Abstract
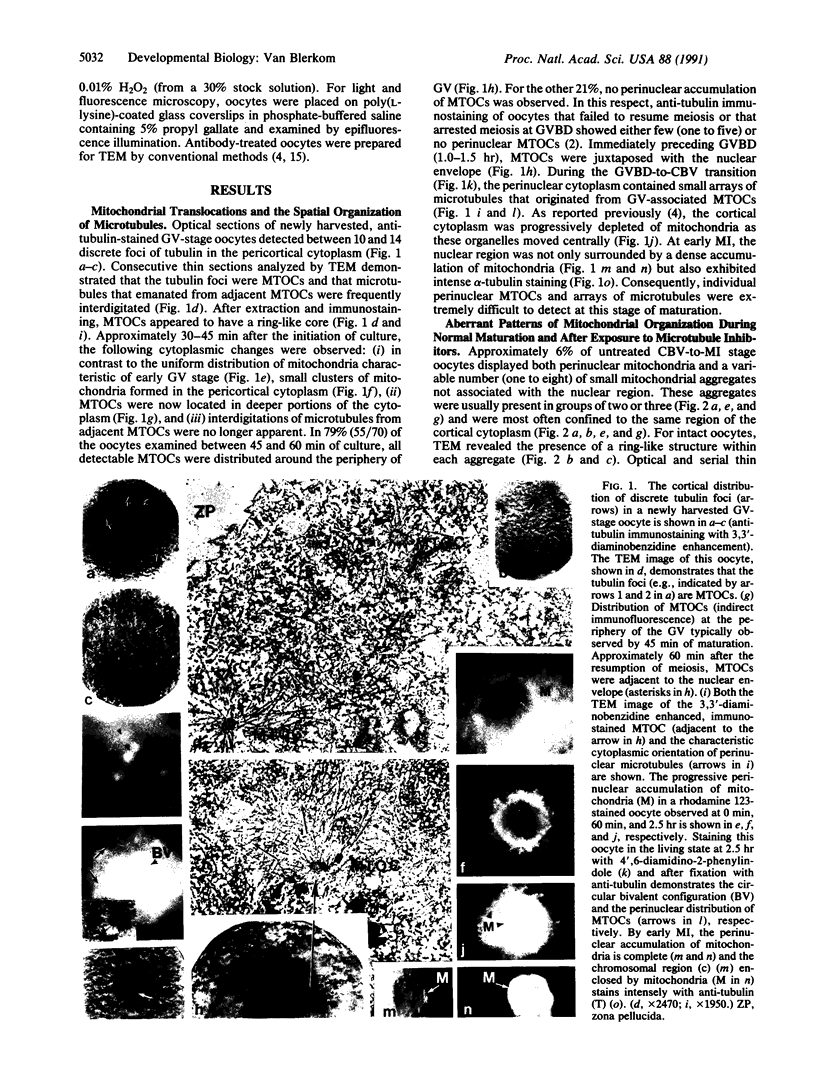
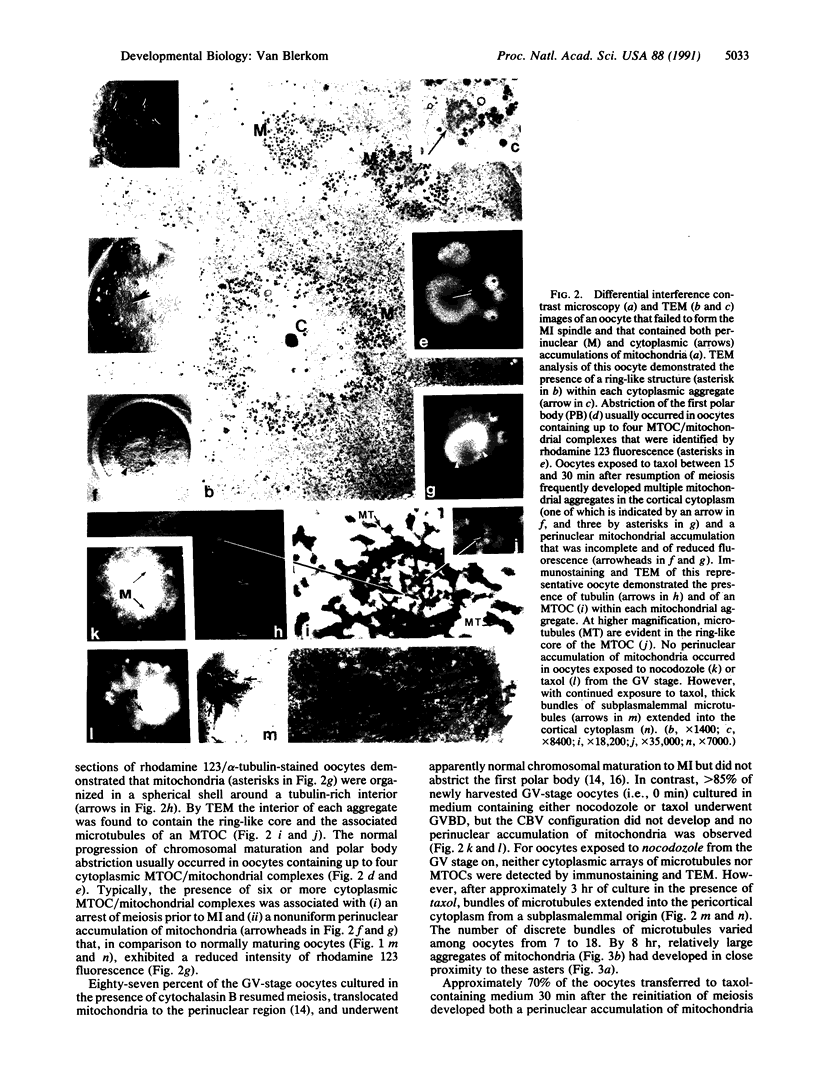
A perinuclear accumulation of mitochondria characterizes the premetaphase I stages of reinitiated meiosis in the laboratory mouse oocyte. The cellular basis of this organelle-specific translocation was examined by fluorescent probe analysis, immunostaining, and immunoelectron microscopy in oocytes cultured at specific stages of meiotic maturation in the presence and absence of drugs that influence the stability of microfilaments and microtubules. The results suggest that a temporal, spatial, and developmental relationship exists between the location of microtubule organizing centers and the progressive translocation of mitochondria to the nuclear region. The findings indicate that mitochondrial translocations are mediated by microtubules and that individual microtubule organizing centers are not only foci for mitochondrial aggregation but may also facilitate the establishment of the circular bivalent configuration.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini D. F. Cytoplasmic reorganization during the resumption of meiosis in cultured preovulatory rat oocytes. Dev Biol. 1987 Mar;120(1):121–131. doi: 10.1016/0012-1606(87)90110-2. [DOI] [PubMed] [Google Scholar]
- Bachvarova R., De Leon V., Johnson A., Kaplan G., Paynton B. V. Changes in total RNA, polyadenylated RNA, and actin mRNA during meiotic maturation of mouse oocytes. Dev Biol. 1985 Apr;108(2):325–331. doi: 10.1016/0012-1606(85)90036-3. [DOI] [PubMed] [Google Scholar]
- Bornslaeger E. A., Mattei P. M., Schultz R. M. Protein phosphorylation in meiotically competent and incompetent mouse oocytes. Mol Reprod Dev. 1988;1(1):19–25. doi: 10.1002/mrd.1080010105. [DOI] [PubMed] [Google Scholar]
- Calarco P. G., Donahue R. P., Szollosi D. Germinal vesicle breakdown in the mouse oocyte. J Cell Sci. 1972 Mar;10(2):369–385. doi: 10.1242/jcs.10.2.369. [DOI] [PubMed] [Google Scholar]
- Ducibella T., Anderson E., Albertini D. F., Aalberg J., Rangarajan S. Quantitative studies of changes in cortical granule number and distribution in the mouse oocyte during meiotic maturation. Dev Biol. 1988 Nov;130(1):184–197. doi: 10.1016/0012-1606(88)90425-3. [DOI] [PubMed] [Google Scholar]
- Heggeness M. H., Simon M., Singer S. J. Association of mitochondria with microtubules in cultured cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3863–3866. doi: 10.1073/pnas.75.8.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maro B., Howlett S. K., Webb M. Non-spindle microtubule organizing centers in metaphase II-arrested mouse oocytes. J Cell Biol. 1985 Nov;101(5 Pt 1):1665–1672. doi: 10.1083/jcb.101.5.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNiven M. A., Porter K. R. Microtubule polarity confers direction to pigment transport in chromatophores. J Cell Biol. 1986 Oct;103(4):1547–1555. doi: 10.1083/jcb.103.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Yanagimachi R., Yanagimachi H. Ultrastructural localization of lectin-binding sites on the zonae pellucidae and plasma membranes of mammalian eggs. J Cell Biol. 1975 Aug;66(2):263–274. doi: 10.1083/jcb.66.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn J. C., Moor R. M. An assessment of the factors causing embryonic loss after fertilization in vitro. J Reprod Fertil Suppl. 1988;36:59–72. [PubMed] [Google Scholar]
- Richter J. D., McGaughey R. W. Patterns of polypeptide synthesis in mouse oocytes during germinal vesicle breakdown and during maintenance of the germinal vesicle stage by dibutyryl cAMP. Dev Biol. 1981 Apr 15;83(1):188–192. doi: 10.1016/s0012-1606(81)80023-1. [DOI] [PubMed] [Google Scholar]
- Rime H., Jessus C., Ozon R. Distribution of microtubules during the first meiotic cell division in the mouse oocyte: effect of taxol. Gamete Res. 1987 May;17(1):1–13. doi: 10.1002/mrd.1120170102. [DOI] [PubMed] [Google Scholar]
- Schliwa M., van Blerkom J. Structural interaction of cytoskeletal components. J Cell Biol. 1981 Jul;90(1):222–235. doi: 10.1083/jcb.90.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapp B. J., Vale R. D., Sheetz M. P., Reese T. S. Single microtubules from squid axoplasm support bidirectional movement of organelles. Cell. 1985 Feb;40(2):455–462. doi: 10.1016/0092-8674(85)90160-6. [DOI] [PubMed] [Google Scholar]
- Schultz R. M., Montgomery R. R., Belanoff J. R. Regulation of mouse oocyte meiotic maturation: implication of a decrease in oocyte cAMP and protein dephosphorylation in commitment to resume meiosis. Dev Biol. 1983 Jun;97(2):264–273. doi: 10.1016/0012-1606(83)90085-4. [DOI] [PubMed] [Google Scholar]
- Terasaki M., Chen L. B., Fujiwara K. Microtubules and the endoplasmic reticulum are highly interdependent structures. J Cell Biol. 1986 Oct;103(4):1557–1568. doi: 10.1083/jcb.103.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Blerkom J., Bell H. Regulation of development in the fully grown mouse oocyte: chromosome-mediated temporal and spatial differentiation of the cytoplasm and plasma membrane. J Embryol Exp Morphol. 1986 Apr;93:213–238. [PubMed] [Google Scholar]
- Van Blerkom J. Extragenomic regulation and autonomous expression of a developmental program in the early mammalian embryo. Ann N Y Acad Sci. 1985;442:58–72. doi: 10.1111/j.1749-6632.1985.tb37505.x. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J. Maturation at high frequency of germinal-vesicle-stage mouse oocytes after cryopreservation: alterations in cytoplasmic, nuclear, nucleolar and chromosomal structure and organization associated with vitrification. Hum Reprod. 1989 Nov;4(8):883–898. doi: 10.1093/oxfordjournals.humrep.a137006. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J. Morphodynamics of nuclear and cytoplasmic reorganization during the resumption of arrested meiosis in the mouse oocyte. Prog Clin Biol Res. 1989;294:33–51. [PubMed] [Google Scholar]
- Van Blerkom J. Occurrence and developmental consequences of aberrant cellular organization in meiotically mature human oocytes after exogenous ovarian hyperstimulation. J Electron Microsc Tech. 1990 Dec;16(4):324–346. doi: 10.1002/jemt.1060160405. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J., Runner M. N. Mitochondrial reorganization during resumption of arrested meiosis in the mouse oocyte. Am J Anat. 1984 Nov;171(3):335–355. doi: 10.1002/aja.1001710309. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M., Fujiwara K. Immunofluorescent anti-tubulin staining of spindles during meiotic maturation of mouse oocytes in vitro. J Cell Sci. 1978 Feb;29:171–188. doi: 10.1242/jcs.29.1.171. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M., Letourneau G. E. RNA synthesis in fully-grown mouse oocytes. Nature. 1976 May 6;261(5555):73–74. doi: 10.1038/261073a0. [DOI] [PubMed] [Google Scholar]