To the Editor
Each year, over 250,000 older U.S. adults experience hip fractures.1 Few randomized studies are available regarding the impact of anesthesia on outcomes after hip fracture surgery,2 and the feasibility of randomized studies in this context has been questioned.3 We carried out a pilot study (ClinicalTrials.gov identifier NCT02190903) at one urban academic health system to explore the feasibility of a prospective, randomized trial to compare outcomes with spinal versus general anesthesia for hip fracture surgery.
After IRB approval, we randomized 12 patients to receive either spinal anesthesia with intravenous sedation or general anesthesia for hip fracture surgery. Inclusion criteria were age over 18; femoral neck or pertrochanteric hip fracture requiring surgery; and ability to speak English and provide written informed consent. Exclusion criteria were pathologic or periprosthetic fracture; concurrent conditions requiring surgery (e.g. multitrauma, acute cholecystitis); Montreal Cognitive Assessment (MOCA)4 score less than 16; delirium at the time of screening; known contraindications to spinal anesthesia or volatile general anesthetics; and pregnancy.
All enrolled patients provided written informed consent and underwent an interview with a trained research coordinator to assess eligibility and to complete a brief medical history interview, the Montreal Cognitive Assessment (MOCA) and a delirium screen using the Confusion Assessment Method (CAM).5 Outcomes were assessed by blinded research coordinators daily on postoperative days (POD) 1 through 5, or through discharge for patients discharged before POD 5. The primary outcome was a new diagnosis of delirium, as determined by a positive CAM screen at any point during POD 1–5, or between randomization and discharge for patients discharged before POD 5. As our primary goal was to assess study feasibility, rather than to make efficacy comparisons, we did not conduct a formal power calculation; rather, we chose a sample size of 12 patients as a sufficient number to yield information for future study planning. Data were analyzed via intention-to-treat using Fisher’s exact test and the Wilcoxon rank-sum test.
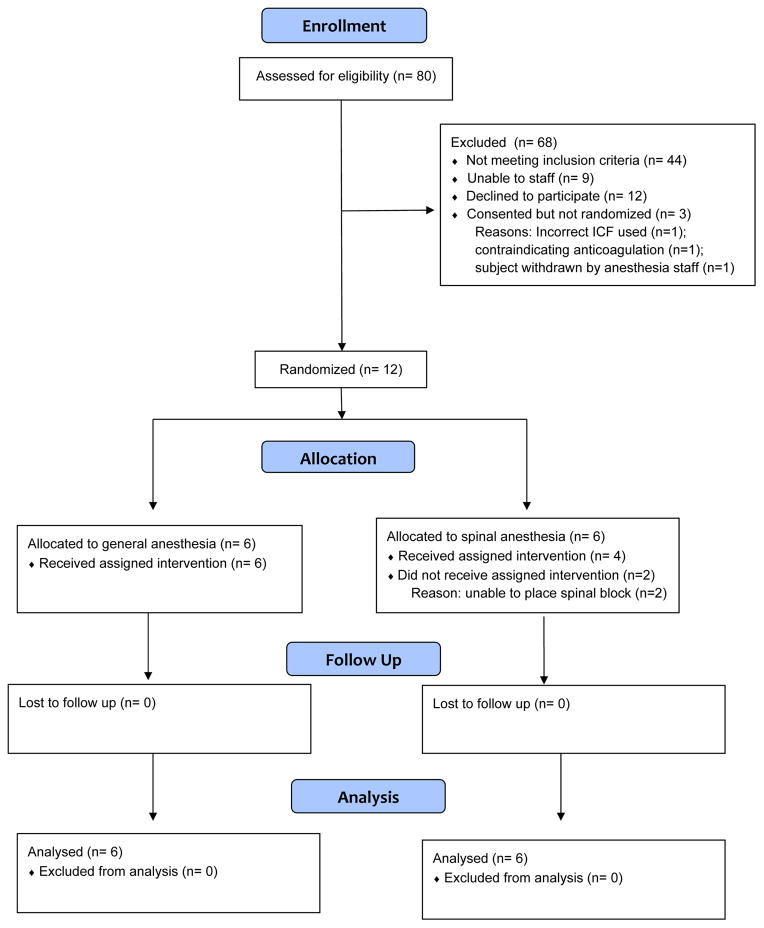
The study CONSORT diagram appears in Figure 1. A total of 80 patients with hip fractures were assessed for eligibility between July 2014 and March 2015; 15 completed informed consent and 12 were randomized. Among 12 randomized patients, 6 were assigned to general anesthesia and 6 were assigned to spinal anesthesia. 5 of 6 (83%) patients in the general anesthesia arm were male, versus 4 of 6 patients in the spinal anesthesia arm (67%, P=1.00). The median age among patients randomized to receive general anesthesia was 62.5 (range, 57 to 88) versus 80.5 years among patients randomized to spinal anesthesia (range, 62 to 92, P = 0.17). 3 of 6 (50%) patients in the general anesthesia arm had one or more major comorbidities versus 2 of 6 (33%) in the spinal anesthesia arm (P =1.00). The general anesthesia arm included 3 (50%) patients with femoral neck fractures and 3 (50%) with pertrochanteric fractures, versus 4 (67%) femoral neck fractures and 2 (33%) pertrochanteric fractures in the spinal anesthesia arm (P=1.00).
Figure 1.
CONSORT diagram
Among patients randomized to receive general anesthesia, 6 of 6 (100%) received general anesthesia; among patients randomized to receive spinal anesthesia, 4 of 6 (67%) received spinal anesthesia; in 2 of 6 patients (33%), the patient received general anesthesia due to inability to place the spinal block. Postoperative delirium occurred in 2 (33%) patients randomized to receive general anesthesia and 0 patients randomized to receive spinal anesthesia (P =0.45). There were no in-hospital deaths; one patient in the general anesthesia arm experienced a non-fatal postoperative myocardial infarction.
This pilot study provides several insights regarding the feasibility of randomized controlled trials to compare outcomes among patients receiving spinal versus general anesthesia for hip fracture surgery. These insights have informed the implementation of the REGAIN trial, a multicenter pragmatic study funded by the Patient Centered Outcomes Research Institute to compare short- and long-term outcomes among 1,600 hip fracture patients randomized to receive spinal versus general anesthesia (ClinicalTrials.gov identifier NCT02507505). First, we successfully randomized 12 out of 36 patients initially assessed as meeting study inclusion criteria (33%), a relatively high rate given the context of an acute traumatic injury in a frail elderly population. Second, as we observed a 33% rate of crossover from spinal to general anesthesia, our pilot study highlights the importance of rigorous patient selection and proper spinal technique for limiting crossover in future studies. Finally, we observed a high degree of acceptance among our clinical staff for the study treatment regimens, further supporting the feasibility of further large-scale randomized comparisons of spinal versus general anesthesia for hip fracture surgery.
Acknowledgments
Funding source: National Institute on Aging (Grant K08AG043548-04 to MDN)
Footnotes
Conflict of Interest: The authors have no conflicts to report.
Sponsor’s Role: The study sponsor had no role in the design, methods, subject recruitment, data collections, analysis or the preparation of the paper.
Author Contributions:
Mark D. Neuman: Obtaining funding; study concept and design; acquisition, analysis, and interpretation of data; writing the first draft of the manuscript; critical revision of the manuscript; final approval of the submitted manuscript.
Samir Mehta: Study concept and design; analysis and interpretation of data; critical revision of manuscript; final approval of the submitted manuscript.
Evan Bannister: Acquisition, analysis and interpretation of data; critical revision of manuscript; final approval of the submitted manuscript.
Patrick Hesketh: Acquisition and analysis of data; critical revision of manuscript; final approval of the submitted manuscript.
Annamarie Horan: Study concept and design; interpretation of data; critical revision of manuscript; final approval of the submitted manuscript.
Nabil Elkassabany: Study concept and design; interpretation of data; critical revision of manuscript; final approval of the submitted manuscript.
References
- 1.Brauer CA, Coca-Perraillon M, Cutler DM, et al. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573–1579. doi: 10.1001/jama.2009.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.K. National Institute for Health and Care Excellence. Managment of hip fracture in adults: patient outcomes following hip fracture. London: U.K. National Institute for Health and Care Excellence; 2012. [Google Scholar]
- 3.White SM, Griffiths R, Moppett I. Type of anaesthesia for hip fracture surgery - the problems of trial design. Anaesthesia. 2012;67:574–578. doi: 10.1111/j.1365-2044.2012.07120.x. [DOI] [PubMed] [Google Scholar]
- 4.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 5.Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]