Abstract
Hepatitis B virus/hepatitis C virus (HBV/HCV) dual infection is common among high-risk individuals. To characterize the virological and immunological features of patients with HBV/HCV dual infection, we enrolled 1,049 individuals who have been identified as injection drug users. Patients were divided into single and dual infection groups according to the serological markers. We found the average HCV RNA level was significantly lower; however, HBV viral load was significantly higher in HBV/HCV dual-infected patients (n = 42) comparing HCV single infection (n = 340) or HBV single infection (n = 136). The level of anti-HBs in patients who experienced spontaneous HBV clearance was higher than that in HCV single-infected patients with HBV spontaneous clearance. The level of anti-HCV E2 in HBV/HCV dual infection was lower than that detected in HCV single infection. Serum levels of IL-6, IL-8, and TNF-α were significantly lower in HBV/HCV dual-infected patients than in patients infected with HBV or HCV alone. Taken together, two viral replications are imbalanced in dual infected patients. The anti-HBs and anti-HCV E2 antibody production were impaired and proinflammatory IL-6, IL-8, and TNF-α also downregulated due to dual infection. These findings will help further understanding the pathogenesis of HBV/HCV dual infection.
Hepatitis B virus/Hepatitis C virus (HBV/HCV) dual infection is common, especially in highly endemic areas and among individuals with a high risk for parenteral infections including injection drug users (IDUs) and patients on hemodialysis1. The incidence of HBV/HCV dual infection is approximately 2–10% in anti-HCV positive and 5–20% in HBsAg individuals2,3. Increasing evidences indicate a greater likelihood of the advancement of chronic hepatitis to cirrhosis and hepatocellular carcinoma (HCC) in patients with HBV/HCV dual infection and the increased difficulty for therapy relative to HBV or HCV single infection3,4,5,6,7.
Reports show that HBV/HCV dual infection may exhibit different virological and immunological profiles from HBV or HCV single infection; however, the described results were often inconsistent and warrant further investigation. For instance, a few studies have shown that HCV replication was more robust in HBV/HCV dual infection compared with HCV single infection8,9,10. Other studies have suggested that HBV infection suppressed HCV replication11,12,13,14,15, or that reciprocal inhibition manifested between HBV and HCV infection16,17,18. Interestingly, some reports have shown that HBV and HCV replicated independently and that there was no direct evidence of replication interference12,19,20,21. Thus, it is likely that HBV and HCV can infect and replicate in the same cells without interference22. A phenomenon known as superinfection exclusion, which is generally restricted to homologous viruses has been reported in many viruses. Moreover, nonrelated viruses have appeared to be able to replicate normally23.
Compared to HBV or HCV single infection, HBV/HCV dual infection may have a more profound impact on the immunological response. Chu et al. reported that the frequency and relative intensity of antibody response to HCV non-structural protein 4 were significantly diminished in HBV/HCV dual infection compared to HCV single infection12. Furthermore, Zampino et al. showed that levels of HCV envelope glycoprotein E2 antibody were lower in HBV/HCV dual-infected individuals than in patients infected with HCV alone, following interferon treatment24. In addition, HBV vaccination in chronic HCV-infected individuals has been shown to induce relatively lower anti-HBs antibody levels25,26.
The immunopathogenesis of viral diseases likely entails cytokine production in response to viral infection by the host27. The co-existence of two chronic viral infections may alter the profile of cytokine production. For example, HCV and human immunodeficiency virus (HIV) co-infection significantly decreased IFN-γ and TNF-α expression, and HBV or HCV core proteins decreased TNF-α, IL-6, IL-12 and increased IL-10 production in vitro28,29. Virus clearance can be mediated by antiviral cytokines such as TNF-α and IFN-γ. Furthermore, it has been shown that decreased production of IFN-γ and TNF-α and elevated IL-10 levels contribute to persistent infection and disease progression30.
Taken together, we believe pathological competition exists for uninfected hepatocytes in the livers of patients with HBV/HCV dual infection. Therefore, we hypothesized that the dual infection places a heavier burden on the immune system of the host. To test this hypothesis, we conducted a comprehensive study to verify our hypothesis and found that an early established viral infection may have an advantage in competing for uninfected hepatocytes. In addition, our studies revealed that dual infection may weaken antibody and cytokine production.
Methods
Patients and study design
A total of 1,049 IDUs who participated in a compulsory rehabilitation program were recruited from August 2014 to May 2015 in Chenzhou city, Hunan Province, China. All participants were naïve to anti-viral treatment. Blood samples were collected for HBV and HCV detection. The patients were divided into three groups according to the HBV and HCV serological markers and viral load as follows: (1) HBV single infection (n = 136), if HBsAg positive and HCV RNA negative; (2) HCV single infection (n = 340), if HCV RNA positive and HBsAg negative; and (3) HBV/HCV dual infection (n = 42), if both HBsAg and HCV RNA positive. Patients infected with hepatitis D virus (HDV), HIV, and syphilis were excluded from the study. Informed consent was obtained from each participant and the study was approved by the Ethics Committee of The First People’s Hospital of Chenzhou according to the Declaration of Helsinki.
Serological tests
Hepatitis B virus surface antigen/antibody, HBV E antigen/antibody, HBV core antibody, and anti-HCV IgG were determined using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Wantai Biological Pharmacy, Beijing, China). Antibodies specific for the HCV E2 were measured using ELISA. Hepatitis C virus E2 plasmid (1b) was a gift from professor Mansun Law (The Scripps Research Institute, USA), HCV E2 protein was expressed in CHO cell and purified. Briefly, 96-well plates were coated with HCV E2 protein overnight at 4 °C. The plates were then incubated with 100 μL of 20-fold diluted serum with blocking buffer and a positive control. Horseradish peroxidase-conjugated anti-human IgG was used as the secondary antibody, as per the protocol described. HCV E2 antibody level was measured at OD450.
HBV and HCV viral load
Hepatitis B virus and HCV viral loads were quantitatively determined by qPCR using a commercially Nucleic Acid Diagnostic Kit (Sansure Biotech, Changsha, China). The results were expressed as copies/mL and international unit/mL for HBV and HCV, respectively.
Cytokine measurement
The pro-inflammatory cytokine IL-1, IL-6, IL-8, TNF-α, and IFN-γ levels were measured by Bio-Plex Pro Assay Quick Guide 4 (Bio-Rad, Hercules, California, United States of America), according to the instructions provided by the manufacturer. The levels of the detected cytokines were further confirmed using ELISA kits (MAXTM Deluxe Set, BioLegend, San Diego, CA, USA).
Statistical analyses
The means of continuous variables were compared using Mann–Whitney U test or Kruskal–Wallis test. Differences in categorical variables were evaluated using the χ2 test or Fisher’s exact test. Correlation was analyzed by Spearman’s correlation. P values were derived from a two-tailed probability, and a P value less than 0.05 (P < 0.05) was considered significant.
Results
Demographic and clinical characteristics of patients
Among the 1,049 enrolled IDUs, 136 patients had HBV single infection (HBsAg+), 340 patients had HCV single infection (HCV RNA+), and 42 patients had dual infection with HBV and HCV (HBsAg+ and HCV RNA+). The median ages of patients with HBV single infection, HCV single infection, and HBV/HCV dual infection were 32.30 years, 39.54 years, and 35.85 years, respectively. Gender was not observed to influence HBV/HCV dual or single infection as there was no difference in gender measurements observed among the different groups. However, higher alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were detected in patients with HCV single infection than in patients with HBV single infection and HBV/HCV dual infection (P = 0.03 and P = 0.003, respectively, Table 1).
Table 1. Demographic characteristics of HBV, HCV and HBV/HCV dual infected patients.
| HBV (n = 136) | HCV (n = 340) | HBV /HCV (n = 42) | P | |
|---|---|---|---|---|
| Age(y) | 32.30(27.30–37.41) | 39.54(32.64–43.98) | 35.85(29.95–42.24) | <0.001 |
| Gender(M/F) | 128/8 | 314/26 | 41/1 | 0.876 |
| ALT(U/L) | 26.80(18.00–44.40) | 33.80(21.40–55.60) | 32.20(23.30–54.40) | 0.03 |
| AST(U/L) | 23.20(17.60–32.60) | 29.00(20.20–39.60) | 25.40(18.90–38.20) | 0.003 |
| HBsAg | + | − | + | |
| anti-HBs | −/+ | −/+ | − | |
| HBeAg | −/+ | −/+ | −/+ | |
| anti-HBe | −/+ | −/+ | −/+ | |
| anti-HBc | −/+ | −/+ | + | |
| HBV DNA | −/+ | − | −/+ | |
| HBV DNA titer(copies/mL) | 3.14(0–178.37) × 102 | 0(0–46.19) × 102 | ||
| anti-HCV | −/+ | −/+ | −/+ | |
| HCV RNA | − | + | + | |
| HCV RNA titer(IU/mL) | 1.18(0.10–8.20) × 104 | 1.97(0.28–18.20) × 103 |
Data expressed as Median (25th–75th percentile).
Chi-square test and Kruskal–Wallis test were used in the analysis; P values were two-tailed.
HBV and HCV replication was imbalanced in HBV/HCV dual infection
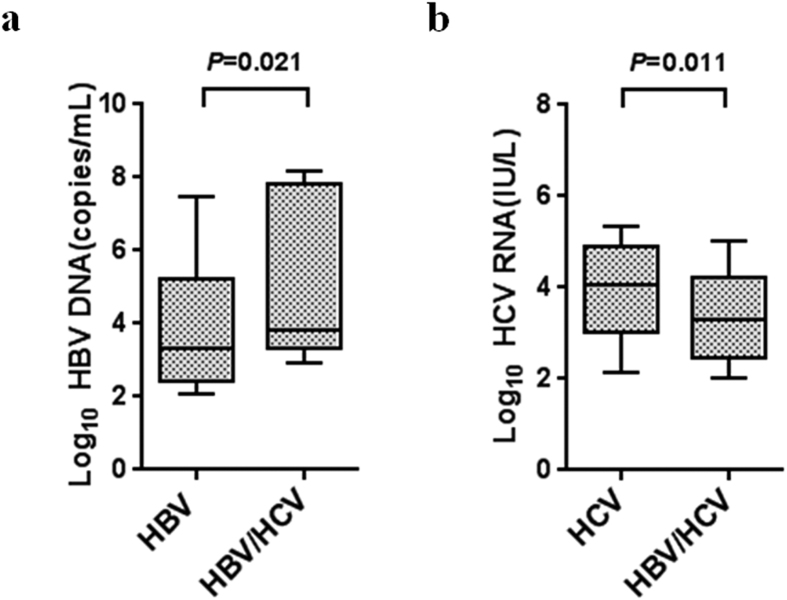
Hepatitis B virus and HCV viral loads in HBV/HCV dual infection, and HBV and HCV single infection were compared. The levels of HCV RNA were significantly lower in patients with HBV/HCV dual infection than in patients with HCV single infection (3.29 [2.44–4.24] versus 4.07 [3.01–4.91] log10 IU/mL, P = 0.011, median (25th–75th percentile)). However, HBV DNA levels were significantly higher in HBV/HCV dual infection than in HBV single infection (3.81 [3.32–7.83] versus 3.33 [2.44–5.21] log10 copies/mL, P = 0.021, median (25th–75th percentile)) (Fig. 1a,b).
Figure 1. Viral replication levels in dual infection with HBV and HCV.
(a) HBV DNA levels were compared between HBV single infection (HBsAg+, n = 136) and HBV/HCV dual infection (HBsAg+, HCV RNA+, n = 42). (b) HCV RNA levels were compared between HCV single infection (HCV RNA+, n = 340) and HBV/HCV dual infection. The data of Fig. 1a and b are expressed as interquartile ranges (IQR) (10th–90th percentile). P values were derived from a two-tailed probability generated from a Mann–Whitney test.
Antibody response was impaired in HBV/HCV dual infection
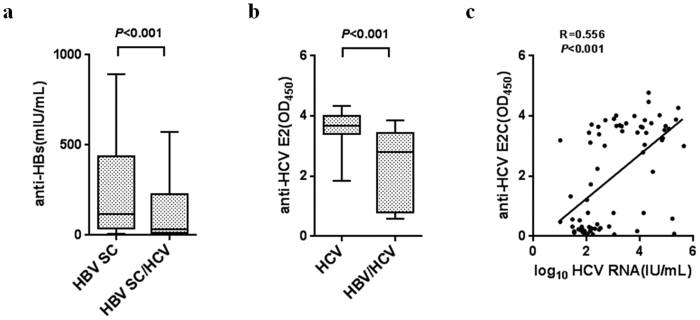
To investigate the impact of HBV/HCV dual infection on HBV and HCV antibody production, we compared the anti-HBs antibody response in individuals with spontaneous HBV and HCV infection with spontaneous HBV clearance due to lack of anti-HBs antibody in chronic HBV and HCV dual infection. The anti-HBs antibody levels in 208 patients who experienced spontaneous HBV clearance (HBsAg−, anti-HBc+ and anti-HBs −/+) and 248 HCV single-infected patients with HBV spontaneous clearance (HBsAg−, anti-HBc+, anti-HBs−/+ and HCV RNA+) were examined. We found that the percentage of anti-HBs antibody titers ≥10 mIU/mL in 208 patients who experienced spontaneous HBV clearance (n = 179, 86.06%) was significantly higher than in the 248 HCV single-infected patients with HBV spontaneous clearance (n = 176, 71.97%) (χ2 = 14.94, P <0.001). Significantly lower anti-HBs antibody levels were observed in the HCV single-infected patients with HBV spontaneous clearance than in the patients who experienced spontaneous HBV clearance (29.14 [7.52–204.26] versus 112.67 [32.01–426.44] mIU/mL, P < 0.001, median (25th–75th percentile) (Fig. 2a). The HCV E2 antibody response in the 32 individuals with HCV single infection and 19 with HBV/HCV dual infection was measured. HCV E2 antibody level was significantly lower in HBV/HCV dual infection (OD value: 2.79 [0.78–3.45] vs. 3.67 [3.40–4.02], P < 0.001, median (25th–75th percentile) Fig. 2b) compared to the level in HCV single infection. There was a significant positive correlation between anti-E2 and HCV RNA levels (R = 0.556, P < 0.001; Fig. 2c).
Figure 2. Antibody production was impaired in HBV/HCV dual infection.
(a) Serum levels of anti-HBs antibody were compared between two groups with HBV spontaneous clearance, patients who experienced spontaneous HBV clearance (HBV SC) (HBsAg−, anti-HBc +, anti-HBs −/+, n = 208) and HCV single-infected patients with HBV spontaneous clearance (HBV SC/HCV) (HBsAg−, anti-HBc+, anti-HBs −/+, HCV RNA+, n = 248). (b) anti-HCV E2 levels were compared between HCV single infection (HCV RNA+, n = 32) and HBV/HCV dual infection (HBsAg+, HCV RNA+, n = 19). (c) Correlation between serum HCV RNA titer and anti-HCV E2 level by Spearman correlation. Data of Fig. 2a and b are expressed as interquartile ranges (IQR) (10th–90th percentile). P values were derived from a two-tailed probability generated from a Mann–Whitney test.
Pro-inflammatory cytokine expression was reduced in HBV/HCV dual infection
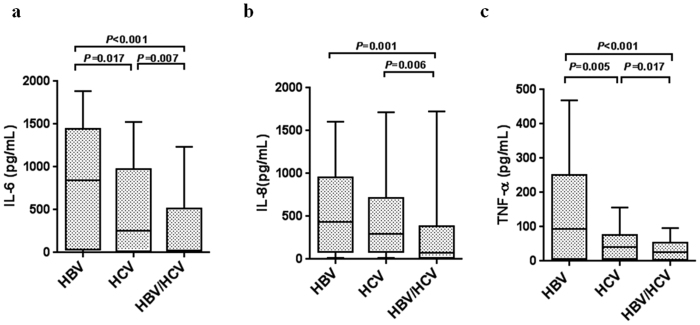
The serum pro-inflammatory IL-1, IL-6, IL-8, and TNF-α and IFN-γ levels were determined in 82 HBV single-infected, 87 HCV single-infected, and 34 HBV/HCV dual-infected individuals. IL-6, IL-8, and TNF-α levels were significantly lower in HBV/HCV dual infection compared to the levels in HBV single infection (22.43 versus 844.18 pg/mL, P < 0.001; 77.25 versus 432.71 pg/mL, P = 0.001; 23.72 versus 93.34 pg/mL, P < 0.001) and HCV single infection (22.43 versus 253.42 pg/mL, P = 0.007; 77.25 versus 297.15 pg/mL, P = 0.006; 23.72 versus 42.40 pg/mL, P = 0.017 (Fig. 3).
Figure 3. HBV/HCV dual infection changed the cytokine expression profiles.
Cytokine levels were measured by Luminex and confirmed by ELISA in HBV single infection (HBsAg+, n = 82), HCV single infection (HCV RNA+, n = 87) and HBV/HCV dual infection (HBsAg+, HCV RNA+, n = 34). (a) IL-6, (b) IL-8, and (c) TNF-α levels were compared among the three groups. Data are expressed as interquartile ranges (IQR) (10th–90th percentile). P values were derived from a two-tailed probability generated from a Mann–Whitney test.
Discussion
In this study, we found that HBV DNA levels were higher and HCV viral load were lower in HBV/HCV dual infection as compared with HBV or HCV single infection. Our results clearly suggest a competition between HBV and HCV infection when the liver is infected with both viruses, and HBV replication is dominant in dual-infected subjects.
The different HBV and HCV replication levels are typically attributed to direct interference9,11,18. However, we believe that the observed difference in the two viral replications derived from a competition for uninfected hepatocytes rather than from viral interference. It is well known that the prevalence of HBV infection is much higher than that of HCV infection in the Chinese general population16,31. Furthermore, our previous study, which examined HBV and HCV infection patterns between IDUs and the general population using the similar cohort with this study, demonstrated that HBV infections shared similar patterns by IDUs and the general populations, and HCV infection exhibited distinct features between two populations32. From these studies, we infer that most of the patients with HBV/HCV dual infection infected HBV were though perinatal, while some of these patients were infected though injection drug use. However, the study herein shows that HCV was acquired at a later age. Therefore, it is conceivable that the HBV/HCV dual-infected individuals were already infected with HBV before contracting HCV infection. As the majority of the hepatocytes are already infected with HBV, these cells are generally resistant to viral superinfection. Only a small number of hepatocytes are uninfected, which may be available for HCV infection. Besides, HBV has a long-lived nuclear form of its genome (covalently closed circular DNA) that is able to persist in the face of potent inhibition of viral replication. In contrast, HCV does not have a long-lived genome form; HCV is therefore much more susceptible to eradication by potent immunity33. Moreover, a lower HCV level may reflect a small number of HCV-infected cells, which is a possible explanation for the dominant HBV replication in our cohort.
Further to the rationale for our study results is an explanation for the higher HBV DNA levels in the dual-infected patients than in HBV single-infected patients. Patients with HBV single infection, especially those who acquired the infection in adulthood, likely experienced spontaneous viral clearance, and the number of infected cells was significantly reduced as the natural course of the virus progressed. On the other hand, the subjects with dual infection were likely to have been repeatedly infected with HBV during transmission of HCV, leading to more HBV-infected cells despite the ongoing viral clearance. Due to no treatment guidelines for HBV/HCV dual-infection patients, so it is important to determine the “dominant” virus by serological and virological testing prior to initiating therapy. For patients with dominant HCV infection, IFN or pegylated IFN in addition to ribavirin can achieve comparable sustained virus response as expected with HCV monoinfection. For patients with dominant HBV infection, pegylated IFN plus ribavirin and a nucleos(t)ide analog appears to be a feasible option2,34,35,36.
Wiedmann et al. showed that chronic HCV-infected patients tended to have poor HBV vaccination response and low anti-HBs antibody levels25. In this study, we found both the percentage of anti-HBs antibody titers ≥10 mIU/mL and anti-HBs levels in the HCV single-infected patients with HBV spontaneous clearance were lower than that found in patients who experienced spontaneous HBV clearance. The results of various studies revealed that higher concentrations of serum antibody may lead to a longer duration of immunity. The anti-HBs titer correlated to the frequency of IFN-γ-producing HBs-specific T cells. Reports show that HBs-specific T cell and antibody responses did not differ between vaccines and HBV-recovered patients37,38. This finding suggests that greater levels of antibody production would lead to enhanced immunity, and that HCV infection may directly or indirectly influence HBV surface antibody production. Therefore, it is necessary to strengthen the HBV vaccine and increase the monitoring of the anti-HBs antibody levels in the high risk population of HCV infection.
Moorman et al. showed an impaired response to HBV vaccination in chronic HCV-infected patients, which was partly attributed to the upregulated expression of PD-1 and PD-L1 on CD4+ T cells26. We also found that the anti-HCV E2 antibody response was weaker in HBV/HCV dual infection than in HCV single infection. The HCV envelope glycoproteins E2 are codified by E2 genomic regions. Envelope glycoprotein E2 comprises two hypervariable regions. The two hypervariable regions are subject to immune pressure, which leads to the formation of escape mutants. Patients infected with HCV develop a humoral immune response against HCV envelope proteins; therefore, anti-HCV E1 and E2 may have the capability of neutralizing HCV infection39. This finding suggests that HBV infection may negatively impact HCV antibody response. Of great consideration, our findings do not support the suggestion that the upregulation of expression of immune checking molecules was partially responsible for lower anti-HBs or anti-HCV E2 levels in the dual infection, since viral proteins are also expressed in single infection if they inhibited the immune response. We believe that the HBV/HCV dual infection placed a heavier burden on the immune system of the host, and that the antibody production has to deal with two viral infections, which leads to a weak antibody response to each virus.
Pro-inflammatory cytokines such as IL-6, IL-8, and TNF-α are involved in HBV- or HCV-induced liver inflammation and treatment outcomes27,40,41,42,43. The expression levels of these cytokines in HBV/HCV dual infection are unclear. Here, we showed that the serum levels of IL-6, IL-8, and TNF-α expression were significantly lower in HBV/HCV dual infection compared with HCV or HBV single infection, which is consistent with an in vitro study that showed that co-culturing HBV and HCV core proteins with human dendritic cells significantly increased the production of immune-suppressive cytokine, IL-10, and decreased the expression of pro-inflammatory cytokines. IL-6, IL-12, and TNF-α. In addition, the results of this study suggested that viral core proteins can synergistically induce the immune tolerance of dendritic cells and overproduce IL-10, which can inhibit the production of pro-inflammatory cytokines such as IL-6 and TNF-α28.
In summary, our study showed that there likely exists competition for uninfected hepatocytes when the liver is infected with both HBV and HCV. The viral dominance in dual infection was largely determined by the virus that firstly established the infection. Hepatitis B replication was dominant in this cohort because HBV was likely the first to infect the majority of liver cells. In addition, dual infection placed a heavier burden on the immune system of the host and weakened the antibody production capacity, leading to a lower level of protective antibody to each virus. Taken together, our data may contribute to further understanding the biology of viral infection and immune response in patients with a dual infection of HBV and HCV.
Additional Information
How to cite this article: Chen, F. et al. HBV/HCV dual infection impacts viral load, antibody response, and cytokine expression differently from HBV or HCV single infection. Sci. Rep. 6, 39409; doi: 10.1038/srep39409 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
The authors gratefully acknowledge all of the participants in this study. This study was in part supported by the Natural Science Foundation of China (Grant numbers: 81471959, 81401367, 81501746), the Natural Science Foundation of Hunan Province (Grant numbers: 2015JJ2132, 2015JJ3112), and the Research Foundation of The First People’s Hospital of Chenzhou (Grant numbers: N2013-012).
Footnotes
Author Contributions Xiaowang Qu and Wenpei Liu contributed to the design of the study. Fei Chen, Jian Zhang, Bo Wen, Shan Luo, Fengfan Guo and Ping Tang performed the experiments and analyzed the data included in the present study. Yingbiao Lin and Wensheng Ou contributed to the reagent and clinical sample preparation to conduct the studies herein. Fei Chen and Xiaowang Qu prepared the manuscript. All authors reviewed the manuscript.
References
- Tyson G. L. et al. Prevalence and predictors of hepatitis B virus coinfection in a United States cohort of hepatitis C virus-infected patients. Hepatology 58, 538–545, doi: 10.1002/hep.26400 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C. J. & Lee S. D. Hepatitis B virus/hepatitis C virus coinfection: epidemiology, clinical features, viral interactions and treatment. Journal of gastroenterology and hepatology 23, 512–520, doi: 10.1111/j.1440-1746.2008.05384.x (2008). [DOI] [PubMed] [Google Scholar]
- Caccamo G., Saffioti F. & Raimondo G. Hepatitis B virus and hepatitis C virus dual infection. World journal of gastroenterology 20, 14559–14567, doi: 10.3748/wjg.v20.i40.14559 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. J., Chen P. J. & Chen D. S. Dual chronic hepatitis B virus and hepatitis C virus infection. Hepatology international 3, 517–525, doi: 10.1007/s12072-009-9147-9 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamma S., Hussain G. & Lau D. T. Current Concepts of HBV/HCV Coinfection: Coexistence, but Not Necessarily in Harmony. Current hepatitis reports 9, 260–269, doi: 10.1007/s11901-010-0060-4 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin J., Law M. G., Bartlett M., Kaldor J. M. & Dore G. J. Causes of death after diagnosis of hepatitis B or hepatitis C infection: a large community-based linkage study. Lancet 368, 938–945, doi: 10.1016/S0140-6736(06)69374-4 (2006). [DOI] [PubMed] [Google Scholar]
- El-Serag H. B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142, 1264–1273 e1261, doi: 10.1053/j.gastro.2011.12.061 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland S. F., Asabe S., Engle R. E., Purcell R. H. & Chisari F. V. Limited hepatitis B virus replication space in the chronically hepatitis C virus-infected liver. Journal of virology 88, 5184–5188, doi: 10.1128/JVI.03553-13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuttler C. G. et al. Suppression of hepatitis B virus enhancer 1 and 2 by hepatitis C virus core protein. Journal of hepatology 37, 855–862 (2002). [DOI] [PubMed] [Google Scholar]
- Fattovich G. et al. Hepatitis C virus infection in chronic hepatitis B virus carriers. The Journal of infectious diseases 163, 400–402 (1991). [DOI] [PubMed] [Google Scholar]
- Pan Y. et al. NS5A protein of HCV enhances HBV replication and resistance to interferon response. Biochemical and biophysical research communications 359, 70–75, doi: 10.1016/j.bbrc.2007.05.052 (2007). [DOI] [PubMed] [Google Scholar]
- Chu C. M., Sheen I. S. & Liaw Y. F. Low‐level hepatitis C viremia and humoral immune response to NS4 in chronic hepatitis B virus‐hepatitis C virus coinfection. Scandinavian Journal of Gastroenterology 39, 778–782, doi: 10.1080/00365520410006332 (2004). [DOI] [PubMed] [Google Scholar]
- Liaw Y.-F. et al. Impact of acute hepatitis C virus superinfection in patients with chronic hepatitis B virus infection☆. Gastroenterology 126, 1024–1029, doi: 10.1053/j.gastro.2004.01.011 (2004). [DOI] [PubMed] [Google Scholar]
- Zarski J. P. et al. Characteristics of patients with dual infection by hepatitis B and C viruses. Journal of hepatology 28, 27–33 (1998). [DOI] [PubMed] [Google Scholar]
- Sagnelli E. et al. HBV superinfection in HCV chronic carriers: a disease that is frequently severe but associated with the eradication of HCV. Hepatology 49, 1090–1097, doi: 10.1002/hep.22794 (2009). [DOI] [PubMed] [Google Scholar]
- Bini E. J. & Perumalswami P. V. Hepatitis B virus infection among American patients with chronic hepatitis C virus infection: prevalence, racial/ethnic differences, and viral interactions. Hepatology 51, 759–766, doi: 10.1002/hep.23461 (2010). [DOI] [PubMed] [Google Scholar]
- Rodriguez-Inigo E. et al. Hepatitis C virus (HCV) and hepatitis B virus (HBV) can coinfect the same hepatocyte in the liver of patients with chronic HCV and occult HBV infection. Journal of virology 79, 15578–15581, doi: 10.1128/JVI.79.24.15578-15581.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G. et al. Replication Inhibition of Hepatitis B Virus and Hepatitis C Virus in Co-Infected Patients in Chinese Population. PloS one 10, e0139015, doi: 10.1371/journal.pone.0139015(2015) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chulanov V. P., Shipulin G. A., Schaefer S. & Gerlich W. H. Kinetics of HBV DNA and HBsAg in acute hepatitis B patients with and without coinfection by other hepatitis viruses. Journal of medical virology 69, 313–323, doi: 10.1002/jmv.10291 (2003). [DOI] [PubMed] [Google Scholar]
- Bellecave P. et al. Hepatitis B and C virus coinfection: a novel model system reveals the absence of direct viral interference. Hepatology 50, 46–55, doi: 10.1002/hep.22951 (2009). [DOI] [PubMed] [Google Scholar]
- Eyre N. S. et al. Hepatitis B virus and hepatitis C virus interaction in Huh-7 cells. Journal of hepatology 51, 446–457, doi: 10.1016/j.jhep.2009.04.025 (2009). [DOI] [PubMed] [Google Scholar]
- Yang D. et al. Complete replication of hepatitis B virus and hepatitis C virus in a newly developed hepatoma cell line. Proceedings of the National Academy of Sciences of the United States of America 111, E1264–1273, doi: 10.1073/pnas.1320071111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. M., Tscherne D. M., Yun S. I., Frolov I. & Rice C. M. Dual mechanisms of pestiviral superinfection exclusion at entry and RNA replication. Journal of virology 79, 3231–3242, doi: 10.1128/JVI.79.6.3231-3242.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampino R. et al. Anti-envelope 1 and 2 immune response in chronic hepatitis C patients: Effects of hepatitis B virus co-infection and interferon treatment. Journal of medical virology 73, 33–37, doi: 10.1002/jmv.20058 (2004). [DOI] [PubMed] [Google Scholar]
- Wiedmann M. et al. Decreased immunogenicity of recombinant hepatitis B vaccine in chronic hepatitis C. Hepatology 31, 230–234, doi: 10.1002/hep.510310134 (2000). [DOI] [PubMed] [Google Scholar]
- Moorman J. P. et al. Impaired hepatitis B vaccine responses during chronic hepatitis C infection: Involvement of the PD-1 pathway in regulating CD4+ T cell responses. Vaccine 29, 3169–3176, doi: 10.1016/j.vaccine.2011.02.052 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C., Liu Y., Chen Z. & Zheng M. Involvement of Interleukin 6 in Hepatitis B Viral Infection. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology 37, 677–686, doi: 10.1159/000430386 (2015). [DOI] [PubMed] [Google Scholar]
- Agrawal A., Samrat S. K., Agrawal B., Tyrrell D. L. & Kumar R. Co-incubation with core proteins of HBV and HCV leads to modulation of human dendritic cells. Viral immunology 27, 412–417, doi: 10.1089/vim.2014.0056 (2014). [DOI] [PubMed] [Google Scholar]
- Rahman S. et al. Unique Cytokine/Chemokine Signatures for HIV-1 and HCV Mono-infection versus Co-infection as Determined by the Luminex(R) Analyses. Journal of clinical & cellular immunology 2, doi: 10.4172/2155-9899.1000104 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y. et al. Role of IL-10-Producing Regulatory B Cells in Chronic Hepatitis B Virus Infection. Digestive diseases and sciences 60, 1308–1314, doi: 10.1007/s10620-014-3358-1 (2014). [DOI] [PubMed] [Google Scholar]
- Nguyen L. H. et al. Ethnic differences in viral dominance patterns in patients with hepatitis B virus and hepatitis C virus dual infection. Hepatology 53, 1839–1845, doi: 10.1002/hep.24308 (2011). [DOI] [PubMed] [Google Scholar]
- Chen F. et al. HBV, HCV and HDV infection shows distinct patterns between injection drug users and general population. Journal of gastroenterology and hepatology, doi: 10.1111/jgh.13460 (2016). [DOI] [PubMed] [Google Scholar]
- Delaney W. E. Molecular virology of chronic hepatitis B and C: Parallels, contrasts and impact on drug development and treatment outcome. Antiviral research 99, 34–48, doi: 10.1016/j.antiviral.2013.04.010 (2013). [DOI] [PubMed] [Google Scholar]
- Chuang W. L. et al. Viral interaction and responses in chronic hepatitis C and B coinfected patients with interferon-alpha plus ribavirin combination therapy. Antiviral therapy 10, 125–133 (2005). [PubMed] [Google Scholar]
- Potthoff A. et al. Sustained HCV-RNA response and hepatitis Bs seroconversion after individualized antiviral therapy with pegylated interferon alpha plus ribavirin and active vaccination in a hepatitis C virus/hepatitis B virus-coinfected patient. European journal of gastroenterology & hepatology 19, 906–909, doi: 10.1097/MEG.0b013e3282094160 (2007). [DOI] [PubMed] [Google Scholar]
- Marrone A. et al. Combined interferon plus lamivudine treatment in young patients with dual HBV (HBeAg positive) and HCV chronic infection. Journal of hepatology 41, 1064–1065, doi: 10.1016/j.jhep.2004.07.009 (2004). [DOI] [PubMed] [Google Scholar]
- Norouzirad R., Shakurnia A. H., Assarehzadegan M. A., Serajian A. & Khabazkhoob M. Serum levels of anti-hepatitis B surface antibody among vaccinated population aged 1 to 18 years in ahvaz city southwest of iran. Hepat Mon 14, e13625, doi: 10.5812/hepatmon.13625 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner J. M., Abdalla A., Gara N., Ghany M. G. & Rehermann B. The hepatitis B vaccine protects re-exposed health care workers, but does not provide sterilizing immunity. Gastroenterology 145, 1026–1034, doi: 10.1053/j.gastro.2013.07.044 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampino R. et al. Anti-envelope 1 and 2 immune response in chronic hepatitis C patients: effects of hepatitis B virus co-infection and interferon treatment. Journal of medical virology 73, 33–37, doi: 10.1002/jmv.20058 (2004). [DOI] [PubMed] [Google Scholar]
- Yang K. et al. Enhanced levels of interleukin-8 are associated with hepatitis B virus infection and resistance to interferon-alpha therapy. International journal of molecular sciences 15, 21286–21298, doi: 10.3390/ijms151121286 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y. et al. Interferon-gamma and Tumor Necrosis Factor-alpha Produced by T Cells Reduce the HBV Persistence Form, cccDNA, Without Cytolysis. Gastroenterology 150, 194–205, doi: 10.1053/j.gastro.2015.09.026 (2016). [DOI] [PubMed] [Google Scholar]
- Akbar H. et al. High baseline interleukine-8 level is an independent risk factor for the achievement of sustained virological response in chronic HCV patients. Infection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases 11, 1301–1305, doi: 10.1016/j.meegid.2011.04.021 (2011). [DOI] [PubMed] [Google Scholar]
- Jablonska J. et al. The correlation between pretreatment cytokine expression patterns in peripheral blood mononuclear cells with chronic hepatitis c outcome. BMC infectious diseases 15, 556, doi: 10.1186/s12879-015-1305-1 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]