Abstract
The egg-laying rates of hens approximately 470 days of age exhibited a positive correlation to blood melatonin levels. The hens with an egg-laying rate <30%, 30~90% and ≥90% had blood melatonin levels of 5.8 ± 2.6, 74.0 ± 32.9 and 445.9 ± 115.3 ng/ml, respectively. When 10 mg of melatonin was implanted into the hens at 300, 360, 470 and 550 days of age, the egg-laying rates increased 4.63 ± 0.46%, 8.38 ± 1.45%, 4.93 ± 0.85% and 7.93 ± 0.91%, respectively, compared to that of the controls. Melatonin implantation in hens at 300–470 days of age was observed to enhance egg production and reduce the rate of appearance of sharpei eggs. Melatonin (10 mg) implanted in hens 360 days of age did not influence the blood levels of progesterone (P4) or the gene expression levels of ovarian follicle stimulating hormone receptor (FSHR), luteinizing hormone receptor (LHR), oestradiol receptor alpha (ERα), superoxide dismutase 2 (SOD2) or melatonin receptor 1 (MT1). In contrast, melatonin significantly elevated the serum oestradiol-17β (E2) content, down-regulated the gene expression of gonadotropin-inhibitory hormone receptor (GnIHR), and enhanced the expression of melatonin receptor 2 (MT2). This result indicates that the improved egg-laying rate by melatonin was the result of increased serum oestradiol and decreased ovarian GnIHR. These alterations may be mediated by MT2 activation.
Several studies have reported that melatonin is capable of regulating the reproductive activities of birds. For example, in jungle bush quail (Perdicula asiatica), the activity of their pineal glands exhibited an inversed relationship with their ovarian performance1. Melatonin administration for 30 days completely suppressed the seasonal gonadal growth in male Indian finches (Estrilda amandava)2. For the white leghorn roosters, melatonin treatment suppressed their LH secretion in a dose- and time-related manner3. Melatonin could also up-regulate the expression of gonadotropin-inhibitory hormone in the avian brain and thus suppressed pituitary gonadotropin secretion4.
Many recent studies, however, reported beneficial effects of melatonin on animal reproduction. Melatonin application promoted the maturation and development of oocytes as well as early embryos in mammals including the mouse5, human6, porcine7, bovine8 and sheep9. In addition to mammals, melatonin has also been shown to have beneficial effects on birds. For example, a melatonin supplement could improve the feeding efficiency of chickens and promote their growth10,11. In birds, melatonin also functions as an immunoenhancement agent12. Its supplementation elevated cellular and humoral immune responses in Japanese quail13 and chicken14,15,16. The signalling pathway analysis showed that both cellular and humoral immunoresponses triggered by melatonin were exclusively mediated by its receptor subtype MT2 (Mel 1b)17.
Egg production is a complex process that not only involves the reproductive system but also depends on the availability of specific nutrients and the efficiency of their utilization. For example, melatonin, as a nutrient, may enhance the egg laying productivity of hens. The egg-laying peak is a period when the egg-laying rate is higher than 90% in hens and often appears in hens before the age of 300 days18,19. After that period, their egg-laying productivity declines with age20. Previous melatonin research has focused on the period around the egg-laying peak or earlier in chickens3,11,16,21,22. There is no report related to the effects of melatonin on hens that are past their egg-laying peak. In the current study, the effects of melatonin on the egg production of these hens will be investigated.
Results
Higher blood melatonin levels in hens 470 days of age were associated with more egg production
The results are listed in Fig. 1. It was shown that hens 470 days of age with an egg-laying rate more than 90% had significantly higher melatonin levels than those with an egg-laying rate below 30% (445.9 ± 115.3 vs 5.8 ± 2.6 ng/ml, respectively).
Figure 1. The association of blood melatonin levels and egg-laying rates in hens (440–470 days old).
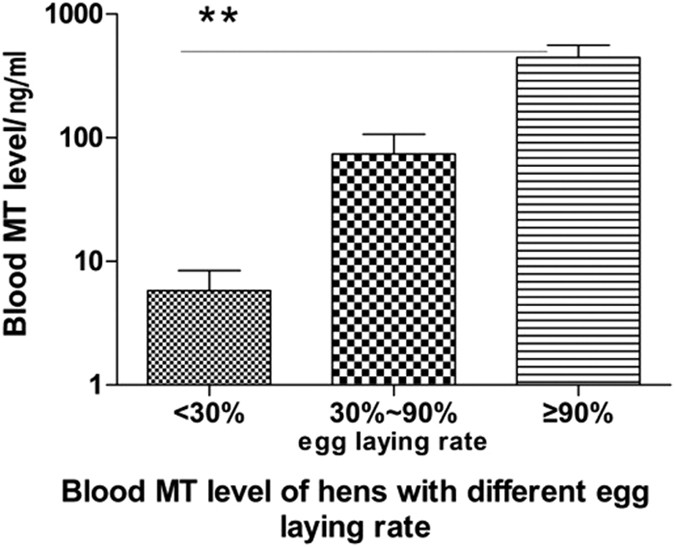
Egg laying numbers were recorded daily for a month before melatonin implantation. The blood was collected from the different groups classified by the egg-laying rates (n = 12). Data were expressed as the means ± SEM. “**” represents extremely significant (p < 0.01).
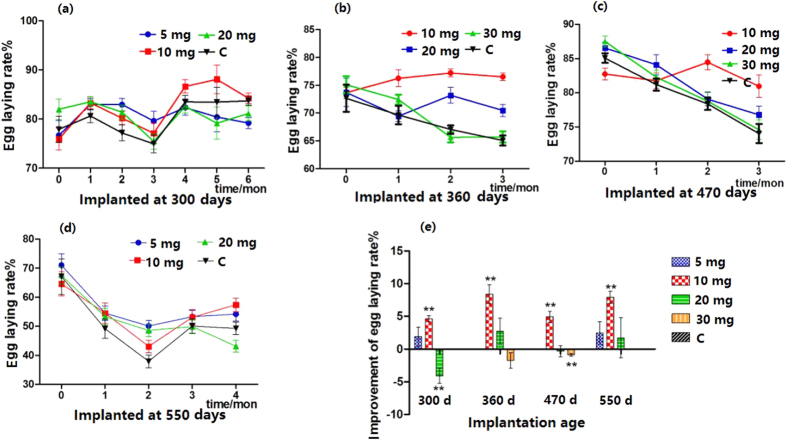
Effect of melatonin implantation on the egg-laying rate
When melatonin (10 mg) was implanted into hens 360 days of age, their egg-laying rates significantly increased compared to those of their untreated counterparts, and this increase lasted for at least 6 months; however, the effects of implantation of 5 mg of melatonin lasted only 3 months (Fig. 2a). Similar effects were observed in the hens 470 and 550 days old (Fig. 2b–d). The results of melatonin (10 mg) treatment were more significant in the hens 550 days old. Throughout the experimental period, the egg-laying rate of old hens with melatonin implantation was significantly higher than those of their untreated counterparts (Fig. 2d). The most effective dose of melatonin implantation was 10 mg/hen, and at this dose, the average egg-laying rate in hens of different ages (from 300–550 days) was uniformly increased at a range of 4.63–8.38% (Fig. 2e). High melatonin levels, for example 20 mg/hen, resulted in a slightly inhibitory effect on the egg-laying rate.
Figure 2. Effect of melatonin implantation on egg-laying rate.
(a–d) Show the egg-laying rate of hens at different ages after melatonin implantation. (e) Shows the summary of the effects of melatonin implantation on the egg-laying rate in hens at different ages. “**” represents extremely significant (p < 0.01).
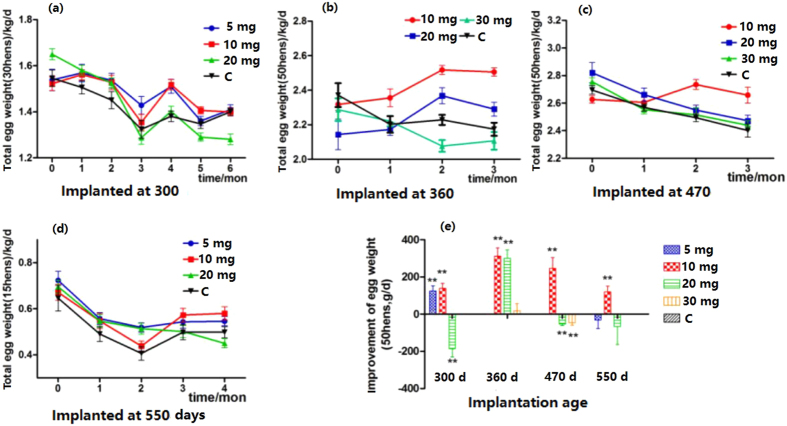
Effects of melatonin implantation on total egg weight
The effects of melatonin on total egg weight were variable depending on the dosages of melatonin used and the age of the hens. The results are listed in Fig. 3. In hens 300 days of age with either 5 or 10 mg of implanted melatonin, the total egg weights were significantly increased compared to the controls after 4 months of treatment (Fig. 3a). In hens 360 days of age, 20 mg of implanted melatonin increased the total egg weight compared to the controls, but 30 mg had the opposite effect (Fig. 3b). The best results were obtained from the hens 470 days of age with 10 mg of implanted melatonin. In this group, the total egg weights were significantly increased on the second month after melatonin application and maintained the highest level among all groups until the end of the study (Fig. 3c). In hens 550 days of age, different doses of implanted melatonin collectively increased their total egg weights at different time points; otherwise, the egg weights were similar to that of the control group (Fig. 3d).
Figure 3. Effect of melatonin implantation on total egg weight.
(a–d) Shows the total weight of eggs laid by hens at different ages after melatonin implantation. (e) Shows the summary of the effects of melatonin implantation on total egg weight (calculated daily). “**” represents significant differences of p < 0.01.
Generally, melatonin implantation at a dosage of 10 mg in the hens 300–470 days old increased their egg weight 3–6 g/hen/day compared to that of their untreated counterparts. This increase lasted at least 3 months and was statistically significant (p < 0.01). However, a high dose of melatonin, for example 20 mg, had the opposite effect (Fig. 3e).
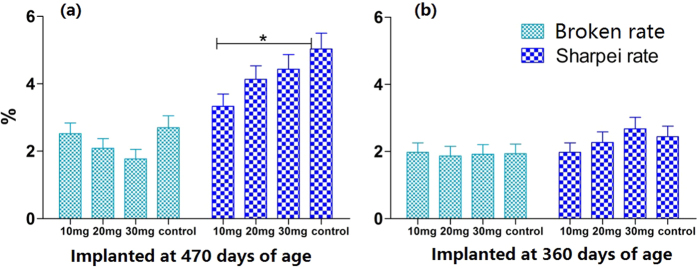
Effects of melatonin implantation on the rates of broken and sharpei eggs
Melatonin implantation had no significant effect on the broken egg rate among the different age groups. The egg sharpei rate in hens 360 days of age implanted with 10 mg of melatonin was slightly lower than that in the control group. However, the egg sharpei rate in hens 470 days of age implanted with 10 mg of melatonin was significantly lower than that of the control (3.3 ± 0.36% vs 5.0 ± 0.47%) (p < 0.05) (Fig. 4a).
Figure 4. Effect of melatonin implantation on egg quality.
(a) Shows the effect of melatonin implanted for 3 months at 470 days of age on egg broken rate and Sharpei rate. (b) Shows the effect of melatonin implanted for 3 months at 360 days of age on egg broken rate and Sharpei rate. Data were expressed as the mean (after 3 months of melatonin treatment) ± SEM. “*” represents significant differences (p < 0.05).
Effects of melatonin implantation on blood E2 and P4 levels
Melatonin implantation at a dose of 10 mg significantly increased the blood level of E2 in hens 360 days of age compared to that in the controls (900.1 ± 34.4 vs 780.0 ± 44.6 pg/ml) (p < 0.05) (Table 1) and had no significant influence on other groups. Melatonin application did not impact the blood P4 levels in any groups.
Table 1. Effect of melatonin implantation on blood E2 and P4 levels.
| Implantation age | Time/month | 10 mg | control | |
|---|---|---|---|---|
| E2 (pg/ml) | 360 d | 0 | — | — |
| 3 | 900.1 ± 34.4a | 780.0 ± 44.6b | ||
| 470 d | 0 | — | — | |
| 3 | 823.3 ± 80.4 | 828.0 ± 83.2 | ||
| P4 (pg/ml) | 360 d | 0 | — | — |
| 3 | 116.3 ± 18.1 | 92.7 ± 10.5 | ||
| 470 d | 0 | 115.3 ± 10.5 | 233.9 ± 80.3 | |
| 3 | 72.1 ± 8.2 | 97.0 ± 15.2 |
In the same line, different small letters mean significance of p < 0.05. Data were expressed as the means ± SEM.
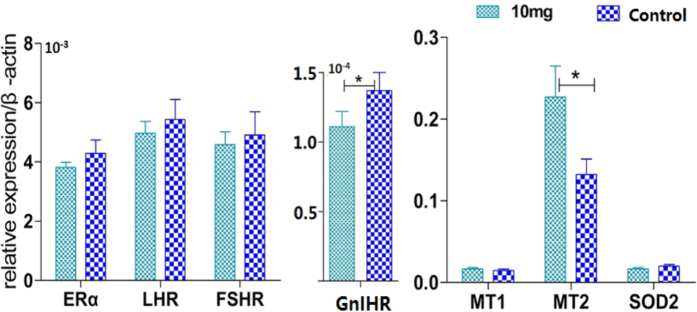
Effect of melatonin implantation on ovarian gene expression
After 3 months of melatonin implantation (10 mg), the expression of MT2 mRNA in small white follicles of hens 360 days old was up-regulated; however, GnIHR expression was down-regulated. Melatonin application had no significant influence on the gene expression levels of MT1, SOD2, ERα, FSHR and LHR (Fig. 5).
Figure 5. Effect of melatonin implantation on ovary gene expression.
The small white follicles were used to analyse the expression of genes that are related to the effects of melatonin on egg laying. The samples were collected 3 months after melatonin implantation in hens 360 days old. Data were expressed as the mean ± SEM. “*” represents significant differences (p < 0.05).
Discussion
There are few reports that are related to the effects of melatonin application on the yield of egg production in hens, especially in hens that are past the duration of their peak egg-laying rate, such as in the hens 470 to 550 days old. In the current study, it was found that in hens of 470 days of age, the egg-laying rate was positively associated with blood melatonin levels. Furthermore, melatonin implantation at a dose of 10 mg significantly improved their egg-laying rate. This phenomenon was also observed in different age groups from 300–550 days old when the suitable melatonin dose (10 mg) was used. A relatively high dose of implanted melatonin (30 mg) had a negative effect on the egg-laying rate. In addition to the increase in the egg-laying rate, melatonin (10 mg) implantation also improved the quality of the eggs, as indicated by the reduced sharpei egg rate.
Many studies have shown that melatonin plays an important role in animal reproduction. Melatonin could enhance the maturation of oocytes and the development of follicles in mammals23,24,25,26,27 and fish28,29. Despite the great differences between birds and mammals, melatonin may play a similar role in the maturation of oocytes and the development of follicles in birds as it does in mammals. Melatonin and its receptors were found to be present in the ovary. MT1 and MT2 were expressed in both the thecal and granulosa layers30. It was reported that MT1 and MT3 were negatively correlated with the total number of eggs yielded in hens 300 days of age that were exposed to different monochromatic light31. We observed that melatonin implantation did not influence the expression of ovarian MT1 but up-regulated the expression of MT2. Interestingly, similar to MT2, GnIHR was also found to be expressed in both the thecal and granulosa layers32. It has been reported that E2 secretion and GnIHR expression regulate egg production and quality33,34,35,36. GnIHR is mainly expressed in the pituitary. It participates in the negative control of luteinizing hormone (LH) and follicle stimulating hormone (FSH)21. GnIHR was also found in the chicken ovary and is expressed in every stage of the follicle. Expression of GnIHR declines throughout sex maturation32. In the current study, melatonin was found to down-regulate expression of GnIHR in the ovary. This result indicated that melatonin-promoted ovulation in hens might be through the GnIHR-LH-FSH pathway.
Even though a positive correlation between GnIHR and MT2 expression in the testes of European starlings has been reported22, the concrete mechanism for this correlation is still not clear. Furthermore, their relationship in the ovary is virtually unknown. Granulosa cells are essential for oocyte maturation and ovarian ovulation. Glucose metabolism in mouse cumulus cells could prevent oocyte ageing37. Coculture with cumulus-derived cells in vitro promotes porcine and sheep oocyte maturation38. Melatonin and its receptor MT1 have been reported to be involved in the downstream reaction to luteinizing hormone and to participate in the regulation of luteinization in the mouse39. In the current study, it seems that the down-regulation of GnIHR expression by melatonin might be mediated by MT2 in the granulosa layers and then promote ovulation.
Reactive oxygen species (ROS) caused by ageing and environmental stress will significantly affect an animal’s reproduction, suppressing the reproductive potential. The ROS caused by reproductive ageing is associated with changes in oocyte mitochondrial dynamics and function and mtDNA quantity and prevents oocyte maturation40. It has been reported that heat shock results in an inhibitory effect on porcine oocyte maturation in vitro by improving the ROS in the oocytes41. Maternal-restraint stress increases oocyte aneuploidy by impairing metaphase I spindle assembly and reducing spindle assembly checkpoint proteins in mice42. To combat the negative effects of the excessive ROS and promote the maturation of oocytes and ovulation, antioxidants are frequently used in the in vitro culture system43,44. Melatonin is a naturally occurring potent free radical scavenger and a broad-spectrum antioxidant45,46. As a result, the use of melatonin to prevent oxidative stress in cell culture or animal studies has been extensively reported43,44. It was reported that under stressful heat conditions, melatonin application improved the feeding efficiency of Japanese quail and reduced their oxidative stress47. Moreover, melatonin could enhance immunity in chickens against disease14,48,49. As a result, these activities of melatonin may also help the hens preserve their egg-laying rate after their egg-laying peak age.
A few studies have investigated the effects of melatonin on the quality of eggs. Some researchers have observed that melatonin supplementation reallocated the calcium distribution between bone and eggshell in laying hens, suggesting that melatonin could strengthen the bone and weaken the eggshell50. Other reports have shown that melatonin application increased the eggshell weight and thickness51. These findings are consistent with our observations that melatonin implantation significantly reduced the sharpei egg rate.
Conclusion
Physiologically, once hens are older than 300 days of age, the hens have passed their egg-laying peak, and the egg-laying rate significantly declines with age20. Prolonging the egg-laying peak in chicken is task that remains to be accomplished. In the current study, we observed that implantation of the appropriate dose of melatonin (10 mg/hen) in laying hens up to 550 days of age significantly increased their egg-laying rate and egg quality. The results indicated that melatonin application may prolong the physiological egg-laying peak. Considering the low cost of melatonin, there may be an application for it in the poultry industry. The mechanistic studies revealed that melatonin implantation up-regulated the gene expression of MT2 and down-regulated GnIHR in the granulosa cells of small white follicles. This finding indicated that melatonin may promote ovulation in hens through the GnIHR-LH-FSH pathway mediated by MT2 activation.
Materials and Methods
Chemicals
The melatonin implants were made by the Specialty Institute, Chinese Academy of Agricultural Sciences.
Animals
We selected 120 hens (Beijing Red No. 1) 300 days old, divided them into four groups, and treated them with either 0, 5, 10 or 20 mg of melatonin implants (C, 5 mg, 10 mg, and 20 mg groups, respectively). We randomly divided 240 hens (120 hens 360 days of age and 120 hens 470 days of age) into four groups at each age and treated them with 0, 10, 20 and 30 mg of melatonin (C, 10 mg, 20 mg, and 30 mg groups, respectively). We divided 60 hens 550 days old into 4 groups, and their treatment was the same as the hens in the 300-day-old groups. Hens of different ages came from different hatches.
Melatonin implants were implanted under the neck skin of the birds. Birds were reared under photostimulatory conditions (16 L:8D) with a corn-soybean meal diet of 110 g/hen/day. One cage (40 × 37 × 35 cm, length × width × height) contained 3 birds. The egg numbers and total egg weight of each group were monitored daily from at least 2 weeks before the treatment started until the termination of the study. All experimental procedures were approved by the animal care committee of the China Agricultural University, and all experiments were carried out in accordance with the relevant guidelines.
Melatonin assay using high-performance liquid chromatography (HPLC)
To evaluate the melatonin level, blood samples (2 ml for each hen) were drawn from the wing sinus of the chicken. The sample preparation and detection were performed as described by Zhao et al.52.
Analysis of progesterone (P4) and oestradiol-17β (E2) levels by radioimmunoassay
Blood samples (2 ml for each hen, n = 20) were drawn from the wing sinus of the chicken 3 months after melatonin implantation. Progesterone (P4) and oestradiol-17β (E2) were detected using radioimmunoassay using the method described by He et al.53.
Gene expression assay using reverse-transcriptional PCR (real-time PCR)
The ovaries of hens 360 days of age (n = 10) were collected after 3 months of melatonin implantation and were immediately frozen in liquid nitrogen for future use. The total RNA extraction, reverse transcription PCR and quantitative real-time PCR were performed as described previously54. A housekeeping gene (β-actin) was used as the normalization control. The relative mRNA expression was calculated using the 2−△△ct method. Primer sequences for the real-time PCR are listed in Table 2.
Table 2. Primers used in this study.
| Primer | GenBank ID | Production length bp | Sequence |
|---|---|---|---|
| β-actin-F | L08165 | 154 | GAGAAATTGTGCGTGACATCAAGG |
| β-actin-R | CACCTGAACCTCTCATTGCCA | ||
| LHR-F | u31987 | 193 | CTCAGGCGGATACACAACGA |
| LHR-R | TCAGAACGGCTTCCAGCAGG | ||
| FSHR-F | NM_205079.1 | 191 | TACCCGTCGTCCATAAGGTGC |
| FSHR-R | GCTCATCCAGGCAGGTTCCATT | ||
| ERα-F | NM—205183.2 | 157 | TATTGATGATCGGCTTAGTCTGGCG |
| ERα-R | CGAGCAGCAGTAGCCAGTAGCA | ||
| GnIHR-F | AB193127 | 139 | CACTGATGCTGCTGACAGACTAC |
| GnIHR-R | CTCATTGAAGTAGCCGTAGATGATGG | ||
| MT1-F | NM_001097538.1 | 127 | CAGGACTGCCCTTGTGCC |
| MT1-R | CACACTTGGCACATCCTGC | ||
| MT2-F | NM_205275.1 | 99 | AACCGACCCGAACTGAACCA |
| MT2-R | CAGCGGCAGTTCTTGCACTT | ||
| SOD2-F | NM_204211.1 | 157 | CGCAAGGCAGAAGCACACTC |
| SOD2-R | CAGCGCCTCTTTGTATTTCTCC |
Statistical analyses
The data were expressed as the means ± SEM. The statistical significance was analysed using an ANOVA followed by the Dunnett’s t-test. The significance level was set at p < 0.05.
Additional Information
How to cite this article: Jia, Y. et al. Melatonin implantation improved the egg-laying rate and quality in hens past their peak egg-laying age. Sci. Rep. 6, 39799; doi: 10.1038/srep39799 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (31372306) and the poultry industry technology system innovation team of Beijing.
Footnotes
Author Contributions Yaxiong Jia, Kuanfeng Zhu and Minghui Yang designed and participated in the entire experiment and wrote the manuscript. Minghui Yang and Liang Wang participated in the data collection and analyses. Yukun Song assisted with the sample collection. Jing Wang performed the melatonin analysis. Wenxiang Qin, Zhiyuan Xu and Yu Chen all participated in the different stages of the study. All experiments were directed by Guoshi Liu. All authors reviewed the manuscript.
References
- Dubey S. & Haldar C. Environmental factors and annual harderian–pineal–gonadal interrelationship in Indian jungle bush quail, Perdicula asiatica. Gen. Comp. Endocr. 1, 17–22 (1997). [DOI] [PubMed] [Google Scholar]
- Gupta B., Haldar-Misra C., Ghosh M. & Thapliyal J. P. Effect of melatonin on gonads, body weight, and luteinizing hormone (LH) dependent coloration of the Indian finch, Lal munia (Estrilda amandava). Gen. Comp. Endocr. 3, 451–456 (1987). [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Aharony T. & Yahav S. The effect of melatonin administration on circulating plasma luteinizing hormone concentration in castrated White Leghorn roosters. Poultry Sci. 9, 1354–1359 (2002). [DOI] [PubMed] [Google Scholar]
- Ubuka T., Bentley G. E., Ukena K., Wingfield J. C. & Tsutsui K. Melatonin induces the expression of gonadotropin-inhibitory hormone in the avian brain. P. Natl Acad. Sci. USA. 8, 3052–3057 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganji R., Nabiuni M & Faraji R. Development of mouse preantral follicle after in vitro culture in a medium containing melatonin. Cell Journal (Yakhteh). 4, 546 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeri C., Sbracia G., Selman H., Antonini G. & Pacchiarotti A. Beneficial effects of melatonin on oocytes and embryo quality in aged IVF patients ed: OXFORD UNIV PRESS GREAT CLARENDON ST, OXFORD OX2 6DP, ENGLAND, 48–49 (2015). [Google Scholar]
- Do L. et al. Melatonin supplementation during in vitro maturation and development supports the development of porcine embryos. Reprod. Domest. Anim. 6, 1054–1058 (2015). [DOI] [PubMed] [Google Scholar]
- Zhao X. et al. Melatonin enhances the in vitro maturation and developmental potential of bovine oocytes denuded of the cumulus oophorus. Zygote. 4, 525–536 (2015). [DOI] [PubMed] [Google Scholar]
- Casao A. et al. Melatonin secretion in sheep cumulus cells and its possible effect on DNA damage during in vitro maturation. ed: Asociación Interprofesional para el Desarrollo Agrario, 368–370 (2013). [Google Scholar]
- Clark W. D. & Classen H. L. The effects of continuously or diurnally fed melatonin on broiler performance and health. Poultry Sci. 11, 1900–1904 (1995). [DOI] [PubMed] [Google Scholar]
- Phetteplace H. W. & Nockels C. F. Melatonin increases cockerel growth ed: FEDERATION AMER SOC EXP BIOL 9650 ROCKVILLE PIKE, BETHESDA, MD 20814-3998 USA, 761 (1985). [Google Scholar]
- Poon A. et al. Evidence for a direct action of melatonin on the immune system. Neurosignals. 2, 107–117 (1994). [DOI] [PubMed] [Google Scholar]
- Moore C. B. & Siopes T. D. Effects of lighting conditions and melatonin supplementation on the cellular and humoral immune responses in Japanese quail Coturnix coturnix japonica. Gen. Comp. Endocr. 1, 95–104 (2000). [DOI] [PubMed] [Google Scholar]
- Brennan C. P., Hendricks G. L., El-Sheikh T. M. & Mashaly M. M. Melatonin and the enhancement of immune responses in immature male chickens. Poultry Sci. 3, 371–375 (2002). [DOI] [PubMed] [Google Scholar]
- Kliger C. A. et al. Effects of photoperiod and melatonin on lymphocyte activities in male broiler chickens. Poultry Sci. 1, 18–25 (2000). [DOI] [PubMed] [Google Scholar]
- Moore C. B. & Siopes T. D. Effect of melatonin supplementation on the ontogeny of immunity in the Large White turkey poult. Poultry Sci. 12, 1898–1903 (2002). [DOI] [PubMed] [Google Scholar]
- Drazen D. L. & Nelson R. J. Melatonin receptor subtype MT2 (Mel 1b) and not mt1 (Mel 1a) is associated with melatonin-induced enhancement of cell-mediated and humoral immunity. Neuroendocrinology. 3, 178–184 (2001). [DOI] [PubMed] [Google Scholar]
- Yaoxing C. & Zixu W. Effect and mechanism of monochromatic light on the peak period of Laying Hens. Journal of China Agricultural University (2007). [Google Scholar]
- Ailian G. et al. Comparison of layer’s health and welfare under different cage conditions. Journal-China Agricultural University 5, 67 (2007). [Google Scholar]
- Lukanov H., Petrov P., Genchev A., Halil E. & Ismail N. Productive performance of easter egger crosses of araucana and schijndelaar roosters with white leghorn hens. Trakia Journal of Sciences. 1, 73 (2016). [Google Scholar]
- Maddineni S. et al. Gonadotrophin-inhibitory hormone receptor expression in the chicken pituitary gland: Potential influence of sexual maturation and ovarian steroids. J. Neuroendocrinol. 9, 1078–1088 (2008). [DOI] [PubMed] [Google Scholar]
- McGuire N. L., Kangas K. & Bentley G. E. Effects of melatonin on peripheral reproductive function: Regulation of testicular GnIH and testosterone. Endocrinology. 9, 3461–3470 (2011). [DOI] [PubMed] [Google Scholar]
- Maganhin C. C. et al. Effects of melatonin on ovarian follicles. Eur. J. Obstet. Gyn. R. B. 2, 178–184 (2013). [DOI] [PubMed] [Google Scholar]
- Do L. et al. Melatonin supplementation during in vitro maturation and development supports the development of porcine embryos. Reprod. Domest. Anim. 6, 1054–1058 (2015). [DOI] [PubMed] [Google Scholar]
- Shi J. M. et al. Melatonin exists in porcine follicular fluid and improves in vitro maturation and parthenogenetic development of porcine oocytes. J. Pineal Res. 4, 318–323 (2009). [DOI] [PubMed] [Google Scholar]
- Wei D. et al. Supplementation with low concentrations of melatonin improves nuclear maturation of human oocytes in vitro. J. Assist. Reprod. Gen. 7, 933–938 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y. M. et al. Melatonin modulates the functions of porcine granulosa cells via its membrane receptor MT2 in vitro. Anim. Reprod. Sci. 17, 164–172 (2016). [DOI] [PubMed] [Google Scholar]
- Moniruzzaman M., Hasan K. N. & Maitra S. K. Melatonin actions on ovaprim (synthetic GnRH and domperidone)-induced oocyte maturation in carp. Reproduction. 4, 285–296 (2016). [DOI] [PubMed] [Google Scholar]
- Maitra S. K., Chattoraj A., Mukherjee L. & Moniruzzaman M. Melatonin: A potent candidate in the regulation of fish oocyte growth and maturation. Gen. Comp. Endocr. 215–222 (2013). [DOI] [PubMed] [Google Scholar]
- Sundaresan N. R. et al. Expression analysis of melatonin receptor subtypes in the ovary of domestic chicken. Vet. Res. Commun. 1, 49–56 (2009). [DOI] [PubMed] [Google Scholar]
- Li D. Y. et al. Expression patterns of melatonin receptors in chicken ovarian follicles affected by monochromatic light. Genet. Mol. Res. 3, 10072–10080 (2015). [DOI] [PubMed] [Google Scholar]
- Maddineni S. et al. Gonadotropin inhibitory hormone (GnIH) receptor gene is expressed in the chicken ovary: Potential role of GnIH in follicular maturation. Reproduction. 2, 267–274 (2008). [DOI] [PubMed] [Google Scholar]
- Lebedeva I. Y., Lebedev V. A., Grossmann R. & Parvizi N. Age-dependent role of steroids in the regulation of growth of the hen follicular wall. Reprod. Biol. Endocrin. 1, 1 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistedt A., Ridderstråle Y., Wall H. & Holm L. Exogenous estradiol improves shell strength in laying hens at the end of the laying period. Acta Vet. Scand. 1, 1 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson A., Mura E., Brunström B., Panzica G. & Halldin K. Selective activation of estrogen receptor alpha in Japanese quail embryos affects reproductive organ differentiation but not the male sexual behavior or the parvocellular vasotocin system. Gen. Comp. Endocr. 2, 150–157 (2008). [DOI] [PubMed] [Google Scholar]
- Hansen K. K., Beck M. M., Scheideler S. E. & Blankenship E. E. Exogenous estrogen boosts circulating estradiol concentrations and calcium uptake by duodenal tissue in heat-stressed hens. Poultry Sci. 6, 895–900 (2004). [DOI] [PubMed] [Google Scholar]
- Li Q. et al. Glucose metabolism in mouse cumulus cells prevents oocyte aging by maintaining both energy supply and the intracellular redox potential. Biol. Reprod. 6, 1111–1118 (2011). [DOI] [PubMed] [Google Scholar]
- Yoon J. D. et al. Effects of coculture with cumulus-derived somatic cells on in vitro maturation of porcine oocytes. Theriogenology. 2, 294–305 (2015). [DOI] [PubMed] [Google Scholar]
- He C. J. et al. Melatonin and its receptor MT1 are involved in the downstream reaction to luteinizing hormone and participate in the regulation of luteinization in different species. J. Pineal Res. 3, 279–290 (2016). [DOI] [PubMed] [Google Scholar]
- Babayev E. et al. Reproductive aging is associated with changes in oocyte mitochondrial dynamics, function, and mtDNA quantity. Maturitas. 14, 121–130 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. et al. Melatonin protects porcine oocyte in vitro maturation from heat stress. J. Pineal Res. 3, 365–375 (2015). [DOI] [PubMed] [Google Scholar]
- Zhou P. et al. Maternal-restraint stress increases oocyte aneuploidy by impairing metaphase I spindle assembly and reducing spindle assembly checkpoint proteins in mice. Biol. Reprod. 3, 83 (2012). [DOI] [PubMed] [Google Scholar]
- Bormann C. L., Ongeri E. M. & Krisher R. L. The effect of vitamins during maturation of caprine oocytes on subsequent developmental potential in vitro. Theriogenology. 5, 1373–1380 (2003). [DOI] [PubMed] [Google Scholar]
- Wang F. et al. Beneficial effect of resveratrol on bovine oocyte maturation and subsequent embryonic development after in vitro fertilization. Fertil. Steril. 2, 577–586 (2014). [DOI] [PubMed] [Google Scholar]
- Manchester L. C. et al. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 4, 403–419 (2015). [DOI] [PubMed] [Google Scholar]
- Reiter R. J., Tan D. X. & Maldonado M. D. Melatonin as an antioxidant: Physiology versus pharmacology. J. Pineal Res. 2, 215–216 (2005). [DOI] [PubMed] [Google Scholar]
- Sahin K., Onderci M., Gursu M. F., Kucuk S. M. & Sahin G. L. Effect of melatonin supplementation on biomarkers of oxidative stress and serum vitamin and mineral concentrations in heat-stressed Japanese quail. The Journal of Applied Poultry Research. 2, 342–348 (2004). [Google Scholar]
- Markowska M., Mrozkowiak A. & Skwarlo-Sonta K. Influence of melatonin on chicken lymphocytes in vitro: Involvement of membrane receptors. Neuro endocrinology letters. 6, 67–72 (2002). [PubMed] [Google Scholar]
- Liu S. et al. Effect of melatonin on changes of lymphocytes and their subtypes of chickens, ducks and quails in different light [J]. Veterinary Science in China. 15 (2006).
- Taylor A. C., Horvat-Gordon M., Moore A. & Bartell P. A. The effects of melatonin on the physical properties of bones and egg shells in the laying hen. PloS one. 2, e55663 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A. et al. Studies on production performance of layers supplemented with dietary melatonin. Indian Journal of Poultry Science. 3, 345–347 (2012). [Google Scholar]
- Zhao Y. et al. Melatonin and its potential biological functions in the fruits of sweet cherry. J. Pineal Res. 1, 79–88 (2013). [DOI] [PubMed] [Google Scholar]
- He C. et al. Melatonin-related genes expressed in the mouse uterus during early gestation promote embryo implantation. J. Pineal Res. 3, 300–309 (2015). [DOI] [PubMed] [Google Scholar]
- Li Y. et al. Melatonin protects porcine oocyte in vitro maturation from heat stress. J. Pineal Res. 3, 365–375 (2015). [DOI] [PubMed] [Google Scholar]