Abstract
Cryopreservation of biological materials such as cells, tissues, and organs is a prevailing topic of high importance. It is employed not only in many research fields but also in the clinical area. Cryopreservation is of great importance for reproductive medicine and clinical studies, as well as for the development of vaccines. Peripheral blood mononuclear cells (PBMCs) are commonly used in vaccine research where comparable and reliable results between different research institutions and laboratories are of high importance. Whereas freezing and thawing processes are well studied, controlled, and standardized, storage conditions are often disregarded. To close this gap, we investigated the influence of suboptimal storage conditions during low-temperature storage on PBMC viability, recovery, and T cell functionality. For this purpose, PBMCs were isolated and exposed with help of a robotic system in a low-temperature environment from 0 up to 350 temperature fluctuation cycles in steps of 50 cycles to simulate storage conditions in large biorepositories with sample storage, removal, and sorting functions. After the simulation, the viability, recovery, and T cell functionality were analyzed to determine the number of temperature rises, which ultimately lead to significant cell damage. All studied parameters decreased with increasing number of temperature cycles. Sometimes after as little as only 50 temperature cycles, a significant effect was observed. These results are very important for all fields in which cell cryopreservation is employed, particularly for clinical and multicenter studies wherein the comparability and reproducibility of results play a crucial role. To obtain reliable results and to maintain the quality of the cells, not only the freezing and thawing processes but also the storage conditions should be controlled and standardized, and any deviations should be documented.
Keywords: : PBMC, cryopreservation, temperature fluctuations, T-cell functionality, viability, recovery
Introduction
Peripheral blood mononuclear cells (PBMCs) are commonly used not only in research of infectious diseases, such as HIV,1,2 malaria,3 and tuberculosis,4 but also in other fields of research such as cancer5 and dementia6,7 As cells of the immune system, they play a crucial role in vaccine development8–10 and clinical trials.11–13 Therefore, large quantities of PBMC samples are often isolated and stored in biobanks. The storage in biorepositories enables multicenter10,14 as well as retrospective studies. Nowadays, a variety of samples such as blood, stem cells, tissues, oocytes, and sperms, as well as environmental specimens15 are stored in biorepositories. Optimal cryopreservation enables storage of samples by maintaining viability and functionality.14 However, every sample reacts with different sensitivity to cryopreservation.
In the past, freezing and thawing processes have been studied extensively. Suboptimal freezing and thawing conditions lead to cell damage. There are two basic damage mechanisms. First, the mechanical damage caused by the formation of intracellular ice crystals,16,17 and second, the osmotic damages due to high intracellular salt concentrations as a result of water loss after crystallization. The loss of water has further consequences, which Farrant described as solution effects.18 Mazur et al. established the two-factor hypothesis wherein they described the optimal freezing rate for successful cryopreservation.19 Based on these findings, the freezing and thawing processes are now mostly standardized and controlled, for example through the use of freezing containers. To avoid biochemical reactions and recrystallization processes, the storage temperature should be below the glass transition temperature of −130°C.19–22
However, in contrast to the freezing and thawing processes, the storage conditions are often much less controlled or standardized. Referring to this issue, some research groups studied the effect of storage time and temperature on cell viability and T cell functionality.23–28 The T cell response as an indicator of effective vaccines plays a crucial role in the development of current vaccines such as for HIV.29 The maintenance of T cell functionality during cryopreservation is therefore very important to produce reliable results.10,26,30–34
Uncontrolled storage can easily lead to multiple temperature changes during sample storage, sorting, and removal.30 In 2013, Germann et al. studied the effect of short temperature rises during deep temperature storage to PBMC viability, recovery, and T cell functionality.30 The cells were first exposed to 400 temperature cycles and then analyzed. They determined a reduction of all sampled parameters after 400 temperature shifts.30
To complement this study, we isolated and froze PBMCs of different human donors and simulated suboptimal storage handling with a robotic system in a low-temperature environment. Therefore, we tried to mimic sample banking practice in a large biorepository. The basis of our hypothesis was that samples in biobanks are generally stored and removed several times a day. In this case, the samples, which are already stored in the biobank, are also involved in the new storage and removal processes and therefore also exposed to temperature rises. In contrast to Germann et al.30 where the influence of temperature fluctuations on cell viability, recovery, and functionality was only determined after completing 400 cycles, we analyzed the effects after every 50th cycle step. This allows us to observe the minimum number of temperature cycles causing significant cell damage.
For this study, the isolated cells were frozen and exposed to 350 temperature fluctuation cycles. Three samples were removed after every 50th cycle step to analyze the influence of temperature fluctuations. PBMC viability and recovery were determined directly after thawing and after an overnight culture. The T cell functionality was quantified by interferon-gamma (IFN-γ) ELISpots.
Materials and Methods
Isolation of PBMCs
PBMCs were isolated from buffy coat samples from healthy cytomegalovirus (CMV)-seropositive donors at the blood donor center, Blutspendezentrale Saar-Pfalz gGmbH Am Klinikum Saarbruecken (Saarbruecken, Germany). They gave written informed consent that the buffy coats could be used for research purposes.
The isolation of PBMCs was performed by using density gradient centrifugation and Ficoll (Histopaque-1007; Sigma-Aldrich, Taufkirchen, Germany) as the PBMC separation medium. It separated the PBMCs from almost all erythrocytes, granulocytes, and dead cells. The PBMCs were collected and washed with phosphate-buffered saline (PBS; Gibco, Karlsruhe, Germany). The remaining erythrocytes were lysed. Then, 2 × 108 PBMCs were incubated in Pharm Lyse (BD Biosciences, Heidelberg, Germany) for 30 minutes in the dark. PBMCs were purified by several washing steps.
Cryopreservation
Isolated PBMCs were frozen in cryomedium IBMT I (Fraunhofer IBMT, Sulzbach, Germany)35 with a final concentration of 1 × 107 cells/mL in 1-mL aliquots. The samples were transferred into precooled (+4°C), freezing isopropanol containers (Mr. Frosty™; Thermo Fisher Scientific, Dreieich, Germany) where the cells were frozen at a controlled cooling rate of −1°C/min from +4°C to −80°C. Twenty-four hours after the freezing process, the frozen cells were transferred into the vapor phase of liquid nitrogen (LN2) below −135°C for storage.
Simulation of suboptimal storage conditions
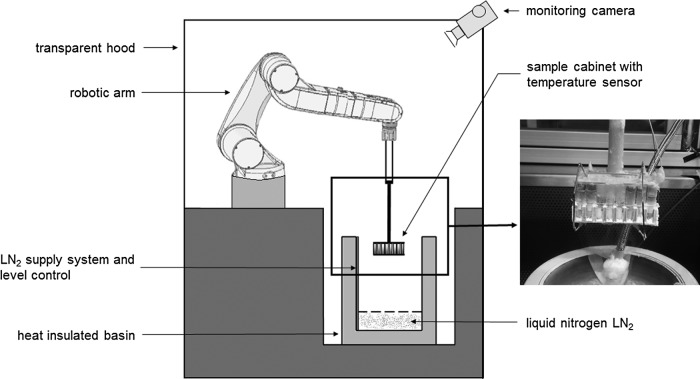
To simulate sample storage, sorting, and removal processes in biorepositories and clinical trials, PBMCs were cycled from below −130°C up to −60°C to 350 times in steps of 50 cycles (0, 50, 100, …, 350) by a robotic system (Fig. 1). The samples were placed into a sample cabinet of the robotic arm. The robotic arm was able to change its vertical direction between two positions. In the first position, the samples were in the gas phase of LN2 below −130°C. By changing its vertical direction to the second position, the samples were exposed for about 5 minutes to room temperature until the temperature inside the samples reached a defined temperature of −60°C.
FIG. 1.
Automated robotic system with robotic arm and sample cabinet. Robotic arm changed vertical position. Samples exposed to room temperature and temperature below −130°C. Temperature shift simulates storage conditions during biobanking. Modified from Germann et al.30
The temperature was measured with a Type T thermocouple embedded inside a reference sample. After warming to a defined temperature of −60°C, the samples were lowered to below −130°C in the vapor phase of LN2. The cooling and warming rates during the experiment correlate with the rates in a normal cryotank when samples are sorted and removed.
Every 50 cycles, three samples were replaced with dummies and transported, maintaining the cooling chain, into the gasphase of LN2 of a cryotank until further analysis. Thus, for every cycle condition (0, 50, 100, …, 350), we had three samples per donor. The cycling process is described in detail in Figure 2.
FIG. 2.
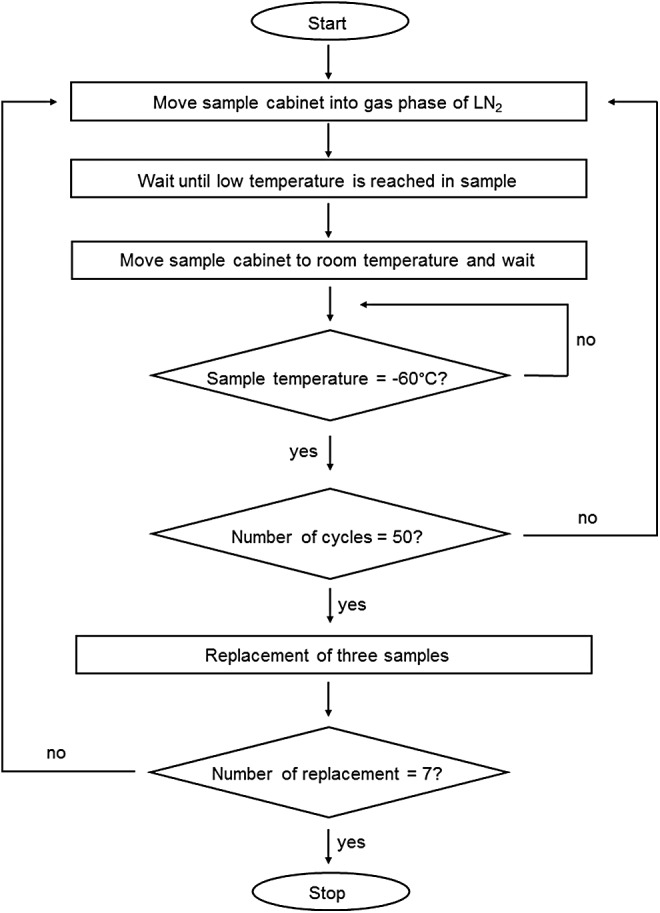
Cycle program in detail. After starting the cycle program, the sample cabinet is moved into the cold gas phase above liquid nitrogen until a temperature below −130°C is reached in the reference sample. Next, the sample cabinet moves up into a room temperature zone until a defined temperature of −60°C is reached in the reference sample. This process is repeated until the last remaining samples are exposed to 350 cycles.
Thawing PBMCs
The frozen PBMCs were thawed in a water bath at 37°C. One milliliter of prewarmed medium (RPMI 1648 medium [Gibco, Darmstadt, Germany] supplemented with 25 mM HEPES buffer [Gibco, Darmstadt, Germany], 1 mM l-glutamine [Gibco, Darmstadt, Germany], 1 × penicillin/streptomycin [PAA; Cölbe, Germany], and 10% heat-inactivated fetal calf serum [FCS; [Gibco, Darmstadt, Germany]) was added to the thawed cells. The cell suspension was transferred to 50-mL tubes (Greiner Bio-One, Frickenhausen, Germany) filled with 8 mL medium (end volume 10 mL). The cells were centrifuged at 400 × g for 6 minutes at room temperature; 1 × 107 cells were resuspended in 10 mL medium and incubated overnight in a cell incubator at 37°C and 5% CO2.
Determination of cell viability and cell recovery
The influence of temperature changes during deep temperature storage on cell viability and cell recovery was determined by using the trypan blue dye exclusion test. The measurement was performed using an automated cell analyzer (ViCell cell analyzer; Beckman Coulter, Krefeld, Germany). The cell viability and cell recovery from three samples per cycle condition per donor were measured three times immediately after thawing as well as after overnight culture.
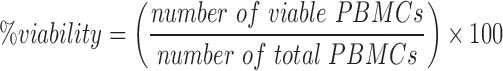 |
Viability:
Recovery (%) directly after thawing:
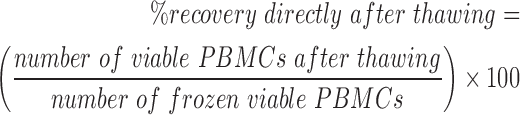 |
Recovery (%) after overnight culture:
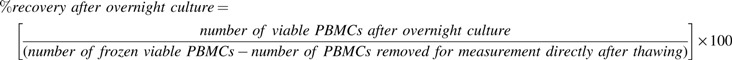
Determination of T cell functionality
IFN-γ ELISpots were performed to determine the influence of temperature changes during deep temperature storage on T cell functionality.
The immune response was stimulated by CMV pp65 peptide pool (cytomegalovirus; JTP, Berlin, Germany), CEF peptide pool (peptide mixture of cytomegalovirus, Epstein-Barr virus, influenza virus; CTL, Aalen, Germany), and PHA (phytohemagglutinin; Thermo Fisher Scientific) as a positive control. Thawing medium containing 0.4% dimethyl sulfoxide (DMSO; Sigma-Aldrich) was used as background control.
After overnight culture, 1 × 105 PBMCs were added into the wells of a precoated anti-human-IFN-γ mAb 1-D1k 96-well plate (Mabtech AB, Hamburg, Germany). To stimulate an immune response, the different peptides were added to a final concentration of 2 μg/mL CMV, 4 μg/mL CEF, and 1 μg/mL PHA and incubated at 37°C and 5% CO2 for 20–22 hours. After incubation, the plate was washed with PBS-Tween (PBS supplemented with 1% FCS and 250 μL Tween 20; Sigma-Aldrich). The production of IFN-γ was detected by using a biotinylated detection antibody (mAb 7-B6-1; Mabtech AB) diluted 1:500 in PBS +10% FCS and incubated for 3 hours and a streptavidin-horseradish peroxidase complex (Mabtech AB) diluted 1:300 in PBS +10% FCS and incubated for 1 hour. After the incubation time, the plate was washed with PBS-Tween and PBS. The spot development was performed by using the Nova Red Substrate Kit (Vector, Burlingame, CA) and stopped after 15 minutes, washing the plate from every site with distilled water. After drying the plate overnight, the spots were evaluated with Immunospot Analyzer (CTL) and the results were specified as spot-forming cells (SFCs per million PBMCs).
Quality assurance
The study was performed in a laboratory, which is under Good Clinical Laboratory Practice. There are standard operating procedures and the staff is well trained. The performance of ELISpot assay is a long-standing process in the laboratory and is periodically evaluated by an External Quality Assessment Program Oversight Laboratory (EQAPOL) and ELISpot Quality Assurance Program.
Statistics
Statistical tests were performed using OriginPro 9.0. All data are shown as mean ± standard deviation. For statistical testing, we used the individual values. In summary, for every cycle condition, we performed 36 individual measurements for viability, 36 for recovery, and triplicates for T cell functionality. After testing the sample population for normal distribution through the Kolmogorov-Smirnov test, statistical differences between storage with and without temperature fluctuations were calculated by using a one-way analysis of variance (ANOVA) Bonferroni test. To quantify the reduction of T cell functionality, a linear regression analysis over the numbers of cycles was performed. A p-value of 0.05 is specified as the significance level.
Results
Influence of temperature cycles on PBMC viability and recovery
To study the influence of temperature changes during deep temperature storage, PBMCs from four CMV-positive donors were isolated and exposed to 0–350 temperature fluctuations in steps of 50 cycles with a robotic system. After the simulation of temperature fluctuations during cell storage, the PBMC viability and recovery were determined immediately after thawing as well as after overnight culture, through a trypan blue exclusion test.
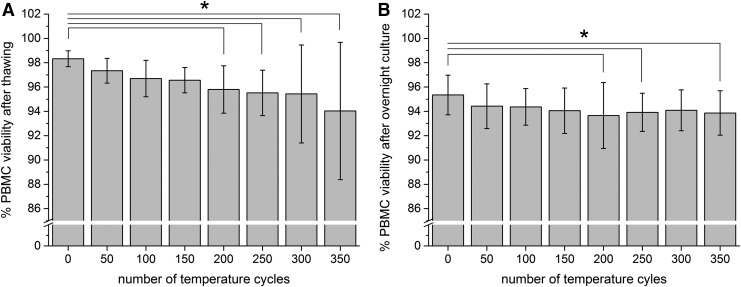
The viability of the samples stored without temperature fluctuations in the vapor phase of LN2 directly after thawing was 98.33% ± 0.65% (Fig. 3A). After 350 temperature cycles, the viability decreased to 94.03% ± 5.65%. The difference became statistically significant after 200 temperature fluctuations (N = 36, one-way ANOVA Bonferroni with p < 0.05, R2 = 0.17051, η2 = 1.0089). After overnight culture, the viability of the samples stored in the gas phase of LN2 was 95.35% ± 1.63% and decreased to 93.87% ± 1.83% after 350 temperature cycles (Fig. 3B). A significant difference was observed after 200, 250, and 350 temperature cycles (N = 36, one-way ANOVA Bonferroni with p < 0.05, R2 = 0.06666, η2 = 1.0019).
FIG. 3.
PBMC viability after thawing (A) and after overnight culture (B). Figures show the PBMC viability after 0–350 temperature cycles. PBMC viability determined directly after thawing and after overnight culture. Figures show mean ± standard deviation. *Statistical difference to 0 cycles with p < 0.05. PBMC, peripheral blood mononuclear cell.
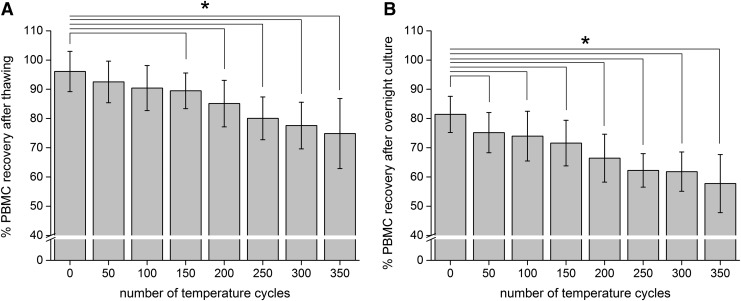
Like the PBMC viability, the recovery decreased with increasing numbers of temperature fluctuations, but to a higher degree. After thawing, the recovery was 96.08% ± 6.91% (0 cycles) and decreased to 74.86% ± 11.97% after 350 cycles (Fig. 4A). A significant difference, after thawing, occurred after 150 temperature cycles (Fig. 4A; N = 36, one-way ANOVA Bonferroni with p < 0.05, R2 = 0.44609, η2 = 1.0096). After an overnight rest, the recovery of samples stored in the gas phase of LN2 was 81.41% ± 6.20% and decreased to 57.74% ± 9.94% after 350 cycles (Fig. 4B). After overnight culture, a significant difference was observed after only 50 temperature fluctuations (Fig. 4B, N = 36, one-way ANOVA Bonferroni with p < 0.05, R2 = 0.502, η2 = 1.0076).
FIG. 4.
PBMC recovery after thawing (A) and after overnight culture (B). Figures show the PBMC recovery after 0–350 temperature cycles. PBMC recovery determined directly after thawing and after overnight culture. Figures show mean ± standard deviation. *Statistical difference to 0 cycles with p < 0.05.
Influence of temperature cycles on T cell functionality
To examine a possible influence of temperature fluctuations on the T cell functionality, IFN-γ ELISpots were performed. To specify positive T cell responses, the average number of SFCs/1 × 106 PBMCs was determined from three replicate wells. Following standardization and validation issues of ELISpot assay,36 we used the definition of responder R>4D and R>55, where R is the SFCs/1 × 106 PBMCs for the reagents (CEF and CMV) and D corresponds to SFCs for background. All samples were positive for both CEF and CMV. Furthermore, we classified the immune response of donors in IFN-γ ELISpot in four different groups to compare the reactivity: negative <55 SFCs/1 × 106 PBMCs, low responder <600 SFCs/1 × 106 PBMCs, medium responder <2000 SFCs/1 × 106 PBMCs, and high responder >2000 SFCs/1 × 106 PBMCs.
After CEF stimulation, none of four donors were classified negative, two donors as low responders, one donor as medium responder, and one donor was classified as high responder (Table 1). After stimulation with CMV, none of four donors were classified negative, two donors as low responders, and two donors as high responders (Table 2).
Table 1.
Interferon-Gamma ELISpot Response of Four Different CMV-Seropositive Donors Stimulated with CEF Peptide Pool After Temperature Cycles
| No. of temperature cycles | Donor 1, high responder | Donor 2, low responder | Donor 3, medium responder | Donor 4, low responder |
|---|---|---|---|---|
| CEF | ||||
| 0 | 4120 ± 365 | 367 ± 6 | 1440 ± 269 | 563 ± 32 |
| 50 | 4223 ± 180 | 317 ± 90 | 1217 ± 99 | 457 ± 71 |
| 100 | 4007 ± 142 | 320 ± 17 | 1170 ± 87 | 570 ± 123 |
| 150 | 3867 ± 55 | 430 ± 26 | 1363 ± 0 | 400 ± 46 |
| 200 | 3660 ± 200 | 323 ± 70 | 897 ± 126 | 417 ± 121 |
| 250 | 3460 ± 105 | 310 ± 115 | 810 ± 92 | 410 ± 62 |
| 300 | 3427 ± 164 | 267 ± 38 | 790 ± 30 | 380 ± 36 |
| 350 | 3797 ± 23 | 260 ± 50 | 930 ± 168 | 347 ± 38 |
Values show mean ± standard deviation of SFCs per 1 × 106 PBMCs from three replicate wells.
PBMCs, peripheral blood mononuclear cells; SFCs, spot-forming cells.
Table 2.
Interferon-Gamma ELISpot Response of Four Different CMV-Seropositive Donors Stimulated with CMV Peptide Pool After Temperature Cycles
| No. of temperature cycles | Donor 1, high responder | Donor 2, low responder | Donor 3, high responder | Donor 4, low responder |
|---|---|---|---|---|
| CMV | ||||
| 0 | 4863 ± 195 | 187 ± 42 | 6280 ± 140 | 237 ± 38 |
| 50 | 4993 ± 133 | 170 ± 36 | 5970 ± 125 | 193 ± 32 |
| 100 | 4807 ± 182 | 210 ± 50 | 5807 ± 29 | 140 ± 35 |
| 150 | 4620 ± 10 | 170 ± 20 | 5960 ± 118 | 200 ± 20 |
| 200 | 4297 ± 272 | 137 ± 31 | 5130 ± 159 | 167 ± 45 |
| 250 | 4073 ± 59 | 197 ± 81 | 5697 ± 172 | 170 ± 20 |
| 300 | 3827 ± 120 | 153 ± 25 | 5363 ± 25 | 177 ± 46 |
| 350 | 4500 ± 108 | 130 ± 10 | 5033 ± 171 | 160 ± 70 |
Values show mean ± standard deviation of SFCs per 1 × 106 PBMCs from three replicate wells. SFC, spot-forming cell.
Donor 1 showed a high response on CEF as well as on CMV stimulation. Except for 350 cycles, the T cell response decreased with increasing numbers of temperature cycles (Tables 1 and 2). For Donor 2, a low responder, a decrease with increasing numbers of temperature cycles after both CEF and CMV stimulation was also observed. Donors 3, apart from 350 cycles, and 4 showed similar behavior (Tables 1, 2). After stimulation with CEF, the decrease of the high responder cells became significant after 250 and 300 cycles and medium responder's cells after 200 cycles. The low responder cells showed no significant degradation.
After stimulation with CMV peptide pool, the decrease in high responder cells of Donor 1 was significant after 200, 250, and 300 temperature cycles and for Donor 3 after 100 cycles. The low responder cells showed no statistically significant decrease.
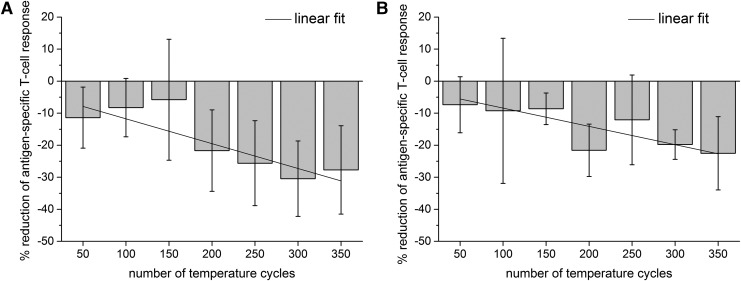
To present the decrease in a clearer manner, we pooled and normalized the data of all donors. A linear regression model was used to calculate the relative reduction per 50 cycles in percent (%). After stimulation with either CEF or CMV peptides, the immune response decreased with increasing numbers of temperature fluctuations. After 50 cycles, the T cell response decreased about −11.39% ± 9.52% after CEF stimulation and about −7.38% ± 8.73% (Fig. 5A). The decrease reached a maximum of −27.70% ± 13.80% after CEF stimulation (Fig. 5A) and −22.52% ± 11.44% after CMV stimulation after 350 temperature cycles (Fig. 5B).
FIG. 5.
Reduction of antigen-specific T cell response. (A) Shows reduction of T cell response after stimulation with CEF peptide pool and (B) after stimulation with CMV peptide pool. To show the reduction in a clearer way, a linear fit was performed and is presented as a line. Figures show reduction of immune response after 50–350 temperature cycles in comparison with 0 cycles. Figures show mean ± standard deviation of four donors. The gradients are significantly different from zero temperature cycles with p < 0.05.
The pooled data of each 50th cycle group show no significant differences (N = 12, one-way ANOVA Bonferroni with p < 0.05, R2 [CEF] = 0.50355, R2 [CMV] = 0.36517). The linear regression model yielded a significant reduction of the gradient of −3.88% ± 0.82% (N = 7, R2 = 0.81607, p = 0.00529) after every 50th cycle step, after stimulation with CEF peptides, and −2.86% ± 0.78% (N = 7, R2 = 0.72575, p = 0.01494) after every 50th cycle step after stimulation with CMV peptides.
Discussion
Cryopreservation below −130°C is widely used in clinical trials,11,12 multicenter studies,10 and in almost every biological and biomedical research field. It enables long-term storage,24,31 maintaining functionality and viability. A variety of samples such as stem cells, blood, oocytes, and sperms, or environmental samples are stored in large amounts in biorepositories. While the freezing and thawing processes are well standardized, the storage conditions are often less controlled. Several research groups showed that suboptimal storage conditions lead to cell damage.26,30,37 Because cryopreservation is becoming increasingly important, our findings are of great interest, particularly for biobanks and clinical trials.
In this study, we showed that a small number of temperature rises from below −130°C up to −60°C during deep temperature storage negatively affects the viability, recovery, and T cell functionality of PBMCs. We further detected the number of such temperature rises, which leads to significant cell damages.
For this purpose, we exposed isolated and frozen PBMCs from four different donors from below −130°C up to −60°C, from 0 to 350 temperature cycles, to mimic storage conditions within sample sorting, removal, and transfer in biobanking and clinical trials. In this process, the samples are not thawed, only reaching a temperature of −60°C. This temperature was chosen because it is a critical temperature where recrystallization processes and biochemical reactions can occur. The incorporation of PBMCs from different donors in this study was chosen to get a first qualitative insight into interperson variability.
However, the question of this study was especially if we can observe the same general trend for all donors. During the simulation of suboptimal storage conditions, we removed samples after every 50th cycle step to determine the influence on PBMC viability and recovery by the trypan blue exclusion test and on T cell functionality by means of IFN-γ ELISpot. The assays and methods in this study show the expected variability when working with cell suspensions, such as potential cell agglomeration, counting errors, transfer losses, and other sources of variability.
We observed high viability and recovery in our control cells continuously stored below −130°C in the gas phase of LN2, demonstrating the suitability of our freezing and thawing processes. Similar control results were reported by some other research groups.24,32,38 This indicates that optimized cell cryopreservation and storage conditions without temperature fluctuations lead to a suitable recovery and maintenance of cell viability and functionality for subsequent immune assays.
We further found that all examined parameters, the viability, the recovery, and the T cell functionality, decreased nearly proportionally with increasing numbers of temperature cycles during deep temperature storage. Obviously, any processes, which lead to cell damages, occur during the warming process from −130°C up to −60°C, for example, biochemical reactions and ice recrystallization processes above the glass transition temperature. They can damage the cell membrane and the cytoskeleton in multiple ways.16,17,21 It is also known that during the freezing and thawing processes, two main cell damage mechanisms take place, which are described by Lovelock.39–42 On the one hand, this is due to the formation of intracellular ice crystals,17 and on the other hand, due to osmotic damages, which are a result of high salt concentration due to water loss during crystallization. The water loss has additional consequences, which are also described as solution effects.18 The phospholipid membrane can be influenced by dehydration of the cell, leading to an increased sensitivity to mechanical effects.43,44 Furthermore, the tertiary structure can be damaged due to the high intracellular salt concentration, which can lead to loss of functionality, including denaturation of proteins. Accordingly, we suspect that these processes also took place during the warming process from below −130°C up to −60°C.
We observed a decrease of recovery and viability after overnight culture in comparison with the measurement directly after thawing. This may not only be due to normal apoptosis and necrosis processes during overnight culture,25,29,45,46 but it may also be caused by preapoptotic cells, which are triggered by cryopreservation to induce apoptosis during overnight culture.23 This difference did not change with increasing numbers of cycles (data not shown). Moreover, Smith et al. showed by simulating transport conditions that the number of apoptotic cells increases and additionally the T cell response decreases.26
The T cell response is a crucial factor in vaccine development.29 Therefore, reliable performance of the ELISpot assay is important. Thus, we also investigated the influence of suboptimal storage on T cell functionality. We observed a high T cell response for the control cells stored below −130°C without temperature fluctuations. Furthermore, we observed that the T cell response of the cells, which were exposed to the temperature rises, decreased with increasing numbers of temperature cycles. Due to the expected variability when working with cell suspensions together with the small intervals of 50 temperature cycles, not every interval data group significantly differs from the one before or after.
Nevertheless, the linear regression analysis, statistically and graphically, shows a significant reduction of the antigen-specific T cell response over increasing number of temperature cycles. We suspect that changes of cell surface molecules and changes of expression levels, which were described by Costantini et al. (2003), are also triggered by the temperature rises from below −130°C up to −60°C.47 Furthermore, Kreher et al. showed that cytokine secretion changed due to freezing and thawing processes.38 This could explain the observed reduction in T cell response in ELISpot.
Above all, the maintenance of T cell functionality during cryopreservation is controversially discussed. Regarding the T cell immune response, there are different observations. Meanwhile, Owen et al. noticed the loss of T cell response, and Kreher et al. observed the maintenance of antigen-specific T cell response.25,38
We suspect that the storage conditions may potentially be responsible for the different observations. Thus, for optimal storage conditions, it is important to avoid temperature fluctuations. The detection and documentation of deviations in deep temperature storage play a crucial role, especially in terms of storage duration and sample history. Samples, which are often stored for several years, could potentially degrade with regard to various examined parameters. Moreover, sample history regarding temperature fluctuations in long-term storage is most often unknown. To understand this retrospectively, the temperature should be recorded over longer periods in regular short intervals. It would be an improvement toward standardization and optimal cryopreservation if updated quality management systems and standard operating procedures would be established.
Although the cell suspension did not thaw completely, but merely warmed up to −60°C, the temperature rises sufficed to lead to multiple cell damages. In summary, we showed that temperature rises during deep temperature storage lead to reduction of viability, recovery, and T cell functionality of PBMCs. The study emphasizes the need for controlled storage, especially in clinical studies and biobanks, to yield comparable and reliable results and to maintain the quality of cryopreserved samples.
Furthermore, to guarantee controlled storage and handling without interruption of the cooling chain, protective hood systems, wherein the working area for sample removal and sorting is cooled down, are recommended. This would reduce the temperature fluctuations to a minimum. Moreover, establishment of an updated quality management system and introduction of standard operating procedures wherein, among others, deviations are documented would lead to a better standardization and quality of the respective samples.
In the future, more attention should be paid to the storage conditions to achieve a high recovery, maintain the viability, and the T cell functionality of cells. Moreover, studies are needed to determine whether other cell types such as stem cells and other biological materials react in a similar way to temperature cycles during cryostorage. In addition, further aspects, including protein degradation, will be included in subsequent studies.
Acknowledgments
The authors thank B. Kemp-Kamke for her excellent technical assistance. This work was supported by the Bill & Melinda Gates Foundation grant number OPP38580_01.
Author Disclosure Statement
No conflicting financial interests exist.
References
- 1.Mlotshwa M, Riou C, Chopera D, et al. . Fluidity of HIV-1-specific T-cell responses during acute and early subtype C HIV-1 infection and associations with early disease progression. J Virol 2010;84:12018–12029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torresi J, Bharadwaj M, Jackson DC, et al. . Neutralising antibody, CTL and dendritic cell responses to hepatitis C virus: A preventative vaccine strategy. Curr Drug Targets 2004;5:41–56 [DOI] [PubMed] [Google Scholar]
- 3.Riccio EK, Neves I, Banic DM, et al. . Cryopreservation of peripheral blood mononuclear cells does not significantly affect the levels of spontaneous apoptosis after 24-h culture. Cryobiology 2002;45:127–134 [DOI] [PubMed] [Google Scholar]
- 4.Sester M, Giehl C, Sester U, et al. . Management of tuberculosis in HIV infection: Where T-cells matter. Eur Respir J 2010;35:475–476 [DOI] [PubMed] [Google Scholar]
- 5.Gilboa E. The promise of cancer vaccines. Nat Rev Cancer 2004;4:401–411 [DOI] [PubMed] [Google Scholar]
- 6.Arosio B, D'Addario C, Gussago C, et al. . Peripheral blood mononuclear cells as a laboratory to study dementia in the elderly. BioMed Res Int 2014;2014:169203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Francesco A, Arosio B, Falconi A, et al. . Global changes in DNA methylation in Alzheimer's disease peripheral blood mononuclear cells. Brain Behav Immun 2015;45:139–144 [DOI] [PubMed] [Google Scholar]
- 8.Bourguignon P, Clement F, Renaud F, et al. . Processing of blood samples influences PBMC viability and outcome of cell-mediated immune responses in antiretroviral therapy-naive HIV-1-infected patients. J Immunol Methods 2014;414:1–10 [DOI] [PubMed] [Google Scholar]
- 9.Wieczorek L, Krebs SJ, Kalyanaraman V, et al. . Comparable antigenicity and immunogenicity of oligomeric forms of a novel, acute HIV-1 subtype C gp145 envelope for use in preclinical and clinical vaccine research. J Virol 2015;89:7478–7493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarzotti-Kelsoe M, Needham LK, Rountree W, et al. . The Center for HIV/AIDS Vaccine Immunology (CHAVI) multi-site quality assurance program for cryopreserved human peripheral blood mononuclear cells. J Immunol Methods 2014;409:21–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris SA, Satti I, Matsumiya M, et al. . Process of assay selection and optimization for the study of case and control samples from a phase IIb efficacy trial of a candidate tuberculosis vaccine, MVA85A. Clin Vaccine Immunol 2014;21:1005–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanchez AM, Denny TN, O'Gorman M. Introduction to a special issue of the Journal of Immunological Methods: Building global resource programs to support HIV/AIDS clinical trial studies. J Immunol Methods 2014;409:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheret A, Nembot G, Melard A, et al. . Intensive five-drug antiretroviral therapy regimen versus standard triple-drug therapy during primary HIV-1 infection (OPTIPRIM-ANRS 147): A randomised, open-label, phase 3 trial. Lancet Infect Dis 2015;15:387–396 [DOI] [PubMed] [Google Scholar]
- 14.Germann A, Schulz JC, Kemp-Kamke B, et al. . Standardized serum-free cryomedia maintain peripheral blood mononuclear cell viability, recovery, and antigen-specific T-cell response compared to fetal calf serum-based medium. Biopreserv Biobank 2011;9:229–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lermen D, Schmitt D, Bartel-Steinbach M, et al. . A new approach to standardize multicenter studies: Mobile lab technology for the German Environmental Specimen Bank. PloS One 2014;9:e105401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bischof JC, Rubinsky B. Large ice crystals in the nucleus of rapidly frozen liver cells. Cryobiology 1993;30:597–603 [DOI] [PubMed] [Google Scholar]
- 17.Takamatsu H, Rubinsky B. Viability of deformed cells. Cryobiology 1999;39:243–251 [DOI] [PubMed] [Google Scholar]
- 18.Farrant J. Water transport and cell survival in cryobiological procedures. Philos Trans R Soc Lond B Biol Sci 1977;278:191–205 [DOI] [PubMed] [Google Scholar]
- 19.Mazur P, Leibo SP, Chu EH. A two-factor hypothesis of freezing injury. Evidence from Chinese hamster tissue-culture cells. Exp Cell Res 1972;71:345–355 [DOI] [PubMed] [Google Scholar]
- 20.Mazur P. Kinetics of water loss from cells at subzero temperatures and the likelihood of intracellular freezing. J Gen Physiol 1963;47:347–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meryman HT. Preservation of living cells. Fed Proc 1963;22:81–89 [PubMed] [Google Scholar]
- 22.Friedler S, Giudice LC, Lamb EJ. Cryopreservation of embryos and ova. Fertil Steril 1988;49:743–764 [DOI] [PubMed] [Google Scholar]
- 23.Fowke KR, Behnke J, Hanson C, et al. . Apoptosis: A method for evaluating the cryopreservation of whole blood and peripheral blood mononuclear cells. J Immunol Methods 2000;244:139–144 [DOI] [PubMed] [Google Scholar]
- 24.Kleeberger CA, Lyles RH, Margolick JB, et al. . Viability and recovery of peripheral blood mononuclear cells cryopreserved for up to 12 years in a multicenter study. Clin Diagn Lab Immunol 1999;6:14–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Owen RE, Sinclair E, Emu B, et al. . Loss of T cell responses following long-term cryopreservation. J Immunol Methods 2007;326:93–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith JG, Joseph HR, Green T, et al. . Establishing acceptance criteria for cell-mediated-immunity assays using frozen peripheral blood mononuclear cells stored under optimal and suboptimal conditions. Clin Vaccine Immunol 2007;14:527–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulz JC, Germann A, Kemp-Kamke B, et al. . Towards a xeno-free and fully chemically defined cryopreservation medium for maintaining viability, recovery, and antigen-specific functionality of PBMC during long-term storage. J Immunol Methods 2012;382:24–31 [DOI] [PubMed] [Google Scholar]
- 28.Weinberg A, Song LY, Wilkening CL, et al. . Optimization of storage and shipment of cryopreserved peripheral blood mononuclear cells from HIV-infected and uninfected individuals for ELISPOT assays. J Immunol Methods 2010;363:42–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kierstead LS, Dubey S, Meyer B, et al. . Enhanced rates and magnitude of immune responses detected against an HIV vaccine: Effect of using an optimized process for isolating PBMC. AIDS Res Hum Retroviruses 2007;23:86–92 [DOI] [PubMed] [Google Scholar]
- 30.Germann A, Oh YJ, Schmidt T, et al. . Temperature fluctuations during deep temperature cryopreservation reduce PBMC recovery, viability and T-cell function. Cryobiology 2013;67:193–200 [DOI] [PubMed] [Google Scholar]
- 31.Hubel A, Spindler R, Skubitz AP. Storage of human biospecimens: Selection of the optimal storage temperature. Biopreserv Biobank 2014;12:165–175 [DOI] [PubMed] [Google Scholar]
- 32.Olemukan RE, Eller LA, Ouma BJ, et al. . Quality monitoring of HIV-1-infected and uninfected peripheral blood mononuclear cell samples in a resource-limited setting. Clin Vaccine Immunol 2010;17:910–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambor A, Garcia A, Berrong M, et al. . Establishment and maintenance of a PBMC repository for functional cellular studies in support of clinical vaccine trials. J Immunol Methods 2014;409:107–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanchez AM, Rountree W, Berrong M, et al. . The External Quality Assurance Oversight Laboratory (EQAPOL) proficiency program for IFN-gamma enzyme-linked immunospot (IFN-gamma ELISpot) assay. J Immunol Methods 2014;409:31–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mairhofer M, Schulz JC, Parth M, et al. . Evaluation of a xeno-free protocol for long-term cryopreservation of cord blood cells. Cell Transplant 2013;22:1087–1099 [DOI] [PubMed] [Google Scholar]
- 36.Janetzki S, Cox JH, Oden N, et al. . Standardization and validation issues of the ELISPOT assay. Methods Mol Biol 2005;302:51–86 [DOI] [PubMed] [Google Scholar]
- 37.Vysekantsev IP, Gurina TM, Martsenyuk VF, et al. . Probability of lethal damages of cryopreserved biological objects during storage. Cryo Letters 2005;26:401–408 [PubMed] [Google Scholar]
- 38.Kreher CR, Dittrich MT, Guerkov R, et al. . CD4+ and CD8+ cells in cryopreserved human PBMC maintain full functionality in cytokine ELISPOT assays. J Immunol Methods 2003;278:79–93 [DOI] [PubMed] [Google Scholar]
- 39.Lovelock JE. The haemolysis of human red blood-cells by freezing and thawing. Biochim Biophys Acta 1953;10:414–426 [DOI] [PubMed] [Google Scholar]
- 40.Lovelock JE. The mechanism of the protective action of glycerol against haemolysis by freezing and thawing. Biochim Biophys Acta 1953;11:28–36 [DOI] [PubMed] [Google Scholar]
- 41.Lovelock JE. The protective action of neutral solutes against haemolysis by freezing and thawing. Biochem J 1954;56:265–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lovelock JE. Haemolysis by thermal shock. Br J Haematol 1955;1:117–129 [DOI] [PubMed] [Google Scholar]
- 43.Crowe JH, Crowe LM, Chapman D. Preservation of membranes in anhydrobiotic organisms: The role of trehalose. Science 1984;223:701–703 [DOI] [PubMed] [Google Scholar]
- 44.Williams RJ, Hope HJ. The relationship between cell injury and osmotic volume reduction. III. Freezing injury and frost resistance in winter wheat. Cryobiology 1981;18:133–145 [DOI] [PubMed] [Google Scholar]
- 45.Glander HJ, Schaller J. Binding of annexin V to plasma membranes of human spermatozoa: A rapid assay for detection of membrane changes after cryostorage. Mol Hum Reprod 1999;5:109–115 [DOI] [PubMed] [Google Scholar]
- 46.Kutscher S, Dembek CJ, Deckert S, et al. . Overnight resting of PBMC changes functional signatures of antigen specific T-cell responses: Impact for immune monitoring within clinical trials. PloS One 2013;8:e76215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Costantini A, Mancini S, Giuliodoro S, et al. . Effects of cryopreservation on lymphocyte immunophenotype and function. J Immunol Methods 2003;278:145–155 [DOI] [PubMed] [Google Scholar]