Abstract
Macrostomy is the anatomical feature present in macrostomatan snakes that permits the ingestion of entire prey with high cross-sectional area. It depends on several anatomical traits in the skeleton and soft tissues, of which the elongation of gnathic complex and backward rotation of the quadrate represent crucial skeletal requirements. Here, the relevance of postnatal development of these skull structures and their relationship with macrohabitat and diet are explored. Contrary to the condition present in lizards and basal snakes that occupy underground macrohabitats, elements of the gnathic complex of most macrostomatan snakes that exploit surface macrohabitats display conspicuous elongation during postnatal growth, relative to the rest of the skull, as well as further backward rotation of the quadrate bone. Remarkably, several clades of small cryptozoic macrostomatans reverse these postnatal transformations and return to a diet based on prey with low cross-sectional area such as annelids, insects or elongated vertebrates, thus resembling the condition present in underground basal snakes. Dietary ontogenetic shift observed in most macrostomatan snakes is directly linked with this ontogenetic trajectory, indicating that this shift is acquired progressively as the gnathic complex elongates and the quadrate rotates backward during postnatal ontogeny. The numerous independent events of reversion in the gnathic complex and prey type choice observed in underground macrostomatans and the presence of skeletal requirements for macrostomy in extinct non-macrostomatan species reinforce the possibility that basal snakes represent underground survivors of clades that had the skeletal requirements for macrostomy. Taken together, the data presented here suggest that macrostomy has been shaped during multiple episodes of occupation of underground and surface macrohabitats throughout the evolution of snakes.
Keywords: snakes, postnatal ontogeny, macrostomy, evolution
1. Introduction
Snakes are gape-limited predators that swallow their prey whole, that is, without mechanical reduction through an intraoral treatment prior to ingestion. Among the wide diversity of extant snakes, basal forms such as scolecophidians (worm-like snakes) and basal alethinophidians (pipe snakes, shieldtail snakes) occupy underground macrohabitats and feed on prey of small size and/or small cross-sectional area, such as insects, earthworms and elongated vertebrates. By contrast, alethinophidian snakes included in the clade Macrostomata (pythons, boas, dwarf boas and colubroids) have developed in extreme this feeding strategy ingesting large prey with large cross-sectional area in relation to their head dimensions. This particular feeding behaviour present in macrostomatans is possible due to an anatomical feature labelled as macrostomy [1,2], which requires complementary notable modifications in skeletal and soft tissue organs such as increased length of the gnathic complex (palatomaxillary arch, suspensorium and mandible) and modifications of the intermandibular soft tissues to allow stretching [1–7]. Macrostomy permits exploiting an enormous diversity of prey types, a fact that has deep implications for the occupation of diverse macrohabitats and evolutionary success of macrostomatan snakes [1]. The acquisition of this astonishing anatomical feature results in one of the most interesting innovations in vertebrate feeding, but much of the evolutionary events that shaped macrostomy remain uncertain.
Studies concerning postnatal ontogeny have become a powerful source of information about evolutionary processes in the skeleton of squamates [8,9]. However, transformations during postnatal ontogeny in snakes remain largely unknown. To date, the few studies of snake postnatal development [10–13] have identified that skull components directly involved in macrostomy experience important changes during postnatal ontogeny. Here, I analyse postnatal transformations in the skull of several representatives of major groups of snakes to investigate the impact of postnatal ontogeny on the evolution of cranial requirements for macrostomy. In order to explore the relation between macrohabitat, diet and skull morphology, I also perform an exhaustive survey comparing these variables in all major groups of living snakes to evaluate their relationship in the evolution of macrostomatan snakes. Finally, I place these results in a phylogenetic context to trace the evolution of macrostomy in the context of the evolution of snake feeding.
2. Material and methods
2.1. Specimens examined
Postnatal transformations were examined in dry and cleared and stained skulls of several lizard and snake groups (electronic supplementary material). Most specimens were studied first hand, but in other cases, I relied on photographs or published descriptions of embryonic and postnatal series. Postnatal sequences utilized represent series of growth stages assembled with available specimens in collections.
2.2. Quantitative analysis
Several cranial bones were measured in selected representatives of the major clades of snakes and simply quantified as ratios and figured with respect to the snout--condyle length, a standard measure that corresponds with the longitudinal measurement of the skull (see electronic supplementary material). Small specimens were measured to the nearest 0.1 mm using an ocular micrometer.
2.3. Feeding morphological–ecological survey
To explore the relationship between the morphological and ecological traits of snake feeding, I surveyed information about skull anatomy, body size (snout--vent length, SVL), macrohabitat and prey type in 147 species representing all major groups of extant snakes (electronic supplementary material, S2). In order to standardize terminology, I first defined accurately each term used in reference to underground (fossorial, cryptozoic) and surface (terrestrial, arboreal and aquatic) macrohabitats and diet types (electronic supplementary material, S1 and S2). This survey is exhaustive with respect to the currently recognized major clades of snakes, including nearly all basal and advanced alethinophidians that occupy underground macrohabitats. Information concerning body size, macrohabitat and prey items was obtained using published studies about natural history of selected species (see electronic supplementary material). In the particular case of food habits, I chose only studies based on gut content of a large number of individuals, with the exception of a few relevant taxa without specific studies about their diet (e.g. Xenopeltis), which were scored using studies that register single records of predation or gut contents. In order to test for significant differences in these traits throughout the phylogeny, I performed a phylogenetic MANOVA [14] using the R package ‘Geiger’ [15]. A phylogeny of extant snakes was taken from the maximum-likelihood phylogenetic tree of Pyron et al. [16].
3. Results
3.1. Postnatal transformations in gnathic complex
3.1.1. Elongation of the palatomaxillary arch, suspensorium and mandible
With increasing postnatal stage, the elements that form the gnathic complex of basal snakes such as scolecophidians, basal alethinophidians and basal macrostomatans (Xenopeltis, Loxocemus) experience isometric growth with respect to the rest of the skull (figure 1b,c; electronic supplementary material, S1 and figure S2), similar to the condition previously described for lizards [17–21]. As in lizards, relative dimensions of the pterygoid, supratemporal, quadrate and mandible of basal snakes increase their length isometrically with respect to the skull length during postnatal growth (figures 1a–c and 3). However, the posterior elongation of the pterygoid, supratemporal and mandible never surpasses the posterior boundary delimited by the occipital–vertebral joint. Also, the quadrate bone grows with the same isometric pattern of the rest of the gnathic elements.
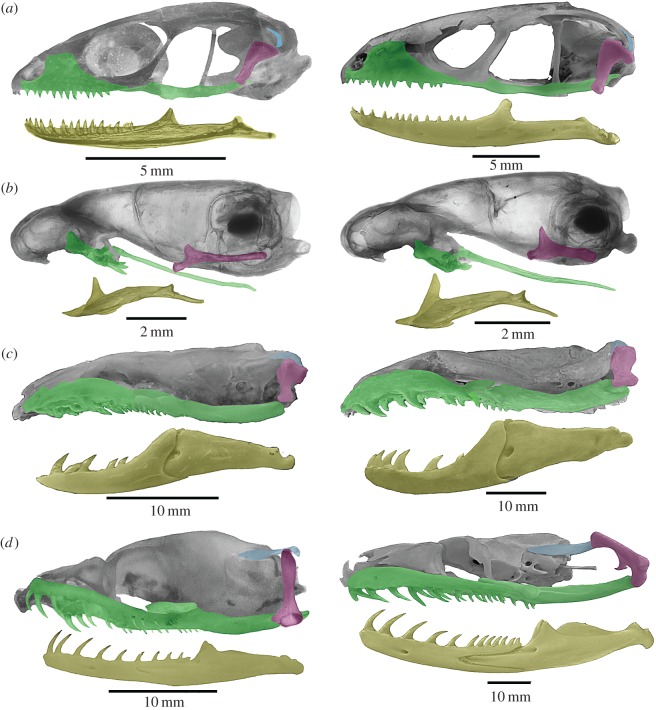
Figure 1.
Postnatal transformations in the gnathic complex in lizards and snakes. (a) Juvenile (SVL 56 mm) and adult (SVL 237 mm) of the anguid lizard Ophiodes intermedius; (b) juvenile (SVL 43 mm) and adult (SVL 131 mm) of the typhlopid scolecophidian snake Amerotyphlops brongersmianus; (c) juvenile (SVL 212 mm) and adult (SVL 895 mm) of the basal alethinophidian snake Anilius scytale; (d) juvenile (SVL 311 mm) and adult (SVL 1620 mm) of the macrostomatan snake Boa constrictor.
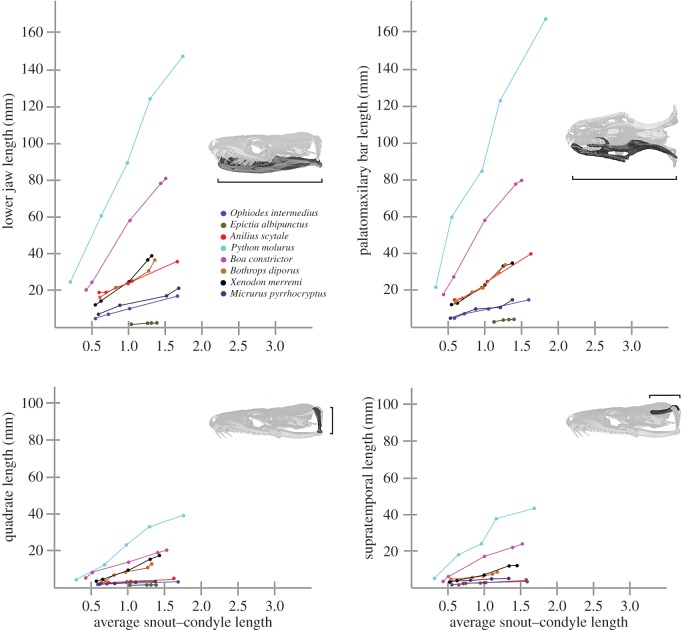
Figure 3.
Metrics of changes in underground and surface snake gnathic complex elements during postnatal ontogeny.
By contrast, the major changes that occur in the skull of most macrostomatan snakes from hatchling to adult stages comprise the allometric elongation of the elements that form the gnathic complex with respect to the rest of the skull (figures 1d and 3; electronic supplementary material, S1 and figure S1). This growth is clearly evidenced by the elongation of the quadrate ramus of the pterygoid bone, supratemporal and mandible beyond the posterior limit marked by the occipital–vertebral joint. The posterior elongation of the supratemporal bone generates the free-ending process, which represents one of the most distinctive traits of the macrostomatan skull. The length of the gnathic complex varies considerably among macrostomatan subclades, and it tends to be more slender in snakes with moderate to large SVL (more than 1500 mm). The quadrate bone also shows a significant growth through the elongation of the quadrate shaft. Notably, some groups of macrostomatans, specifically small ones, are an exception because these small snakes exhibit isometric growth of the gnathic complex in relation to skull length, resembling the condition present in lizards and basal snakes (figures 2 and 3; electronic supplementary material, S1 and figure S3). This shift involves the reversion of key characters for macrostomy like the shortening or absence of the free-ending process of the supratemporal bone and shortening of quadrate bone, resulting in a stout quadrate that displays a strikingly similar shape to that observed in basal alethinophidians.
Figure 2.
Postnatal transformations in the gnathic complex of cryptozoic macrostomatans. (a) Lateral view of the skull of a juvenile (SVL 114 mm) and adult (SVL 277 mm) of the dipsadine colubroid Atractus reticulatus; (b) juvenile (SVL 182 mm) and adult (SVL 921 mm) of the elapid Micrurus pyrrhocryptus. Scale bars, 2 mm.
3.1.2. Quadrate rotation
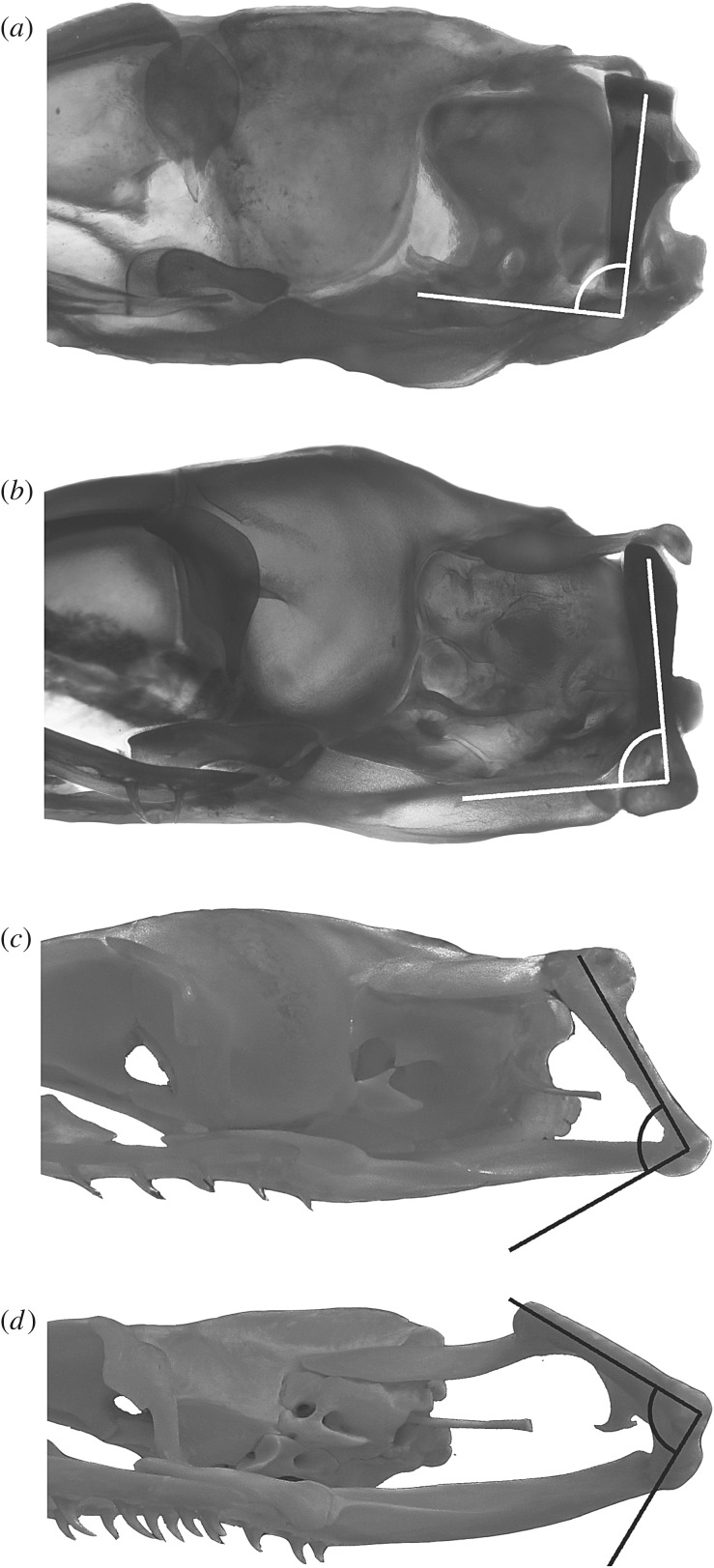
The quadrate of macrostomatan snakes experiences a noteworthy backward rotation process during embryonic development [22–25]. In late embryonic stages, the ossifying quadrate undergoes a counter-clockwise rotation to a vertical position, which is typical of postembryonic stages in basal alethinophidians, basal macrostomatans and hatchling individuals of core macrostomatans. This rotatory process seems to be absent in scolecophidians because the quadrate bone is rostroventrally slanted in all postnatal stages [26].
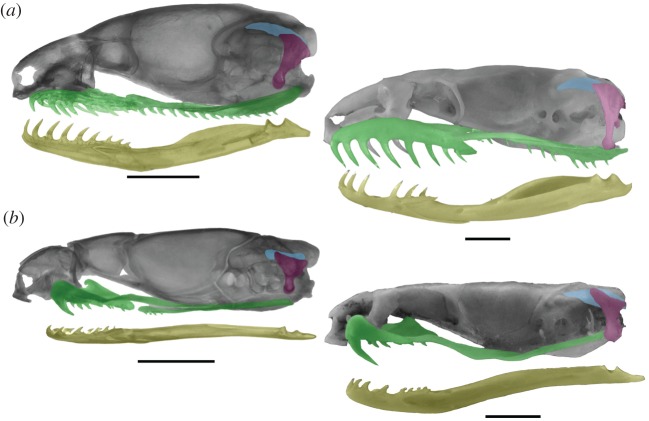
Remarkably, adults of most macrostomatans exhibit a further backward inclined position of the quadrate with respect of the vertical or slightly inclined position exhibited by hatchings (figure 1d; electronic supplementary material, S1 and figure S4). Available ontogenetic series indicate that this position is the result of the continuation during the postnatal development of the rotatory process initiated in embryonic stages. Late embryos and postnatal individuals of the macrostomatan Boa constrictor (figure 4) show this condition, where the quadrate exhibits an intermediate rotation process in a vertical position with respect of the rest of the skull. During subsequent postnatal stages this backwards rotation continues, reaching the caudoventral inclined orientation that characterizes adult individuals (figure 4d; electronic supplementary material, S1 and figure S4). It is important to note that macrostomatans without the elongation of the gnathic complex also lack the postnatal rotation process of the quadrate bone (figure 2; electronic supplementary material, S1 and figure S3).
Figure 4.
Rotation of the quadrate bone during postnatal development in the macrostomatan snake Boa constrictor. (a) Late embryo (SVL 299 mm); (b) hatchling (SVL 311 mm); (c) juvenile (SVL 463 mm); (d) adult (SVL 1620 mm). Not to scale.
3.2. A comparative survey among morphological and ecological traits
The performed comparative survey supports previous observations [1,27] that claimed a close relationship between morphological and ecological traits concerning snake feeding.
Basal groups (scolecophidians, basal alethinophidians), which exploit underground macrohabitats (fossorial, cryptozoic), exhibit a small gape size due to their short gnathic complex and a rostroventrally or vertically oriented quadrate (figure 5; electronic supplementary material, S2). Almost invariably, these small-sized (SVL < 1000 mm) underground species ingest small prey with a small cross-sectional area such as insects, earthworms or elongated vertebrates. Remarkably, macrostomatan species that occupy underground macrohabitats, which also lack the postnatal changes in gnathic complex, mirror basal snakes in these feeding aspects. Results of the phylogenetic MANOVA analysis indicate there are significant differences in prey type and macrohabitat depending on SVL (F = 8.274 3, 126 p = 0.00429) and do not simply reflect the phylogenetic structure of the species sample. In other words, small underground snakes, independent of their phylogenetic position, lack postnatal transformations in gnathic complex and consume prey with small cross-sectional area throughout their whole lifespan.
Figure 5.
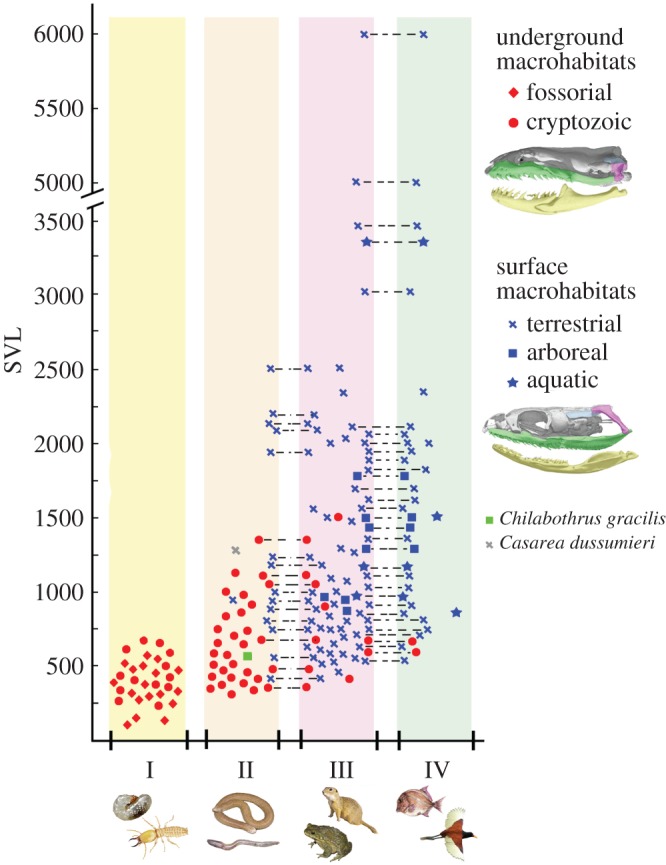
Graphical summary of the ecological morphological survey performed between body size (SVL), prey type and macrohabitat preferences of a set of adult extant snakes. Each point represents one species and points joined by a dotted line represent a single species with two prey type preferences.
Nearly all snakes that occupy surface macrohabitats (terrestrial, aquatic and arboreal) belong to the clade Macrostomata (figure 5; electronic supplementary material, S2). These snakes display the postnatal elongation of the gnathic complex and the quadrate rotation, and thus acquire in adult stages a large gape size that allows the ingestion of an extraordinary array of large prey with a large cross-sectional area. However, juvenile surface macrostomatans exhibit a small gape size owing to their early postnatal stage, consuming prey with small cross-sectional area. Notably, adult individuals of the few small macrostomatan species that occupy surface macrohabitats and were postulated as paedomorphic forms (e.g. Casarea dussumieri, Chilabothrus gracilis) lack the postnatal changes of the gnathic complex, and also consume prey items with a small cross-sectional area (figure 5; electronic supplementary material, S2).
4. Discussion
4.1. Postnatal ontogeny and dietary shift
It is widely acknowledged that most macrostomatan snakes experience an ontogenetic dietary shift, which implies a dietary change from small ectothermic prey with a small cross-sectional area as juveniles to bulky often endothermic prey with large cross-sectional area as adults. This shift necessarily implies an increase in gape size and other changes in behavioural and physiological characteristics [1,28–30]. Here, I argue that crucial factors for gape size such as length of components of gnathic complex and quadrate orientation undergo dramatic changes during postnatal ontogeny in macrostomatans. Hence, postnatal changes experienced by the gnathic complex constitute intrinsic anatomical factors that lead the ontogenetic dietary shift. Accordingly, the ability to ingest prey with large cross-sectional area is acquired progressively as lengthening of gnathic complex and backward quadrate rotation occurs during the course of postnatal ontogeny of surface macrostomatan snakes. Underground snakes, as well as the few surface macrostomatans that lack these changes during skull postnatal ontogeny, do not experience an ontogenetic dietary shift, thus highlighting the relevance of postnatal changes in the gnathic complex described here to the achievement of an ontogenetic dietary shift.
4.2. Macrostomy and ‘regressed macrostomatans'
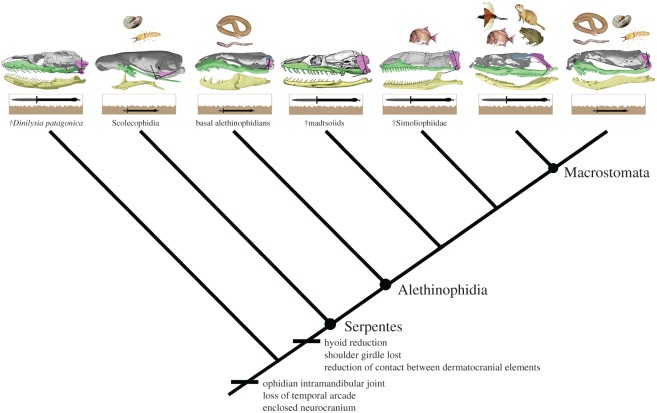
Miniaturization, which is a process usually attributed to paedomorphosis, is frequently correlated with burrowing habits in squamate reptiles [31–33]. This process generates shortening of the gnathic complex and verticalization of the quadrate bone in cryptozoic macrostomatans [34], and thus a regression of the crucial osteological requirements for macrostomy analysed herein. Based on the results of this study, the effects of miniaturization in the gnathic complex of cryptozoic macrostomatans can be inferred to have arisen through the truncation of the postnatal ontogenetic trajectory described above. Taking into account that at least 10 distantly related subclades of cryptozoic macrostomatan snakes lost skeletal requirements for macrostomy independently (electronic supplementary material, S1 and figure S5), this phenomenon appears to be recurrent in extant clades of snakes that occupy underground macrohabitats. Furthermore, recent molecular and combined phylogenetic analyses [16,35–37] yielded intriguing results where macrostomatan snakes with well-developed cranial requirements for macrostomy are nested within basal alethinophidians that lack these requirements and vice versa. Taken together, this body of information indicates that loss and reappearance of cranial requirements for macrostomy could have occurred several times in the history of snakes. Thus, scolecophidians and basal alethinophidians could be considered as miniaturized underground survivors (‘regressed macrostomatans’) of clades that formerly occupy surface macrohabitats as was discussed recently [26,38,39]. Although this hypothesis clashes with the traditional perspective, according to which the structure of the gnathic complex in basal snakes is ancestral, the presence of skeletal requirements for macrostomy in non-macrostomatan fossil snakes with large body size supports this interpretation. The stem snake Dinilysia patagonica exhibits a postnatal growth pattern of the supratemporal and lower jaws that positioned the mandibular articulation beyond the posterior limit marked by the occipital–vertebral joint [13]. Also, cranial requirements for macrostomy are present in Cretaceous marine simoliophiid snakes [40–42], a clade that is positioned outside Macrostomata in many phylogenetic analyses [36,37,43–45]. Moreover, pycnodontiform fish remains were reported as a gut content in the simoliophiid Pachyrhachis problematicus [40,46], a kind of prey that certainly represented a challenge for feeding because these fishes had deep, rounded and laterally compressed bodies.
4.3. The evolution of macrostomy
The overwhelming majority of living snake species belong to Macrostomata, making them one of the most diverse lineages of extant reptiles. Thus, the ability to consume entire bulky prey allowed by macrostomy arguable constitutes the most relevant key innovation that has fuelled their evolutionary success. Beyond the anatomical particularities of the gnathic complex analysed above, there are other skeletal transformations necessary to achieve this astonishing feeding behaviour, such as an intramandibular joint, reduction of bony contact between skull elements to permit unilateral movements of the upper jaw, loss of contact between hyoid and skull, an enclosed neurocranium, loss of the temporal arcade and loss of contact between ribs with sternum [1–4]. Remarkably, pressures imposed by underground macrohabitats have been postulated as triggers of most of these skeletal transformations via heterochronic processes and body elongation [31,34,47,48]. Therefore, underground macrohabitats in the early history of snakes were necessary to acquire a highly kinetic skull and reduce constraints for the transport of entire bulky prey into the stomach. Additionally, a large digestive tract to accommodate entire large prey was probably acquired during this early underground phase [49]. Given the findings of this research, it appears that a surface phase was crucial for releasing the constraints imposed by underground macrohabitats and permitting the elongation of the gnathic complex and the backward rotation of the quadrate bone during postnatal growth to expand gape size for exploitation of large prey. Thus, different episodes of occupation of underground and surface macrohabitats coupled with changes in behaviour and diet have shaped the skull morphology of macrostomatan snakes (figure 6). Regrettably, the appearance of other relevant transformations related to macrostomy such as stretching capabilities and reorganization of intermandibular tissues and adductor musculature are harder to explain with the available data. However, the retention of a well-developed aponeurotic system in the jaw adductor musculature of cryptozoic colubroids and its reduction observed during the postnatal development in terrestrial forms [50] invites consideration of the idea that the evolution of anatomical systems other than the skeleton have been also driven by the alternation of underground--surface macrohabitat occupation.
Figure 6.
Simplified phylogenetic tree of snakes [45] showing the information discussed in this work about body size, macrohabitat, prey type preferences and osteological requirements for macrostomy. Note the early appearance of crucial osteological requirements for macrostomy and the elongation of the gnathic complex present in non-macrostomatan simoliophiids.
Finally, the growing fossil record of snakes reveals that non-macrostomatan snakes with the capacity to consume large prey have existed [51,52], and that other species of miniaturized macrostomatan snakes have lost the skeletal requirements for macrostomy [45], thus indicating that the diversity of snake feeding anatomy was more complex than previously thought. Further investigations of the postnatal trajectory of other anatomical systems involved in macrostomy and more efforts towards the discovery of fossil snakes surely will shed light on this captivating evolutionary issue.
Supplementary Material
Supplementary Material
Acknowledgements
I thank Margarita Chiaraviglio, Santiago Brizuela, David Kizirian, Lauren Vonnahme and Gregory Schneider for providing valuable specimens and photographs. Mariana Chuliver, Andrew Cuff, Anjali Goswami, Martín Ezcurra and Facundo Llompart provided invaluable assistance with the statistics. Hussam Zaher for discussions and photographs of many snake specimens. I am also grateful to Marissa Fabrezi and Anjali Goswami for many helpful discussions and suggestions. Krister Smith always helped with English grammar. The manuscript also benefitted from reviews by two anonymous reviewers.
Data accessibility
Electronic supplementary material S1: specimens examined, macrohabitat and prey type definitions, morphometric data, phylogenetic MANOVA results, supplementary figures and references for electronic supplementary materials. Electronic supplementary material S2: summary of diet, prey type, body size and macrohabitat data for snake species included in the comparative survey. All electronic supplementary materials are available from the Dryad Digital Repository http://dx.doi.org/10.5061/dryad.1g626 [53].
Competing interests
I declare I have no competing interests.
Funding
This work was supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and Agencia Nacional de Promoción Científica y Tecnológica (PICT 2013-220 and PICT Bicentenario 616).
References
- 1.Cundall D, Greene HW. 2000. Feeding in snakes. In Feeding: form, function and evolution in tetrapod vertebrates (ed. Schwenk K.), pp. 293–333. San Diego, CA: Academic Press. [Google Scholar]
- 2.Cundall DF, Irish F. 2008. The snake skull. In Biology of the reptilia, Vol. 20, morphology H, the skull of Lepidosauria (eds Gans C, Gaunt AS, Adler K), pp. 349–692. Ithaca, NY: Society for the Study of Amphibians and Reptiles. [Google Scholar]
- 3.Gans C. 1961. The feeding mechanism of snakes and its possible evolution. Am. Zool. 1, 217–227. (doi:10.1093/icb/1.2.217217-227) [Google Scholar]
- 4.Rieppel O. 1980. The evolution of the ophidian feeding system. Zool. J. (Anat.) 103, 551–564. [Google Scholar]
- 5.Young BA. 1998. The comparative morphology of the intermandibular connective tisssue in snakes (Reptilia: Squamata). Zool. Anz. 237, 59–84. [Google Scholar]
- 6.Close M, Cundall D. 2014. Snake lower jaw skin: extension and recovery of a hyperextensible keratinized integument. J. Exp. Zool. 321, 78–97. (doi:10.1002/jez.1839) [DOI] [PubMed] [Google Scholar]
- 7.Close M, Perni S, Franzini-Armstrong C, Cundall D. 2014. Highly extensible skeletal muscle in snakes. J. Exp. Biol. 217, 2445–2448. (doi:10.1242/jeb.097634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rieppel O. 1992. Studies on skeleton formation in reptiles. I. The postembryonic development on the skeleton in Cyrtodactylus pubisulcus (Reptilia: Gekkonidae). J. Zool. 227, 87–100. (doi:10.1111/j.1469-7998.1992.tb04346.x) [Google Scholar]
- 9.Maisano JA. 2001. A survey of the state of ossification in neonatal squamates. Herpetol. Monogr. 15, 135–157. (doi:10.2307/1467041) [Google Scholar]
- 10.Rossman CE. 1980. Ontogenetic changes in skull proportions of the diamondback water snake, Nerodia rhombifer. Herpetologica 36, 42–46. [Google Scholar]
- 11.Young BA. 1989. Ontogenetic changes in the feeding system of the red-sided garter snake, Thamnophis sirtalis parietalis. I. Allometric analysis. J. Zool. 218, 365–381. (doi:10.1111/j.1469-7998.1989.tb02550.x) [Google Scholar]
- 12.Monteiro LR. 1998. Ontogenetic changes in the skull of Corallus caninus (L. 1758) and Corallus enhydris (L. 1758) (Serpentes-Boidae), an allometric study. The Snake 28, 51–58. [Google Scholar]
- 13.Scanferla A, Bhullar BAS. 2014. Postnatal development of the skull of Dinilysia patagonica (Squamata-Stem Serpentes). Anat. Rec. 297, 560–573. (doi:10.1002/ar.22862.) [DOI] [PubMed] [Google Scholar]
- 14.Garland T Jr, Dickerman AW, Janis CM, Jones JA. 1993. Phylogenetic analysis of covariance by computer simulation. Syst. Biol. 42, 265–292. (doi:10.1093/sysbio/42.3.265) [Google Scholar]
- 15.Harmon LJ, Weir JT, Brock CD, Glor RE, Challenger W. 2008. GEIGER: investigating evolutionary radiations. Bioinformatics 24, 129–131. (doi:10.1093/bioinformatics/btm538) [DOI] [PubMed] [Google Scholar]
- 16.Pyron RA, Burbrink FT, Wiens JJ. 2013. A phylogeny and updated classification of Squamata, including 4161 species of lizards and snakes. BMC Evol. Biol. 13, 93 (doi:10.1186/1471-2148-13-93) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barahona F, Barbadillo LJ. 1998. Inter- and intraspecific variation in the postnatal skull of some lacertid lizards. J. Zool. 245, 393–405. (doi:10.1111/j.1469-7998.1998.tb00114.x) [Google Scholar]
- 18.Bell CJ, Evans SE, Maisano JA. 2003. The skull of the gymnophthalmid lizard Neusticurus ecpleopus (Reptilia: Squamata). Zool. J. Linn. Soc. 139, 283–304. (doi:10.1046/j.1096-3642.2003.00077.x) [Google Scholar]
- 19.Torres-Carvajal O. 2003. Cranial osteology of the Andean lizard Stenocercus guentheri (Squamata: Tropiduridae) and its postembryonic development. J. Morphol. 255, 94–113. (doi:10.1002/jmor.10051) [DOI] [PubMed] [Google Scholar]
- 20.Tarazona OA, Fabrezi M, Ramírez-Pinilla MP. 2008. Cranial morphology of Bachia bicolor (Squamata: Gymnophthalmidae) and its postnatal development. Zool. J. Linn. Soc. 152, 775–792. (doi:10.1111/j.1096-3642.2007.00364.x) [Google Scholar]
- 21.Bhullar BAS. 2012. A phylogenetic approach to ontogeny and heterochrony in the fossil record: cranial evolution and development in anguimorphan lizards (Reptilia: Squamata). J. Exp. Zool. B 318, 521–530. (doi:10.1002/jez.b.22456) [DOI] [PubMed] [Google Scholar]
- 22.Kamal AM. 1966. On the process of rotation of the quadrate cartilage in Ophidia. Anat. Anz. 118, 87–90. [Google Scholar]
- 23.Haluska F, Alberch P. 1989. The cranial development of Elaphe obsoleta (Ophidia, Colubridae). J. Morphol. 178, 37–55. (doi:10.1002/jmor.1051780104) [DOI] [PubMed] [Google Scholar]
- 24.Rieppel O, Zaher H. 2001. The development of the skull in Acrochordus granulatus (Schneider) (Reptilia: Serpentes), with special consideration of the otico-occipital complex. J. Morphol. 249, 252–266. (doi:10.1002/jmor.1053) [DOI] [PubMed] [Google Scholar]
- 25.Boughner JC, Buchtova M, Fu K, Diewert VW, Hallgrimsson B, Richman JM. 2007. Embryonic development of Python sebae. I: staging criteria and macroscopic skeletal morphogenesis of the head and limbs. Zoology 110, 212–230. (doi:10.1016/j.zool.2007.01.005) [DOI] [PubMed] [Google Scholar]
- 26.Kley NJ. 2006. Morphology of the lower jaw and suspensorium in the Texas blindsnake, Leptotyphlops dulcis (Scolecophidia: Leptotyphlopidae). J. Morphol. 267, 494–515. (doi:10.1002/jmor.10414) [DOI] [PubMed] [Google Scholar]
- 27.Greene HW. 1983. Dietary correlates of the origin and radiation of snakes. Am. Zool. 23, 431–441. (doi:10.1093/icb/23.2.431) [Google Scholar]
- 28.Mushinsky HR. 1987. Foraging ecology. In Snakes: ecology and evolutionary biology (eds Seigel RA, Collins JT, Novak SS), pp. 302–334. New York, NY: McGraw-Hill. [Google Scholar]
- 29.Arnold SJ. 1993. Foraging theory and prey-size-predator-size relations in snake. In Snakes: ecology and behaviour (eds Seigel RA, Collins JT), pp. 87–116. New York, NY: McGraw-Hill. [Google Scholar]
- 30.Rodríguez-Robles JA, Bell CJ, Greene HW. 1999. Gape size and evolution of diet in snakes: feeding ecology of erycine boas. J. Zool. 248, 49–58. (doi:10.1111/j.1469-7998.1999.tb01021.x) [Google Scholar]
- 31.Rieppel O. 1984. Miniaturization of the lizard skull: its functional and evolutionary implications. In The structure, development and evolution of reptiles (ed. Freguson MWJ.), pp. 503–520. London, UK: The Zoological Society of London, Academic Press. [Google Scholar]
- 32.Rieppel O. 1996. Miniaturization in tetrapods: consequences for skull morphology. Symp. Zool. Soc. Lond. 69, 47–61. [Google Scholar]
- 33.Olori JC, Bell CJ. 2012. Comparative skull morphology of uropeltid snakes (Alethinophidia: Uropeltidae) with special reference to disarticulated elements and variation. PLoS ONE 7, e32450 (doi:10.1371/journal.pone.0032450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savitsky AH. 1983. Coadapted character complexes among snakes: fossoriality, piscivory and durophagy. Am. Zool. 23, 397–409. (doi:10.1093/icb/23.2.397) [Google Scholar]
- 35.Vidal N, Delmas AS, Hedges SB. 2007. The higher-level relationships of alethinophidian snakes inferred from seven nuclear and mitochondrial genes. In Biology of the boas and pythons (eds Henderson RW, Powell R), pp. 27–33. Eagle Mountain, UT: Eagle Mountain Publishing. [Google Scholar]
- 36.Reeder TW, Townsend TM, Mulcahy DG, Noonan BP, Wood PL Jr, Sites JW Jr, Wiens JJ. 2015. Integrated analyses resolve conflicts over squamate reptile phylogeny and reveal unexpected placements for fossil taxa. PLoS ONE 10, e0118199 (doi:10.1371/journal.pone.0118199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsiang AY, Field DJ, Webster TH, Behlke ADB, Davis MB, Racicot RA, Gauthier JA. 2015. The origin of snakes: revealing the ecology, behavior, and evolutionary history of early snakes using genomics, phenomics, and the fossil record. BMC Evol. Biol. 15, 87 (doi:10.1186/s12862-015-0358-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rieppel O, Maisano JA. 2007. The skull of the rare malaysian snake Anomochilus leonardi Smith, based on high-resolution x-ray computed tomography. Zool. J. Linn. Soc. 149, 671–685. (doi:10.1111/j.1096-3642.2007.00276.x) [Google Scholar]
- 39.Rieppel O. 2012. ‘Regressed’ macrostomatan snakes. Fieldiana (Life and Earth Sci.) 2012, 99–103. (doi:10.3158/2158-5520-5.1.99) [Google Scholar]
- 40.Lee MSY, Caldwell MW. 1998. Anatomy and relationships of Pachyrhachis problematicus, a primitive snake with limbs. Phil. Trans. R. Soc. Lond. B 353, 1521–1552. (doi:10.1098/rstb.1998.0308) [Google Scholar]
- 41.Rieppel O, Zaher H, Tchernov E, Polcyn MJ. 2003. The anatomy and relationships of Haasiophis terrasanctus, a fossil snake with well-developed hind limbs from the mid-Cretaceous of the Middle East. J. Paleont. 77, 536–558. (doi:10.1017/S0022336000044231) [Google Scholar]
- 42.Rieppel O, Head JJ. 2004. New specimens of the fossil snake genus Eupodophis Rage & Escuillié, from Cenomanian (Late Cretaceous) of Lebanon. Mem. Soc. Ital. Sci. Nat e Mus. Civ. Storia Nat. Milano 32, 1–26. [Google Scholar]
- 43.Lee MSY, Scanlon JD. 2002. Snake phylogeny based on osteology, soft anatomy and ecology. Biol. Rev. 77, 333–401. (doi:10.1017/S1464793102005924) [DOI] [PubMed] [Google Scholar]
- 44.Palci A, Caldwell MW, Nydam RL. 2013. Reevaluation of the anatomy of the cenomanian (Upper Cretaceous) hind-limbed marine fossil snakes Pachyrhachis, Haasiophis, and Eupodophis. J. Vert. Paleont. 33, 1328–1342. (doi:10.1080/02724634.2013.779880) [Google Scholar]
- 45.Scanferla A, Smith KT, Schaal S. 2016. Revision of the cranial anatomy and phylogenetic relationships of the Eocene minute boas Messelophis variatus and Messelophis ermannorum (Serpentes, Booidea). Zool. J. Linn. Soc. 176, 182–206. (doi:10.1111/zoj.12300) [Google Scholar]
- 46.Haas G. 1979. On a new snakelike reptile from the Lower Cenomanian of Ein Jabrud, near Jerusalem. Bull. Mus. Nation. d'Hist. Nat. 4, 51–64. [Google Scholar]
- 47.Irish FJ. 1989. The role of heterochrony in the origin of a novel bauplan: evolution of the ophidian skull. Géobios 22 (Suppl. 2), 227–233. (doi:10.1016/S0016-6995(89)80024-5) [Google Scholar]
- 48.Rieppel O. 1988. A review of the origin of snakes. Evol. Biol. 22, 37–130. (doi:10.1007/978-1-4613-0931-4_2) [Google Scholar]
- 49.Shine R, Wall M. 2008. Interactions between locomotion, feeding and bodily elongation during the evolution of snakes. Biol. J. Linn. Soc. 95, 293–304. (doi:10.1111/j.1095-8312.2008.01046.x) [Google Scholar]
- 50.Zaher H. 1994. Comments on the evolution of the jaw adductor musculature of snakes. Zool. J. Linn. Soc. 111, 339–384. (doi:10.1006/zjls.1994.1027) [Google Scholar]
- 51.Scanlon JD. 2006. Skull of the large non-macrostomatan snake Yurlunggur from the Australian Oligo-Miocene. Nature 439, 839–842. (doi:10.1038/nature04137) [DOI] [PubMed] [Google Scholar]
- 52.Wilson JA, Mohabey DM, Peters SE, Head JJ. 2010. Predation upon hatchling dinosaurs by a new snake from the Late Cretaceous of India. PLoS Biol. 8, e1000322 (doi:10.1371/journal.pbio.1000322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scanferla A. 2016. Data from: Postnatal ontogeny and the evolution of macrostomy in snakes. Dryad Digital Repository (doi:10.5061/dryad.1g626) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Electronic supplementary material S1: specimens examined, macrohabitat and prey type definitions, morphometric data, phylogenetic MANOVA results, supplementary figures and references for electronic supplementary materials. Electronic supplementary material S2: summary of diet, prey type, body size and macrohabitat data for snake species included in the comparative survey. All electronic supplementary materials are available from the Dryad Digital Repository http://dx.doi.org/10.5061/dryad.1g626 [53].