Abstract
Natural selection favours a restricted host breadth in disease vector mosquitoes, indicating that there is an adaptive value associated with maintaining plasticity in host preference. One mechanism to maintain such plasticity is via the detection of generic cues by conserved peripheral olfactory pathways, which when perceived in different host odour contexts enable the identification of and discrimination among potential host species. Here, we show that the context of an odour cue shapes host perception in mosquitoes, by altering the release rate of the generic host-related volatile (R)-1-octen-3-ol, within its natural range, and in the background odour of known hosts and non-hosts. This result highlights that host recognition is contextual and dependent on quantitative and qualitative differences in odour blends and the olfactory codes evoked. From the perspective of vector management, understanding the perception of odour blends and their context is essential to the process of developing synthetic blends for the optimal attraction of mosquitoes in efforts to control and monitor populations.
Keywords: (R)-1-octen-3-ol, chemical analysis, behaviour, electrophysiology, Anopheles coluzzii, Aedes aegypti and Culex quinquefasciatus
1. Background
Inherent host preference is often a marked characteristic of mosquito disease vectors [1–3], and while blood-feeding preference is inherent in mosquitoes [1,3], it can be modulated by host availability [4,5]. The plasticity of host preference, a key trait regulating disease transmission by anthropophilic mosquitoes [6], underlies the ability of mosquitoes to adapt to varying ecological conditions [1,3,5]. Natural selection on the malaria vectors Anopheles gambiae sensu lato, and the arbovirus vectors Aedes aegypti and Culex quinquefasciatus has, however, been shown to favour a restricted host breadth [3], indicating an adaptive value to maintaining plasticity [1]. We hypothesize that such plasticity could be maintained through the use of generic cues, which are common to many host species and detected by conserved olfactory receptor neuron pathways, placed in different host odour contexts to identify and discriminate among potential host species.
Mosquitoes locate their hosts primarily through olfaction [3,7]. A sequence of behaviours contribute to host discrimination and selection, including activation, long- and short-range attraction, and landing on the host [8]. Minute fluctuations in carbon dioxide (CO2) concentration elicit activation and attraction in host-seeking mosquitoes, which constitutes the initial recognition of a potential host [8–11]. Host odours take on a more prominent role in short-range attraction and landing [8,12]. Close to the host, mosquito behavioural responses to complex host odours are more robust than to single host volatiles, indicating that volatile blends play a crucial role in the coding for host odour recognition [7,13–15]. Although species-specific host volatiles may be involved in host discrimination and selection by anthropophilic mosquitoes [16,17], an increasing body of research suggests that host perception relies on a number of generic host volatiles and their relative proportions [7,9,18–20]. To process qualitative and quantitative differences among host blends, mosquitoes use coincidence detection to distinguish among hosts [7], similar to what has been shown for herbivorous insects [21]. Such coincidence detection has been shown to be essential for the behavioural response to one of the best-characterized generic host-related volatiles, (R)-1-octen-3-ol [7,22,23]. While not active by itself, (R)-1-octen-3-ol requires the simultaneous detection of CO2 to elicit behavioural attraction [22]. This has, however, only been shown for release rates that exceed the natural emission of (R)-1-octen-3-ol from known hosts [22]. The advantage of coincidence detection is that it is a flexible odour coding system, which allows for adaptation to alternative host species [21].
In this study, we investigate how the context of an odour cue shapes host perception in mosquitoes. We demonstrate that an individual host compound is interpreted in the context of the host odour. By altering the release rate of the generic host-related volatile (R)-1-octen-3-ol within its natural range, we demonstrate that blend context plays an important role in host and non-host recognition.
2. Material and methods
2.1. Insects
Aedes aegypti (Rockefeller strain), An. coluzzii (Suakoko strain) and C. quinquefasciatus (Thai strain) were reared at 27 ± 2°C, 70 ± 2% relative humidity (RH) under a 12 L : 12 D period, as previously described [22,24]. For all experiments, 4- to 10-day post-emergence sugar-fed adult female mosquitoes were used.
2.2. Volatile collections
Headspace volatile extracts were collected from humans, chickens and cattle hair. Human body volatiles were collected as previously described [25], with minor modifications. Briefly, naked volunteers were placed in customized heat-sealed bags (2 × 1.75 m; Melitta, Helsingborg, Sweden), with only their heads protruding. Empty bags of the same size were used as controls. Synthetic air (20.9% O2 and 79.1% N2, Strandmöllen AB, Ljungby, Sweden) was introduced into the bags at a rate of 6.5 l min−1. Pumps (reversed aquarium pumps; Rena 301, Rena, USA) extracted the air at 0.9 l min−1 through seven columns containing 40 mg Porapak Q (PQ; 80/100 mesh, Alltech, Deerfield, IL, USA) over 2.5 h. Volatiles were collected from a live chicken (Gammalsvensk dvärghöna) placed on a metal mesh in a desiccator covered with a black cloth to keep it calm. Charcoal filtered air was introduced (1 l min−1) via a Teflon tube and pumped out of the desiccator via a glass splitter connected to four PQ adsorbent columns (0.25 l min−1 each) over 1 h. Volatiles from Holstein cattle were collected by placing 20 g of freshly cut hair, approximately one third of the hair on a single cow, in a glass wash bottle. Charcoal filtered air was drawn by pumps (0.1 l min−1) through the bottle onto a PQ adsorbent column over 24 h. Trapped volatiles were desorbed by eluting each column with 600 µl of pentane (puriss p.a., Sigma-Aldrich Chemie GmbH, Steinheim, Germany). The volatile collections from each group and species were pooled and concentrated under a gentle stream of nitrogen to contain 0.25 min equivalents µl−1 for use in further experiments. Before use, the adsorbent columns were rinsed with 1 ml each of methanol, dichloromethane and pentane. Heptyl acetate (1 µg, 99.8%; Aldrich, St Louis, MO, USA) was added to each extract as an internal quantification standard before concentration.
2.3. Single sensillum recordings
The maxillary palps of Ae. aegypti, An. coluzzii and C. quinquefasciatus are covered with capitate peg sensilla, variously described as peg sensilla or basiconic sensilla, each housing three olfactory receptor neurons [22,26–28]. In all species, the intermediate amplitude neuron, referred to as the B cell, has been shown to respond to (R)-1-octen-3-ol [22,27,28]. Electrophysiological recordings from the B cells were made as previously described [22].
2.4. Stimulation and stimuli
An Agilent 6890 gas chromatograph (GC; Agilent Technology, Santa Clara, CA, USA) fitted with a fused silica capillary column (30 m × 0.25 mm i.d.) coated with non-polar HP-5 stationary phase (d.f. = 0.25 µm) was used for the separation of volatiles in the collected extracts, with hydrogen gas as the mobile phase (Q = 45 cm s−1). Aliquots of each sample (2 µl) were injected splitless for 30 s at 225°C. The GC oven temperature was programmed from 30°C (3 min hold), followed by a ramp of 8°C min–1 to 225°C, and held isothermal for 10 min. The GC was fitted with a make-up gas-fed (4 psi N2) four-way cross (Graphpack® 3D/2 Crosspiece Sulfinert™, Gerstel, Mülheim an der Ruhr, Germany) at the end of the column, delivering half of the effluent to the flame ionization detector, and the other half to the air stream passing over the maxillary palp via a Gerstel ODP-2 transfer line maintained at 135°C for 15 min and increased at 8°C min–1. Bioactive compounds were identified by injection on a combined Agilent 6890N GC and 5975 mass spectrometer (GC-MS; Agilent Technology) fitted with an HP-5MS column (dimensions as above), and using the same programme as for the GC-SSR analyses, but with helium as the mobile phase (Q = 35 cm s−1). The active compounds were identified by comparison with reference mass spectra in our custom-made and commercially available libraries (NIST05 and Wiley). Identified compounds were confirmed by parallel injections of synthetic reference compounds with authentic samples on the GC-MS. In addition, the bioactive compound (R)-1-octen-3-ol was quantified by the extracted ion current profile of m/z 57 [29] in the human extract, and in the cattle extract, by ratio compared with the internal standard. For verification of bioactive amounts, synthetic (R)-1-octen-3-ol (99%; courtesy of Dr James Logan) [22] dissolved in redistilled hexane was injected into the GC-SSR at concentrations ranging from 0.001 to 100 ng µl−1.
2.5. Landing bioassay
Behavioural responses of mosquitoes to (R)-1-octen-3-ol were observed in a no-choice landing assay by using a membrane feeding apparatus (Discovery Workshops, Accrington, Lancashire, UK) as previously described [30]. Volatile extracts (10 µl; 2.5 min equivalents) were applied on a collagen membrane, and regulated at 37°C for Ae. aegypti and An. coluzzii, and 42°C for C. quinquefasciatus. Experiments were conducted at 27 ± 2°C and 68 ± 2% RH, under either white or red light. Eight treatments (n = 10 for each species) were tested: (i) human hand rubbing for 1 min; (ii) cattle hair extract; (iii) chicken extract; (iv) 0.1 ng of (R)-1-octen-3-ol; (v) 5 ng of (R)-1-octen-3-ol; (vi) human hand rubbing with a cattle equivalent of (R)-1-octen-3-ol (5 ng); (vii) chicken extract with a human equivalent of (R)-1-octen-3-ol (0.1 ng); and (viii) hexane (control). For all treatments, the total volume added to the membrane was 20 µl, and where this was not provided by the treatments themselves, the remainder was supplied by the solvent, hexane. Initial control tests verified that human hand rubbing was not significantly different (ANOVA, F = 0.06, d.f. = 1, p = 0.80) from the human body extract (10 µl; 2.5 min equivalents) in a two-choice assay (data not shown).
Twenty individual mosquitoes were kept in an experimental cage (30 × 30 × 30 cm; Bugdorm, MegaView Science, Taiwan) under experimental climatic conditions for 24 h prior to the experiment. The number of landing mosquitoes was observed every 30 s for 10 min. Pure CO2 was pulsed into the cage for 4 s, once every minute (0.2 l min−1). The numbers of mosquitoes landing every 30 s reached equilibrium after 6 min, and the number of mosquitoes landing after 10 min was used for all further comparisons. Experiments were performed in the last 4 h of photophase for Ae. aegypti and during scotophase for An. coluzzii and C. quinquefasciatus.
2.6. Statistical analysis
Repeated measures two-way ANOVA, followed by a Bonferroni post hoc test was performed to compare the physiological activity among the species. A general linear model (GLM) two-way ANOVA, followed by Bonferroni post hoc test was used to calculate the significance within the eight different treatments of each species in the landing bioassay. The repeated measures two-way ANOVA was carried out with Graph Pad Prism v. 5.01 for Mac (GraphPad Software, La Jolla, CA, USA), while the GLM two-way ANOVA was analysed using Minitab v. 16.1.0 (Minitab Statistical Software, State College, PA, USA).
3. Results
3.1. Detection of the natural emission rates of (R)-1-octen-3-ol
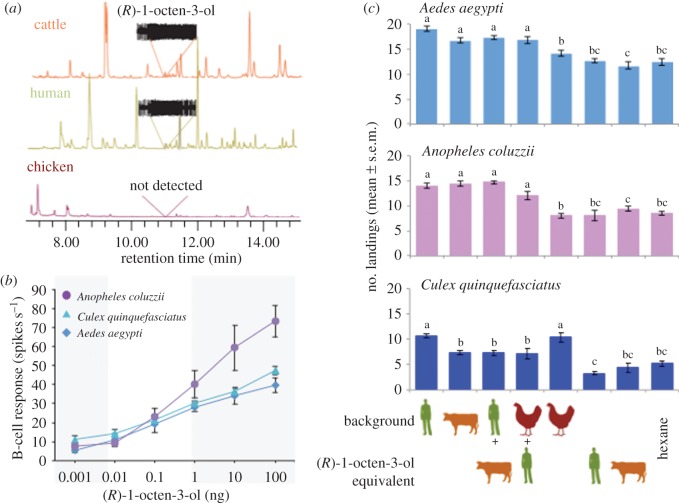
The GC-SSR (figure 1a) and GC-MS analyses identified (R)-1-octen-3-ol as a key natural ligand for one of the olfactory receptor neurons of the capitate peg sensillum, the B cell. (R)-1-octen-3-ol was present in the cattle hair (0.6 ± 0.35 ng min−1 equivalent) and human extracts (0.06 ± 0.04 ng min−1 equivalent), but was not detected in the chicken extract (figure 1a). Considering the difference in the methods used to collect the headspace of cattle (shaved hair), compared to human and chicken (whole body), we may be underestimating the release rate of (R)-1-octen-3-ol for the cow. Dose–response analysis, using GC-SSRs, revealed a similar B cell response among the three mosquito species to (R)-1-octen-3-ol (F = 3.522, d.f. = 2, p = 0.063). The response to (R)-1-octen-3-ol that approximates the human release rate (0.01–0.1 ng) reflects the threshold of detection for the B cell in these mosquitoes, while that associated with cattle hair (0.5–1.0 ng) is well within the detection range of the OSN (F = 86.98, d.f. = 5, p < 0.001; figure 1b; white panel).
Figure 1.
Females of Aedes aegypti, Anopheles coluzzii and Culex quinquefasciatus respond physiologically and behaviourally to (R)-1-octen-3-ol. (a) Representative odourant profiles of cattle (orange), human (green) and chicken (dark red) extracts as generated by gas chromatography (GC). (R)-1-octen-3-ol was present in the cattle and human extracts but was not detected (nd) in the chicken extracts. Traces above the human and cattle extract represent the elicited responses from the (R)-1-octen-3-ol sensitive neuron (the B cell) in Ae. aegypti, using GC-single sensillum recordings (SSR). (b) Averaged GC-SSRs from Ae. aegypti (light blue), Cu. quinquefasciatus (dark blue) and An. coluzzii (purple) to synthetic (R)-1-octen-3-ol (n = 5) show a dose-dependent relationship. The white panel indicates the range of natural release of (R)-1-octen-3-ol from human and cattle. (c) Landing bioassays were performed to observe the role of (R)-1-octen-3-ol in host-seeking behaviour (n = 10). The different extracts were tested alone and in combination with human and cattle equivalents of (R)-1-octen-3-ol (0.1 and 5 ng over 10 min, respectively). Human and cattle equivalents of (R)-1-octen-3-ol were also tested individually, as was the solvent hexane. Letters above the bars denote significant difference between treatments within species (GLM ANOVA, p < 0.05).
3.2. Behavioural response to (R)-1-octen-3-ol
The landing behaviour of mosquitoes was examined in the presence of different odour extracts. Aedes aegypti and An. coluzzii landed significantly more often on the human hand rubbing and the cattle hair extract than on both the chicken extract and the control (F = 30.85, d.f. = 3, p < 0.001; F = 52.82, d.f. = 3, p < 0.001; figure 1c). In both species, addition of a human equivalent (0.1 ng) of (R)-1-octen-3-ol to chicken extract increased landing to a level comparable to the human and cattle extracts (F = 8.09, d.f. = 3, p < 0.001; F = 25.22, d.f. = 3, p < 0.001). Human hand rubbing with a cattle equivalent (5 ng) of (R)-1-octen-3-ol, however, did not increase the landing above that of the cattle extract alone (F = 1.24, d.f. = 1, p < 0.281; F = 0.23, d.f. = 1, p < 0.634; figure 1c).
Culex quinquefasciatus showed similar landing responses in the presence of the human hand rubbing and chicken extracts, while the behaviour towards the cattle extract and the extracts (human and chicken) with added (R)-1-octen-3-ol did not differ significantly from the control (F = 1.64, d.f. = 3, p < 0.22; figure 1c). Interestingly, when the human equivalent of (R)-1-octen-3-ol was added to the chicken extract, a reduction in landing was observed compared with chicken extracts alone (F = 17.06, d.f. = 7, p < 0.001; figure 1c). The control and (R)-1-octen-3-ol alone (0.1 and 5 ng) were not significantly different from each other in all species (F = 0.70, d.f. = 2, p < 0.504; F = 1.04, d.f. = 2, p < 0.368; F = 1.32, d.f. = 2, p < 0.284; figure 1c). A two-choice assay verified that human body extract was not significantly different from hand rubbing (F = 0.06, d.f. = 1, p < 0.80).
4. Discussion
The mechanism by which the breadth of mosquito host choice is regulated is a product of the combination of sensory adaptation to preferred hosts over evolutionary time and the phenotypic plasticity of the sensory response [7,31]. Here, we demonstrate that the detection of (R)-1-octen-3-ol by the olfactory system and its perception within a blend plays a vital role in host selection and discrimination. Odour differences among hosts, both qualitative and quantitative, significantly affect mosquito behavioural output, highlighting that accurate host recognition results from the perception of generic volatiles in the context of the host odour blend. The data provided here, and in our companion paper [11], emphasize that generic host volatiles provide host recognition cues for mosquitoes, and that the detection and perception of such volatiles provide mosquitoes with a flexible, yet constrained, coding system for host finding.
4.1. Detection of (R)-1-octen-3-ol at natural release rates
The peripheral olfactory system is conserved across species and is detecting (R)-1-octen-3-ol within the ecologically relevant release rates of the compound by potential mammalian hosts. The fact that (R)-1-octen-3-ol was not detected in the chicken extracts is in line with previous studies on chickens and other bird species [32–34]. The observed increase in sensitivity of An. coluzzii B cells to the highest two concentrations tested may be related to the combination of the increased transcript abundance and sensitivity of its cognate odorant receptor, AgOr8 [27,35], compared with both Ae. aegypti (AaOr8 [36,37]) and C. quinquefasciatus (CqOr118 and CqOr113 [38]). Any observed differential behaviours in response to the natural release rates of (R)-1-octen-3-ol are thus not linked to differential peripheral coding, but are more likely to be a result of combinatorial coding in higher olfactory centres, similar to what has been described in herbivorous insects [21].
4.2. Blend perception regulates host choice
The landing response of Ae. aegypti, An. coluzzii and C. quinquefasciatus to the natural headspace volatiles of the preferred and non-preferred host species, but not to the single components therein, underlines that individual components of a host blend, such as (R)-1-octen-3-ol (this study), are often not recognized as a host cue when perceived outside the context of the blend [21]. Insects rely on ratio and coincidence detection by olfactory receptor neurons tuned to generic and host-specific volatiles [21]. Such an odour coding system provides flexibility to accommodate for variation in the ratio of volatiles within preferred host blends. For instance, Ae. aegypti and An. coluzzii continue to land following the increase in the ratio of (R)-1-octen-3-ol in human headspace to the cattle equivalent. However, C. quinquefasciatus did not respond to the headspace of their preferred hosts, human and chicken, after supplementation with cattle or human equivalent doses of (R)-1-octen-3-ol, respectively. Conversely, Ae. aegypti and An. coluzzii responded to the headspace of their non-preferred host, chicken, after supplementation with a human equivalent dose of (R)-1-octen-3-ol. Combined, these results emphasize that host recognition is contextual and depends on quantitative and qualitative differences in blends and the olfactory codes they evoke. Similar patterns have been observed in studies on herbivorous insects [21], suggesting that the ratios of host volatiles play a key role in blend perception and host recognition.
5. Conclusion
While the role of generic host volatiles in species-specific, particularly human, host selection by mosquitoes has been indicated in previous work [3,7,11], this study highlights the importance of generic host volatiles, within their natural release rates, in modulating interspecific host recognition. The cross-species comparison revealed the importance of analysing the response properties and tuning of olfactory receptor neurons together with how this affects the behavioural output. The study also emphasized the importance of analysing the behavioural response to generic host volatiles in the context of the host blend. From a vector control perspective, this is essential when developing synthetic blends for optimal attraction of mosquitoes in efforts to control and monitor populations.
Acknowledgements
We thank Dr James Logan for his gift of the (R)-1-octen-3-ol. We also thank Dr Teun Dekker for his critical reading of the manuscript.
Data accessibility
All data that is relevant to this study is reported within the article.
Authors' contributions
R.I. and S.R.H. contributed to the conception and design of this study. S.M. and G.B. acquired the data. All authors were involved in the analysis and interpretation of the data. R.I., S.R.H. and S.M. wrote, and G.B. critically revised, the manuscript. All authors approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Funding
This study was supported by the Linnaeus initiative ‘Insect Chemical Ecology, Ethology and Evolution, IC-E3’ (Formas, Swedish University of Agricultural Sciences) and a PhD scholarship to S.M. from the High Education Commission (HEC) of Pakistan.
References
- 1.Lyimo IN, Ferguson HM. 2009. Ecological and evolutionary determinants of host species choice in mosquito vectors. Trends Parasitol. 25, 189–196. (doi:10.1016/j.pt.2009.01.005) [DOI] [PubMed] [Google Scholar]
- 2.Scott TW, Takken W. 2012. Feeding strategies of anthropophilic mosquitoes result in increased risk of pathogen transmission. Trends Parasitol. 28, 114–121. (doi:10.1016/j.pt.2012.01.001) [DOI] [PubMed] [Google Scholar]
- 3.Takken W, Verhulst NO. 2013. Host preferences of blood-feeding mosquitoes. Annu. Rev. Entomol. 58, 433–453. (doi:10.1146/annurev-ento-120811-153618) [DOI] [PubMed] [Google Scholar]
- 4.Dekker T, Takken W, Braks MAH. 2001. Innate preference for host-odor blends modulates degree of anthropophagy of Anopheles gambiae sensu lato (Diptera: Culicidae). J. Med. Entomol. 38, 868–871. (doi:10.1603/0022-2585-38.6.868) [DOI] [PubMed] [Google Scholar]
- 5.Lefèvre T, Gouagna LC, Dabiré KR, Elguero E, Fontenille D, Renaud F, Costantini C, Thomas F. 2009. Beyond nature and nurture: phenotypic plasticity in blood-feeding behavior of Anopheles gambiae ss when humans are not readily accessible. Am. J. Trop. Med. Hyg. 81, 1023–1029. (doi:10.4269/ajtmh.2009.09-0124) [DOI] [PubMed] [Google Scholar]
- 6.Govella NJ, Chaki PP, Killeen GF. 2013. Entomological surveillance of behavioural resilience and resistance in residual malaria vector populations. Malar. J. 12, 124 (doi:10.1186/1475-2875-12-124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takken W, Knols BGJ. 1999. Odor-mediated behavior of afrotropical malaria mosquitoes. Annu. Rev. Entomol. 44, 131–157. (doi:10.1146/annurev.ento.44.1.131) [DOI] [PubMed] [Google Scholar]
- 8.Cardé RT. 2015. Multi-cue integration: how female mosquitoes locate a human host. Curr. Biol. 25, R793–R795. (doi:10.1016/j.cub.2015.07.057) [DOI] [PubMed] [Google Scholar]
- 9.Geier M, Bosch OJ, Boeckh J. 1999. Influence of odour plume structure on upwind flight of mosquitoes towards hosts. J. Exp. Biol. 202, 1639–1648. [DOI] [PubMed] [Google Scholar]
- 10.Dekker T, Takken W, Cardé RT. 2001. Structure of host-odour plumes influences catch of Anopheles gambiae s.s. and Aedes aegypti in a dual-choice olfactometer. Physiol. Entomol. 26, 124–134. (doi:10.1046/j.1365-3032.2001.00225.x) [Google Scholar]
- 11.Majeed S, Hill SR, Dekker T, Ignell R. Submitted. Detection and perception of generic host volatiles by mosquitoes: I. Impaired responses to CO2 constrains host-seeking behaviour. [DOI] [PMC free article] [PubMed]
- 12.De Jong R, Knols BGJ. 1996. Selection of biting sites by mosquitoes. In Olfaction in mosquitoes-host interaction (eds Bock G, Cardew G), pp. 89–103. Chichester, UK: Ciba Foundation Symposium 200, John Wiley & Sons. [DOI] [PubMed] [Google Scholar]
- 13.Smallegange RC, Takken W. 2010. Host-seeking behaviour of mosquitoes: responses to olfactory stimuli in the laboratory. In Olfaction in vector-host interactions. (eds Takken W, Knols BGJ), pp. 143–180. Wageningen, The Netherlands: Wageningen Academic Publishers. [Google Scholar]
- 14.Qiu YT, Smallegange RC, Van Loon JJA, Takken W. 2011. Behavioural responses of Anopheles gambiae sensu stricto to components of human breath, sweat and urine depend on mixture composition and concentration. Med. Vet. Entomol. 25, 247–255. (doi:10.1111/j.1365-2915.2010.00924.x) [DOI] [PubMed] [Google Scholar]
- 15.Okumu FO, et al. 2010. Development and field evaluation of a synthetic mosquito lure that is more attractive than humans. PLoS ONE 5, e8951 (doi:10.1371/journal.pone.0008951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukabana WR, Takken W, Coe R, Knols BGJ. 2002. Host-specific cues cause differential attractiveness of Kenyan men to the African malaria vector Anopheles gambiae. Malar. J. 1, 17 (doi:10.1186/1475-2875-1-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smallegange RC, Bukovinszkine-Kiss G, Otieno B, Mbadi PA, Takken W, Mukabana WR, Van Loon JJA. 2012. Identification of candidate volatiles that affect the behavioural response of the malaria mosquito Anopheles gambiae sensu stricto to an active kairomone blend: laboratory and semi-field assays. Physiol. Entomol. 37, 60–71. (doi:10.1111/j.1365-3032.2011.00827.x) [Google Scholar]
- 18.Smallegange RC, Qiu YT, van Loon JJA, Takken W. 2005. Synergism between ammonia, lactic acid and carboxylic acids as kairomones in the host-seeking behaviour of the malaria mosquito Anopheles gambiae sensu stricto (Diptera: Culicidae). Chem. Senses 30, 145–152. (doi:10.1093/chemse/bji010) [DOI] [PubMed] [Google Scholar]
- 19.Smallegange RC, Qiu YT, Bukovinszkiné-Kiss G, Van Loon JJA, Takken W. 2009. The effect of aliphatic carboxylic acids on olfaction-based host-seeking of the malaria mosquito Anopheles gambiae sensu stricto. J. Chem. Ecol. 35, 933–943. (doi:10.1007/s10886-009-9668-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verhulst NO, Mukabana WR, Takken W, Smallegange RC. 2011. Human skin microbiota and their volatiles as odour baits for the malaria mosquito Anopheles gambiae ss. Entomol. Exp. Appl. 139, 170–179. (doi:10.1111/j.1570-7458.2011.01119.x) [Google Scholar]
- 21.Bruce TJA, Pickett JA. 2011. Perception of plant volatile blends by herbivorous insects—finding the right mix. Phytochemistry 72, 1605–1611. (doi:10.1016/j.phytochem.2011.04.011) [DOI] [PubMed] [Google Scholar]
- 22.Cook JI, Majeed S, Ignell R, Pickett JA, Birkett MA, Logan JG. 2011. Enantiomeric selectivity in behavioural and electrophysiological responses of Aedes aegypti and Culex quinquefasciatus mosquitoes. Bull. Entomol. Res. 101, 541–550. (doi:10.1017/s0007485311000162) [DOI] [PubMed] [Google Scholar]
- 23.Takken W, Kline D. 1989. Carbon dioxide and 1-octen-3-ol as mosquito attractants. J. Am. Mosq. Control Assoc. 5, 311–316. [PubMed] [Google Scholar]
- 24.Hill SR, Hansson BS, Ignell R. 2009. Characterization of antennal trichoid sensilla from female southern house mosquito, Culex quinquefasciatus Say. Chem. Senses 34, 231–252. (doi:10.1093/chemse/bjn080) [DOI] [PubMed] [Google Scholar]
- 25.Harraca V, Ryne C, Birgersson G, Ignell R. 2012. Smelling your way to food: can bed bugs use our odour? J. Exp. Biol. 215, 623–629. (doi:10.1242/jeb.065748) [DOI] [PubMed] [Google Scholar]
- 26.Majeed S, Hill SR, Ignell R. 2014. Impact of elevated CO2 background levels on the host-seeking behaviour of Aedes aegypti. J. Exp. Biol. 217, 598–604. (doi:10.1242/jeb.092718) [DOI] [PubMed] [Google Scholar]
- 27.Lu T, et al. 2007. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr. Biol. 17, 1533–1544. (doi:10.1016/j.cub.2007.07.062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Syed Z, Leal WS. 2007. Maxillary palps are broad spectrum odorant detectors in Culex quinquefasciatus. Chem. Senses 32, 727–738. (doi:10.1093/chemse/bjm040) [DOI] [PubMed] [Google Scholar]
- 29.Garland WA, Power ML. 1981. Quantitative selected ion monitoring (QSIM) of drugs and/or metabolites in biological matrices. J. Chromatogr. Sci. 19, 392–434. (doi:10.1093/chromsci/19.8.392) [DOI] [PubMed] [Google Scholar]
- 30.Dube FF, Tadesse K, Birgersson G, Seyoum E, Tekie H, Ignell R, Hill SR. 2011. Fresh, dried or smoked? repellent properties of volatiles emitted from ethnomedicinal plant leaves against malaria and yellow fever vectors in Ethiopia. Malar. J. 10, 375 (doi:10.1186/1475-2875-10-375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowen M. 1991. The sensory physiology of host-seeking behavior in mosquitoes. Annu. Rev. Entomol. 36, 139–158. (doi:10.1146/annurev.en.36.010191.001035) [DOI] [PubMed] [Google Scholar]
- 32.Campagna S, Mardon J, Celerier A, Bonadonna F. 2012. Potential semiochemical molecules from birds: a practical and comprehensive compilation of the last 20 years’ studies. Chem. Senses 37, 3–25. (doi:10.1093/chemse/bjr067) [DOI] [PubMed] [Google Scholar]
- 33.Bernier UR, Allan SA, Quinn BP, Kline DL, Barnard DR, Clark GG. 2008. Volatile compounds from the integument of White Leghorn Chickens (Gallus gallus domesticus L.): candidate attractants of ornithophilic mosquito species. J. Sep. Sci. 31, 1092–1099. (doi:10.1002/jssc.200700434) [DOI] [PubMed] [Google Scholar]
- 34.Allan SA, Bernier UR, Kline DL. 2006. Laboratory evaluation of avian odors for mosquito (Diptera: Culicidae) attraction. J. Med. Entomol. 43, 225–231. (doi:10.1093/jmedent/43.2.225) [DOI] [PubMed] [Google Scholar]
- 35.Wang G, Carey AF, Carlson JR, Zwiebel LJ. 2010. Molecular basis of odor coding in the malaria vector mosquito Anopheles gambiae. Proc. Natl Acad. Sci. USA 107, 4418–4423. (doi:10.1073/pnas.0913392107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bohbot JD, Dickens JC. 2009. Characterization of an enantioselective odorant receptor in the yellow fever mosquito Aedes aegypti. PLoS ONE 4, e7032 (doi:10.1371/journal.pone.0007032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bohbot JD, Durand NF, Vinyard BT, Dickens JC. 2013. Functional development of the octenol response in Aedes aegypti. Front. Physiol. 4, 39 (doi:10.3389/fphys.2013.00039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hill SR, Majeed S, Ignell R. 2015. Molecular basis for odorant receptor tuning: a short C-terminal sequence is necessary and sufficient for selectivity of mosquito Or8. Insect Mol. Biol. 24, 402–412. (doi:10.1111/imb.12176) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data that is relevant to this study is reported within the article.