Abstract
Ketosis in patients with type 2 diabetes mellitus (T2DM) is overlooked due to atypical symptoms. The objective of this study is to evaluate the value of hemoglobin A1c (HbA1c) as a screening tool for ketosis in T2DM patients. This retrospective study consisted of 253 T2DM patients with ketosis at Shanghai 10th People’s Hospital during a period from January 1, 2011 to June 30, 2015. A control group consisted of 221 T2DM patients without ketosis randomly selected from inpatients during the same period. Receiver operating characteristic curve (ROC) analysis was used to examine the sensitivity and specificity of HbA1c as an indicator for ketosis. Higher HbA1c levels were correlated with ketosis. In patients with newly diagnosed T2DM, the area under the curve (AUC) was 0.832, with 95% confidence interval (CI) 0.754–0.911. The optimal threshold was 10.1% (87 mmol/mol). In patients with previously diagnosed T2DM, the AUC was 0.811 (95% CI: 0.767–0.856), with an optimal threshold of 8.6% (70 mmol/mol). HbA1c is a potential screening tool for ketosis in patients with T2DM. Ketosis is much more likely with HbA1c values at ≥10.1% in patients with newly diagnosed T2DM and HbA1c values at ≥8.6% in patients with previously diagnosed T2DM.
Ketosis-prone type 2 diabetes is defined as the A-β+ ketosis-prone diabetes (KPD) subgroup1. This subgroup is a major factor driving the increasing prevalence of KPD2,3,4,5,6,7. The term “ketosis-prone type 2 diabetes (T2DM)” is often used to describe the A-β+ patients who present with new onset diabetes, unprovoked diabetic ketoacidosis (DKA)8,9 and acidosis10,11,12. As a result, the prevalence of ketosis-prone T2DM could be grossly underestimated. In comparison with DKA in type 1 diabetes mellitus (T1DM), DKA in T2DM is more intractable7,13. DKA in T2DM patients is more likely to develop into severe forms13 and also requires higher doses of insulin and longer durations of treatment7. T2DM patients with ketosis but no acidosis often do not present with overt clinical symptoms. As such, failure to recognize ketosis also likely contributes to the worse outcomes7.
HbA1c reflects average blood glucose over the past 2–3 months14. Several reports have indicated the utility of HbA1c in predicting the development of diabetic retinopathy and nephropathy15,16,17,18. The mean HbA1c is reported to be higher than 10% in T2DM patients with ketosis9,19,20,21. Considering the fact that ketosis is the end result of prolonged uncontrolled diabetes22,23, we hypothesized that HbA1c could be used as a screening tool for ketosis in T2DM patients.
Results
Patient characteristics
In comparison to the control subjects, the ketosis group had a higher percentage of males (66.8% vs. 63.8%, P = 0.494; Table 1) and was younger (50.9 ± 18.1 vs. 55.0 ± 16.6, P = 0.01). Patients with ketosis also had higher HbA1c (11.5% ± 2.4% vs. 8.5% ± 2.0%, P < 0.001), higher fasting plasma glucose (FPG) and 2h-postprandial plasma glucose (PG) levels (P < 0.001), lower fasting C-peptide levels (P < 0.001), and lower 2h-postprandial insulin and C-peptide levels (P < 0.001). No significant differences were found in body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), hemoglobin (Hb), arterial pH, bicarbonate, osmolality, fasting insulin, serum creatinine (sCr), blood urine nitrogen (BUN), uric acid (UA), glutamic-pyruvic transaminase (ALT), glutamic-oxalacetic transaminase (AST), low density lipoprotein (LDL), and high density lipoprotein (HDL) levels between the two groups, with the exception of cholesterol (TC) (4.9 ± 1.5 vs. 4.6 ± 1.1, P = 0.006), triglycerides (TG) (1.4 (1.0, 2.5) vs. 1.4 (1.0, 2.1), P = 0.016), and free fatty acid (FFA) levels (0.6 ± 0.3 vs. 0.5 ± 0.2, P < 0.001). Among patients with ketosis, subjects with a known history of T2DM had lower HbA1c than in subjects with newly diagnosed T2DM (12.3 ± 2.0 vs. 11.1 ± 2.5, P < 0.001; Supplemental Table S1).
Table 1. Clinical Characteristics of Patients in the Ketosis and Control groups.
| Characterizes | All (N = 474) | Type 2 diabetes (N = 221) | Type 2 diabetes + Ketosis (N = 253) | P-Value |
|---|---|---|---|---|
| Age (years) | 52.8 ± 17.5 | 55.0 ± 16.6 | 50.9 ± 18.1 | 0.010a |
| Gender (Male) | 310 (65.4%) | 141 (63.8%) | 169 (66.8%) | 0.494 |
| Diabetes history (year) | ||||
| 0 | 129 (27.2%) | 40 (18.1%) | 89 (35.2%) | — |
| 1~10 | 218 (46.0%) | 113 (51.1%) | 105 (41.5%) | — |
| 10+ | 127 (26.8%) | 68 (30.8%) | 59 (23.3%) | — |
| BMI (kg/m2) | 25.1 ± 4.5 | 25.3 ± 4.5 | 25.0 ± 4.6 | 0.542 |
| Plasma ketones (mmol/l) | 0.2 (0.0, 1, 8) | 0.0 (0.0, 0.1) | 1.7 (0.9, 3.4) | <0.001a |
| Urine ketones (ml/l) | ||||
| 0 (0.8) | 226 (47.7%) | 221 (100%) | 5 (2.0%) | |
| 1+ (1.5) | 25 (5.3%) | 0 | 25 (9.9%) | |
| 2+ (4.0) | 65 (13.7%) | 0 | 65 (25.7%) | |
| 3+ (>8.0) | 63 (13.3%) | 0 | 63 (24.9%) | |
| 4+ (>8.0) | 95 (20%) | 0 | 95 (37.5%) | |
| HbA1c (%) | 10.1 ± 2.7 | 8.5 ± 2.0 | 11.5 ± 2.4 | <0.001a |
| Hemoglobin (g/l) | ||||
| Male | 143.4 ± 12.2 | 143.9 ± 9.8 | 142.9 ± 13.8 | 0.520 |
| Female | 129.9 ± 12.5 | 130.3 ± 7.0 | 129.5 ± 16.1 | 0.712 |
| Admission glucose (mmol/l) | 16.5 ± 7.7 | 12.0 ± 4.9 | 20.1 ± 7.7 | <0.001a |
| FPG (mmol/l) | 9.7 ± 4.0 | 8.2 ± 3.1 | 11.0 ± 4.2 | <0.001a |
| 2h-PG (mmol/l) | 18.4 ± 5.3 | 17.0 ± 4.9 | 19.9 ± 5.4 | <0.001a |
| Fasting insulin (pmol/l) | 10.0 (6.0, 17.1) | 10.3 (6.2, 18.1) | 10.0 (5.8, 16.8) | 0.180 |
| 2h-postprandial insulin (pmol/l) | 22.8 (13.2, 38.2) | 30.6 (17.6, 53.8) | 17.2 (10.2, 29.3) | <0.001a |
| Fasting C-peptide (nmol/l) | 1.7 (1.0, 2.3) | 1.9 (1.3, 2.7) | 1.4 (0.8, 2.0) | <0.001a |
| 2h-postprandialC-peptide (nmol/l) | 3.4 (2.3, 5.4) | 4.7 (3.1, 6.9) | 2.6 (1.8, 3.9) | <0.001a |
| TC (mmol/l) | 4.8 ± 1.4 | 4.6 ± 1.1 | 4.9 ± 1.5 | 0.006a |
| TG (mmol/l) | 1.4 (1.0, 2.3) | 1.4 (1.0, 2.1) | 1.4 (1.0, 2.5) | 0.016a |
| LDL (mmol/l) | 2.8 ± 1.0 | 2.7 ± 0.9 | 2.8 ± 1.1 | 0.372 |
| HDL (mmol/l) | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.4 | 0.713 |
| FFA (mmol/l) | 0.6 ± 0.3 | 0.5 ± 0.2 | 0.6 ± 0.3 | <0.001a |
| Arterial PH | 7.4 ± 0.1 | 7.4 ± 0.1 | 7.4 ± 0.1 | 0.057 |
| Bicarbonate (mmol/l) | 22.3 (17.8, 24.8) | 25.5 (23.6, 26.4) | 22.3 (17.8, 24.7) | 0.232 |
| BE (mmol/l) | −1.4 (−5.8, 0.7) | 1.4 (−0.1, 2.7) | −1.6 (−6.0, 0.6) | 0.005a |
| Osmolality (mOsm/kg) | 296.6 ± 10.6 | 296.5 ± 6.4 | 296.6 ± 13.0 | 0.859 |
| sCr (umol/l) | 68.04 ± 28.60 | 70.4 ± 29.6 | 66.0 ± 27.6 | 0.097 |
| BUN (mmol/l) | 6.05 ± 2.56 | 6.1 ± 2.2 | 6.0 ± 2.9 | 0.916 |
| AST (U/L) | 27.1 (23.8, 30.4) | 23.6 (20.6, 26.8) | 30.1 (24.5, 35.7) | 0.197 |
| ALT (U/L) | 33.4 (27.4, 39.5) | 32.6 (21.6, 43.5) | 34.7 (28.1, 41.2) | 0.301 |
Continuous normal distribution variables are presented as means ± standard deviation (SD); continuous skew distribution variables are presented as medians (interquartile ranges); categorical data are given as numbers in percentage. BMI: body mass index; FPG: fasting plasma glucose; 2h-PG: 2 hours postprandial plasma glucose; TC: total cholesterol; TG: triglycerides; LDL: low density lipoprotein; HDL: high density lipoprotein; FFA: free fatty acids; BE: base excess; sCr: serum creatinine; BUN: blood urine nitrogen; AST: glutamic-oxalacetic transaminase; ALT: glutamic-pyruvic transaminase. aP < 0.05.
Relationship between HbA1c and ketosis
Higher HbA1c was positively correlated with urine ketones (r = 0.54, P < 0.001) as well as plasma ketones (r = 0.58, P < 0.001). HbA1c was plotted in quartiles with the HbA1c levels set at <7.9%, 7.9–9.8%, 9.8–11.9%, and ≥11.9%. As expected, the occurrence of ketosis increased rapidly with increasing levels of HbA1c (12.3%, 45.0%, 67.2% and 86.3%, per HbA1c quartile respectively) and exhibited a sevenfold increase from the lowest to the highest quartile (Fig. 1). In the multivariate model 1 that included age, gender and C-reactive protein (CRP) as co-variables, HbA1c was significantly associated with ketosis (odds ratio (OR) = 1.87, 95% confidence interval (CI) 1.64 to 2.13, P < 0.001; Table 2). In the multivariate model 2 with BMI, smoking, drinking, and duration of diabetes as additional co-variables, the association between HbA1c and ketosis remained (OR = 1.88, 95% CI 1.64 to 2.15, P < 0.001; Table 2).
Figure 1. The prevalence of type 2 diabetic ketosis with increasing levels of HbA1c.
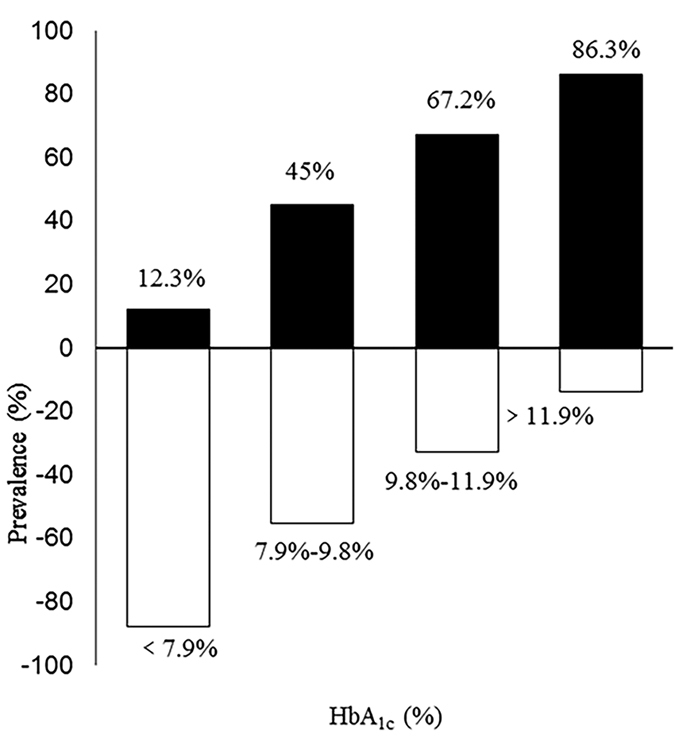
HbA1c was plotted in quartiles with the HbA1c levels set at ≤7.9%, 7.9–9.8%, 9.8–11.9%, and ≥11.9%. Black bars = proportions of patients with type 2 diabetic ketosis. White bars = proportions of type 2 diabetes patients without ketosis.
Table 2. Parameters of the multiple logistic regression model.
| Variables | βc | SE | Wald | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Model 1 | ||||||
| HbA1c | 0.65 | 0.07 | 91.32 | 0.001 | 1.87 | (1.64–2.13) |
| Age | −0.01 | 0.01 | 1.44 | 0.231 | 0.99 | (0.98–1.01) |
| Gender | 0.05 | 0.27 | 0.03 | 0.866 | 1.05 | (0.61–1.79) |
| CRP | 0.04 | 0.01 | 16.83 | 0.001 | 1.04 | (1.02, 1.05) |
| Model 2 | ||||||
| HbA1c | 0.63 | 0.07 | 85.03 | 0.001 | 1.88 | (1.64, 2.15) |
| Age | −0.01 | 0.01 | 0.97 | 0.325 | 0.99 | (0.97, 1.01) |
| Gender | 0.11 | 0.31 | 0.14 | 0.708 | 1.12 | (0.62, 2.04) |
| CRP | 0.04 | 0.01 | 15.02 | 0.001 | 1.04 | (1.02, 1.06) |
Adjusted variables in model 1: HbA1c, age, gender and CRP. Adjusted variables in model 2: HbA1c, age, gender, BMI, smoking, drinking, CRP and diabetes duration. βc: Regression coefficient. OR: odds ratio.
Determination of optimal HbA1c thresholds
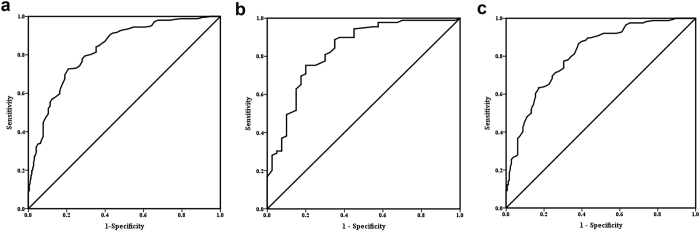
In the receiver operating characteristics (ROC) analysis, area under the curve (AUC) was 0.827 (95% CI: 0.791–0.864) for the overall analysis that included all subjects, 0.832 (95% CI: 0.754 to 0.911) in patients with newly diagnosed T2DM, and 0.811 (95% CI: 0.767 to 0.856) in patients with a known T2DM history (Fig. 2). In patients with a known T2DM history, a HbA1c threshold of 8.6% (70 mmol/mol) resulted in the highest Youden index, with 86.59% sensitivity, 62.00% specificity, and 0.22 negative likelihood ratio (LR) (Table 3). In patients with newly diagnosed T2DM, a HbA1c threshold of 11.0% (97 mmol/mol) resulted in the highest Youden index, with 75.30% sensitivity and 80.00% specificity. A HbA1c threshold of 10.1% (87 mmol/mol) seemed optimal with the second highest Youden index, with 88.76% sensitivity, 65.00% specificity, and 0.17 negative LR (Table 4). In subjects with a known T2DM diagnosis, the adjusted OR for having ketosis in individuals with HbA1c levels greater than or equal to 8.6% (70 mmol/mol) vs. lower than 8.6% was 12.49 (95% CI: 6.35 to 24.56) (Supplemental Table S2). In subjects with newly diagnosed T2DM, the adjusted OR (95% CI) for having ketosis in individuals with HbA1c levels greater than or equal to 10.1% (87 mmol/mol) vs. lower than 10.1% was 27.58 (95% CI: 7.77 to 97.88) (Supplemental Table S2).
Figure 2. Receiver operating characteristics curve of HbA1c in screening for diabetic ketosis in type 2 diabetes patients.
(a) Total group: area under curve (AUC) were 0.827 (95% confidence interval (CI) 0.791 to 0.864). (b) The subgroup of patients with newly diagnosed type 2 diabetes: AUC were 0.832 (95% CI 0.754 to 0.911), cut-off point = 10.1%, sensitivity = 88.76%, specificity = 65.00%. (c) The subgroup of patients with previously diagnosed of type 2 diabetes: AUC were 0.811 (95% CI 0.767 to 0.856), cut-off point = 8.6%, sensitivity = 86.59%, specificity = 62.00%.
Table 3. Sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, negative likelihood ratio and Youden index comparing various thresholds of HbA1c with the ADA criteria for diabetic ketosis in the subgroup of patients with previously diagnosed T2DM (n = 345).
| HbA1c Thresholds | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | +LR | −LR | Youden index |
|---|---|---|---|---|---|---|---|
| 8.3% (67 mmol/mol) | 89.63 (83.94, 93.51) | 57.00 (49.62, 63.90) | 65.33 (58.90, 71.25) | 85.83 (78.39, 91.06) | 2.08 (1.75, 2.48) | 0.18 (0.11, 0.29) | 0.466 |
| 8.4% (68 mmol/mol) | 88.41 (82.54, 92.53) | 58.00 (50.73, 64.96) | 65.61 (59.12, 71.56) | 84.68 (77.22, 90.05) | 2.11 (1.76, 2.52) | 0.20 (0.13, 0.31) | 0.464 |
| 8.5% (69 mmol/mol) | 87.80 (81.84, 92.04) | 60.00 (52.95, 67.07) | 66.67 (60.13, 72.62) | 84.50 (77.19, 89.81) | 2.21 (1.83, 2.66) | 0.20 (0.13, 0.31) | 0.478 |
| 8.6% (70 mmol/mol) | 86.59 (80.46, 91.04) | 62.00 (54.62, 68.64) | 67.30 (60.70, 73.28) | 83.58 (76.32, 88.97) | 2.27 (1.87, 2.76) | 0.22 (0.15, 0.33) | 0.486 |
| 8.7% (72 mmol/mol) | 84.76 (78.41, 89.51) | 62.00 (55.18, 69.16) | 67.15 (60.48, 73.19) | 81.88 (74.57, 87.47) | 2.26 (1.85, 2.75) | 0.24 (0.17, 0.36) | 0.468 |
| 8.8% (73 mmol/mol) | 81.10 (74.38, 86.39) | 62.00 (55.18, 69.16) | 67.17 (60.35, 73.34) | 78.91 (71.58, 84.77) | 2.26 (1.83, 2.78) | 0.30 (0.21, 0.41) | 0.431 |
| 8.9% (74 mmol/mol) | 79.88 (73.05, 85.33) | 66.00 (58.56, 72.28) | 67.88 (60.99, 74.07) | 78.29 (71.05, 84.14) | 2.33 (1.88, 2.89) | 0.31 (0.22, 0.42) | 0.459 |
Values in parentheses are 95% confidence intervals.
PG: admission plasma glucose. PPV: positive predictive value. NPV: negative predictive value. +LR: positive likelihood ratio. −LR: negative likelihood ratio.
Table 4. Sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, negative likelihood ratio and Youden index comparing various thresholds of HbA1c with the ADA criteria for diabetic ketosis in the subgroup of patients with newly diagnosed T2DM (n = 129).
| HbA1c Thresholds | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | +LR | −LR | Youdenindex |
|---|---|---|---|---|---|---|---|
| 9.7% (83 mmol/mol) | 91.01 (83.03, 95.6) | 55.00 (39.82, 69.3) | 81.82 (72.99, 88.27) | 73.33 (55.35, 86.02) | 2.02 (1.43, 2.87) | 0.16 (0.08, 0.34) | 0.460 |
| 9.8% (84 mmol/mol) | 89.89 (81.68, 94.79) | 55.00 (39.82, 69.3) | 81.63 (72.74, 88.14) | 70.97 (53.25, 84.06) | 2.00 (1.41, 2.83) | 0.18 (0.09, 0.36) | 0.449 |
| 9.9% (85 mmol/mol) | 89.89 (81.68, 94.79) | 60.00 (44.57, 73.68) | 83.33 (74.52, 89.58) | 72.73 (55.61, 85.1) | 2.25 (1.53, 3.31) | 0.17 (0.00, 0.33) | 0.499 |
| 10.0% (86 mmol/mol) | 89.89 (81.68, 94.79) | 63.00 (47.00, 75.81) | 84.21 (75.46, 90.31) | 73.53 (56.71, 85.58) | 3.00 (1.60, 3.60) | 0.16 (0.08, 0.31) | 0.529 |
| 10.1% (87 mmol/mol) | 88.76 (80.36, 93.96) | 65.00 (49.45, 77.92) | 84.95 (76.18, 90.94) | 72.22 (55.86, 84.30) | 2.54 (1.65, 3.89) | 0.17 (0.09, 0.32) | 0.538 |
| 10.2% (88 mmol/mol) | 86.52 (77.74, 92.27) | 65.00 (49.45, 77.92) | 84.62 (75.69, 90.73) | 68.42 (52.45, 81.01) | 2.47 (1.61, 3.80) | 0.21 (0.12, 0.37) | 0.515 |
| 10.3% (89 mmol/mol) | 84.27 (75.19, 90.52) | 65.00 (49.45, 77.92) | 84.27 (75.19, 90.52) | 65.00 (49.45, 77.92) | 2.41 (1.56, 3.71) | 0.24 (0.14, 0.41) | 0.493 |
Values in parentheses are 95% confidence intervals.
PG:admission plasma glucose. PPV:positive predictive value. NPV:negative predictive value. +LR: positive likelihood ratio. −LR: negative likelihood ratio.
Oral glucose tolerance tests (OGTTs) analysis
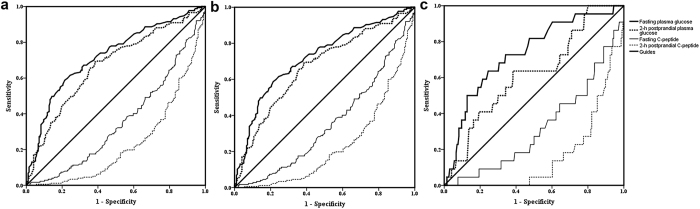
In the overall analysis that included subjects with ketosis regardless of having acidosis or not, the AUC was 0.712 (95% CI: 0.664 to 0.760) for FPG, 0.666 (0.613 to 0.720) for 2-h postprandial PG, 0.337 (0.287 to 0.388) for fasting C-peptide, and 0.243 (0.196 to 0.290) for 2-h postprandial C-peptide (Fig. 3a). In the subset with ketosis but not acidosis, the AUC was 0.771 (0.661 to 0.762) for FPG, 0.672 (0.616 to 0.727) for 2-h postprandial PG, 0.354 (0.301 to 0.407) for fasting C-peptide, and 0.252 (0.202 to 0.301) for 2-h postprandial C-peptide in the subset of patients with ketosis without acidosis (Fig. 3b). In the subset with ketoacidosis, the AUC was 0.717 (0.623 to 0.810) for FPG, 0.631 (0.514 to 0.747) for 2-h postprandial PG, 0.241 (0.152 to 0.330) for fasting C-peptide, and 0.184 (0.112 to 0.255) for 2-h postprandial C-peptide (Fig. 3c).
Figure 3. Receiver operating characteristics curve of oral glucose tolerance tests (OGTTs) in screening for type 2 diabetic ketosis.
(a) Total group: area under curve (AUC) were 0.712 (95% CI 0.664 to 0.760) for fasting plasma glucose (FPG), 0.666 (0.613 to 0.720) for 2-h postprandial plasma glucose (PG), 0.337 (0.287 to 0.388) for fasting C-peptide and 0.243 (0.196 to 0.290) for 2-h postprandial C-peptide. (b) The subset of patients with diabetic ketosis without acidosis: AUC were 0.771 (0.661 to 0.762) for FPG, 0.672 (0.616 to 0.727) for 2-h postprandial PG, 0.354 (0.301 to 0.407) for fasting C-peptide and 0.252 (0.202 to 0.301) for 2-h postprandial C-peptide. (c) The subset of patients with diabetic ketoacidosis: 0.717 (0.623 to 0.810) for FPG, 0.631 (0.514 to 0.747) for 2-h postprandial PG, 0.241 (0.152 to 0.330) for fasting C-peptide and 0.184 (0.112 to 0.255) for 2-h postprandial C-peptide.
Discussion
In this study we found a significant association between higher HbA1c values with ketosis in T2DM patients. The optimal threshold for screening ketosis was 10.1% (87 mmol/mol) and 8.6% (70 mmol/mol) in patients with newly diagnosed T2DM and in patients with a known T2DM history, respectively. These results provide a pragmatic tool to screen for ketosis in patients with T2DM.
The mean HbA1c of T2DM patients reported in this study is similar to that reported in previous studies9,19,20,21. DKA was demonstrated to be associated with increased HbA1c levels which reflect both fasting and postprandial hyperglycemia24 in T1DM23,25,26,27 and T2DM28. Our results provide further evidence to support the relevance of HbA1c levels and risk of ketosis in T2DM. In addition, Cheng PC et al. have demonstrated that serum albumin concentration, which is inversely associated with HbA1c29,30,31, is inversely associated with the risk of ketosis in patients with T2DM29. However, few research studies have concentrated on the value of HbA1c as a screening tool for ketosis in T2DM. The pathogenesis of ketosis likely involves decreasing effective concentrations of insulin as well as increased concentrations of glucagon, cortisol, growth hormone and catecholamines, which promote lipolysis and ketogenesis32,33 and trigger ketonemia and DKA. Moreover, insulin deficiency and increased counterregulatory hormones inhibit glucose utilization in peripheral tissues, promoting gluconeogenesis and glycogenolysis, thereby exacerbating hyperglycemia34. In addition, there are some interactions between hyperglycemia and disturbances in lipid metabolism. We found that patients with diabetic ketosis had high plasma FFA, TC, and TG levels. Excess FFA in the liver stimulate gluconeogenesis35. Thus, dyslipidemia and disturbances in glucose metabolism can be distinct consequences of the same cause. Hyperglycemia coexists with ketosis rather than as a cause of it. In our opinion, measures of glucose metabolism could reflect lipid metabolism to some degree. This is in line with the America Diabetes Association (ADA)’s recommendation that plasma glucose is a key diagnostic criteria for DKA34. Previous studies also have indicated that HbA1c can provide valuable supplementary information about the extent of circulating lipids in both T1DM and T2DM in addition to its primary role in monitoring long-term glycemic control36,37,38,39,40. The observed correlation of HbA1c with ketosis in the current study provides additional evidence that links HbA1c with disturbances in lipid metabolism in T2DM patients.
The Youden index in ROC analysis is commonly used to measure overall diagnostic performance41,42. In the subgroup of patients with a known T2DM history, the cutoff value with the highest Youden index was 8.6% (70 mmol/mol), with a high sensitivity (86.59%) and moderate specificity (62.00%). The low negative LR (0.217) indicates good discriminatory performance and a lower rate of false negatives43. In the subgroup of patients with newly diagnosed T2DM, the highest Youden index was obtained at a cutoff of 11.0%, with 75.30% sensitivity and 80.00% specificity. Considering the severe adverse outcomes of diabetic ketoacidosis, such as death, and the heavy economic burden of hospitalization34, we placed particular emphasis on sensitivity in the current study, and set the HbA1c threshold at 10.1% (87 mmol/mol; with second highest Youden index); at this cutoff, the analysis yielded higher sensitivity (88.76%), moderate specificity (65.00%), and low negative LR (0.173). Distinct optimal HbA1c thresholds between the two subgroups may relate to fewer changes in the counter regulatory hormone system in patients with newly diagnosed T2DM than in those with long standing diabetes44. Furthermore, almost all patients with a known diagnosis of T2DM had initiated medications for diabetes, which may additionally influence the optimal threshold value.
It is note worthy that HbA1c showed better performance than OGTTs in the current study. In addition, OGTTs require that patients fast for at least 8 hours before examination, and short term dietary control or physical exercise can influence the results. This test is also an expensive and lengthy procedure that requires additional manpower and professional expertise. Moreover, there are stringent requirements for processing blood during OGTTs including rapid processing as well as separation and storage of plasma or serum at 4 °C18. In contrast, HbA1c levels can be checked at any time of a day without fasting and accurately reflect long term glycemic control without susceptibility to short term changes in diet or exercise. In addition to be more cost-effective, HbA1c is more reproducible than OGTTs45. Also, blood samples for HbA1c measurement can be maintained at 4 °C for up to a week18. Importantly, instant blood or urine ketone measurements can determine those with ketosis but are unable to recognize those at high risk of developing ketosis. In contrast, the ability of HbA1c to reflect glycometabolic status over several months may allow identification of patients who are at high risk for developing ketosis.
Although this study has addressed some knowledge gaps in the use of HbA1c to screen for ketosis, there are several limitations. Most importantly, this is a retrospective case-control study, which does not provide evidence as strong as randomized controlled trials. Furthermore, the design of this study could have generated selection bias: all subjects were from one hospital and of a single ethnic background (Chinese Han), which limits the generalizability of the study findings. Also, all subjects in the current study were inpatients; as a result, whether the findings can be extrapolated to outpatients needs to be verified. Due to less severity of diseases in the outpatient setting, we believe that a lower HbA1c threshold may appropriate in outpatients. Finally, confounding factors such as the various comorbidities among the ketosis and control group patients may weaken the study findings.
In conclusion, HbA1c is a useful tool to screen T2DM patients at high-risk for ketosis. We believe that plasma and urine ketones should be monitored carefully while appropriate treatments should be implemented in patients with newly diagnosed T2DM with HbA1c at ≥10.1% (87 mmol/mol) and in patients with a known T2DM history having a HbA1c value at ≥8.6% (70 mmol/mol) at the time of admission.
Materials and Methods
Ethics statement
The study protocol was approved by the Research Ethics Review Committee of Tongji University. Methods used in the present study were carried out in accordance with approved guidelines and regulations. It conformed to the provisions of the Declaration of Helsinki.
Study population
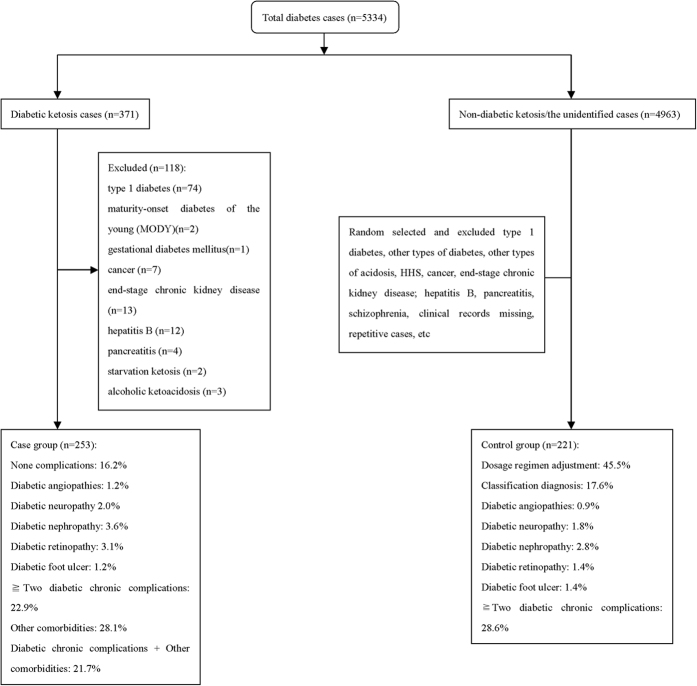
This retrospective case–control study was conducted at the Department of Endocrinology and Metabolism, Shanghai 10th People’s hospital in China, a 1,860-bed comprehensive teaching hospital, from January 1, 2011 to June 30, 2015. In the initial screening step of the study, we identified a total of 5,334 T2DM patients receiving medical treatment as inpatients and they did not undergo any surgical procedure. Ketosis without anaemia was verified in 371 out of the 5,334 cases. After excluding 118 cases (due to comorbid conditions listed in Fig. 1), 253 cases (213 ketosis without acidosis and 40 with DKA) were included in the data analysis. We randomly selected 221 cases without anaemia from the remaining 4,963 as the control group. Comorbid conditions of the control subjects are listed in Fig. 4. Within the entire study sample of 474 subjects, 129 had newly diagnosed T2DM and the remaining 345 had an established diagnosis of T2DM.
Figure 4. Diagram demonstrating the case and control selection and exclusion criteria used in this study.
Definitions and diagnostic criteria
The diagnosis of T2DM was established based on the 2014 ADA guidelines2. The diagnosis of ketosis was based on positive serum ketones (serum β-hydroxybutyrate level at >0.3 mmol/l) or moderate to large urine ketones (≥4 ml/L(2+))34. Ten patients with hypertonicity, ketosis and acidosis46,47 were also included in the ketosis group. Both blood ketones and urine ketones were required to be negative for inclusion as a control subject.
Differential diagnosis
T1DM was identified by known history, uninterrupted insulin treatment, positive beta-cell autoantibodies and undetectable/low levels of plasma C-peptide during oral glucose tolerance test2. Two patients with maturity-onset diabetes of the young (MODY) were excluded on the basis of a prior diagnosis. One case of gestational diabetes mellitus (GDM) was also excluded from data analysis. Hyperglycemic hyperosmolar state (HHS) was identified based on the ADA criteria34. Anemia, caner, pancreatitis, end-stage chronic kidney disease, and hepatitis B were identified through clinical history, laboratory test results, or imaging studies. Starvation ketosis and alcoholic ketosis were distinguished by a history of chronic starvation or alcoholism and low plasma glucose concentrations or hypoglycemia48.
Laboratory measurements and anthropometric index
All anthropometric and laboratory measures were obtained upon admission. The anthropometric information included age, gender, BMI, SBP, DBP, HR, and history of T2DM. Laboratory results included serum ketones (β-hydroxybutyrate), urine ketones (acetoacetic acid and acetone), Hb, HbA1c, PG, insulin, C-peptide, liver function tests (ALT and AST), renal function tests (sCr and BUN), UA, CRP, lipid profile (TG, TC, LDL, HDL, and FFA), arterial pH, base excess (BE), bicarbonate, electrolyte levels, and osmolality (Table 1). Serum ketones were measured using a MediSense hand-held device (Abbott Corporation, Abbott Park, IL, USA). Urine ketones were measured using the nitroprusside method (Semi-automatic urine analyzer, Cobas u411, Roche, Germany). HbA1c was measured by high performance liquid chromatography (HLC-723G8, Tosoh, Japan). OGTTs were conducted prior to any treatment in all but 40 patients with diabetic ketoacidosis (after correction of acidosis). Insulin and C-peptide were measured during the OGTT. The formula, [sodium (mEq/l) × 2 + glucose (mg/dl)/18]34,49, was used to calculate effective osmolality.
Multivariable model
The adjusted variables included in Model 1 were HbA1c, age, gender, and CRP. The variables included in Model 2 were HbA1c, age, gender, CRP, BMI, smoking, drinking, and diabetes duration. PG, insulin, and C-peptide levels were not included as independent variables due to the strong correlation with HbA1c. HbA1c data were further divided into two factions depending on the HbA1c threshold and was included in the multivariate models as a binary variable.
Statistical methods
Continuous variables are presented as mean and standard deviation (SD) upon normal distribution, and as medians and interquartile ranges upon skew distribution. Categorical data are presented as percentages. Comparison of continuous variables between the cases and controls were conducted using Student’s t-test upon equal variance between the two groups, and otherwise using Mann-Whitney U test. Comparison of categorical variables between the cases and controls was conducted using a Chi-squared test (χ2-test). Rank correlations between HbA1c and blood or urine ketones were determined using Spearman correlation coefficients. A step-wise binary logistic regression analysis was conducted to explore the risk factors for type 2 diabetic ketosis. ROC analysis was conducted to examine the sensitivity and specificity of HbA1c and to determine the optimal HbA1c threshold in order to discriminating ketosis from the entire sample. All statistical analyses were carried out using SPSS17.0 software (SPSS Inc., Chicago, IL, USA). Results were considered to be statistically significant at two-tailed P values less than 0.05.
Additional Information
How to cite this article: Zhu, B. et al. HbA1c as a Screening tool for Ketosis in Patients with Type 2 Diabetes Mellitus. Sci. Rep. 6, 39687; doi: 10.1038/srep39687 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We thank Professor Li Ming Wen from School of Public Health, University of Sydney, Australia for his kind support for editing and proofreading this manuscript. This work was supported by the National Natural Science Foundation of China (grant No. 81570782).
Footnotes
Author Contributions B.Z. contributed to the study conception and design: collected, analyzed, and interpreted data, drafted the manuscript, reviewed and edited the manuscript, and final approved the final version to be published. L.B., M.Z., A.G., L.Z., and S.R. contributed to data collection, data analysis and interpretation, and reviewed and edited the manuscript. J.L. and S.Q. contributed to the study conception and design, data collection, data analysis and interpretation, drafting of the manuscript and reviewed and edited the manuscript, approved the final version to be published and helped to obtain funding. J.L. and S.Q. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- Balasubramanyam A., Nalini R., Hampe C. S. & Maldonado M. Syndromes of ketosis-prone diabetes mellitus. Endocr Rev 29, 292–302 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes, A. Diagnosis and classification of diabetes mellitus. Diabetes Care 37 Suppl 1, S81–90 (2014). [DOI] [PubMed] [Google Scholar]
- Andel M. et al. A multinational, multi-centre, observational, cross-sectional survey assessing diabetes secondary care in Central and Eastern Europe (DEPAC Survey). Diabet Med 25, 1195–1203 (2008). [DOI] [PubMed] [Google Scholar]
- Dabelea D. et al. Trends in the prevalence of ketoacidosis at diabetes diagnosis: the SEARCH for diabetes in youth study. Pediatrics 133, e938–945 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu A. et al. Ketoacidosis at diabetes onset is still frequent in children and adolescents: a multicenter analysis of 14,664 patients from 106 institutions. Diabetes Care 32, 1647–1648 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu A. et al. Ketoacidosis at onset of type 1 diabetes mellitus in children–frequency and clinical presentation. Pediatr Diabetes 4, 77–81 (2003). [DOI] [PubMed] [Google Scholar]
- Newton C. A. & Raskin P. Diabetic ketoacidosis in type 1 and type 2 diabetes mellitus: clinical and biochemical differences. Arch Intern Med 164, 1925–1931 (2004). [DOI] [PubMed] [Google Scholar]
- Umpierrez G. E. Ketosis-prone type 2 diabetes: time to revise the classification of diabetes. Diabetes Care 29, 2755–2757 (2006). [DOI] [PubMed] [Google Scholar]
- Umpierrez G. E., Smiley D. & Kitabchi A. E. Narrative review: ketosis-prone type 2 diabetes mellitus. Ann Intern Med 144, 350–357 (2006). [DOI] [PubMed] [Google Scholar]
- Pinero-Pilona A., Litonjua P., Aviles-Santa L. & Raskin P. Idiopathic type 1 diabetes in Dallas, Texas: a 5-year experience. Diabetes Care 24, 1014–1018 (2001). [DOI] [PubMed] [Google Scholar]
- Umpierrez G. E. et al. Immunogenetic analysis suggests different pathogenesis for obese and lean African-Americans with diabetic ketoacidosis. Diabetes Care 22, 1517–1523 (1999). [DOI] [PubMed] [Google Scholar]
- Umpierrez G. E. et al. Hyperglycemic crises in urban blacks. Arch Intern Med 157, 669–675 (1997). [PubMed] [Google Scholar]
- Barski L. et al. Comparison of diabetic ketoacidosis in patients with type-1 and type-2 diabetes mellitus. Am J Med Sci 345, 326–330 (2013). [DOI] [PubMed] [Google Scholar]
- Nathan D. M., Turgeon H. & Regan S. Relationship between glycated haemoglobin levels and mean glucose levels over time. Diabetologia 50, 2239–2244 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki R. et al. Incidence and progression of diabetic retinopathy in Japanese adults with type 2 diabetes: 8 year follow-up study of the Japan Diabetes Complications Study (JDCS). Diabetologia 54, 2288–2294 (2011). [DOI] [PubMed] [Google Scholar]
- McCance D. R. et al. Comparison of tests for glycated haemoglobin and fasting and two hour plasma glucose concentrations as diagnostic methods for diabetes. BMJ 308, 1323–1328 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapp R. J. et al. Longitudinal association of glucose metabolism with retinopathy: results from the Australian Diabetes Obesity and Lifestyle (AusDiab) study. Diabetes Care 31, 1349–1354 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation. WHO Guidelines Approved by the Guidelines Review Committee Geneva (2011). [PubMed] [Google Scholar]
- Haaland W. C. et al. A-beta-subtype of ketosis-prone diabetes is not predominantly a monogenic diabetic syndrome. Diabetes Care 32, 873–877 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauvais-Jarvis F. et al. Ketosis-prone type 2 diabetes in patients of sub-Saharan African origin: clinical pathophysiology and natural history of beta-cell dysfunction and insulin resistance. Diabetes 53, 645–653 (2004). [DOI] [PubMed] [Google Scholar]
- Sobngwi E. et al. Ketosis-prone type 2 diabetes mellitus and human herpesvirus 8 infection in sub-saharan africans. JAMA 299, 2770–2776 (2008). [DOI] [PubMed] [Google Scholar]
- Kitabchi A. E., Umpierrez G. E., Murphy M. B. & Kreisberg R. A. Hyperglycemic crises in adult patients with diabetes: a consensus statement from the American Diabetes Association. Diabetes Care 29, 2739–2748 (2006). [DOI] [PubMed] [Google Scholar]
- Fredheim S. et al. Diabetic ketoacidosis at the onset of type 1 diabetes is associated with future HbA1c levels. Diabetologia 56, 995–1003 (2013). [DOI] [PubMed] [Google Scholar]
- Bonora E. et al. Plasma glucose levels throughout the day and HbA(1c) interrelationships in type 2 diabetes: implications for treatment and monitoring of metabolic control. Diabetes Care 24, 2023–2029 (2001). [DOI] [PubMed] [Google Scholar]
- Govan L. et al. The effect of deprivation and HbA1c on admission to hospital for diabetic ketoacidosis in type 1 diabetes. Diabetologia 55, 2356–2360 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govan L. et al. Achieved levels of HbA1c and likelihood of hospital admission in people with type 1 diabetes in the Scottish population: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetes Care 34, 1992–1997 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewers A. et al. Predictors of acute complications in children with type 1 diabetes. JAMA 287, 2511–2518 (2002). [DOI] [PubMed] [Google Scholar]
- Longo-Mbenza C. O. E. B. & Blanco-Blanco E. Glycosylated haemoglobin is markedly elevated in new and known diabetes patients with hyperglycaemic ketoacidosis. Afr Health Sci 14, 526–532 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P. C., Hsu S. R. & Cheng Y. C. Association between Serum Albumin Concentration and Ketosis Risk in Hospitalized Individuals with Type 2 Diabetes Mellitus. J Diabetes Res 2016, 1269706 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Segade S., Rodriguez J., Mayan D. & Camina F. Plasma albumin concentration is a predictor of HbA1c among type 2 diabetic patients, independently of fasting plasma glucose and fructosamine. Diabetes Care 28, 437–439 (2005). [DOI] [PubMed] [Google Scholar]
- Bae J. C. et al. Association between Serum Albumin, Insulin Resistance, and Incident Diabetes in Nondiabetic Subjects. Endocrinol Metab (Seoul) 28, 26–32 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiasson J. L. et al. Diagnosis and treatment of diabetic ketoacidosis and the hyperglycemic hyperosmolar state. CMAJ 168, 859–866 (2003). [PMC free article] [PubMed] [Google Scholar]
- Miles J. M., Haymond M. W., Nissen S. L. & Gerich J. E. Effects of free fatty acid availability, glucagon excess, and insulin deficiency on ketone body production in postabsorptive man. J Clin Invest 71, 1554–1561 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitabchi A. E., Umpierrez G. E., Miles J. M. & Fisher J. N. Hyperglycemic crises in adult patients with diabetes. Diabetes Care 32, 1335–1343 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G., Chen X., Capulong E. & Mozzoli M. Effects of free fatty acids on gluconeogenesis and autoregulation of glucose production in type 2 diabetes. Diabetes 50, 810–816 (2001). [DOI] [PubMed] [Google Scholar]
- Kunikullaya K. P. U. K. & Goturu J. Glycosylated Haemoglobin (HbA1c) - A Marker of Circulating Lipids in Type 2 Diabetic Patients. J Clin Diagn Res 8, 20–23 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad Khan H. Clinical significance of HbA1c as a marker of circulating lipids in male and female type 2 diabetic patients. Acta Diabetol 44, 193–200 (2007). [DOI] [PubMed] [Google Scholar]
- Ladeia A. M. et al. Lipid profile correlates with glycemic control in young patients with type 1 diabetes mellitus. Prev Cardiol 9, 82–88 (2006). [DOI] [PubMed] [Google Scholar]
- Faulkner M. S. et al. Total homocysteine, diet, and lipid profiles in type 1 and type 2 diabetic and nondiabetic adolescents. J Cardiovasc Nurs 21, 47–55 (2006). [PMC free article] [PubMed] [Google Scholar]
- Chan W. B. et al. Triglyceride predicts cardiovascular mortality and its relationship with glycaemia and obesity in Chinese type 2 diabetic patients. Diabetes Metab Res Rev 21, 183–188 (2005). [DOI] [PubMed] [Google Scholar]
- Zou K. H., Hall W. J. & Shapiro D. E. Smooth non-parametric receiver operating characteristic (ROC) curves for continuous diagnostic tests. Stat Med 16, 2143–2156 (1997). [DOI] [PubMed] [Google Scholar]
- Zweig M. H. & Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem 39, 561–577 (1993). [PubMed] [Google Scholar]
- Biggerstaff B. J. Comparing diagnostic tests: a simple graphic using likelihood ratios. Stat Med 19, 649–663 (2000). [DOI] [PubMed] [Google Scholar]
- Bolli G. et al. Abnormal glucose counterregulation in insulin-dependent diabetes mellitus. Interaction of anti-insulin antibodies and impaired glucagon and epinephrine secretion. Diabetes 32, 134–141 (1983). [DOI] [PubMed] [Google Scholar]
- Lacher D. A., Hughes J. P. & Carroll M. D. Estimate of biological variation of laboratory analytes based on the third national health and nutrition examination survey. Clin Chem 51, 450–452 (2005). [DOI] [PubMed] [Google Scholar]
- Scott A. R. Joint British Diabetes Societies for Inpatient, C. & group, J. h. h. g. Management of hyperosmolar hyperglycaemic state in adults with diabetes. Diabet Med 32, 714–724 (2015). [DOI] [PubMed] [Google Scholar]
- Wilson J. F. In clinic. Diabetic ketoacidosis. Ann Intern Med 152, ITC1-1, ITC1-2, ITC1-3, ITC1-4, ITC1-5, ITC1-6, ITC1-7, ITC1-8, ITC1-9, ITC1-10, ITC11-11, ITC11-12, ITC11-13, ITC11-14, ITC11-15, table of contents; quiz ITC11-16 (2010). [DOI] [PubMed] [Google Scholar]
- Umpierrez G. E. et al. Differences in metabolic and hormonal milieu in diabetic- and alcohol-induced ketoacidosis. J Crit Care 15, 52–59 (2000). [DOI] [PubMed] [Google Scholar]
- Kitabchi A. E. et al. Management of hyperglycemic crises in patients with diabetes. Diabetes Care 24, 131–153 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.