence of trifluoroacetic acid (Htfa) as modulator results in the formation of the new metal–organic framework (MOF) named DUT-126 (DUT = Dresden University of Technology). The nature and concentration of modulators are found to be decisive synthetic parameters affecting the topology of the formed product. DUT-126 (hbr) extends the series of polymorphs differing in topology, namely DUT-67 (reo), DUT-68 (bon) and DUT-69 (bct) to four, where DUT-67 and DUT-68 show the same eight-connected secondary building units as in DUT-126. In DUT-126, linker molecules have a peculiar orientation, resulting in hbr topology, which is described for the first time in this work for MOFs. DUT-126 contains three pore types, including two micropores surrounding mesoporous channels. DUT-126 is stable against hydrolysis and features permanent porosity with a specific surface area of 1297 m2 g−1 and a total pore volume of 0.48 cm3 g−1, calculated from the nitrogen physisorption isotherm measured at 77 K.
This article is part of the themed issue ‘Coordination polymers and metal–organic frameworks: materials by design’.
Keywords: zirconium, metal–organic framework, topology, polymorphism, 2, 5-thiophenedicarboxylic acid
1. Introduction
Since their discovery in 2008 [1], Zr-based metal–organic frameworks (MOFs) have become highly emergent materials combining advantages of various classes of porous solids [2]. For example, the well-defined crystal structures of MOFs, or rather the crystallographically defined pore sizes and their tunable pore-design, allow their use in separation processes based on molecular sieving [3,4]. The functionalization possibilities for both organic ligand and metal cluster in combination with high porosity opens a wide action field for catalysis [5–8]. Owing to the high affinity of the Zr4+-cation to oxygen, the chemical and thermal stability of the produced carboxylate-based frameworks is higher in comparison to other MOFs [9]. In combination with facile functionalization, this opens a new application field in adsorption-based heat transformation processes [10].
In particular, materials with reduced cluster connectivity [10–32] are of great interest because functionalization can be directed to the SBU site increasing catalytic activity, topological diversity and influencing in this way polarity and, as a consequence, hydrophilicity of the material [3].
For example, in the Zr-ndc (ndc = 2,6-naphthalene dicarboxylate) system, the connectivity of the cluster, as well as topology of the framework could be affected by the amount of modulator (acetic acid) used in the synthesis. Consequently, numerous crystal structures with three-dimensional networks, such as DUT-52 (12-connected framework, fcu topology), DUT-53 (eight-connected framework), or even two-dimensional net (DUT-84, double sheets) [16] were obtained.
Also in the Zr-tdc system, proper choice of the synthetic conditions yields frameworks with varying underlying topologies and cluster connectivity from 8 to 12 in DUT-67 (reo), DUT-68 (bon) and DUT-69 (bct) [15]. Interestingly, the analogue of the last one could also be synthesized using 1H-pyrazole-3,5-dicarboxylic acid as linker resulting in MOF-802 [10]. Direct comparison of the DUT-67 and DUT-68 structures shows that a different orientation of two thiophenedicarboxylates in the environment of one from two independent clusters leads to the formation of another polymorph with a completely different pore system, textural properties and framework topology. Such drastic changes in the properties are caused by only minor changes in the synthesis procedure, namely increasing the amount of the acetic acid as modulator. Thus, the examples demonstrate the paramount importance of the modulator in the synthesis of Zr-based MOFs. But not only the amount of the modulator, but also the nature of the monocarboxylic acid has a crucial influence on the structure formation.
In this contribution, we report that using trifluoracetic acid instead of acetic acid as modulator in the synthesis in the Zr-tdc system leads to a new porous polymorph, named DUT-126. The crystal structure of the compound was solved ab initio from the powder X-ray diffraction (PXRD) pattern. DUT-126 has 8,8-connected binodal framework, which can be referred to as hbr topological type. To the best of our knowledge, this topology occurs for the first time for MOF materials. The pore accessibility of the DUT-126 was probed by nitrogen physisorption experiments. Application of the Brunauer--Emmett--Teller (BET) equation to the adsorption isotherm results in the apparent BET surface area of 1297 m2 g−1 and pore volume of 0.48 m3 g−1.
2. Experimental section
(a). General remarks
ZrCl4 (98% purity) was purchased from Sigma Aldrich and 2,5-thiophenedicarboxylic acid (H2tdc) from TCI. The solvents N,N-dimethylformamide (DMF) (p.a. purity), N-methyl-2-pyrrolidione (NMP) (99% purity) and ethanol (99% purity) were purchased from ABCR GmbH. The trifluoroacetic acid (Htfa, 99%) was purchased from Alfa Aesar. All chemicals were used without further purification.
Nitrogen physisorption isotherms were measured at 77 K up to 1 bar on BELSORP-max (MicrotracBEL, Japan) apparatus. The water vapour physisorption measurements were carried out at 298 K on a Hydrosorb 1000 apparatus (Quantachrome Co). All samples were activated in a vacuum at 373 K for at least 12 h prior the measurements.
PXRD measurements were performed at room temperature on a STOE STADI P diffractometer with Cu-Kα1 radiation (λ = 1.54059 Å).
Scanning electron microscopy (SEM) images were taken with a ZEISS DSM-982 Gemini apparatus.
The thermogravimetric analysis and differential thermal analysis (TG/DTA) were carried out on a STA 409 PC Luxx (Netzsch) thermal analyser with air as the carrier gas and a heating rate 2 K min−1.
The 1H-NMR spectra were measured at 500.13 MHz on a Bruker DRX 500 P. The deuterated dimethyl sulfoxide was used as reference. For dissolving, 15 mg of DUT-126 were added to the same amount of CsF and three drops of concentrated deuterated hydrochloric acid (DCl). After 6 h, 0.7 ml of the deuterated DMSO was added. For neutralization of the in situ generated hydrofluoric acid, K2CO3 was used. The mixture was finally filtered over cotton wool and transferred into an NMR tube for analysis.
(b). Synthesis of DUT-126
A solution of ZrCl4 (230 mg, 1 mmol) in DMF and NMP mixture (1 : 1, 50 ml) was sonicated for 10 min. 2,5-Thiophendicarboxylic acid (H2tdc) (110 mg, 0.67 mmol) was added afterwards and the mixture was sonicated for an additional 10 min. After that, the trifluoroacetic acid (50 equivalents, 3810 µl) was added. The resulting solution was transferred into a pressure tube and heated at 120°C for two weeks. After cooling down, the resulting precipitate was washed several times with DMF and dried in a vacuum at 120°C. Yield: 73%.
Analytical data for dried DUT-126 (Zr6O4(OH)4(tdc)4(tfa)4 (DMF)2)·1.6tfa·0.25DMF: calculated C 23.36%, H 1.30%, S 5.94%, N 1.46%, found from elemental analysis C 23.14%, H 1.46%, S 5.82%, N 1.68% 1H-NMR (500 MHz, DMSO/DCl): δ/ppm = 7.68 (s, 2H, tdc), δ/ppm = 2.85, 2.68 (s, 3H, DMF), δ/ppm = 7.9 (s, 1H, DMF).
(c). Powder X-ray diffraction study
A high-resolution PXRD pattern was measured on a laboratory powder diffractometer STOE STADI P, equipped with CuKα1 monochromatic radiation and gas-filled 0D detector in transmission geometry using a capillary set-up as a rotating sample holder. The data were collected in the 2θ range of 1.5–90°. The PXRD pattern was indexed using DICVOL91 program [33]. The obtained cell was imported into the Material Studio 5.0 program [34], where Pawley refinement was performed using the Reflex module. After analysis of the extinction conditions, the space group P6/mmm was used for the structure solution. The positions of the Zr6O4(OH)4 clusters were located using the simulated annealing technique. After that, the ligand molecules were modelled in the visualization module of the program. Further, the geometrical optimization without optimization of the unit cell was performed using Universal Force Field. The resulting structure was used as a starting model for the Rietveld refinement. Because of the poor data-to-parameter ratio as well as reflection overlap at higher 2θ angles, some functional groups like carboxylates and thiophene moieties were refined as rigid bodies. The final structural model was obtained after hybrid Rietveld refinement with energy using the Reflex module of Materials Studio software (energy contribution 1%). The final Rietveld plot is given in figure 1.
Figure 1.

Rietveld plot for DUT-126.
Structural data are as follows: Zr6O34C38S4H26N2F12, hexagonal, P6/mmm, a = 37.5757(2) Å, c = 19.5244(1) Å, V = 23 873.97(7) Å3, z = 9; zero point line shift 0.00555°; profile function: Thompson–Cox–Hastings, U = 0.23898, V = −0.11824, W = 0.01767, X = 0.62810, Y = 0.08960; Asymmetry correction function: Berar–Baldinozzi P1 = −7.98812, P2 = −2.70412, P3 = 15.84250, P4 = 5.38100; preferred orientation function: Rietveld–Toraya a* = 0.56616, b* = 0.81145, c* = 0.14493, G1 = 4.20142, G2 = 0.99000; lattice strain: A = 0.90506, B = 0.90506, C = 0.00447; Global isotropic temperature factors U = 0.06990; Refined motion groups 23, refined degree of freedom 57; Rw = 21.88%, Rp = 15.64%.
3. Results and discussion
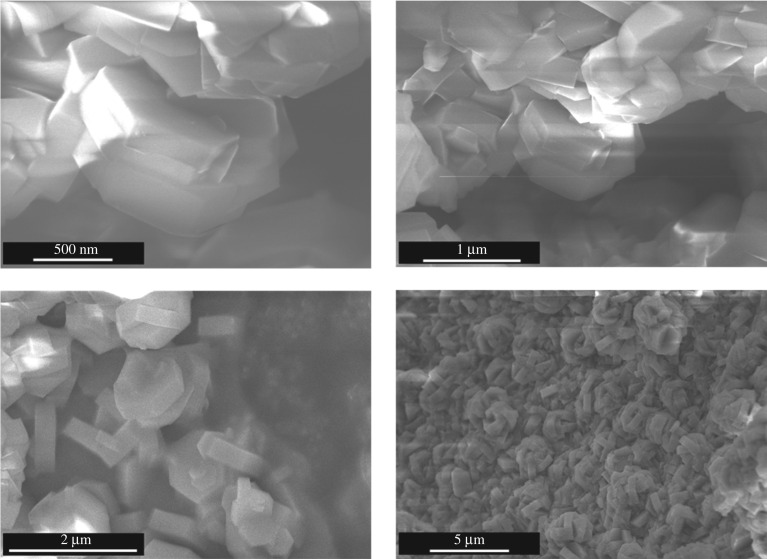
DUT-126 (with the composition Zr6O4(OH)4(tdc)4(tfa)4(DMF)2) could be synthesized as a microcrystalline powder in the solvothermal reaction of ZrCl4 and H2tdc in DMF in the presence of 50 equivalents of trifluoracetic acid. As the crystals suitable for single crystal X-ray diffraction study could not be obtained even by increasing the amount of the modulator, the crystal structure was solved ab initio from PXRD data. The above-mentioned composition of the framework was derived from the set of analytical methods, namely elemental analysis, TG, and 1H NMR. DUT-126 crystallizes in the hexagonal crystal system. This finding was confirmed by the hexagonal morphology of the crystallites, observed in the SEM measurements (figure 2).
Figure 2.
SEM images of DUT-126 of different resolutions.
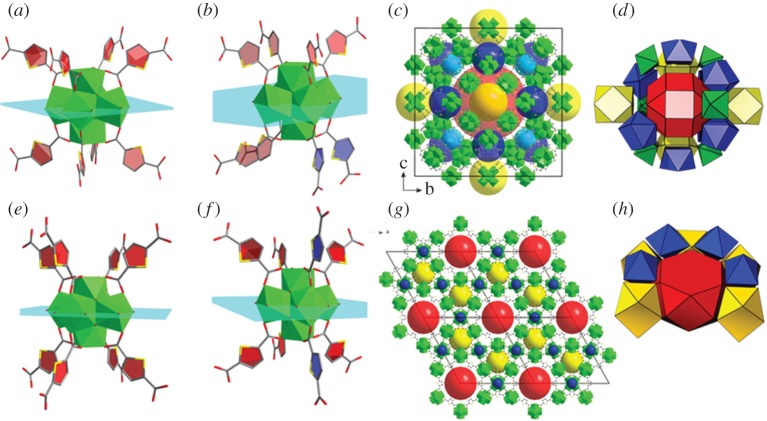
The crystal structure was refined in the P6/mmm space group with two symmetry independent Zr6O4(OH)4 secondary building units (SBUs), similar to that reported earlier for the DUT-68 structure. The interconnection of the SBUs by tdc2– linkers results in a binodal 8,8-connected three-dimensional framework showing the same connectivity as it was observed in DUT-68 [15]. However, the detailed analysis of the clusters environment shows significant differences between these two frameworks. In both SBUs, the modulator molecules are located in the equatorial plane (EqP) of the Zr6 octahedron (figure 3). In SBU1 (in DUT-68 and DUT-126), the thiophene-based ligands are oriented in such a way that the sulfur atoms point to the plane of terminal ligands (figure 3a,e). But, in the SBU2 of DUT-68, two adjacent linkers, located on the same side of the EqP (marked blue in figure 3b), are turned by 180°, leading to the formation of the cubic framework with reo topology (figure 3c).
Figure 3.
Crystal structures of DUT-68 and DUT-126: (a) SBU1 of DUT-68; (b) SBU2 of DUT-68; (c) pore packing for DUT-68; (d) topological representation of DUT-68 as natural tiling; (e) SBU1 of DUT-126; (f) SBU2 of DUT-126; (g) pore packing for DUT-126; (h) topological representation of DUT-126 as natural tiling.
In DUT-126, the linker orientation in SBU2 results in a topology different from reo. Here, two linkers located from the different sides of the EqP are turned away from the plane with reference to the sulfur atoms (figure 3f). Hence, 8 of 12 coordination places of the cluster are occupied by linker molecules. The remaining four coordination sites at the Zr clusters are occupied by trifluoroacetate anions and solvent.
Thus, under preservation of the same connectivity as in DUT-68, a completely different topology and pore system is formed in DUT-126. Topological analysis [35] results in a net that can be referred to as hbr topology according to the RCSR database [36]. To the best of our knowledge, this topology is recognized here for the first time in MOF chemistry.
Using the Zeo++ software [37], three types of pores could be identified in DUT-126, if DMF and tfa− anions are omitted in the calculations: one-dimensional hexagonal channels with a diameter of 22.8 Å running along [001] direction (figure 3g, red polyhydron; electronic supplementary material, figure S1); smaller pores with diameter of 11.8 Å (figure 3h, yellow polyhedron; electronic supplementary material, figure S1); and small octahedral pores with diameter of 5.3 Å (figure 3h, blue polyhedron; electronic supplementary material, figure S1).
The differences in topology, pore sizes and pore connectivity are reflected in different textural properties of the compound with the same network connectivity, namely DUT-67(Zr) (reo), DUT-68(Zr) (bon) and DUT-126(Zr) (hbr).
The evaluation of the crystallographic porosity of the framework reveals that 69% of the unit cell is solvent accessible. This results in a geometrical surface area of 2088 m2 g−1 and pore volume of 0.73 cm3 g−1, which are higher than that of DUT-67 and DUT-68 (table 1). After activation, DUT-126 is accessible for nitrogen at 77 K (figure 4). Owing to the presence of large hexagonal channels (d = 22.8 Å), the nitrogen isotherm differs from the ‘Type I’ characteristic for DUT-67. Similar to the isotherm of DUT-68, the isotherm of DUT-126 is of Type IVb [38] and features a step, indicating the presence of small mesopores.
Table 1.
Textural properties of eight-connected Zr-tdc MOFs.
| DUT-126 | DUT-67 | DUT-68 | |
|---|---|---|---|
| solvent accessible void, % | 69 | 66 | 60 |
| theoretical pore volume, Vpore, cm3 g−1 | 0.73 | 0.55 | 0.5 |
| theoretical surface area, SSA, m2 g−1 | 2088 | 1767 | 1786 |
| experimental pore volume, Vpore cm3 g−1 | 0.48 | 0.44 | 0.41 |
| experimental surface area, SSA, m2 g−1 | 1297 | 1064 | 891 |
water pore filling degree 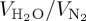 (p/p0 = 0.97), in % (p/p0 = 0.97), in % |
75 | 93 | 88 |
Figure 4.
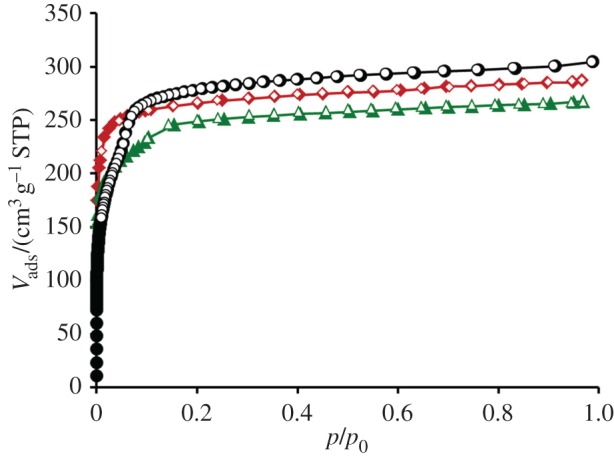
Nitrogen adsorption (filled symbols) and desorption (open symbols) isotherms measured at 77 K of DUT-126 (black circles), DUT-67 (red diamonds) and DUT-68 (green triangles).
The specific pore volume derived from the nitrogen physisorption isotherm at p/p0 = 0.95 is 0.48 cm3 g−1 and the BET area is determined to be 1297 m2 g−1.
To provide information about the hydrophobicity of DUT-126, water vapour physisorption was investigated at 298 K. The water isotherm shows an S-shape, similar to the isotherms of DUT-67 and DUT-68 (figure 5). In the p/p0 range between 0.45 and 0.55 the isotherm has a step, where the adsorbed amount increases almost twice, indicating the mesopore filling.
Figure 5.
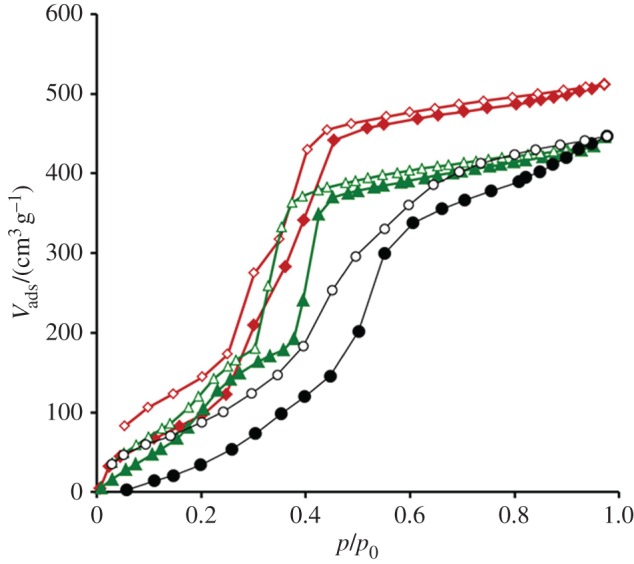
Water vapour adsorption (solid symbols) and desorption (blank symbols) isotherms, measured at 298 K for DUT-126 (black circles), DUT-67 (red diamonds) and DUT-68 (green triangles).
The water vapour uptake in saturation is 447 cm3 g−1 (STP), which corresponds to a pore volume of 0.36 cm3 g−1. Therefore, the degree of the pore filling with water is 75%. This value is significantly lower than that of DUT-67 and DUT-68 (table 1), pointing towards a more hydrophobic character of DUT-126. The enhanced hydrophobicity may originate from the trifluoroacetate modulator molecules, which are coordinated to the metal cluster [3,39] and located inside the hexagonal channels and the middle-sized pores.
Despite its hydrophobic properties, the isotherm of DUT-126 has a hysteresis, which does not close even at low relative pressures. This behaviour can be an indicator of a partial degradation of the framework during the water desorption process caused by strong capillary forces acting during water removal. If DUT-126 is stored under water for 3 days, no decrease in crystallinity was observed (electronic supplementary material, figure S3), indicating high stability of the framework against hydrolysis.
After exchange of water to ethanol and drying at 85°C, DUT-126 shows reduced porosity. A higher activation temperature (135°C) causes even larger porosity drop and loss of mesoporous character and crystallinity (electronic supplementary material, figures S2 and S4). TG/DTA measurement of DUT-126 activated at 120°C performed under air atmosphere shows that the material does not release coordinated DMF molecules below 180°C and tfa molecules below 250°C (electronic supplementary material, figure S5). Therefore, the release of terminal ligands in vacuum, obviously causing the decomposition of the framework, starts at significantly lower temperatures.
4. Summary and conclusion
Using a fluorinated modulator (trifluoroacetic acid) in the synthesis of Zr(IV) and tdc2− based MOFs, a new compound (DUT-126) could be synthetized. Considering only the network structure and arrangement of tdc and Zr-clusters, it is a polymorph of DUT-67, DUT-68 and DUT-69 reported earlier, and therefore a fourth alternative to build a network from the Zr6 and tdc building blocks discovered up to now.
DUT-126 consists of a binodal 8,8-connected framework, forming an hbr network topology. The resulting pore system contains three kinds of pores: micropores with a diameter of 5.8 Å, middle-sized pores with a diameter of 11.8 Å and mesopores in the form of channels with a diameter of 22.8 Å. The compound is stable in water and shows permanent porosity, if activated from DMF in vacuum at temperatures lower then 180°C. The BET area, calculated from nitrogen physisorption measurements at 77 K, is 1297 m2 g−1 and the total pore volume amounts to 0.48 cm3 g−1. The presence of fluorinated modulator molecules coordinated to the cluster leads to a more hydrophobic character of DUT-126, compared with its analogues, according to the water vapour physisorption isotherm.
Supplementary Material
Supplementary Material
Data accessibility
CCDC 1477065 contains the structural data for DUT-126. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre at https://summary.ccdc.cam.ac.uk/structure-summary-form.
Authors' contributions
F.D. synthesis and characterization of the compound, V.B. and J.G. crystal structure solution, I.S. interpretation of adsorption data. All authors contributed to manuscript preparation.
Competing interests
We declare we have no competing interests.
Funding
I.S., J.G. and S.K. were funded by Technische Universität Dresden. V.B. is supported by BMBF (Project N.05K16OD3). F.D. is funded by DFG (Grant KA 1698/19-1).
References
- 1.Cavka JH, Jakobsen S, Olsbye U, Guillou N, Lamberti C, Bordiga S, Lillerud KP. 2008. A New zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 130, 13 850–13 851. ( 10.1021/ja8057953) [DOI] [PubMed] [Google Scholar]
- 2.Bai Y, Dou Y, Xie L-H, Rutledge W, Li J-R, Zhou H-C. 2016. Zr-based metal-organic frameworks: design, synthesis, structure, and applications. Chem. Soc. Rev. 45, 2327–2367. ( 10.1039/C5CS00837A) [DOI] [PubMed] [Google Scholar]
- 3.Wang B, Huang H, Lv X-L, Xie Y, Li M, Li J-R. 2014. Tuning CO2 selective adsorption over N2 and CH4 in UiO-67 analogues through ligand functionalization. Inorg. Chem. 53, 9254–9259. ( 10.1021/ic5013473) [DOI] [PubMed] [Google Scholar]
- 4.Hu Z, Nalaparaju A, Peng Y, Jiang J, Zhao D. 2016. Modulated hydrothermal synthesis of UiO-66(Hf)-type metal–organic frameworks for optimal carbon dioxide separation. Inorg. Chem. 55, 1134–1141. ( 10.1021/acs.inorgchem.5b02312) [DOI] [PubMed] [Google Scholar]
- 5.Sawano T, Thacker NC, Lin Z, McIsaac AR, Lin W. 2015. Robust, chiral, and porous BINAP-based metal–organic frameworks for highly enantioselective cyclization reactions. J. Am. Chem. Soc. 137, 12 241–12 248. ( 10.1021/jacs.5b09225) [DOI] [PubMed] [Google Scholar]
- 6.Arrozi USF, Wijaya HW, Patah A, Permana Y. 2015. Efficient acetalization of benzaldehydes using UiO-66 and UiO-67: substrates accessibility or Lewis acidity of zirconium. Appl. Catal. A Gen. 506, 77–84. ( 10.1016/j.apcata.2015.08.028) [DOI] [Google Scholar]
- 7.Yang D, Odoh SO, Wang TC, Farha OK, Hupp JT, Cramer CJ, Gagliardi L, Gates BC. 2015. Metal–organic framework nodes as nearly ideal supports for molecular catalysts: NU-1000- and UiO-66-supported iridium complexes. J. Am. Chem. Soc. 137, 7391–7396. ( 10.1021/jacs.5b02956) [DOI] [PubMed] [Google Scholar]
- 8.Chen D, Xing H, Wang C, Su Z. 2016. Highly efficient visible-light-driven CO2 reduction to formate by a new anthracene-based zirconium MOF via dual catalytic routes. J. Mater. Chem. A 4, 2657–2662. ( 10.1039/C6TA00429F) [DOI] [Google Scholar]
- 9.Burtch NC, Jasuja H, Walton KS. 2014. Water stability and adsorption in metal–organic frameworks. Chem. Rev. 114, 10 575–10 612. ( 10.1021/cr5002589) [DOI] [PubMed] [Google Scholar]
- 10.Furukawa H, Gándara F, Zhang Y-B, Jiang J, Queen WL, Hudson MR, Yaghi OM. 2014. Water adsorption in porous metal–organic frameworks and related materials. J. Am. Chem. Soc. 136, 4369–4381. ( 10.1021/ja500330a) [DOI] [PubMed] [Google Scholar]
- 11.Bon V, Senkovskyy V, Senkovska I, Kaskel S. 2012. Zr(IV) and Hf(IV) based metal-organic frameworks with reo-topology. Chem. Commun. 48, 8407–8409. ( 10.1039/C2CC34246D) [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Hoang T, Ma S. 2012. Biomimetic catalysis of a porous iron-based metal–metalloporphyrin framework. Inorg. Chem. 51, 12 600–12 602. ( 10.1021/ic301923x) [DOI] [PubMed] [Google Scholar]
- 13.Feng D, Gu Z-Y, Li J-R, Jiang H-L, Wei Z, Zhou H-C. 2012. Zirconium-metalloporphyrin PCN-222: mesoporous metal–organic frameworks with ultrahigh stability as biomimetic catalysts. Angew. Chem. Int. Ed. 51, 10 307–10 310. ( 10.1002/anie.201204475) [DOI] [PubMed] [Google Scholar]
- 14.Morris W, Volosskiy B, Demir S, Gándara F, McGrier PL, Furukawa H, Cascio D, Stoddart JF, Yaghi OM. 2012. Synthesis, structure, and metalation of two new highly porous zirconium metal–organic frameworks. Inorg. Chem. 51, 6443–6445. ( 10.1021/ic300825s) [DOI] [PubMed] [Google Scholar]
- 15.Bon V, Senkovska I, Baburin IA, Kaskel S. 2013. Zr- and Hf-based metal–organic frameworks: tracking down the polymorphism. Cryst. Growth Design 13, 1231–1237. ( 10.1021/cg301691d) [DOI] [Google Scholar]
- 16.Bon V, Senkovska I, Weiss MS, Kaskel S. 2013. Tailoring of network dimensionality and porosity adjustment in Zr- and Hf-based MOFs. CrystEngComm 15, 9572–9577. ( 10.1039/C3CE41121D) [DOI] [Google Scholar]
- 17.Feng D, Chung W-C, Wei Z, Gu Z-Y, Jiang H-L, Chen Y-P, Darensbourg DJ, Zhou H-C. 2013. Construction of ultrastable porphyrin zr metal–organic frameworks through linker elimination. J. Am. Chem. Soc. 135, 17 105–17 110. ( 10.1021/ja408084j) [DOI] [PubMed] [Google Scholar]
- 18.Jiang H-L, Feng D, Wang K, Gu Z-Y, Wei Z, Chen Y-P, Zhou H-C. 2013. An exceptionally stable, porphyrinic Zr metal–organic framework exhibiting pH-dependent fluorescence. J. Am. Chem. Soc. 135, 13 934–13 938. ( 10.1021/ja406844r) [DOI] [PubMed] [Google Scholar]
- 19.Mondloch JE, et al. 2013. Vapor-phase metalation by atomic layer deposition in a metal–organic framework. J. Am. Chem. Soc. 135, 10 294–10 297. ( 10.1021/ja4050828) [DOI] [PubMed] [Google Scholar]
- 20.Feng D, Gu Z-Y, Chen Y-P, Park J, Wei Z, Sun Y, Bosch M, Yuan S, Zhou H-C. 2014. A highly stable porphyrinic zirconium metal–organic framework with shp-a topology. J. Am. Chem. Soc. 136, 17 714–17 717. ( 10.1021/ja510525s) [DOI] [PubMed] [Google Scholar]
- 21.Gutov OV, et al. 2014. Water-stable zirconium-based metal–organic framework material with high-surface area and gas-storage capacities. Chem. Eur. J. 20, 12 389–12 393. ( 10.1002/chem.201402895) [DOI] [PubMed] [Google Scholar]
- 22.Wang R, Wang Z, Xu Y, Dai F, Zhang L, Sun D. 2014. Porous zirconium metal–organic framework constructed from 2D → 3D interpenetration based on a 3,6-connected kgd net. Inorg. Chem. 53, 7086–7088. ( 10.1021/ic5012764) [DOI] [PubMed] [Google Scholar]
- 23.Wei Z, et al. 2014. Rigidifying fluorescent linkers by metal–organic framework formation for fluorescence blue shift and quantum yield enhancement. J. Am. Chem. Soc. 136, 8269–8276. ( 10.1021/ja5006866) [DOI] [PubMed] [Google Scholar]
- 24.Zhang M, Chen Y-P, Bosch M, Gentle T, Wang K, Feng D, Wang ZU, Zhou H-C. 2014. Symmetry-guided synthesis of highly porous metal–organic frameworks with fluorite topology. Angew. Chem. Int. Ed. 53, 815–818. ( 10.1002/anie.201307340) [DOI] [PubMed] [Google Scholar]
- 25.Feng D, et al. 2015. A highly stable zeotype mesoporous zirconium metal–organic framework with ultralarge pores. Angew. Chem. Int. Ed. 54, 149–154. ( 10.1002/anie.201409334) [DOI] [PubMed] [Google Scholar]
- 26.Kalidindi SB, et al. 2015. Chemical and structural stability of zirconium-based metal–organic frameworks with large three-dimensional pores by linker engineering. Angew. Chem. Int. Ed. 54, 221–226. ( 10.1002/anie.201406501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin Q, Bu X, Kong A, Mao C, Zhao X, Bu F, Feng P. 2015. New heterometallic zirconium metalloporphyrin frameworks and their heteroatom-activated high-surface-area carbon derivatives. J. Am. Chem. Soc. 137, 2235–2238. ( 10.1021/jacs.5b00076) [DOI] [PubMed] [Google Scholar]
- 28.Liu T-F, et al. 2015. Topology-guided design and syntheses of highly stable mesoporous porphyrinic zirconium metal–organic frameworks with high surface area. J. Am. Chem. Soc. 137, 413–419. ( 10.1021/ja5111317) [DOI] [PubMed] [Google Scholar]
- 29.Reinsch H, Stassen I, Bueken B, Lieb A, Ameloot R, De Vos D. 2015. First examples of aliphatic zirconium MOFs and the influence of inorganic anions on their crystal structures. CrystEngComm 17, 331–337. ( 10.1039/C4CE01457J) [DOI] [Google Scholar]
- 30.Wang S, Wang J, Cheng W, Yang X, Zhang Z, Xu Y, Liu H, Wu Y, Fang M. 2015. A Zr metal-organic framework based on tetrakis(4-carboxyphenyl) silane and factors affecting the hydrothermal stability of Zr-MOFs. Dalton Trans. 44, 8049–8061. ( 10.1039/C5DT00421G) [DOI] [PubMed] [Google Scholar]
- 31.Wang TC, et al. 2015. Ultrahigh surface area zirconium MOFs and insights into the applicability of the BET theory. J. Am. Chem. Soc. 137, 3585–3591. ( 10.1021/ja512973b) [DOI] [PubMed] [Google Scholar]
- 32.Yuan S, Lu W, Chen Y-P, Zhang Q, Liu T-F, Feng D, Wang X, Qin J, Zhou H-C. 2015. Sequential linker installation: precise placement of functional groups in multivariate metal–organic frameworks. J. Am. Chem. Soc. 137, 3177–3180. ( 10.1021/ja512762r) [DOI] [PubMed] [Google Scholar]
- 33.Boultif A, Louer D. 1991. Indexing of powder diffraction patterns for low-symmetry lattices by the successive dichotomy method. J. Appl. Crystallogr. 24, 987–993. ( 10.1107/S0021889891006441) [DOI] [Google Scholar]
- 34.2009. Material Studio 5.0. San Diego, CA: Accelrys Software Inc. [Google Scholar]
- 35.Blatov VA, Shevchenko AP, Proserpio DM. 2014. Applied topological analysis of crystal structures with the program package ToposPro. Cryst. Growth Design 14, 3576–3586. ( 10.1021/cg500498k) [DOI] [Google Scholar]
- 36.O'Keeffe M, Peskov MA, Ramsden SJ, Yaghi OM. 2008. The Reticular chemistry structure resource (RCSR) database of, and symbols for, crystal nets. Acc. Chem. Res. 41, 1782–1789. ( 10.1021/ar800124u) [DOI] [PubMed] [Google Scholar]
- 37.Willems TF, Rycroft CH, Kazi M, Meza JC, Haranczyk M. 2012. Algorithms and tools for high-throughput geometry-based analysis of crystalline porous materials. Microporous Mesoporous Mater. 149, 134–141. ( 10.1016/j.micromeso.2011.08.020) [DOI] [Google Scholar]
- 38.Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, Sing Kenneth SW. 2015. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 87, 1051–1069. ( 10.1515/pac-2014-1117) [DOI] [Google Scholar]
- 39.Schaate A, Roy P, Godt A, Lippke J, Waltz F, Wiebcke M, Behrens P. 2011. Modulated synthesis of Zr-based metal–organic frameworks: from nano to single crystals. Chem. Eur. J. 17, 6643–6651. ( 10.1002/chem.201003211) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
CCDC 1477065 contains the structural data for DUT-126. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre at https://summary.ccdc.cam.ac.uk/structure-summary-form.