Abstract
Extant dog and wolf DNA indicates that dog domestication was accompanied by the selection of a series of duplications on the Amy2B gene coding for pancreatic amylase. In this study, we used a palaeogenetic approach to investigate the timing and expansion of the Amy2B gene in the ancient dog populations of Western and Eastern Europe and Southwest Asia. Quantitative polymerase chain reaction was used to estimate the copy numbers of this gene for 13 ancient dog samples, dated to between 15 000 and 4000 years before present (cal. BP). This evidenced an increase of Amy2B copies in ancient dogs from as early as the 7th millennium cal. BP in Southeastern Europe. We found that the gene expansion was not fixed across all dogs within this early farming context, with ancient dogs bearing between 2 and 20 diploid copies of the gene. The results also suggested that selection for the increased Amy2B copy number started 7000 years cal. BP, at the latest. This expansion reflects a local adaptation that allowed dogs to thrive on a starch rich diet, especially within early farming societies, and suggests a biocultural coevolution of dog genes and human culture.
Keywords: domestication, palaeogenomics, amylase, dog, Neolithic
1. Introduction
In western Eurasia, the Neolithic transition took place between 11 500 and 6000 cal. BP (before present), leading to the shift from hunting and gathering to farming [1,2]. At this time, the dog, domesticated during the Upper Palaeolithic [3–6], had accompanied humans for several millennia. The antiquity of this close proximity has already been highlighted by archaeological and genomic approaches [4,7–11]; but the impact of human lifestyle and diet changes on dog genetic characteristics, during the Neolithic transition, is still being investigated. This is of crucial importance in understanding the development of early farming societies, early domestic canid physiological changes and the genomic transformations towards modern dog genotypes and phenotypes.
According to the morphology of archaeological specimens, the genomics of modern canids and experimental domestications, early dogs experienced selective pressures involving behavioural, morphological and physiological traits [3,10–15]. A comparison of genome-wide patterns of genetic variation from a large group of dogs and wolves identified genomic regions affected by directional selection during dog domestication [16]. These included several genes involved in digestion and energy metabolism, most likely connected to a diet change in the dog's lineages [16]. In particular, it was noted that selection had targeted a series of duplications of the gene coding for pancreatic amylase (Amy2B). This led to a several fold copy number increase in modern dog breeds in comparison with their wild ancestor, the wolf, that is associated with a higher amylase activity [16,17]. Whereas Amy2B copy numbers vary widely in dogs (4–34 copies), both at a breed and individual level [18], the copy number range is much lower (two to eight copies) across wolf populations with 60% of the wolves bearing only two copies [17]. This suggests that dogs have adapted to a diet richer in starch, relative to the carnivorous wolf diet [16].
Present-day canids present three patterns with regard to the Amy2B copy number variation [16,17]: (i) 60% of the wolves, and most of the dingos bear two copies of the gene, (ii) a second pattern shows dogs and wolves with two to eight copies of Amy2B, and (iii) and a third pattern consists of dogs that bear more than eight copies of Amy2B.
The question of a link between the increase of the Amy2B copy number in dogs and the Neolithic transition has been previously raised [16–18]. However, this question remains unanswered as we can only hypothesize that the increase could have provided a strong adaptive advantage within a farming context, and we cannot exclude that it occurred much later, as a result of the more recent selection of specialized lineages [19–21].
Palaeogenetics provides a unique opportunity to shed light on this question by investigating the landscape relative to the Amy2B copy number variation in ancient canid populations. In this study, we examined the Amy2B copy number of ancient Eurasian dogs by highlighting the Amy2B gene expansion from the 7th millennium cal. BP to the Bronze Age (ca 4000 cal. BP). We showed Amy2B gene expansion in dog samples coming from archaeological sites corresponding to early farming contexts located in Western Europe, Southeastern Europe and Southwest Asia.
2. Material and methods
2.1. Dog morphotype samples
We attempted to study the Amy2B copy number, using ancient DNA (aDNA) analysis from the tooth and bone remains of 88 different canids from 30 archaeological sites in Western Europe, Romania, Russia, Estonia, Israel, Turkmenistan and the Iranian Plateau, from the Upper Palaeolithic to the Bronze Age. In total, aDNA results were obtained from 13 individuals from eight archaeological sites in Europe and Turkmenistan (see the electronic supplementary material). The osteological distinction between the domestic dog (Canis familiaris) and the wolf (Canis lupus), its wild ancestor, can be difficult, due to the regional and temporal variability of wolf morphology [22] and to the morphological proximity between the two forms in the early steps of domestication; therefore, we used a series of osteological traits to separate them [3,23,24]. Dogs differ from wolves by their overall significantly smaller size, a smaller brain-case volume, a shorter snout, tooth crowding and a higher frequency of dental defects. All the individuals used in this study belonged to the domestic form, according to one or several of these criteria.
When possible, measurements were taken from mandibles, particularly the five dimensions frequently measurable in broken archaeological specimens (dimensions #8, 10, 11, 19, 20, after [25]; electronic supplementary material, table S1—only measurements for individuals providing aDNA results are reported). The data obtained for our archaeological Holocene canids were then compared with the data derived from (i) a series of Pleistocene wolf mandibles from Arcy-sur-Cure (France) [23] dated between 100 000 and 60 000 years BP, prior to any suspicion of domestication; (ii) a series of Pleistocene canid mandibles from Předmostí (Czech Republic) [26], attributed to the wolf and dated to 27 000–26 000 BP; (iii) a series of modern Eurasian wolf mandibles from the National Museum of Natural History, Paris [23]; and (iv) a series of modern wolf mandibles from Southeastern Europe [27] (electronic supplementary material, figure S1). It was noted that the length of the tooth row (dimension #8 [25]) was significantly different between the Holocene canids and the four series of wolf (Mann–Whitney tests corrected for Bonferroni, p < 0.05). The only individual in the Holocene series, located at the very margin of the modern wolves' variation interval (CH1075; electronic supplementary material, figure S1), evidenced a colour mutation typical to domestic animals from one of our previous studies on the same material [15]. Therefore, the canid series analysed in this study can be identified to be the domestic form C. familiaris.
2.2. Ancient DNA
All the aDNA procedures were conducted at the French National Platform of Paleogenetics (PALGENE, CNRS, ENS de Lyon) using facilities and tools specific to aDNA analyses, while following adequate controls [28–33].
2.2.1. Ancient DNA extraction
The external surface of the bones was scratched with a sterile scalpel to produce a clean piece, which was then reduced to powder with a sterile hammer. The powder (150–300 mg) was then digested for 18 h at 55°C with agitation in 4.7 ml of buffer (0.5 M EDTA (ethylene diamine tetra acetic acid), pH = 8.0), 50 µl of proteinase K (1 mg ml−1) and 250 µl of 0.5% N-lauryl-sarcosyl [28]. A silica-based method modified from Rohland & Hofreiter [32] was used to retrieve the aDNA. Mock extractions were performed in order to rule out contamination from reagents. In addition, cross-contamination was monitored by combining the aDNA from our samples with the aDNA from other species (i.e. owls, fish and sheep) for each extraction session.
2.2.2. Ancient DNA pre-amplification and quantitation
In order to restore sufficient aDNA quantity for each sample, we co-amplified the nuclear fragment of the Amy2B gene alongside a fragment of a nuclear reference gene present in two diploid copy numbers (C7orf28b), in a multiplex polymerase chain reaction (PCR). Such pre-amplification procedures have been shown to improve the sensitivity of quantitative PCR (qPCR) analysis on modern [34,35] and aDNA [36]. We followed previous recommendations to perform robust and highly accurate targeted pre-amplification in combination with qPCR [34–36].
Both fragment sequences were amplified using dog specific primers [16,18,37]:
— Amy2B—fragment of 76 bp: forward 5′-CCAAACCTGGACGGACATCT-3′ and reverse 5′-TATCGTTCGCATTCAAGAGCAA-3′.
— C7orf28b-3—fragment of 60 bp: forward 5′-GGGAAACTCCACAAGCAATCA-3′ and reverse 5′-GAGCCCATGGAGGAAATCATC-3′.
Both fragments matched in size and were designed to exclude a potential amplification bias in degraded DNA. These two pairs of primers were then mixed in a single tube. The reaction was performed in a 25 µl reaction volume containing 2.5 µl of 10× Taq buffer, 2 mM of MgCl2, 0.025 mg of BSA (Roche, 20 mg ml−1), 2.5 units of Taq polymerase (AmpliTaq Gold, Applied Biosystems), 250 µM of each dNTP (Sigma) and finally 0.5 µM of each primer. Four volumes of aDNA extract were used for each amplification: 0.5 µl, 1.0 µl, 2.0 µl and 4.0 µl. The cycling conditions were one activation step at 95°C for 2 min followed by 20 cycles of denaturation at 95°C for 30 s, then 60°C for 4 min. We systematically added two different controls in all PCR assays: an aerosol control (tube kept open throughout the manipulation to monitor airborne contaminations) and a PCR-mix control (to monitor contamination of reagents).
Amplification products were quantified using the Quantifluor® dsDNA System (Promega). This system enables the sensitive quantification of small amounts of double stranded DNA thanks to a fluorescent DNA-binding dye.
2.3. Quantitative polymerase chain reaction
DNA copy number variation was quantified using Multiplex TaqMan assays (primers described above). The following probes matched the target Amy2B gene and reference housekeeping gene C7orf28b [16,18,37]: Amy2B probe-6FAM–TTTGAGTGGCGCTGGG-MGBNFQ [33,34]; C7orf28b-3 probe-VIC-CACCTGCTAAACAGC-MGBNFQ.
We followed the same experimental design as previously published [16,18]: the reaction was performed in a 25 µl reaction volume containing 12.5 µl of Taqman Genotyping master mix (Applied Biosystems), 0.9 µM of each primer, 0.25 µM of each probe and 2 ng of DNA. The cycling conditions were one step at 50°C for 2 min, one step at 95°C for 10 min, followed by 40 cycles of one step at 95°C for 15 s and one step at 60°C for 1 min. All reactions were run in triplicate for each sample in the same qPCR plate. We systematically added three qPCR-mix controls to monitor contamination of reagents in each assay and three aerosol controls to monitor airborne contaminations during plate preparation.
2.3.1. First tests on present-day canids
In present-day wolves, the amylase copy number variation ranges from two to eight copies, with 60% of wolves bearing only two copies [17]. In order to choose a wolf reference sample to account for inter-plate variability in subsequent studies, we performed an independent qPCR on 16 wolves to evaluate the number of Amy2B copies (electronic supplementary material, table S2a), following the previous protocol. The same protocol was also used to test 16 present-day dogs (electronic supplementary material, table S2b).
Modern DNA work was performed in a distinct laboratory (IGDR, CNRS-UMR6290, Rennes, France). The modern DNA samples came from the biobank Cani-DNA_CRB, in IGDR-CNRS, Rennes.
2.3.2. Quantitative polymerase chain reaction on ancient canids
The pre-amplification step was independently repeated before every qPCR attempt for each sample, so that each set of qPCR results (e.g. qPCR results of two different plates for the same sample) derived from independent pre-amplification. Pre-amplification controls relating to samples tested for qPCR were systematically added in the assay.
We followed the protocol described above using a present-day wolf sample as a reference to account for inter-plate variability (sample reference 8278—cani-DNA Biobank IGDR, CNRS-UMR6290, Rennes, France). Whenever possible, three positive full replicates (i.e. pre-amplification + qPCR in triplicates) were analysed for each sample and each gene.
2.3.3. Quantitative polymerase chain reaction analysis
Data were analysed using the CopyCaller software (Applied Biosystems), and relative quantitative ratios (RQ) were estimated for each sample and each run. Copy numbers for each target were normalized to the reference modern wolf (sample references 8278—two amylase copies). Raw copy number data were rounded to the nearest whole number. The confidence value of the associated predicted copy number was calculated for each sample (for more details, see: https://tools.thermofisher.com/content/sfs/manuals/cms_062369.pdf).
3. Results
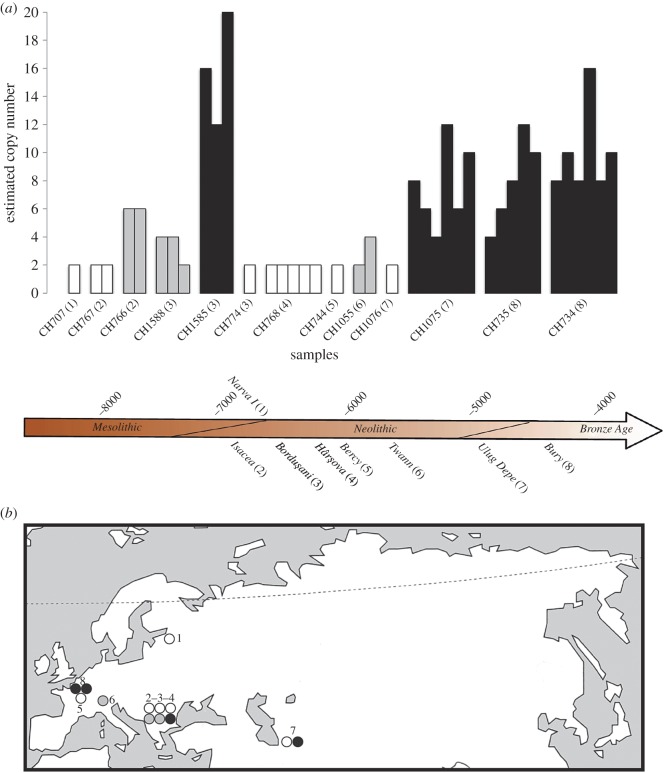
For each aDNA sample, nuclear fragments of the Amy2B gene and a reference gene present in two diploid copy numbers (C7orf28b) were co-amplified by a qPCR procedure. In order to estimate the Amy2B copy number, RQ between these two genes were estimated for each sample and then normalized to the reference modern wolf (bearing two Amy2B copies). The protocol was tested on 16 present-day dogs and 16 present-day wolves. Results showed that the 16 wolves all evidenced two copies of the Amy2B gene (confidence ≥0.93; electronic supplementary material, table S2a), whereas the 16 dogs presented between 4 and 16 Amy2B copy numbers (confidence ≥ 0.93; electronic supplementary material, table S2b). Reproducible results (at least two full experiment replicates) were then obtained for nine ancient dogs, and a single result was obtained for four further specimens (electronic supplementary material, tables S3 and S4). RQ values varied between 0.78 and 10.04 (electronic supplementary material, table S4), indicating that the diploid Amy2B copy numbers varied between 2 and 20 (figure 1a).
Figure 1.
Distribution of estimated Amy2B diploid gene copy numbers for each specimen through space and time. (a) Distribution of estimated Amy2B diploid gene copy numbers for each specimen and replicate through time: two copies (white), two to eight copies (grey) and more than eight copies (black). (b) Geographical distribution of the estimated Amy2B diploid gene copy number variation throughout Eurasia between the Upper Palaeolithic and the Bronze Age: two copies (white), two to eight copies (grey) and more than eight copies (black).
Two Romanian samples (Isaccea, Hârşova—sites 2 and 4; figure 1b; electronic supplementary material, table S3) presented reproducible and concordant results indicating an estimated diploid Amy2B copy number of two (figure 1a; electronic supplementary material, table S4). Four more samples from Estonia (Narva I—site 1; figure 1b; electronic supplementary material, table S3), Romania (Borduşani—site 3; figure 1b; electronic supplementary material, table S3), Turkmenistan (Ulug Depe—site 7; figure 1b; electronic supplementary material, table S3) and north France (Bercy—site 5; figure 1b; electronic supplementary material, table S3) also bore two Amy2B copies, although a replicate was unobtainable (figure 1a; electronic supplementary material, table S4).
The three samples from Romania (Isaccea and Borduşani—sites 2 and 3; figure 1b; electronic supplementary material, table S3) and Switzerland (Twann—site 6; figure 1b; electronic supplementary material, table S3) carried up to six Amy2B copies (figure 1a; electronic supplementary material, table S4).
Finally, four samples from Turkmenistan (CH1075; Ulug Depe—site 7; figure 1b; electronic supplementary material, table S3), Romania (CH1585; Borduşani—site 3; figure 1b; electronic supplementary material, table S3) and north France (CH734 and CH735; Bury—site 8; figure 1b; electronic supplementary material, table S3) carried more than eight Amy2B copies (figure 1a; electronic supplementary material, table S4). Among these four samples we observed Amy2B copy number variations between samples and among replicates (figure 1a). The two samples from north France (CH735) and Turkmenistan (CH1075) evidenced between 4 and 12 estimated copy numbers. The third sample, from north France (CH734), presented between 8 and 16 Amy2B copy numbers. The fourth sample, from Romania (CH1585), presented the highest estimated Amy2B copy number, varying between 12 and 20. These four samples presented high RQ value variations between replicates (three to six replicates, with variances of 3.07, 2.11, 2.63, 4.53 for CH734, CH735, CH1075, CH1585, respectively; electronic supplementary material, table S4).
The four dogs showing Amy2B gene expansion (more than eight copies) came from several regions of Europe and Southwest Asia (i.e. CH1585, Borduşani, Romania, 7th millennium cal. BP; CH1075, Ulug Depe, Turkmenistan, mid- to late 5th millennium cal. BP; CH735 and Ch734, Bury, France, mid- to late 4th millennium cal. BP; see the electronic supplementary material), but no link could be established between the number of gene copy and a given geographical area. We also compared the mandibles of these four individuals (electronic supplementary material, table S1 and figure S1). The first one (CH1585) had a very short tooth row and showed oligodontia. The other three were larger but with no dental defects. No link between the number of gene copies and the morphological characteristics (i.e. size and mandible shape; electronic supplementary material, table S1 and figure S1) could be found. These results were unable to correlate the Amy2B gene expansion to a particular ancient dog population or morphotype.
4. Discussion
We obtained results for 13 of 88 samples. This success rate (15%) can be explained by aDNA degradation: (i) the estimated number of copies can differ between replicates and, therefore, must be interpreted as a minimum number of copies that could be detected and (ii) inhibition was observed in amplification curves from the majority of failed amplification attempts. We highlighted the difficulty to precisely estimate the high copy number, as it is already established that the ability to distinguish copy numbers decreases as they increase [38]. Consequently, the confidence values are often lower for high copy number samples even under optimal experimental conditions, due to the compression of the ΔCT sub distributions for high copy numbers [38].1 This explains some of the high copy numbers within sample variance calculated for the four individuals showing more than eight Amy2B copies. This phenomenon was amplified by the fact that we worked with aDNA (due to inhibition and degradation) and that two genes were targeted (reference C7orf28b and Amy2B). The pre-amplification step was necessary to restore a sufficient amount of aDNA but did not guarantee equal preservation of both targeted fragments. Enzymatic reparation of the aDNA extracts as well as droplet digital PCR (ddPCR) could be explored to improve detection efficiency. In particular, ddPCR has been shown to reduce mean coefficients of variation by 37–86% and improve reproducibility by a factor of 7 [39].
This study is, to our knowledge, the first report of qPCR being used to estimate the copy number variation from aDNA; this has led to three main issues.
4.1. Antiquity of the Amy2B gene expansion
Four of our ancient dogs displayed a high number of Amy2B copies (more than eight), indicating an expansion of this gene as early as the 7th millennium cal. BP in Romania (Borduşani) and the 5th millennium cal. BP in France (Bury) and Turkmenistan (Ulug Depe). These three sites correspond to a late stage in the transition to farming (Late Neolithic/Bronze Age). The Amy2B expansion probably allowed dogs to thrive on a starch rich diet, in comparison with the mostly carnivorous diet of wolves [18]. This constituted an important selective advantage for dogs feeding on human leftovers within a farming context. However, the scarcity of data anterior to the Neolithic does not allow us to assess whether this expansion took place before the Neolithic transition, or emerged during the Neolithic under new selection pressures related to the development of agriculture.
Currently, only a few dog lineages, such as the dingo (two copies) and the Siberian husky (three to four copies), show an unusual lack of Amy2B copy number. These dogs come from regions with no, or recent, agricultural practices [17,18,37]. This supports the hypothesis that the development of a dog's capability to digest starch efficiently does not result from a relaxation of the natural selection pressures related to domestication. It is more likely to result from an adaptation to the shift of human food habits during the Neolithic.
4.2. Persistence of a small number of copies in ancient dogs
We found ancient dogs from early farming contexts with two copies of the Amy2B gene at Isaccea, Hârşova, Borduşani (Romania), Ulug Depe (Turkmenistan) and Bercy (north France). Low copy number is an exceptional situation in present-day dogs and is only found in lineages associated with recent nomadic hunter–gatherers, such as the dingo and the Siberian husky. These two lineages also appear as basal on phylogenetic trees of extant dog breeds [40,41], probably as a result from a lack of recent admixture with other dog breeds due to geographical and cultural isolation [4]. Our early farming series suggests that the low Amy2B copy number present in their genome could stem from an ancient gene pool.
4.3. The Amy2B copy number was not fixed in early dogs
The two dog series from Borduşani and Hârşova can be considered together, as they are contemporary (mid- to late 7th millennium cal. BP) and belong to two neighbouring sites in southeast Romania. The archaeozoological series also displayed identical exploitations of animals [42,43]. The Borduşani/Hârşova set includes dogs bearing either two, two to eight or more than eight Amy2B copies, indicating a strong variability in the number of copies of Amy2B that could exist concurrently in the same population.
On a wider geographical level, our results show that dogs bearing the Amy2B copy number expansion came from various regions. Similarly, we found no link between the number of gene copies and specific morphotypes; though it is expected that the adaptation to a starchy diet would not only impact digestive functions but also morphological traits linked to biting and chewing (e.g. teeth, skull and mandible conformation [44]). These observations are congruent with the situation in modern dogs, where there is no fixation of the number of Amy2B copies in a given breed [16,17].
5. Conclusion
In this study, we have provided evidence for an increase of the amylase gene copy number in ancient dog genomes, with a firm ante quem during the 7th millennium cal. BP in Southeastern Europe. We have demonstrated that the modern capability of numerous dogs to digest starch does not result from the selection of lineages during Classical Antiquity or the nineteenth century selection of modern breeds [19–21]; but began, at the latest, during the Neolithic, between the 10th and 7th–5th millennium cal. BP, at least in various regions of West and East Europe and Southwest Asia. We also demonstrated, on the basis of archaeological remains that the Amy2B copy number increase was not fixed in all dogs from Neolithic farming societies. In addition, we showed the relatively late persistence of only two copies of the Amy2B gene in ancient dogs, well beyond the first appearance of farming. This situation is uncommon in modern dog lineages and could not have been demonstrated without ancient data.
Further analyses, on larger samples of ancient Eurasian dogs and wolves from the Palaeolithic to the Bronze Age, would help define the precise chronology and rhythm of the Amy2B expansion during early dog breeding. It will also help to pinpoint the date(s) and the location(s) of the first occurrence(s) of the Amy2B expansion (i.e. more than eight copies).
In humans, the pattern of variation in copy numbers of the human amylase gene (AMY1) is consistent with a history of diet-related selection pressures [45]: higher AMY1 copy numbers and protein levels likely improve the digestion of starchy diets. Human starch consumption increased significantly during the Neolithic transition and is correlated with a gradual increase of AMY1 copy numbers [46,47].
The history of the Amy2B expansion in dogs suggests that the genes responsible for digestion in both humans and dogs probably underwent similar changes. It is reasonable to speculate that other equally compelling examples of the biocultural coevolution of human culture and dog genes, involved in metabolisms, immunity and brain processes [48,49], are yet to be identified within the dog domestication process.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We are indebted to Catherine André (IGDR, CNRS-UMR6290) for providing samples from the Cani-DNA_CRB, which is part of the CRB-Anim infrastructure, ANR-11-INBS-0003, funded by the French National Research Agency in the frame of the ‘Investing for the Future’ programme. We would like to thank the following archaeologists: Stéphanie Bréhard (MNHN Paris), Philippe Chambon (UMR7055, CNRS, site Bury), Rose-Marie Arbogast (CNRS, UMR7044), Dragomir Popovici (The National Museum of Romanian History, sites Hârşova and Borduşani), Cristian Micu (ICEM, Tulcea, site Isaccea), Jörg Schibler (University of Basel) and the Twann Museum (site Twann) for the material they generously provided. We also thank Maud Pionnier-Capitan for the osteological measures she provided. We are grateful to Bariza Blanquier (SFR Biosciences) for her advice and Antoine Louchart for reading and correcting this article. We express gratitude to Jill Cucchi who kindly proofread the English. Finally, we thank PALGENE (French National Platform of Paleogenetics).
Footnotes
Application Note: Analysis of Copy Number Variation: Design Pipeline and Validation of TaqMan® Copy Number Assays. Publication 135AP03-0.
Data accessibility
All supporting data are made available either as electronic supplementary material or in the paper.
Author's contributions
Conceptualization: M.O., C.Hi; methodology: M.O., E.A., M.-L.A., A.T.; investigation: M.O., F.B., L.L.; resources: A.T., A.B., M.M., M.V.S., L.S., J.D.V., L.L.; visualization: M.O.; writing—original draft: M.O., A.T., J.D.V., C.Hi.; writing—review and editing: M.O., A.T., E.A., M.L.A., A.B., M.M., M.S., L.S., J.D.V., C.Hi, C.H.
Competing interests
We declare we have no competing interests.
Funding
The following organizations have supported this work: CNRS, ENS de Lyon, Nestlé Purina, CNCS-UEFISCDI for the grant provided for field archaeology and zooarchaeology (project numbers PN-II-ID-PCE-2011–3-1015) and ZIN RAS (state topic N 01201351185).
References
- 1.Harris DR.1996. Origins and spread of agriculture and pastoralism in Eurasia. London, UK: UCL Press; Redefining nature: ecology, culture and domestication, pp. 437–463. Oxford, UK: Berg.
- 2.Tresset A, Vigne JD. 2011. Last hunter-gatherers and first farmers of Europe. C. R. Biol. 334, 182–189. (doi:10.1016/j.crvi.2010.12.010) [DOI] [PubMed] [Google Scholar]
- 3.Pionnier-Capitan M, Bemilli C, Bodu P, Celerier G, Ferrie JG, Fosse P, Garcia M, Vigne JD. 2011. New evidence for Upper Palaeolithic small domestic dogs in South-Western Europe. J. Archaeol. Sci. 38, 2123–2140. (doi:10.1016/j.jas.2011.02.028) [Google Scholar]
- 4.Larson G, et al. 2012. Rethinking dog domestication by integrating genetics, archeology and biogeography. Proc. Natl Acad. Sci. USA 109, 8878–8883. (doi:10.1073/pnas.1203005109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thalmann O, et al. 2013. Complete mitochondrial genomes of ancient canids suggest a European origin of domestic dogs. Science 342, 871–874. (doi:10.1126/science.1243650) [DOI] [PubMed] [Google Scholar]
- 6.Frantz L, et al. 2016. Genomic evidence of a dual origin of domestic dogs. Science 352, 1228–1231. (doi:10.1126/science.aaf3161) [DOI] [PubMed] [Google Scholar]
- 7.Valla F. 1975. La Sépulture H. 104 de Mallaha (Eynan) et le problème de la domestication du chien en Palestine. Paléorient 3, 287–292. (doi:10.3406/paleo.1975.4205) [Google Scholar]
- 8.Davis SJM, Valla FR. 1978. Evidence for domestication of the dog 12 000 years ago in the Natufian of Israel. Nature 276, 608–610. (doi:10.1038/276608a0) [Google Scholar]
- 9.Tchernov E, Valla F. 1977. Two new dogs and other Natufian dogs from the Southern Levant. J. Archaeol. Sci. 24, 65–95. (doi:10.1006/jasc.1995.0096) [Google Scholar]
- 10.Li Y, vonHoldt BM, Reynolds A, Boyko AR, Wayne RK, Wu DD, Zhang YP. 2013. Artificial selection on brain expressed genes during the domestication of dog. Mol. Biol. Evol. 30, 1867–1876. (doi:10.1093/molbev/mst088) [DOI] [PubMed] [Google Scholar]
- 11.Wang GD, et al. 2013. The genomics of selection in dogs and the parallel evolution between dogs and humans. Nat. Commun. 4, 1860 (doi:10.1038/ncomms2814) [DOI] [PubMed] [Google Scholar]
- 12.Vigne JD. 2011. The origins of animal domestication and husbandry: a major change in the history of humanity and the biosphere. C. R. Biol. 334, 171–181. (doi:10.1016/j.crvi.2010.12.009) [DOI] [PubMed] [Google Scholar]
- 13.Cadieu E, et al. 2009. Coat variation in the domestic dog is governed by variants in three genes. Science 326, 150–153. (doi:10.1126/science.1177808) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karlsson EK, et al. 2007. Efficient mapping of mendelian traits in dogs through genome-wide association. Nat. Genet. 39, 1321–1328. (doi:10.1038/ng.2007.10) [DOI] [PubMed] [Google Scholar]
- 15.Ollivier M, et al. 2013. Evidence of coat color variation sheds new light on ancient canids. PLoS ONE 8, e75110 (doi:10.1371/journal.pone.0075110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Axelsson E, et al. 2013. The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature 495, 360–364. (doi:10.1038/nature11837) [DOI] [PubMed] [Google Scholar]
- 17.Freedman AH, et al. 2014. Genome sequencing highlights the dynamic early history of dogs. PLoS Genet. 10, e1004016 (doi:10.1371/journal.pgen.1004016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arendt M, Fall T, Lindblad-Toh K, Axelsson E. 2014. Amylase activity is associated with AMY2B copy numbers in dog: implications for dog domestication, diet and diabetes. Anim. Genet. 45, 716–722. (doi:10.1111/age.12179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clutton-Brock J. 1995. Origins of the dog: domestication and early history. In The domestic dog (ed. Serpell J.), pp. 7–20. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 20.Bennet D, Campbell G, Timm RB. 2016. The dogs of Roman Vindolanda, Part I: morphometric techniques useful in differentiating domestic and wild canids. Archeofauna 25, 79–106. [Google Scholar]
- 21.Harcourt RA. 1974. The dog in prehistoric and early historic Britain. J. Archaeol. Sci. 1, 151–175. (doi:10.1016/0305-4403(74)90040-5) [Google Scholar]
- 22.Druzhkova AS, Thalmann O, Trifonov VA, Leonard JA, Vorobieva NV, Ovodov ND, Graphodatsky AS, Wayne RK. 2013. Ancient DNA analysis affirms the canid from Altai as a primitive dog. PLoS ONE 8, e57754 (doi:10.1371/journal.pone.0057754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pionnier-Capitan M. 2010. La domestication du chien en Eurasie: étude de la diversité passée, approches ostéoarchéologiques, morphométriques et paléogénétiques. Thesis of ENS de Lyon.
- 24.Vigne JD. 2005. L'humérus de chien magdalénien de Erralla (Gipuzkoa, Espagne) et la domestication tardiglaciaire du loup en Europe. Munibe 51, 279–287. [Google Scholar]
- 25.von den Driesch A. 1976. A guide to the measurement of animal bones from archaeological sites. Harvard University, Peabody Museum of Archaeology and Ethnology, Peabody Museum Bulletin 1.
- 26.Germonpré M, Laznickova-Galetova M, Losey RJ, Räikkoönen J, Sablin MV. 2015. Large canids at the Gravettian Predmosti’ site, the Czech Republic: the mandible. Quat. Int. 359–360, 261–279. (doi:10.1016/j.quaint.2014.07.012) [Google Scholar]
- 27.Dimitrijević V, Vuković S. 2015. Was the dog locally domesticated in the Danube Gorges? Morphometric study of dog cranial remains from four Mesolithic–Early Neolithic archaeological sites by comparison with contemporary wolves. Int. J. Osteoarchaeol. 25, 1–30. (doi:10.1002/oa.2260) [Google Scholar]
- 28.Loreille O, Orlando L, Patou-Mathis M, Philippe M, Taberlet P, Hänni C. 2001. Ancient DNA analysis reveals divergence of the cave bear, Ursus spelaeus, and brown bear, Ursus arctos, lineages. Curr. Biol. 11, 200–203. (doi:10.1016/S0960-9822(01)00046-X) [DOI] [PubMed] [Google Scholar]
- 29.Calvignac S, Terme JN, Hensley SM, Jalinot P, Greenwood AD, Hänni C. 2008. Ancient DNA identification of early 20th century simian T-cell leukemia virus type 1. Mol. Biol. Evol. 25, 1093–1098. (doi:10.1093/molbev/msn054) [DOI] [PubMed] [Google Scholar]
- 30.Orlando L, Darlu P, Toussaint M, Bonjean D, Otte M, Hänni C. 2006. Revisiting Neandertal diversity with a 100,000 year old mtDNA sequence. Curr. Biol. 16, 400–402. (doi:10.1016/j.cub.2006.05.019) [DOI] [PubMed] [Google Scholar]
- 31.Krause J, et al. 2007. The derived FOXP2 variant of modern humans was shared with Neandertals. Curr. Biol. 17, 1908–1912. (doi:10.1016/j.cub.2007.10.008) [DOI] [PubMed] [Google Scholar]
- 32.Rohland N, Hofreiter M. 2007. Ancient DNA extraction from bones and teeth. Nat. Protoc. 2, 1756–1762. (doi:10.1038/nprot.2007.247) [DOI] [PubMed] [Google Scholar]
- 33.Chassaing O, Hänni C, Berrebi P. 2011. Distinguishing species of European sturgeons Acispenser spp. Using microsatellite allele sequences. J. Fish Biol. 78, 208–226. (doi:10.1111/j.1095-8649.2010.02852.x) [DOI] [PubMed] [Google Scholar]
- 34.Andersson D, Akrap N, Svec D, Godfrey T, Kubista M, Ladberg G, Stahlberg A. 2015. Properties of targeted preamplification in DNA and cDNA quantification. Expert Rev. Mol. Diagn. 795, 1085–1100. (doi:10.1586/14737159.2015.1057124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korenkova V, Scott J, Nodosadova V, Jindrichova M, Langerova L, Svec D, Sidova M, Sjöback R. 2015. Pre-amplification in the context of high-throughput qPCR gene expression experiment. BMC Mol. Biol. 16, 5 (doi:10.1186/s12867-015-0033-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Del Gaudi S, Cirillo A, Di Bernardo G, Galderisi U, Thanassoulas T, Pitsios T, Cipollaro M. 2013. Preamplification procedure for the analysis of ancient DNA samples. Sci. World J. 2013, 734676 (doi:10.1155/2013/734676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arendt M, Cairns KM, Ballard JWO, Savolainen P, Axelsson E. 2016. Diet adaptation in dog reflects spread of prehistoric agriculture. Heredity 117, 301–306. (doi:10.1038/hdy.2016.48) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Livak K, Schmittgen T. 2001. Analysis of relative gene expression data using real-time quantitation PCR and the 2– ΔΔCt method. Methods 25, 402–408. (doi:10.1006/meth.2001.1262) [DOI] [PubMed] [Google Scholar]
- 39.Hindson CM, et al. 2013. Absolute quantification by droplet digital PCR versus analog real-time PCR. Nat. Methods 10, 1003–1005. (doi:10.1038/nmeth.2633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.vonHoldt BM, et al. 2010. Genome-wide SNP and haplotype analyses reveal a rich history underlying dog domestication. Nature 464, 898–902. (doi:10.1038/nature08837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parker HG, et al. 2004. Genetic structure of the purebred domestic dog. Science 304, 1160–1164. (doi:10.1126/science.1097406) [DOI] [PubMed] [Google Scholar]
- 42.Balasse M, Bălăşescu A, Tornero C, Frémondeau D, Hovsepyan R, Gillis R, Popovici D. In press Investigating the scale of herding in Chalcolithic pastoral communities settled along the Danube river in the 5th millennium BC: a case study at Borduşani-Popină and Hârşova-tell (Romania). Quat. Int. (doi:10.1016/j.quaint.2015.07.030) [Google Scholar]
- 43.Bréhard S, Radu V, Martin A, Bălăşescu A. 2014. Food supply strategies in the Romanian Eneolithic: sheep/goat husbandry and fiching activities from Hârşova and Borduşani-Popină (5th Millennium BC). Eur. J. Archaeol. 17, 407–433. (doi:10.1179/1461957113Y.0000000051) [Google Scholar]
- 44.Ellis JL, Thomason J, Kebreab E, Zubair K, France J. 2009. Cranial dimensions and forces of biting in the domestic dog. J. Anat. 214, 362–373. (doi:10.1111/j.1469-7580.2008.01042.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perry GH, et al. 2007. Diet and the evolution of human amylase gene copy number variation. Nat. Genet. 39, 1256–1260. (doi:10.1038/ng2123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lazaridis L, et al. 2014. Ancient human genomes suggest three ancestral populations for present-day Europeans. Nature 513, 409–413. (doi:10.1038/nature13673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olade I, et al. 2014. Derived immune and ancestral pigmentation alleles in a 7,000 year old Mesolithic European. Nature 507, 225–228. (doi:10.1038/nature12960) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gerbault P, Liebert A, Itan Y, Powell A, Currat M, Burger J, Swallow DM, Thomas M. 2011. Evolution of lactase persistence: an example of niche construction. Phil. Trans. R. Soc. B 366, 863–877. (doi:10.1098/rstb.2010.0268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagazawa M, Mitsui S, En S, Ohtani N, Ohta M, Sakuma Y, Onaka T, Mogi K, Kikusui T. 2015. Oxytocin-gaze positive loop and the coevolution of human-dog bonds. Science 358, 333–336. (doi:10.1126/science.1261022) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All supporting data are made available either as electronic supplementary material or in the paper.