Abstract
Background: Low-level laser therapy (LLLT) or photobiomodulation (PBM) is a possible treatment for brain injury, including traumatic brain injury (TBI). Methods: We review the fundamental mechanisms at the cellular and molecular level and the effects on the brain are discussed. There are several contributing processes that have been proposed to lead to the beneficial effects of PBM in treating TBI such as stimulation of neurogenesis, a decrease in inflammation, and neuroprotection. Both animal and clinical trials for ischemic stroke are outlined. A number of articles have shown how transcranial LLLT (tLLLT) is effective at increasing memory, learning, and the overall neurological performance in rodent models with TBI. Results: Our laboratory has conducted three different studies on the effects of tLLLT on mice with TBI. The first studied pulsed against continuous laser irradiation, finding that 10 Hz pulsed was the best. The second compared four different wavelengths, discovering only 660 and 810 nm to have any effectiveness, whereas 732 and 980 nm did not. The third looked at varying regimens of daily laser treatments (1, 3, and 14 days) and found that 14 laser applications was excessive. We also review several studies of the effects of tLLLT on neuroprogenitor cells, brain-derived neurotrophic factor and synaptogenesis, immediate early response knockout mice, and tLLLT in combination therapy with metabolic inhibitors. Conclusions: Finally, some clinical studies in TBI patients are covered.
Keywords: : traumatic brain injury, photobiomodulation, stroke, low-level laser therapy, brain disorders
Introduction
Traumatic brain injury (TBI) can include skull fracture, intracranial hemorrhage, elevated intracranial pressure, and/or cerebral contusion. Unlike stroke, the prevalence of which is tied with an increasing age of onset, TBI is much more common in younger populations. Not only does TBI have a large impact on the healthcare industry but also causes severe socioeconomic problems throughout the world. Every year in the United States, there are nearly 2 million head injuries resulting in 283,000 hospitalizations, 53,000 of which lead to death.1–3 Consequently, Americans living with TBI-related disabilities cost an estimated $56 billion yearly.4 In 2001, the World Health Organization (WHO) projected that within 5 years, motor vehicle accidents, one of the largest causes of TBI, would be ranked just behind ischemic heart disease and unipolar major depression as a cause of morbidity and mortality.5
Although the understanding of the pathophysiology underlying the damage following severe brain injury has improved, current treatment options remain limited.6 The processes and mechanisms that underlie TBI are incredibly complex and are still not well understood. After the initial impact, multiple pathways are activated that result in secondary injury, which can spread throughout the brain. These injury processes may include inflammation, an ionic imbalance, excitotoxic damage, oxidative stress, increased vascular permeability, and mitochondrial dysfunction.7 In turn, these secondary injuries result in brain edema and an increase in intracranial pressure. These physiological changes and disruptions cause neuronal death and a spread of ischemic necrosis, while worsening motor and cognitive impairment follows. Researchers and clinicians should prioritize efforts to improve the outcome and treatment options for TBI patients.8
Recently, transcranial low-level laser therapy (tLLLT) or photobiomodulation (PBM) has garnered a greater interest as an alternative to existing approaches to treat TBI as the search for conventional therapeutic treatments has been relatively unsuccessful. There are a number of articles showing the beneficial effects of tLLLT such as reducing brain damage and recovery times in stroke models. These promising results may soon lead to tLLLT becoming a more widely used treatment for TBI.
Mechanisms of tLLLT
The discussion of tLLLT has moved past its biological effects into a search for how light energy—specifically from lasers or light-emitting diodes (LED)—works at the cellular and organism levels. With a wide range of different applications of LLLT, it is necessary to find and understand the optimal parameters for each. Heat absorption, one of several unlikely mechanistic explanations for tLLLT, is closely tied to the use of lasers, but because the lasers used in tLLLT do not meaningfully raise brain temperature, photothermal effects from light energy do not play a significant role in explaining the benefits of tLLLT. Rather, photochemistry is becoming the accepted hypothesis for explaining the biological effects of light absorption in cells and tissues.9 These effects rely on the absorption of light by chromophores within cells to produce the many biological effects such as an increase of adenosine triphosphate (ATP), DNA, and RNA, release of nitric oxide (NO), cytochrome c oxidase (CCO) activity, a regulation of reactive oxygen species (ROS), and changes to the organelle membrane activity in mitochondria.10–14 In order for the photons to effectively reach their target area, the penetration of light through the tissue must be maximized by choosing an appropriate wavelength. This “optical window” ranges from 600 nm to 1200 nm, involving almost exclusively, red and near-infrared (NIR) light.15
Mitochondria are the most important cellular organelles to study, when trying to understand the cellular response of LLLT. Conventionally known as the “powerhouse of the cell,” mitochondria not only supply the cell with energy but are also involved in cellular signaling, cell differentiation, cell death, along with cellular metabolism and proliferation. Complex IV on the mitochondrial inner membrane, or CCO, is considered to be the crucial chromophore in the cellular response to LLLT as shown by the action spectra (a plot of the rate of the physiological activity plotted against wavelength of light).16,17 CCO is a large protein complex with two copper and two iron centers.18 The way in which light interacts and ultimately affects CCO is not precisely known, but will certainly involve a complex series of interactions that will result in a change in redox states. LLLT produces a shift toward higher oxidation in the overall cell redox potential11,17 and briefly increases the level of ROS.19,20 This change in the redox state of the mitochondria regulates several transcription factors. These include redox factor-1 (Ref-1), cAMP response element (CREB), activator protein 1 (AP-1), p53, nuclear factor kappa B (NF-κB), hypoxia-inducible factor (HIF-1), and HIF-like factor. The activation and regulation of redox-sensitive genes and transcription factors are thought to be caused by ROS induced from LLLT.19 An important feature of LLLT is its biphasic dose–response curve.21,22 This can be explained in other terms: a small amount of light can be good, more may lose the beneficial effect, and too much light may be harmful. This effect can be explained by two of the LLLT signaling mediators, ROS12 and NO.14 Both of these species can have positive effects at low concentration, but can have adverse effects at a high concentration.
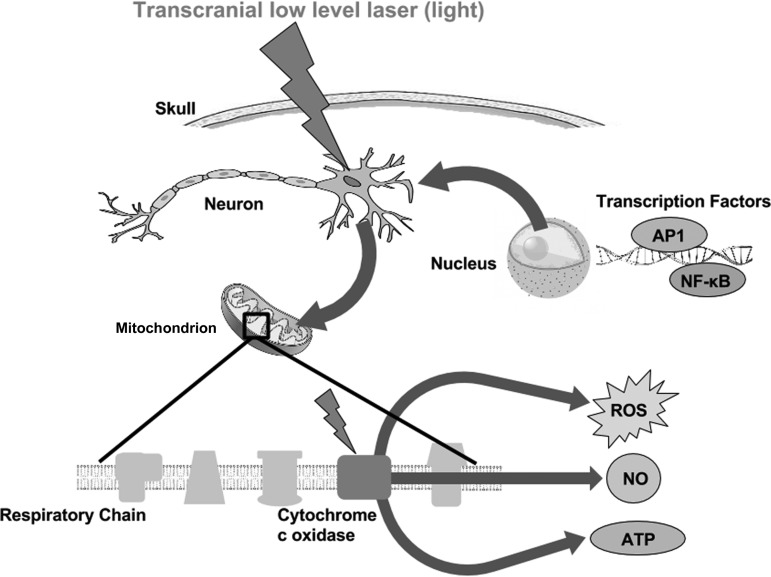
tLLLT may cause a separation (photodissociation) between NO and CCO.13,23 Normally, cellular respiration is downregulated when NO binds with CCO and inhibits it from binding to the Fe and Cu centers. The NO displaces the oxygen from CCO, thus decreasing cellular respiration and decreasing the production rate of ATP.10 Therefore, by breaking NO from CCO, LLLT is able to prevent this inhibition from occurring. In turn, both ATP levels and blood flow increase (NO is a vasodilator).24 With an increase in blood flow, improved oxygenation is found in damaged areas of the brain. Figure 1 depicts the molecular mechanisms and signaling pathways that are thought to occur post-LLLT.
FIG. 1.
Molecular mechanisms of transcranial LLLT. Light passes through the scalp and skull, where it is then absorbed by cytochrome c oxidase in the mitochondrial respiratory chain of the cortical neurons in the brain. Cell signaling and messenger molecules are upregulated as a result of stimulated mitochondrial activity, including ROS, NO, and ATP. These signaling molecules activate transcription factors, including NF-κB and AP-1, which enter the nucleus and cause transcription of a range of new gene products. AP-1, activator protein 1; ATP, adenosine triphosphate; BDNF, brain-derived neurotrophic factor; LLLT, low-level laser therapy; NF-κB, nuclear factor kappa B; NGF, nerve growth factor; NO, nitric oxide; ROS, reactive oxygen species.
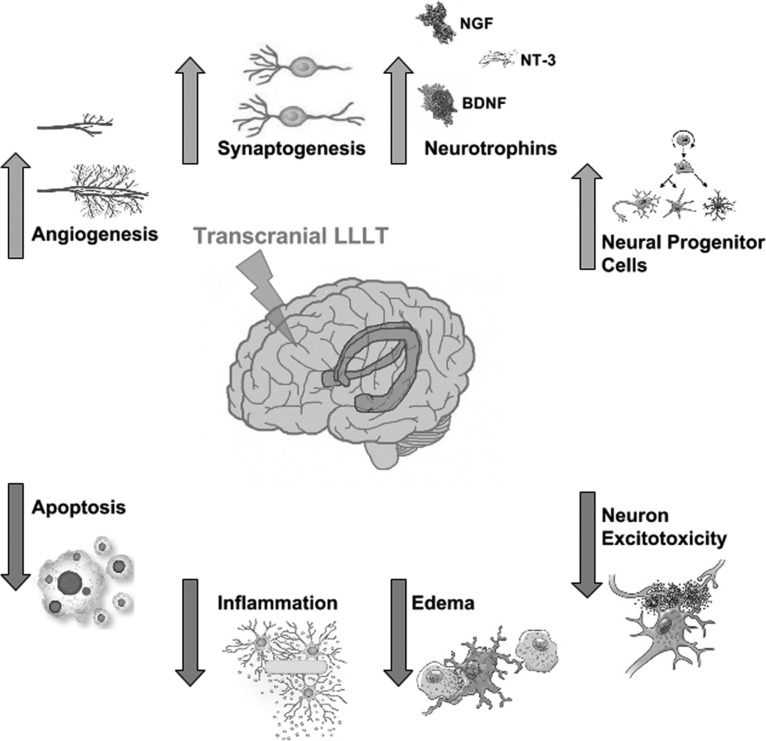
When using LLLT as a method to treat disorders of the brain, more specific mechanisms need to be closely examined. Brain-specific functional mechanisms of tLLLT for TBI are illustrated in Fig. 2. The cytoprotective effects of LLLT are thought to prevent injured neurons from dying and reduce the number of neuronal cells undergoing death as has been shown for toxins such as cyanide,25 tetrodotoxin,26 and methanol.27 This protective effect may include several mediators such as Bcl2, heat shock proteins,28 survivin,29 and superoxide dismutase.30 The decrease of proinflammatory mediators from dendritic cells31 along with the increase in anti-inflammatory mediators [interleukin (IL)-10 and transforming growth factor β]32 is thought to cause the anti-inflammatory effect of LLLT.33,34
FIG. 2.
Functional mechanisms of transcranial LLLT. The gene transcription process described in Fig. 1 can lead to decreases in neuronal apoptosis and excitotoxicity, and lessening of inflammation and edema, which will help reduce progressive brain damage. Increases in angiogenesis and expression of neurotrophins leading to activation of neural progenitor cells, and increased synaptogenesis may all contribute to the brain repairing itself from damage sustained in the trauma.
Last, neurogenesis and neuroplasticity (synaptogenesis) may both be other mechanisms that contribute to the beneficial effects of tLLLT in the brain.35 These processes are initiated by the increase in neurotrophin expression as in brain-derived neurotrophic factor (BDNF) and nerve growth factor.
tLLLT for Stroke and TBI
Apart from ischemic heart disease, stroke is the leading cause of death worldwide. The approved treatment for stroke is to apply tissue plasminogen activator within 3 h of stroke onset.36,37 Although this method is very effective at clearing blood clots, the small time window that exists for effective treatment diminishes the ability to treat most patients. Because of this short time frame, other treatment options for stroke victims must be considered.
Low-level laser therapy (LLLT) has been investigated as an alternative treatment for stroke, and LLLT has been shown to have a neuroprotective effect,38,39 while regulating several biological processes.40–42 Light can penetrate several tissues, including both the scalp and skull, and reach into the brain. Several clinical and preclinical studies have shown that this process can lead to an improved recovery from stroke.43 In these studies, stroke was induced in rat and rabbit models and showed that intervention by tLLLT within 24 h could have meaningful beneficial effects. For the rat models, stroke was induced by permanent middle cerebral artery occlusion by insertion of a filament into the carotid artery or by craniotomy.44,45 Stroke induction in the rabbit models were induced by the small clot embolic model by injecting a microclot made from blood from a donor rabbit.46 These studies along with the treatments and results are listed in Table 1.
Table 1.
Reports of Transcranial Low-Level Laser Therapy Used for Stroke in Animal Models
| Subject | Stroke model | Parameters | Effect | References |
|---|---|---|---|---|
| Rat | MCAO | 660 nm; 8.8 mW; 2.64 J/cm2; pulse frequency of 10 kHz; Laser applied at cerebrum at 1, 5, and 10 min | Suppression of NOS activity and upregulation of TGF-β1 | 44 |
| Rat | MCAO | 808 nm; 7.5 mW/cm2; 0.9 J/cm2; 3.6 J/cm2 at cortical surface; CW and pulse wave at 70 Hz, 4 mm diameter | Administration of LLLT 24 h after stroke onset induced functional benefit and neurogenesis induction | 45 |
| Rabbit | RSCEM | 808 ± 5 nm; 7.5 W/cm2, 2-min duration 3 h after stroke and 25 mW/cm2 10-min duration 1 or 6 h after stroke | Improved behavioral performance and durable effect after LLLT within 6 h from stroke onset | 46 |
| Rat | MCAO | 808 nm; 0.5 mW/cm2; 0.9 J/cm2 on brain 3 mm dorsal to the eye and 2 mm anterior to the ear | LLLT applied at different locations on the skull improved neurological function after acute stroke | 47 |
| Rabbit | RSCEM | 808 nm; 7.5 mW/cm2; 0.9 J/cm2; 3.6 J/cm2 at cortical surface; CW; 300 min; pulse at 1 kHz, 2 min at 100 Hz | LLLT administered 6 h after embolic stroke resulted in clinical improvements in rabbits | 48 |
CW, continuous wave; LLLT, low-level laser therapy; MCAO, middle cerebral artery occlusion; NOS, nitric oxide synthase; RSCEM, rabbit small clot embolic model; TGF-β1, transforming growth factor β1.
Three clinical trials of tLLLT have been conducted in human patients who had suffered from an acute stroke. The first study, NEST-1, enrolled 120 patients between the ages of 40–85 years with a diagnosis of ischemic stroke involving a neurological deficit that could be measured. The purpose of this first clinical trial was to demonstrate the safety and effectiveness of laser therapy for stroke within 24 h.49 Transcranial PBM significantly improved outcome in human stroke patients, when applied at ∼18 h poststroke, over the entire surface of the head (20 points in the 10/20 EEG system) regardless of stroke location.49 Only one LLLT was administered, and 5 days later, there was significantly greater improvement in the Real- but not in the Sham-treated group (p < 0.05, NIH Stroke Severity Scale). This significantly greater improvement was still present at 90 days poststroke, where 70% of the patients treated with Real LLLT had successful outcome, while only 51% of controls did. The second clinical trial, NEST-2, enrolled 660 patients, aged 40–90 years, who were randomly assigned to one of two groups (331 to LLLT, 327 to sham).50 Beneficial results (p < 0.04) were found for the moderate and moderate–severe (but not for the severe) stroke patients, who received the Real laser protocol.50–52 These results suggest that the overall severity of the individual stroke should be taken into consideration in future studies, and very severe patients are unlikely to recover with any kind of treatment. The last clinical trial, NEST-3, was planned for enrollment of 1000 patients. The study was prematurely terminated by the data safety monitoring board for futility—that is, on an Interim Analysis, no significant difference was observed between those receiving the real intervention versus the sham intervention. If the study had been continued, a lack of statistical significance would have been expected.53 The parameters and results for these three clinical trials are listed in Table 2.
Table 2.
Reports of Transcranial Low-Level Laser Therapy Used for Stroke in Clinical Trials
| Clinical trial for stroke | No. of subjects | Eligibility criteria | Parameters of treatment | Effect | References |
|---|---|---|---|---|---|
| NEST-1 | 120 | Patients: between 40 and 85 years of age; clinical diagnosis of ischemic stroke; measurable neurological deficit; NeuroThera Laser System within 24 h of stroke onset. 79 cases received the Real and 41 received the Sham. | 808 nm; 700 mW/cm2 on shaved scalp with cooling; 1 J/cm2 at cortical surface; 20 predetermined location 2 min each. | Greater improvement in the Real-treated group, but not in the Sham-treated group (p < 0.05, NIH Stroke Severity Scale). | 49 |
| 10 placements on the left and right side of the head (regardless of the side of the stroke); no midline placements. | |||||
| NEST-2 | 660 | Patients: between 40 and 90 years of age; clinical diagnosis of ischemic stroke within 24 h of onset; NIH stroke scale of 7–22. | 808 nm; 700 mW/cm2 on shaved scalp with cooling; 1 J/cm2 at cortical surface; 20 predetermined location 2 min each as described for NEST-1. | Beneficial results (p < 0.04) were found for the moderate and moderate-severe (but not for the severe) stroke patients. Mortality and adverse event rates were not adversely affected by TLT. | 50 |
| 331 cases received Real tLLLT and 327 received Sham tLLLT | |||||
| NEST-3 | 1000 | Patients: between 40 and 80 years of age; clinical diagnosis of ischemic stroke within 24 h of onset; NIH stroke scale of 7–17 | 808 nm; 700 mW/cm2 on shaved scalp with cooling; 1 J/cm2 at cortical surface; 20 predetermined location 2 min each as described for NEST-1 and NEST-2 | The study was terminated prematurely after 600 (out of 1000) patients by the DSMB due to an expected lack of statistical significance | 54 |
DSMB, data safety monitoring board; NIH, National Institutes of Health; NEST, NeuroThera Efficacy and Safety Trial; TLT, transcranial laser therapy.
Studies of tLLLT for TBI in Mice
With the success of tLLLT for stroke has come an influx of researchers testing this technique in different animal models of TBI. Oron et al.55 examined the effects of LLLT for TBI in mice. A weight-drop device was used to induce a closed-head injury in the mice. An 808 nm diode laser with two energy densities (1.2–2.4 J/cm2 over 2 min of irradiation with 10 and 20 mW/cm2) was delivered to the head 4 h after TBI was induced. Neurobehavioral function was assessed by the neurological severity score (NSS) with a range of 0 to 10, where the lowest number (0) reflects normal function. There was no significant difference in NSS between the power densities (10 vs. 20 mW/cm2), nor was there a significant difference between the control and laser-treated group after 24 and 48 h post-TBI. However, there was a significant improvement of a 27% lower NSS in the laser-treated group after 5 days to 4 weeks. The laser-treated group also showed a smaller loss of cortical tissue than the sham group.55
Oron et al.56 then looked at the long-term effects of varying treatments of different therapies administered at varying time points in mice with internal (closed-head) injury. Again, a weight-drop device was used along with an 808 nm Ga-Al-As diode laser with an energy density of 1.2 J/cm2 (power density of 10 mW/cm2). Treatments were given at 4, 6, and 8 h postinjury transcranially. Laser treatments at 10 mW/cm2 at 100 Hz and 600 Hz and continuous wave (CW) 4 h postinjury were conducted in a complementary experiment. For the laser-treated group at 6 h postinjury, the NSS was 3.4 times better (lower) than in the nonirradiated control group. For the laser-treated group at 8 h postinjury, the NSS was 1.8 times better (lower) than the control group. Both groups were evaluated on day 56. The NSS for the all three varying frequency treatments (100 Hz, 600 Hz and CW) had 3.5 times greater difference when compared to the nontreated control group. The mice receiving tLLLT with pulsed wave (PW) at 100 Hz 4 h postinjury made a full recovery (NSS of 0) by day 56. When compared to the control group, the CW and PW laser-treated groups had significant smaller lesion size in the brain.56
Khuman et al.12 demonstrated that tLLLT could improve cognitive function in controlled cortical impact (CCI) mice. The CCI was created by a 3 mm flat-tipped pneumatic piston moving at a velocity of 6 m/sec to a depth of 0.6 mm into the brain exposed by a craniotomy. The mice were assigned to one of two groups: either an open craniotomy laser-treated group or a transcranial laser-treated group. Within the open craniotomy group, the mice were irradiated with a low-level laser light with a wavelength of 800 nm at varying energy levels (0, 30, 60, 105, 120, and 210 J/cm2) at 60–80 min post-CCI. The group treated transcranially were exposed to low-level laser light with an energy level of 60 J/cm2 at varying timing regimens (60–80 min post-CCI, 4 h post-CCI, or once a day for 7 days after CCI). The Morris water maze (MWM) was utilized to assess cognitive function improvement, while motor function was evaluated using the wire grip test. Brain edema, lesion size, and nitrosative stress were also evaluated using a nitrotyrosine ELISA test. Mice in both groups (transcranially or open craniotomy) treated with lasers with an energy level of 60 J/cm2 showed significant improvements in both the response time to the hidden platform and probe trial performance. An anti-inflammatory effect was also noted in both groups, however, there was no significant difference found in the motor function (days 1–7), lesion size (14 days), brain edema (24 h), and nitrosative stress (24 h) between the LLLT groups and the control.12
Quirk et al.57 evaluated the neuroprotective effects of NIR light using an in vivo rodent model of TBI induced by CCI and characterized the changes at the behavioral and biochemical levels. Rats were divided into three different groups: a severe TBI group, a sham surgery group, and a group receiving anesthetization only. Rats in each group were then administered either with or without NIR light. They received two 670 nm LED treatments (5 min, 50 mW/cm2, 15 J/cm2) per day for 72 h (biochemical analysis assay) or for 10 days (behavioral assay). During the recovery period, rats were tested for locomotor and behavioral activities using a TruScan device. At the 72-h mark, frozen brain tissue was collected and evaluated for apoptotic markers and measured for reduced glutathione (GSH) levels. Significant differences between TBI with and without NIR (TBI+/−) light and between the sham surgery with and without NIR (S+/−) light were observed. In rats exposed to NIR light, there was a significant decrease in the proapoptotic marker Bax along with a smaller increase in antiapoptotic markers and GSH levels.57
Moreira et al.58 assessed the effects of low-level laser phototherapy following direct cortical cryogenic injury (CI) to the brain of rodents. The rats were irradiated with 780 and 600 nm lasers at energy levels of 3 and 5 J/cm2. This study found that laser-treated lesions had smaller tissue loss at 6 h, had significantly higher amount of viable neurons at 24 h, and had fewer leukocytes and lymphocytes in first 24 h, while the GFAP staining increased in the control group (but not the tLLLT group) by 14 days. It was concluded that LLLT facilitated wound healing in the brain following CI by controlling brain damage, preventing neuronal death, and reducing severe astrogliosis58
Effect of Different Laser Wavelengths in tLLLT for TBI
The following three sections will discuss and summarize studies conducted in our laboratory where we have investigated the use of LLLT to treat TBI in animal models.
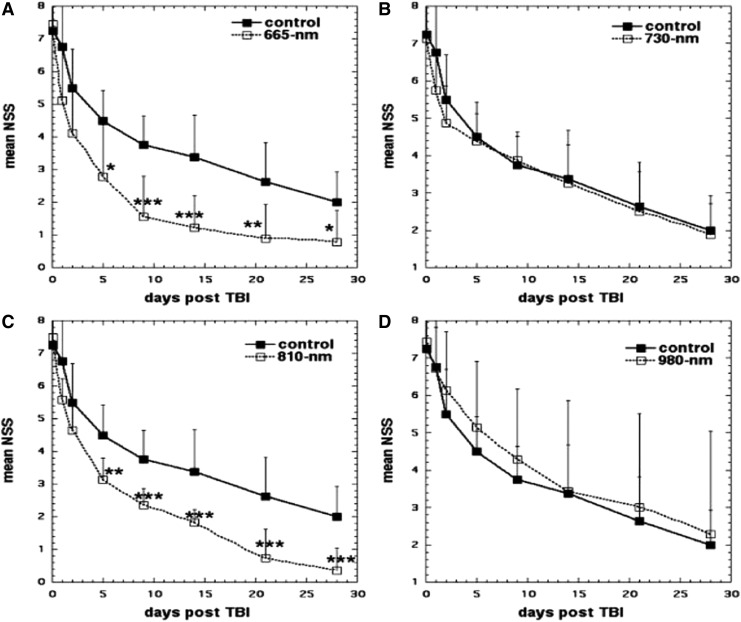
Wu et al.59 explored the effect that varying laser wavelengths of LLLT had on closed-head TBI in mice. Mice were randomly assigned to the LLLT-treated group or the sham group as a control. Closed-head injury was induced by a weight-drop apparatus. To analyze the severity of the TBI, the NSS was measured and recorded. The injured mice were then treated with varying wavelengths of laser (665, 730, 810, or 980 nm) at an energy level of 36 J/cm2 at 4 h directed onto the scalp. The 665 and 810 nm groups showed significant improvement in NSS when compared to the control group from day 5 to 28. Results are shown in Fig. 3. Conversely, the 730 and 980 nm groups did not show a significant improvement in NSS and these wavelengths did not produce similar beneficial effects as in the 665 and 810 nm LLLT groups.59 The tissue chromophore CCO is proposed to be responsible for the underlying mechanism that produces the many PBM effects that are the byproduct of LLLT. CCO has absorption bands around 665 and 810 nm, while it has low absorption bands at the wavelength of 730 nm.23 It should be noted that this particular study found that the 980 nm wavelength did not produce the positive effects as the 665 and 810 nm wavelengths did, but other previous studies did find that the 980 nm wavelength was an active one for LLLT. Wu et al. proposed these dissimilar results may be due to the variance in the energy level, irradiance, and so on between the other studies and this particular study.59
FIG. 3.
Effect of different wavelengths in tLLLT in closed head TBI in mice. (A) Sham-treated control versus 665 nm laser. (B) Sham-treated control versus 730 nm laser. (C) Sham-treated control versus 810 nm laser. (D) Sham-treated control versus 980 nm laser. Points are means of 8–12 mice and bars are SD. *p < 0.05; **p < 0.01; ***p < 0.001 (one-way analysis of variance). NSS, neurological severity score; SD, standard deviation; TBI, traumatic brain injury; tLLLT, transcranial LLLT.
Effect of Pulsing tLLLT for TBI
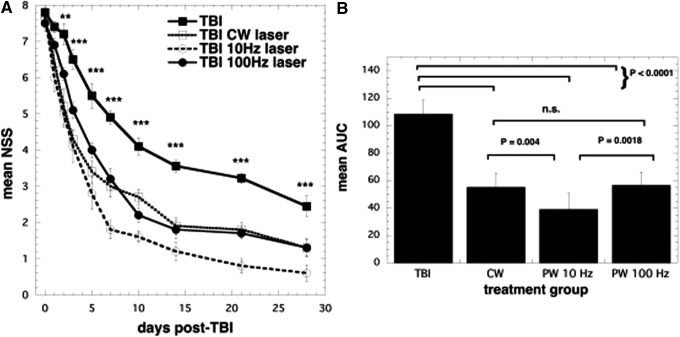
A number of studies have investigated the best range of parameters for laser treatment (total energy delivered, irradiance, or power density) and the best wavelengths of lasers that should be used in LLLT for the treatment of TBI and other brain disorders. However, a consensus on whether treatment should be given by CW light or PW light, including the parameters for this light, has not been reached. Ando et al.60 used the 810 nm wavelength parameters from the previous study and varied the pulse modes of the laser in a mouse model of TBI. These modes consisted of either PW laser at 10 Hz or 100 Hz or CW laser. For the mice, TBI was induced with a CCI device by open craniotomy. A single treatment with an 810 nm Ga-Al-As diode laser with a power density of 50 mW/m2 and an energy density of 36 J/cm2 was given by tLLLT to the closed head in mice for a duration of 12 min at 4 h post-CCI. At 48 h to 28 days post-TBI, all laser-treated groups had significant decreases in the measured NSS when compared to the control. Although all laser-treated groups had similar NSS improvement rates up to day 7, the PW 10 Hz group began to show greater improvement beyond this point as seen in Fig. 4. On day 28, the forced swim test for depression and anxiety was used and showed a significant decrease in the immobility time for the PW 10 Hz group. In the tail suspension test, which measures depression and anxiety, there was also a significant decrease in the immobility time on day 28, and (in contrast to the forced swim test) this was also seen on day 1, in the PW 10 Hz group.
FIG. 4.
Effects of pulsing in transcranial LLLT for CCI-TBI in mice. (A) Time course of NSS of mice with TBI receiving either control (no laser treatment), or 810 nm laser (36 J/cm2 delivered at 50 mW/cm2) with a spot size of 0.78 cm2 in either CW, PW 10 Hz, or PW 100 Hz modes. Results are expressed as mean ± SEM **p < 0.01 and ***p < 0.001 versus the other conditions. (B) Mean areas under the NSS time curves in the two-dimensional coordinate system over the 28-day study for the four groups of mice. Results are mean ± SD (n = 10). AUC, area under the curve; CCI, controlled cortical impact; CW, continuous wave; n.s., not significant; PW, pulsed wave.
Both these test results indicate an antidepressant effect of tLLLT. It should be noted that severe depression is a major symptom of TBI in human patients. For the PW 10 Hz group on days 15 and 28, the lesion size in the tLLLT-treated TBI mice significantly decreased when compared to the nonlaser-treated control group. These results may suggest a neuroprotective effect of tLLLT. Overall, the beneficial effects of tLLLT for TBI, including the antidepressant effects, the degree of injury, and the neuroprotective effects, were found to be more effective with the 10 Hz PW frequency compared to both the 100 Hz frequency and the CW. Ando et al. hypothesized that the PW 10 Hz laser irradiation frequency may be the best frequency to affect the entire brain.60 The pulsed light may have resonance with existing brain waves such as theta waves that oscillate at 4–10 Hz, found in the hippocampus (or a similar region).62
Effects of tLLLT Regimen for TBI
Over the whole history of all the reported LLLT studies, there has been found to exist a biphasic dose–response relationship that persists. This applies to not only cell culture studies but also to preclinical studies in animal models and clinical studies of LLLT.63 Through many previous studies, including the ones discussed above, it is largely accepted that there is an optimal level of energy density and power density and an optimum treatment regimen that is needed to create the most beneficial effects with tLLLT. Choosing suboptimal parameters may lead to a reduction in the beneficial effects and therefore a less than effective treatment, or may even cause negative effects or adverse reactions.40
Xuan et al.64 studied the effectiveness of varying treatment repetition regimens of tLLLT on the neurobehavioral and vestibulomotor function and studied neuroprotection and neurogenesis by histomorphological analysis and histological evidence. They used an 810 nm laser to deliver LLLT to CCI mouse models of TBI. The mice were split into three groups and received tLLLT (CW 810 nm laser, 25 mW/cm2 power density, 18 J/cm2 energy density) once at 4 h post-CCI, at 3 total treatments (once a day for 3 days), or at 14 total treatments (once a day for 14 days). They found that tLLLT may have beneficial effects as a treatment of TBI when treated with a single laser treatment and to a greater degree with three laser treatments. These two groups showed significant improvement in the NSS, motion tests, and in the wire grip test. However, in the group given 14 treatments, there was no significant improvement in any area. This article also discussed that using tLLLT (once or thrice) on mice with TBI could lead to improved neural function, smaller lesion size, and could possibly protect against neuronal damage in the brain.64
tLLLT Has More Effect on IEX Knockout Mice
Mild injury to the brain can sometimes trigger secondary brain injury that can lead to severe postconcussion syndrome. The mechanism behind these series of events is not well understood. Zhang et al.65 showed that secondary brain injury occurs to a worse degree in mice that had been genetically engineered to lack “Immediate Early Response” gene X-1 (IEX-1) when exposed to a gentle head impact (this injury is thought to closely resemble mild TBI in humans). This secondary injury could be characterized by widespread leukocyte infiltrates and extensive cell death that lead to large amounts of tissue loss. A similar insult in wild-type mice (mice with intact IEX-1) did not produce any secondary injury. Exposing IEX-1 knockout mice to LLLT 4 h postinjury suppressed proinflammatory cytokine expression of IL-Iβ and IL-6, but upregulated TNF-α. The lack of IEX-1 decreased ATP production, but exposing the injured brain to LLLT elevated ATP production back to near normal levels. The protective effect of LLLT may be attributed to enhanced ATP production and the regulation of proinflammatory mediators. This new model of IEX knockout mice could be a good tool for investigating the pathways of secondary brain injury as well as the mechanisms behind the beneficial effects of LLLT.65
tLLLT in Combination with Metabolic Inhibitors
Vascular damage occurs in injured brains and frequently leads to hypoxia, a result that often explains poor results in the clinic. Dong et al.66 found that neurons cultured under hypoxia led to high levels of glycolysis, reduced levels of ATP generation, increased formation of ROS, and increased levels of apoptosis. The adverse effects of hypoxia were almost completely reversed after treatment with LLLT. LLLT maintained the mitochondrial membrane potential, constrained cytochrome c leakage in cells succumbing to hypoxia, and protected these cells from apoptosis. The beneficial effects of LLLT were strengthened by combining the treatment with metabolic substrates such as pyruvate and/or lactate, both in vivo and in vitro. This combinatorial treatment was able to reverse memory and learning deficits in TBI-injured mice back to normal levels as well as leaving the hippocampal region completely protected from tissue loss; a stark contrast to control TBI mice that exhibited severe tissue loss from secondary brain injury. Dong et al. concluded that metabolic modulators could work in concert with LLLT to improve its beneficial effects in tissues that have low energy output, such as injured brain tissue.66
tLLLT Increases Neuroprogenitor Cells
Previous studies have shown that treating mice with CCI-induced TBI, with LLLT using an 810-nm laser at 4 h post-TBI, could significantly improve the NSS, while decreasing the lesion size. Xuan et al. hypothesized that tLLLT could improve performance on the MWM for learning and memory and increase neurogenesis in the hippocampus and subventricular zone (SVZ) after CCI TBI in mice.67 TBI was created by subjecting the mice to a single right lateral CCI using a pneumatic impact device with 3 mm flat-tip rod at a speed of 4.8 m/sec with an impact depth of 2 mm. TBI mice were given one of two treatments: one laser treatment 4 h post-TBI or three daily applications (once per day). Both the 1 day and three daily laser treatment groups showed improvement in neurological performance as measured by the wire grip test and by the motion test especially at 3 and 4 weeks post-TBI. Improvements were found in visible and hidden platform latency and probe tests in MWM at 4 weeks. At 4 days post-TBI, caspase-3 expression was found at lower levels in the region of the lesion and double-stained BrdU-NeuN (neuroprogenitor cells) was increased in the dentate gyrus (DG) of the hippocampus and the SVZ. Xuan et al. suggested that tLLLT may improve TBI both by reducing cell death in the lesion and also by stimulating neurogenesis in the hippocampus and the SVZ.67
tLLLT Increases BDNF and Synaptogenesis
Other studies have shown that applying NIR light to the head of animals with TBI improves neurological function, lessens the size of the brain lesion, reduces inflammation in the brain, and stimulates the formation of new neurons. In the study by Xuan et al.,68 they examined the expression of BDNF levels in CCI-TBI mice treated with tLLLT. CCI-TBI mice were subjected to two treatment regimens: either once 4 h post-TBI or were given three daily applications with 810 nm CW laser with an energy density of 36 J/cm2 at a power density of 50 mW/cm2. The NSS improved when compared to the untreated mice on day 14 and improved further by days 21 and 28. The mice given daily treatments for 3 days showed an even greater improvement when compared to the single treatment group. After the mice were sacrificed on days 7 and 28 for analysis, it was found that BDNF was upregulated by laser treatment in the DG of the hippocampus and the SVZ, but not in the perilesional cortex (lesion). A marker of synaptogenesis, Synapsin-1, was up-regulated in the lesion and the SVZ, but not in the DG. Upregulation was seen at day 28 but not at day 7. Xuan et al. found that the benefit of LLLT to the brain is partly mediated by stimulation of BDNF production, which may encourage synaptogenesis. In turn, these benefits of BDNF suggest that tLLLT may have a broader application to neurodegenerative and psychiatric disorders.68
tLLLT in Humans with TBI
Based on postmortem observations, transcranial application of NIR light can penetrate through human scalp, skull, meninges, and brain to a depth of ∼40 mm (808 nm laser system).69 An estimated 2–3% of NIR laser light applied transcranially in human cadavers has also been reported at 1–2 cm depth.70 In 2011, Naeser et al. published two clinical case reports of improved cognition in chronic TBI patients following a series of treatments with red/NIR LEDs applied transcranially.71 In the first case study, the patient reported that her ability to concentrate on tasks for a longer period of time (time able to work at computer) increased from 20 min to 3 h, she had a better ability to remember what she read, decreased sensitivity when receiving haircuts in the spots where LLLT was applied, and improved mathematical skills after undergoing LLLT. A 500 mW CW red/NIR LED with a power density of 25.8 mW/cm2 (area of 19.29 cm2) was applied to the forehead for a typical duration of 10 min (13.3 J/cm2). After applying the red/NIR LED LLLT treatment, both TBI patients reported an improvement in sleep and a better ability to control their social behavior.71 Using red/NIR LED cluster heads, each with a power density of 22.2 mW/cm2 (area of 22.48 cm2), the second patient had statistically significant improvements compared to prior neuropsychological testing, after 9 months of nightly, self-administered home tLED treatments. She returned to work after 4 months of the tLED treatments, whereas she had been on disability for 5 months before that time. The patient had a two standard deviation (SD) increase in tests of inhibition and inhibition accuracy (9th percentile to 63rd percentile) on the Stroop test for executive function and a one SD increase on the Wechsler Memory scale test for the logical memory test (83rd percentile to 99th percentile).71
Both these reported case studies showed significant improvement in the patient's states of wellbeing, despite variability in a number of different areas, including time of injury (2 vs. 7 years) and cause of injury (motor vehicle accident vs. injuries resulting from rugby, sky diving, military deployment, and accidental injury).
Naeser et al.72 conducted an open protocol study that examined whether scalp application of red and NIR light could improve cognition in patients with chronic, mild traumatic brain injury (mTBI). This study had 11 participants ranging from the age of 26 to 62 years (6 males, 5 females) who had persistent cognitive dysfunction resulting from mTBI. The participants' injuries were caused by motor vehicle accidents, sports-related events, and for one participant, IED blast injury. The tLLLT consisted of 18 sessions (Monday, Wednesday, and Friday for 6 weeks) and started from 10 months to 8 years post-TBI. A total of 11 LED clusters (5.25 cm in diameter, 500 mW, 22.2 mW/cm2, 13 J/cm2) were applied for about 10 min per session (5 or 6 LED placements per set, Set A and then Set B, in each session). Neuropsychological testing was performed pre-LED application and 1 week, 1 month, and 2 months after the final treatment. Naeser et al. found that there was a significant positive linear trend observed for the Stroop Test for executive function, in trial 3 inhibition (p = 0.004); Stroop, trial 4 inhibition switching (p = 0.003); California Verbal Learning Test (CVLT)-II, total trials 1–5 (p = 0.003); and CVLT-II, long delay free recall (p = 0.006). Improved sleep and fewer post-traumatic stress disorder (PTSD) symptoms, if present beforehand, were observed after treatment. Participants and family members also reported better social function and a better ability to perform interpersonal and occupational activities. Although these results are significant, further placebo-controlled studies will be needed to ensure the reliability of these data.72
Nawashiro et al.61 quantified cerebral blood flow using single-photon emission computed tomography with N-isopropyl-p-iodoamphetamine (IMP-SPECT). In this case study, a single patient who was in a vegetative state following severe head trauma was administered 146 LED treatments over a period of 73 days with a device consisting of a grid of 23 diodes, 820 nm LED, each 13 mW, with a power density of 11.4 mW/cm2, an energy density of 20.5 J/cm2, and was held 5 mm from the head for 30 min per treatment. After giving treatments bilaterally to the forehead, a 20% increase in blood flow in the left anterior frontal lobe was observed. It was also noted that the patient showed slight movement in the left arm after treatment.
More controlled clinical studies with much larger numbers of patients will be needed to better understand factors that may affect treatment response following a series of tLLLT treatments with brain injuries of different etiologies (TBI or stroke) and different degrees of severity. Collaboration among researchers will be necessary to achieve consistency of tLLLT treatment protocols and parameters applied, as well as measurement of the effects. For example, data from sophisticated brain imaging techniques before and after a series of tLLLT treatments in a specific patient population could help with understanding brain changes that may be occur following a series of tLLLT treatments with that disorder. Some brain imaging techniques include the following: (1) structural magnetic resonance imaging (MRI) scans, which could show potential changes in cortical thickness post-tLED—for example, perhaps in the hippocampus areas, if thinning was present pre-tLLLT; (2) diffusion tensor imaging MRI to measure axonal damage in specific pathways before entry and potential changes post-tLED; (3) Task-related functional MRI to measure cortical activation during tasks requiring focused attention and inhibition, including reaction times; (4) resting-state functional connectivity MRI to measure functional connectivity in the intrinsic networks of the brain; and (5) brain imaging to measure changes in regional, cerebral blood flow utilizing PET or IMP-SPECT scans. Improvements observed with these brain imaging techniques would be expected to parallel changes in behavior, such as improved executive function and verbal memory, as well as changes in mental status, including PTSD and depression. The improvements in behavior are also expected to translate into better quality of life for these patients as well as their families. Long-term tLLLT treatments may be necessary to maintain the gains made. These are all factors that require further research.
Conclusions
tLLLT has strong evidence for many beneficial effects on TBI and stroke in both animal models and human patients. Both acute stroke and acute TBI have a growing number of studies being published showing that tLLLT is an effective means of treating both. The many benefits of tLLLT are thought to be based on several different biological mechanisms. Near-infrared PBM functions by improving mitochondrial energy production by stimulating the enzyme CCO and increasing ATP synthesis. Laser therapy can result in neuroprotection and help prevent the spread of brain cell death after injury as shown by the long-term development of smaller lesion areas in mice treated with LLLT, when delivered at 4 h post-TBI. Protection against toxins, increased cellular proliferation, and reduction in apoptosis and anti-inflammatory and antiedema effects may also contribute to the mechanisms that underlie the beneficial effects of PBM. The most exciting prospect is the possibility that tLLLT may stimulate both neurogenesis (the ability of the brain to repair itself) and synaptogenesis (encourage cells to form new synaptic connections). This could lead to the application of tLLLT as a treatment modality for neurodegenerative diseases such as Alzheimer's disease and Parkinson's disease. Well-funded, controlled studies are necessary.
Acknowledgments
MRH was supported by U.S. NIH grant R01AI050875.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bruns J, Jr, Hauser WA. The epidemiology of traumatic brain injury: a review. Epilepsia 2003;44 Suppl 10:2–10 [DOI] [PubMed] [Google Scholar]
- 2.Kraus JF, McArthur DL. Epidemiologic aspects of brain injury. Neurol Clin 1996;14:435–450 [DOI] [PubMed] [Google Scholar]
- 3.Sosin DM, Sniezek JE, Thurman DJ. Incidence of mild and moderate brain injury in the United States, 1991. Brain Inj 1996;10:47–54 [DOI] [PubMed] [Google Scholar]
- 4.Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: a public health perspective. J Head Trauma Rehabil 1999;14:602–615 [DOI] [PubMed] [Google Scholar]
- 5.Finfer SR, Cohen J. Severe traumatic brain injury. Resuscitation 2001;48:77–90 [DOI] [PubMed] [Google Scholar]
- 6.Vink R, Nimmo AJ. Multifunctional drugs for head injury. Neurotherapeutics 2009;6:28–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zink BJ, Szmydynger-Chodobska J, Chodobski A. Emerging concepts in the pathophysiology of traumatic brain injury. Psychiatr Clin North Am 2010;33:741–756 [DOI] [PubMed] [Google Scholar]
- 8.Marklund N, Hillered L. Animal modelling of traumatic brain injury in preclinical drug development: where do we go from here? Br J Pharmacol 2011;164:1207–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutherland JC. Biological effects of polychromatic light. Photochem Photobiol 2002;76:164–170 [DOI] [PubMed] [Google Scholar]
- 10.Antunes F, Boveris A, Cadenas E. On the mechanism and biology of cytochrome oxidase inhibition by nitric oxide. Proc Natl Acad Sci U S A 2004;101:16774–16779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karu T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B 1999;49:1–17 [DOI] [PubMed] [Google Scholar]
- 12.Khuman J, Zhang J, Park J, Carroll JD, Donahue C, Whalen MJ. Low-level laser light therapy improves cognitive deficits and inhibits microglial activation after controlled cortical impact in mice. J Neurotrauma 2012;29:408–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lane N. Cell biology: power games. Nature 2006;443:901–903 [DOI] [PubMed] [Google Scholar]
- 14.Mungrue IN, Stewart DJ, Husain M. The Janus faces of iNOS. Circ Res 2003;93:e74. [DOI] [PubMed] [Google Scholar]
- 15.Shi L, Sordillo LA, Rodriguez-Contreras A, Alfano R. Transmission in near-infrared optical windows for deep brain imaging. J Biophotonics 2016;9:38–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karu TI. Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life 2010;62:607–610 [DOI] [PubMed] [Google Scholar]
- 17.Karu TI, Pyatibrat LV, Kolyakov SF, Afanasyeva NI. Absorption measurements of a cell monolayer relevant to phototherapy: reduction of cytochrome c oxidase under near IR radiation. J Photochem Photobiol B 2005;81:98–106 [DOI] [PubMed] [Google Scholar]
- 18.Capaldi RA, Malatesta F, Darley-Usmar VM. Structure of cytochrome c oxidase. Biochim Biophys Acta 1983;726:135–148 [DOI] [PubMed] [Google Scholar]
- 19.Chen AC, Arany PR, Huang YY, Tomkinson EM, Sharma SK, Kharkwal GB, et al. Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS One 2011;6:e22453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma SK, Kharkwal GB, Sajo M, Huang YY, De Taboada L, McCarthy T, et al. Dose response effects of 810 nm laser light on mouse primary cortical neurons. Lasers Surg Med 2011;43:851–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai T, Huang YY, Hamblin MR. Photodynamic therapy for localized infections—state of the art. Photodiagnosis Photodyn Ther 2009;6:170–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obrenovitch TP, Urenjak J. Is high extracellular glutamate the key to excitotoxicity in traumatic brain injury? J Neurotrauma 1997;14:677–698 [DOI] [PubMed] [Google Scholar]
- 23.Karu TI, Pyatibrat LV, Afanasyeva NI. Cellular effects of low power laser therapy can be mediated by nitric oxide. Lasers Surg Med 2005;36:307–314 [DOI] [PubMed] [Google Scholar]
- 24.Lohr NL, Keszler A, Pratt P, Bienengraber M, Warltier DC, Hogg N. Enhancement of nitric oxide release from nitrosyl hemoglobin and nitrosyl myoglobin by red/near infrared radiation: potential role in cardioprotection. J Mol Cell Cardiol 2009;47:256–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang HL, Whelan HT, Eells JT, Meng H, Buchmann E, Lerch-Gaggl A, et al. Photobiomodulation partially rescues visual cortical neurons from cyanide-induced apoptosis. Neuroscience 2006;139:639–649 [DOI] [PubMed] [Google Scholar]
- 26.Wong-Riley MT, Liang HL, Eells JT, Chance B, Henry MM, Buchmann E, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem 2005;280:4761–4771 [DOI] [PubMed] [Google Scholar]
- 27.Eells JT, Henry MM, Summerfelt P, Wong-Riley MT, Buchmann EV, Kane M, et al. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc Natl Acad Sci U S A 2003;100:3439–3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coombe AR, Ho CT, Darendeliler MA, Hunter N, Philips JR, Chapple CC, et al. The effects of low level laser irradiation on osteoblastic cells. Clin Orthod Res 2001;4:3–14 [DOI] [PubMed] [Google Scholar]
- 29.Hemvani N, Chitnis DS, Bhagwanani NS. Helium-neon and nitrogen laser irradiation accelerates the phagocytic activity of human monocytes. Photomed Laser Surg 2005;23:571–574 [DOI] [PubMed] [Google Scholar]
- 30.Malinovskaya SL, Monich VA, Artifeksova AA. Effect of low-intensity laser irradiation and wideband red light on experimentally ischemized myocardium. Bull Exp Biol Med 2008;145:573–575 [DOI] [PubMed] [Google Scholar]
- 31.Chen AC, Huang YY, Sharma SK, Hamblin MR. Effects of 810-nm laser on murine bone-marrow-derived dendritic cells. Photomed Laser Surg 2011;29:383–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rocha Junior AM, Vieira BJ, de Andrade LC, Aarestrup FM. Low-level laser therapy increases transforming growth factor-beta2 expression and induces apoptosis of epithelial cells during the tissue repair process. Photomed Laser Surg 2009;27:303–307 [DOI] [PubMed] [Google Scholar]
- 33.Bossini PS, Fangel R, Habenschus RM, Renno AC, Benze B, Zuanon JA, et al. Low-level laser therapy (670 nm) on viability of random skin flap in rats. Lasers Med Sci 2009;24:209–213 [DOI] [PubMed] [Google Scholar]
- 34.Corazza AV, Jorge J, Kurachi C, Bagnato VS. Photobiomodulation on the angiogenesis of skin wounds in rats using different light sources. Photomed Laser Surg 2007;25:102–106 [DOI] [PubMed] [Google Scholar]
- 35.Pearson-Fuhrhop KM, Kleim JA, Cramer SC. Brain plasticity and genetic factors. Top Stroke Rehabil 2009;16:282–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marler J. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995;333:1581–1587 [DOI] [PubMed] [Google Scholar]
- 37.Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, et al. Heart disease and stroke statistics—2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2006;113:e85–e151 [DOI] [PubMed] [Google Scholar]
- 38.Oron U, Yaakobi T, Oron A, Mordechovitz D, Shofti R, Hayam G, et al. Low-energy laser irradiation reduces formation of scar tissue after myocardial infarction in rats and dogs. Circulation 2001;103:296–301 [DOI] [PubMed] [Google Scholar]
- 39.Yaakobi T, Shoshany Y, Levkovitz S, Rubin O, Ben Haim SA, Oron U. Long-term effect of low energy laser irradiation on infarction and reperfusion injury in the rat heart. J Appl Physiol (1985) 2001;90:2411–2419 [DOI] [PubMed] [Google Scholar]
- 40.Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng 2012;40:516–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conlan MJ, Rapley JW, Cobb CM. Biostimulation of wound healing by low-energy laser irradiation. A review. J Clin Periodontol 1996;23:492–496 [DOI] [PubMed] [Google Scholar]
- 42.Mirsky N, Krispel Y, Shoshany Y, Maltz L, Oron U. Promotion of angiogenesis by low energy laser irradiation. Antioxid Redox Signal 2002;4:785–790 [DOI] [PubMed] [Google Scholar]
- 43.Leung MC, Lo SC, Siu FK, So KF. Treatment of experimentally induced transient cerebral ischemia with low energy laser inhibits nitric oxide synthase activity and up-regulates the expression of transforming growth factor-beta 1. Lasers Surg Med 2002;31:283–288 [DOI] [PubMed] [Google Scholar]
- 44.Oron A, Oron U, Chen J, Eilam A, Zhang C, Sadeh M, et al. Low-level laser therapy applied transcranially to rats after induction of stroke significantly reduces long-term neurological deficits. Stroke 2006;37:2620–2624 [DOI] [PubMed] [Google Scholar]
- 45.Zhang L, Chen J, Li Y, Zhang ZG, Chopp M. Quantitative measurement of motor and somatosensory impairments after mild (30 min) and severe (2 h) transient middle cerebral artery occlusion in rats. J Neurol Sci 2000;174:141–146 [DOI] [PubMed] [Google Scholar]
- 46.Lapchak PA, Salgado KF, Chao CH, Zivin JA. Transcranial near-infrared light therapy improves motor function following embolic strokes in rabbits: an extended therapeutic window study using continuous and pulse frequency delivery modes. Neuroscience 2007;148:907–914 [DOI] [PubMed] [Google Scholar]
- 47.Detaboada L, Ilic S, Leichliter-Martha S, Oron U, Oron A, Streeter J. Transcranial application of low-energy laser irradiation improves neurological deficits in rats following acute stroke. Lasers Surg Med 2006;38:70–73 [DOI] [PubMed] [Google Scholar]
- 48.Lapchak PA, Wei J, Zivin JA. Transcranial infrared laser therapy improves clinical rating scores after embolic strokes in rabbits. Stroke 2004;35:1985–1988 [DOI] [PubMed] [Google Scholar]
- 49.Lampl Y, Zivin JA, Fisher M, Lew R, Welin L, Dahlof B, et al. Infrared laser therapy for ischemic stroke: a new treatment strategy: results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1). Stroke 2007;38:1843–1849 [DOI] [PubMed] [Google Scholar]
- 50.Zivin JA, Albers GW, Bornstein N, Chippendale T, Dahlof B, Devlin T, et al. Effectiveness and safety of transcranial laser therapy for acute ischemic stroke. Stroke 2009;40:1359–1364 [DOI] [PubMed] [Google Scholar]
- 51.Huisa BN, Stemer AB, Walker MG, Rapp K, Meyer BC, Zivin JA, et al. Transcranial laser therapy for acute ischemic stroke: a pooled analysis of NEST-1 and NEST-2. Int J Stroke 2013;8:315–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stemer AB, Huisa BN, Zivin JA. The evolution of transcranial laser therapy for acute ischemic stroke, including a pooled analysis of NEST-1 and NEST-2. Curr Cardiol Rep 2010;12:29–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lapchak PA, Boitano PD. Transcranial near-infrared laser therapy for stroke: how to recover from futility in the NEST-3 clinical trial. Acta Neurochir Suppl 2016;121:7–12 [DOI] [PubMed] [Google Scholar]
- 54.Zivin JA, Sehra R, Shoshoo A, Albers GW, Bornstein NM, Dahlof B, et al. NeuroThera(R) Efficacy and Safety Trial-3 (NEST-3): a double-blind, randomized, sham-controlled, parallel group, multicenter, pivotal study to assess the safety and efficacy of transcranial laser therapy with the NeuroThera(R) Laser System for the treatment of acute ischemic stroke within 24 h of stroke onset. Int J Stroke 2014;9:950–955 [DOI] [PubMed] [Google Scholar]
- 55.Oron A, Oron U, Streeter J, de Taboada L, Alexandrovich A, Trembovler V, et al. Low-level laser therapy applied transcranially to mice following traumatic brain injury significantly reduces long-term neurological deficits. J Neurotrauma 2007;24:651–656 [DOI] [PubMed] [Google Scholar]
- 56.Oron A, Oron U, Streeter J, De Taboada L, Alexandrovich A, Trembovler V, et al. Near infrared transcranial laser therapy applied at various modes to mice following traumatic brain injury significantly reduces long-term neurological deficits. J Neurotrauma 2012;29:401–407 [DOI] [PubMed] [Google Scholar]
- 57.Quirk BJ, Desmet KD, Henry M, Buchmann E, Wong-Riley M, Eells JT, et al. Therapeutic effect of near infrared (NIR) light on Parkinson's disease models. Front Biosci (Elite Ed) 2012;4:818–823 [DOI] [PubMed] [Google Scholar]
- 58.Moreira MS, Velasco IT, Ferreira LS, Ariga SK, Barbeiro DF, Meneguzzo DT, et al. Effect of phototherapy with low intensity laser on local and systemic immunomodulation following focal brain damage in rat. J Photochem Photobiol B 2009;97:145–151 [DOI] [PubMed] [Google Scholar]
- 59.Wu Q, Xuan W, Ando T, Xu T, Huang L, Huang YY, et al. Low-level laser therapy for closed-head traumatic brain injury in mice: effect of different wavelengths. Lasers Surg Med 2012;44:218–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ando T, Xuan W, Xu T, Dai T, Sharma SK, Kharkwal GB, et al. Comparison of therapeutic effects between pulsed and continuous wave 810-nm wavelength laser irradiation for traumatic brain injury in mice. PLoS One 2011;6:e26212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nawashiro H, Wada K, Nakai K, Sato S. Focal increase in cerebral blood flow after treatment with near-infrared light to the forehead in a patient in a persistent vegetative state. Photomed Laser Surg 2012;30:231–233 [DOI] [PubMed] [Google Scholar]
- 62.Rondina R, 2nd, Olsen RK, McQuiggan DA, Fatima Z, Li L, Oziel E, et al. Age-related changes to oscillatory dynamics in hippocampal and neocortical networks. Neurobiol Learn Mem 2016;134 Pt A:15–30 [DOI] [PubMed] [Google Scholar]
- 63.Huang YY, Chen AC, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy. Dose Response 2009;7:358–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xuan W, Vatansever F, Huang L, Wu Q, Xuan Y, Dai T, et al. Transcranial low-level laser therapy improves neurological performance in traumatic brain injury in mice: effect of treatment repetition regimen. PLoS One 2013;8:e53454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Q, Zhou C, Hamblin MR, Wu MX. Low-level laser therapy effectively prevents secondary brain injury induced by immediate early responsive gene X-1 deficiency. J Cereb Blood Flow Metab 2014;34:1391–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dong T, Zhang Q, Hamblin MR, Wu MX. Low-level light in combination with metabolic modulators for effective therapy of injured brain. J Cereb Blood Flow Metab 2015;35:1435–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xuan W, Vatansever F, Huang L, Hamblin MR. Transcranial low-level laser therapy enhances learning, memory, and neuroprogenitor cells after traumatic brain injury in mice. J Biomed Opt 2014;19:108003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xuan W, Agrawal T, Huang L, Gupta GK, Hamblin MR. Low-level laser therapy for traumatic brain injury in mice increases brain derived neurotrophic factor (BDNF) and synaptogenesis. J Biophotonics 2015;8:502–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tedford CE, DeLapp S, Jacques S, Anders J. Quantitative analysis of transcranial and intraparenchymal light penetration in human cadaver brain tissue. Lasers Surg Med 2015;47:312–322 [DOI] [PubMed] [Google Scholar]
- 70.Wan S, Parrish JA, Anderson RR, Madden M. Transmittance of nonionizing radiation in human tissues. Photochem Photobiol 1981;34:679–681 [DOI] [PubMed] [Google Scholar]
- 71.Naeser MA, Saltmarche A, Krengel MH, Hamblin MR, Knight JA. Improved cognitive function after transcranial, light-emitting diode treatments in chronic, traumatic brain injury: two case reports. Photomed Laser Surg 2011;29:351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Naeser MA, Zafonte R, Krengel MH, Martin PI, Frazier J, Hamblin MR, et al. Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: open-protocol study. J Neurotrauma 2014;31:1008–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]